Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Serum RIPK1, Acute Lung Injury, and Outcomes in Severe Traumatic Brain Injury: A Multicenter Prospective Study
Authors Cai L, Dou X, Dong W, Zou K, Zhang L, Hong H, Zhang X, Liu J, Tian D, Wu X, Zhang J
Received 24 October 2024
Accepted for publication 6 March 2025
Published 20 March 2025 Volume 2025:21 Pages 385—405
DOI https://doi.org/10.2147/TCRM.S502775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor De Yun Wang
Liang Cai,1,* Xianghong Dou,2,* Wensheng Dong,1 Kangqin Zou,1 Lixin Zhang,3 Huayong Hong,3 Xiaole Zhang,3 Jin Liu,4 Da Tian,4 Xiaoyu Wu,4 Jianhua Zhang1
1Department of Neurosurgery, The second People’s Hospital of Lianyungang Affiliated to Kangda College of Nanjing Medical University, Lianyungang, Jiangsu, 222000, People’s Republic of China; 2Department of Neurology, Donghai County People’s Hospital, Lianyungang, Jiangsu, 222300, People’s Republic of China; 3Department of Neurosurgery, The Hangzhou Ninth People’s Hospital, Hangzhou, Zhejiang, 311225, People’s Republic of China; 4Department of Neurosurgery, The Lishui People’s Hospital, Lishui, Zhejiang, 323000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianhua Zhang, Email [email protected]
Background: Receptor-interacting protein kinase-1 (RIPK1), a regulator of necroptosis, is involved in acute brain injury and acute lung injury (ALI). Here, serum RIPK1 levels were measured after severe traumatic brain injury (sTBI), with an endeavor to unveil its prognostic implications and mediation effects of ALI.
Methods: In this multicenter prospective study, serum RIPK1 levels were gauged in 100 healthy individuals and 158 sTBI patients in need of decompressive craniectomy for brain herniation. The collected materials encompassed the Glasgow Coma Scale (GCS), pupil enlargement status, basal cisternal shapes, ALI, etc. The extended Glasgow outcome scale (GOSE) was employed for estimating neurological impairments at posttraumatic 180-day mark. Multifactorial analytical methods were applied to assess relevancies.
Results: Patients, as opposed to controls, had markedly raised serum RIPK1 levels, with the even substantially higher levels in those with lower GCS scores, bilateral pupil enlargement or obliterated basal cisterns. Using restricted cubic spline, RIPK1 levels were linearly related to occurrent risks of the four outcome variables of interest, that is 180-day death, overall survival, poor prognosis (GOSE scores 1– 4) and ALI. RIPK1 levels independently predicted these outcome variables. RIPK1 levels had noninteractional effects with age, sex, hypertension, diabetes, smoking and alcohol habits in terms of its association with these outcome variables. RIPK1 levels exhibited high discriminatory efficiency for these outcome variables under the receiver operating characteristic curve. RIPK1 levels, via partial mediation by ALI, were associated with death and poor prognosis of patients.
Conclusion: Elevated serum RIPK1 levels of patients with sTBI may be highly related to trauma severity, and risks of poor outcomes and ALI; and ALI partially explains the links between serum RIPK1 levels, death and poor prognosis, substantializing serum RIPK1 as a serological prognostic predictor of good prospect in sTBI.
Keywords: traumatic brain injury, RIPK1, outcomes, biomarkers, mediations
Introduction
Severe traumatic brain injury (sTBI) is undisputedly acknowledged as one of the most fatal traumatic forms.1 Trauma severity is conventionally assessed via Glasgow coma scale (GCS) in clinical application.2 Pupil enlargements or abnormal cisterns are indicative of possibility of brain hernia, and in this setting, patients may be at risk of high mortality and disability rate.3 Brain trauma is capable of provoking an array of cascading molecular events, including inflammatory disequilibrium, mitochondrial functional disbalances, oxidative disorders, neuronal demise, brain edema formation and blood–brain barrier damage.4 In addition to local brain injuries, remote damages and even systemic injuries, such as systemic inflammatory response syndrome, acute cardiac injury and acute lung injury (ALI), are the common adverse affairs following sTBI, which are powerfully pertinent to adverse outcomes of patients.5–9 Because blood samples are characterized by the easy obtainability and high clinical applicability, blood markers have been preferable for recent clinical exploratory researches with respect to their prognostic significances in sTBI.10–12
Receptor-interacting protein kinase-1 (RIPK1), a sort of Ser/Thr kinase, is affirmably implicated in numerous pathophysiological processes, including inflammation, apoptosis, necrosis and necroptosis.13–15 RIPK1 can be localized in almost all main cell types in the central nervous system, including glial cells and neurons.16 The noteworthy fact is that RIPK1, which was promptly elevated after acute brain injury, may pose a major threat to neurological function via increasing neuroinflammation and damaging neurons in experimental traumatic, ischemic and hemorrhagic brain diseases.17–23 Also, RIPK1 can be prominently observable in alveolar cells.24 At state of ALI, its expressions by lung cells were pronouncedly promoted, and its inhibition could dramatically attenuate pulmonary injury through repressing inflammatory response and lessening cell demise.25 Thus, it is inferred that blood RIPK1 may be linked to acute brain injury and ALI. The current study is deployed to unravel serum RIPK1 as a predictor of death, neurological outcome and ALI in a cohort of patients with sTBI, as well as to further unveil mediation function of ALI on association of serum RIPK1 with poor prognosis.
Materials and Methods
Study Design, Settings, Participant Enlistments and Ethical Consents
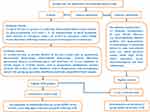
During the period of between March 2020 and April 2023, a multicenter, observational, analytic study was implemented in the following three comprehensive hospitals, ie, (1) the Second People’s Hospital of Lianyungang, (2) the Hangzhou Ninth People’s Hospital and (3) the Lishui People’s Hospital. In essence, this study consisted of two sub-studies. One was a cross-sectional study, in which enrollments of sTBI patients, along with controls, were achieved and post-sTBI alteration of serum RIPK1 levels was disclosed; and the other was a prospective cohort study, in which we completed an assessment of serum RIPK1 as a potential predictor of death, poor neurological functional outcome and ALI in sTBI. The details regarding subject recruitments and study processes were delineated in Figure 1. This study was fulfilled in compliance with the ethical tenets set forth in the Declaration of Helsinki and the institutional ethical terms. The approval to current study protocol was granted by the Institutional Review Committees of the aforementioned three hospitals (No. 2022k045, 2021–163, 2020–001). Controls and patients’ lawful representatives were notified of study contents and then signed their respective informed consent forms.
Data Gathering, Clinical Evaluation and Outcome Metrics
Once patients were transferred to emergency center, some basic information, including age, gender, tobacco-smoking habit, hobby of alcohol-drinking, hypertension, diabetes, time to hospital admission since trauma and traumatic causes, was asked. Traumatic causes were of two types: traffic accidents and others. Pupils were observed for judging pupillary status as bilateral or unilateral enlargement. Non-invasive blood pressure measurements were done and the readings were subsequently registered. All head computed tomography scans were promptly completed, and the radiologically positive appearances were categorized into two classifications, namely, mass lesions and non-mass lesion bleedings. The former included intracerebral, epidural and subdural hematomas, and the latter contained intraventricular hemorrhage and subarachnoid hemorrhage. Under presence of multiple intracranial hematomas, they were classified in accordance with the predominant hematoma.26 According to the previous criteria, basal cistern statuses were divided into obliterated and compressed, and midline shift extents were categorized into <5 mm, 5–10 mm and >10 mm.27 Post-resuscitation GCS was applied to assess consciousness situations. Careful observation for in-hospital seizure ictus and ALI were implemented. In accordance with the international consensus criteria, patients were considered as having ALI, when they presented with acute emergences of the phenomena as the ratio of partial pressure of arterial oxygen to fractional inspired oxygen equal to or below 300 with demonstration of bilateral infiltrates via chest radiograph and exclusion of left arterial hypertension.28 Brain herniation in the current study specifically refers to uncal herniation, which was manifested as coma, pupillary enlargement, and signs of brain stem compression on computerized tomography scans.29 As neurosurgeons preferred, decompressive craniectomy was performed using the conventional standardized procedures30 or the modified methods31 after evacuating possible hematoma. In application of structured interviews at a base of Extended Glasgow outcome scale (GOSE), neurological function of patients was assessed at 180-day mark following trauma via telephone visits, and patients with the scores of 1–4 were recognized as displaying a poor prognosis.32
Blood Obtainments, Sample Processing and Immune Analysis
Peripheral venous blood of patients was drawn via their respective median cubital vein within fifteen minutes after their arrivals at emergency center, while those of controls were garnered using the same methods as patients once their recruitments into this study. All blood specimens were promptly placed in 5 mL gel-containing biochemistry tubes. Once blood clot was observable, centrifugation was commenced. A portion of supernatant was extracted and afterwards put into Eppendorf tubes for preservation at – 80°C condition until subsequent detection of serum RIPK1 levels. For the sake of avoidance of RIPK1 protein from decomposing, a batch of blood samples, which were gained within recent three months, were taken out from freezer and, after melting, every sample was twice detected for quantifying serum RIPK1 levels by employing the sandwich enzyme-linked immunosorbent assay kit (Item number, YB73405Hu; Shanghai Yubo Biotechnology Co. Ltd, China). As for this kit, its lowest detection concentration was 0.094 ng/mL, detection range varied from 0.156 to 10 ng/mL, and both its inter-assay and intra-assay precision coefficients of variation were below 10%. All measurements were done of serum RIPK1 levels by the professional technician, who was not permitted to touch clinical information. Statistical assessments were ultimately conducted by using mean values of the dual measurements.
Statistical Analysis
The SPSS statistical package version 20.0 (SPSS Inc., Chicago, Illinois, USA) was run for routinely handling data. Data are presented as two types, respectively, as qualitative and quantitative variables. The formers in form of frequencies (proportions) were compared for intergroup distinctions by applying the Pearson’s Chi-square test or Fisher’s exact test as deemed applicable. The latters, which were displayed as means (standard deviations, SDs) if normally distributed by the Kolmogorov–Smirnov test or Shapiro–Wilk test as appropriate and as medians (percentiles 25th-75th) if non-normally distributed, were in comparison for their disparities between two groups or among several groups by employing the independent t-test, Mann–Whitney U-test or Kruskal–Wallis H-test as considered appropriate. The selected outcome variables of interest were 180-day death, overall survival and poor prognosis (GOSE scores of 1–4), alongside with ALI. The statistical processes were that univariate analysis was scheduled to figure out significantly different variables, these significantly distinct variables were next consolidated into multivariable models, including binary logistic regression model and multivariable Cox proportional hazard model, and then multivariable assessments were finalized for yielding independent predictors. By applying the Log rank test, differences in terms of 180-day survival time were investigated among the four subgroups divided according to lower and upper quartiles and median value of serum RIPK1 levels. By aidance of the R software (version 3.5.1; https://www.r-project.org), the two auxiliary statistical analyses, ie, the restricted cubic spline (RCS) assessment and subgroup analysis, were achieved for discerning linearities and interactivity, as well as the results were represented by plotting relevant figures. Receiver operating characteristic (ROC) curve analysis was operated, and sample size estimation was done in the context of the MedCalc statistical software version 17.4 (MedCalc Software, Mariakerke, Belgium). The discriminatory efficiencies were shown as area under curve (AUC) with 95% confidence interval (CI). Considering various statistical methods, such as ROC curve analysis, multivariate analysis, two-group comparison, bivariate correlation analysis and more, were applied here, 100 healthy individuals and 158 sTBI patients were sufficient for statistical analysis because the calculated minimum sample size was 89. The GraphPad Prism 9.0 (GraphPad Software, Inc., Boston, MA, USA) was used to paint several common graphs, such as ROC curves, violin plots, bar charts and correlograms. In order to ascertain whether ALI in part may mediate associations of serum RIPK1 level with death and poor prognosis, mediation analysis was performed with result exhibition in form of drawing. The two-sided P < 0.05 was recognized as having statistical disparity.
Results
Participant Selections and Individual Characteristics
In this observational analytic epidemiological survey, there was a final enrollment of an accumulative of 100 healthy controls and 158 sTBI patients who underwent decompressive craniectomy owing to brain herniation. By statistical assessment, a revelation of negligible distinctions was discovered in terms of age, ratio of male to female, and tobacco smoking and alcohol drinking habits between controls and patients (all P>0.05). In terms of demographics of patients, age spanned from 19 to 78 years (lower-upper quartiles, 38–54 years; median, 43 years) and there were ninety-two males and sixty-six females, therefore the ratio of male to female being 1.39. Adverse life habits included cigarette smoking (52 cases, 32.9%) and alcohol consumption (44 cases, 27.9%). An aggregate of 34 patients (21.5%) and 21 patients (13.3%) were hypertensive and diabetic separately. Patients arrived at emergency center from 0.5 to 24 hours, with a median value of 5.9 hours (lower-upper quartiles, 4.8–8.1 hours). Trauma was chiefly induced by traffic accidents (88 cases, 55.7%). GCS score varied from 3 to 8 points, with a median value of 6 points (lower-upper quartiles, 4–7 points). Forty-three patients (27.2%) were of bilateral pupillary enlargement, and the remaining 115 subjects (72.8%) had unilateral pupillary enlargement. Fluctuations of systolic and diastolic artery blood pressures were from 87 to 179 mmHg (median, 133 mmHg; lower-upper quartiles, 114–144 mmHg) and from 48 to 112 mmHg (median, 77 mmHg; lower-upper quartiles, 69–86 mmHg) separately. Abnormal basal cisterns were of two types (obliterated, 32 cases; compressed, 126 cases). Midline shift statuses were divided into three sections, ie, <5 mm, 5–10 mm and >10 mm, which, respectively, comprised 42, 70 and 46 patients. Mass hemorrhagic lesions were composed of epidural hematoma (53 cases, 33.5%), subdural hematoma (108 subjects, 68.5%) and intracerebral hematoma (64 patients, 40.5%). Non-mass bleeding foci were subarachnoid hemorrhage (104 individuals, 65.8%) and intraventricular hemorrhage (17 persons, 10.8%). All patients got therapy of decompressive craniectomy, with two modes as conventional (130 cases, 82.3%) and modified (28 patients, 17.7%). A collective of 30 patients (19.0%) were diseased of seizure.
Alteration of Serum RIPK1 Levels Post-sTBI and Its Relevance to Injury Extent
These patients in our study, in contrast to our designated controls, had an obvious exhibition of markedly raised serum RIPK1 levels (P<0.001; Figure 2A). In order to accurately unveil metrics in close relation to traumatic severity, GCS, pupillary statuses and abnormal basal cisterns were chosen as the three approaches. In Figure 2B, patients presenting with bilateral pupillary enlargement exhibited extremely elevated serum RIPK1 levels, as compared to those with sufferings from unilateral pupillary enlargement (P<0.001). As plotted in Figure 2C, patients with obliterated basal cisterns, in comparison to those with compressed basal cisterns, had substantially higher serum RIPK1 levels (P<0.001). Also, serum RIPK1 levels were dramatically highest in patients with GCS score 3, were gradually decreased in those with GCS scores from 4 to 7, and displayed substantially lowest serum RIPK1 levels in those with the development of GCS 8 (P<0.001; Figure 2D).
Serum RIPK1 Levels and Post-Traumatic 180-Day Death
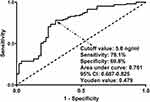
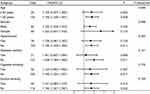
As outlined in Figure 3, there was an existence of linearity relationship between serum RIPK1 levels and likelihood of 180-day death post-sTBI (P for nonlinear > 0.05) in the context of RCS. In addition, serum RIPK1 levels, under ROC curve, took occupation of forceful discrimination efficiency for death at 180-day mark following sTBI, with AUC above 0.75 and with use of the Youden method, an optimal threshold value of serum RIPK1 levels was successfully identifiable, thereby generating the medium-high sensitivity and specificity values (Figure 4). Apart from serum RIPK1 levels, other variables in Table 1 were powerfully distinct between non-survivor and survivors in these patients diseased of sTBI (all P<0.05). In particular, the deceased, as opposed to the living, tended to process significantly decreased GCS scores, were likely to occupy substantially increased percentages of bilateral pupillary enlargement, obliterated basal cisterns, excessive midline shift, subarachnoid hemorrhage, intracerebral hematoma and ALI, as well as were prone to hold markedly elevated blood glucose levels and serum RIPK1 levels (all P<0.05; Table 1). With incorporation of the foregoing nine variables of obvious disparity into the multiple factorial model, the variables of independent association with 180-day death following sTBI were GCS score [odds ratio (OR), 0.721; 95% CI, 0.533–0.976; P=0.034], bilateral pupillary enlargement (OR, 4.524; 95% CI, 1.173–17.446; P=0.028), obliterated basal cisterns (OR, 5.968; 95% CI, 1.122–31.751; P=0.036), ALI (OR, 3.638; 95% CI, 1.272–10.406; P=0.016) and serum RIPK1 levels (OR, 1.090; 95% CI, 1.007–1.181; P=0.033). In application of subgroup analysis, associations of serum RIPK1 levels with death were non-interactionally affected by other traditional parameters, such as age, gender, hypertension, diabetes, tobacco smoking and alcohol consumption (all P interaction > 0.05; Figure 5).
 |
Table 1 Baseline Features in Association with 180-Day Mortality Following Severe Traumatic Brain Injury |
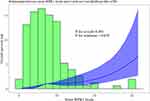
By aidance of RCS, serum RIPK1 levels were linearly related to probability of 180-day overall survival after sTBI (P for nonlinear above 0.05; Figure 6). Serum RIPK1 levels were divided into four segmentations based on their lower-upper quartiles and median value. As a subsequence, overall survival proportion was dramatically reduced over raised serum RIPK1 levels (P less than 0.001; Figure 7). Statistically, serum RIPK1 levels, coupled with GCS scores, bilateral pupillary enlargement, obliterated basal cisterns, midline shift, subarachnoid hemorrhage, intracerebral hematoma, ALI and blood glucose levels, were firmly linked to 180-day overall survival (all P values were less than 0.05; Table 2). When the former nine significantly distinct factors on univariate analyses were given entry into the multivariable model, there was a statistical demonstration that GCS score [hazard ratio (HR), 0.750; 95% CI, 0.595–0.946; P=0.015], bilateral pupillary enlargement (HR, 3.101; 95% CI, 1.320–7.287; P=0.009), obliterated basal cisterns (HR, 2.468; 95% CI, 1.098–5.548; P=0.029), ALI (HR, 3.204; 95% CI, 1.433–6.384; P=0.004) and serum RIPK1 levels (HR, 1.048; 95% CI, 1.004–1.093; P=0.031) appeared as the variables of independent association with 180-day overall survival post-sTBI. By means of subgroup analysis, there were not substantial interactivities between serum RIPK1 levels and other selected parameters, such as age, gender, hypertension, diabetes, tobacco smoking and alcohol consumption, in terms of overall survival association (all P interaction > 0.05; Figure 8).
 |
Table 2 Baseline Features in Association with 180-Day Overall Survival Post-Severe Traumatic Brain Injury |
Serum RIPK1 Levels and Post-Traumatic 180-Day Poor Prognosis
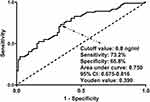
In the whole group of patients diagnosed with sTBI, GOSE scores at 180 days spanned from 1 to 8 (median, 3; lower-upper quartiles, 1–5); and GOSE scores from 1 to 8 were owned by 57, 16, 20, 12, 18, 17, 13 and 5 ill individuals independently. As outlined in Figure 9, there was a significant reduction in serum RIPK1 levels with promoted GOSE scores (P<0.001). In addition, the all patients were dichotomized in accordance with GOSE scores, a cumulative of 105 cases had the development of a poor prognosis (GOSE scores of 1–4) at 180-day duration following sTBI. A statistical revelation by running RCS was that serum RIPK1 levels held linear correlation with risk of posttraumatic 180-day poor prognosis (P for nonlinear > 0.05; Figure 10). A poor prognosis was efficaciously anticipated by serum RIPK1 levels based on the background of the ROC curve module, and the certain cutoff of serum levels forecasted poor prognosis with the maximum Youden index (Figure 11). As listed in Table 3, as compared to patients who presented with good prognosis, those with the development of poor prognosis held significantly declined GCS scores, occupied substantially elevated percentages of bilateral pupillary enlargement, obliterated basal cisterns, excessive midline shift, subarachnoid bleedings, intraventricular accumulation of hemorrhage and ALI, as well as possessed markedly raised serum RIPK1 levels, blood glucose levels and blood leucocyte counts (all P values below 0.05). By application of multivariable model, which contained the aforementioned statistically significantly disparate variables on univariate analyses, an affirmable finding was discovered that GCS score (OR, 0.465; 95% CI, 0.322–0.671; P=0.004), bilateral pupillary enlargement (OR, 4.597; 95% CI, 1.462–14.454; P=0.009), obliterated basal cisterns (OR, 5.353; 95% CI, 1.160–24.704; P=0.032), ALI (OR, 2.803; 95% CI, 1.326–15.926; P=0.017) and serum RIPK1 levels (OR, 1.141; 95% CI, 1.020–1.275; P=0.021) emerged as the independent predictors for prognosticating an adverse outcome in this host of patients with sufferings of sTBI. Moreover, no statistical interactional effects were revealed between serum RIPK1 levels and several conventional parameters, namely, age, gender, hypertensive statues, diabetic state, cigarette smoking condition and alcohol drinking habit (all P interaction >0.05; Figure 12).
 |
Table 3 Baseline Factors in Relation to 180-Day Poor Prognosis Following Severe Traumatic Brain Injury |
Interconnection Between Serum RIPK1 Levels and ALI, as Well as Mediation Impacts of ALI on Association of Serum RIPK1 Levels with Death and Poor Prognosis
RCS was operated to assess linearity connection between serum RIPK1 levels and risk of ALI in this cohort of patients with sufferings from sTBI and the statistical results showed that the statistically explainable linear relation was existent (P for nonlinear > 0.05; Figure 13). As delineated in Figure 14, serum RIPK1 levels were of obvious significance in being predictive of ALI following sTBI by employment of ROC curve analysis and meanwhile, serum RIPK1 levels were screened in the help of the Youden method and revealed was an optimal cutoff value, which distinguished possibility of ALI with satisfactory accuracy. Alternatively, in contrast to patients without ALI, those with ALI were possessive of profoundly diminished GCS scores, possessed pronouncedly enhanced chances of diabetic condition, bilateral pupillary enlargement, obliterated basal cisterns, excessive midline shift, subarachnoid bleedings and intracerebral hematoma, as well as took possession of markedly promoted serum RIPK1 levels and blood glucose levels (all P values were less than 0.05; Table 4). The ninth variables of significant disparity between patients with ALI and those without based on univariate analysis were incorporative in multivariable model and accordingly, there was an interesting ascertainment that GCS score (OR, 0.642; 95% CI, 0.499–0.825; P=0.001), bilateral pupillary enlargement (OR, 4.511; 95% CI, 1.129–18.030; P=0.033), obliterated basal cisterns (OR, 3.663; 95% CI, 1.010–13.289; P=0.038) and serum RIPK1 levels (OR, 1.196; 95% CI, 1.081–1.323; P=0.011) were independent prognosticators of ALI following sTBI. Also, other factors, such as age, gender, hypertensive disease, diabetic illness, alcohol drinking habit and tobacco smoking hobby, were non-statistically significantly influential of associations of serum RIPK1 levels with possibility of ALI (all P interaction >0.05; Figure 15). In Figures 16 and 17, ALI in part mediated association of serum RIPK1 levels with poor prognosis. And, association of serum RIPK1 levels with death was also partially mediated by ALI (Figures 18 and 19).
 |
Table 4 Baseline Factors in Relevance to Acute Lung Injury After Severe Traumatic Brain Injury |
Discussion
As far as we are aware, there has not been a revelation of study about circulating levels of RIPK1 in humans subjected to acute brain injury. The main findings of our study are that (1) serum RIPK1 levels were obviously higher in patients with sTBI than in controls; (2) serum RIPK1 levels were independently associated with the four outcome variables of interest in sTBI studies, namely, 180-day death, overall survival, poor prognosis (GOSE scores 1–4) and ALI; (3) serum RIPK1 levels possessed efficacious prognostic capability under ROC curve; (4) ALI in part mediated associations of serum RIPK1 levels with death and poor prognosis of patients with sTBI. Thus, serum RIPK1 may be feasible as a prognostic biomarker of sTBI.
In this study, we selected a most lethal type of acute brain injury diseases. In detail, this group of patients were diseased of sTBI, accompanied by brain herniation and warranting urgent treatments by decompressive craniectomy. In the first step, a significant elevation of serum RIPK levels after incident sTBI was statistically authenticated. Next, death, overall survival and poor prognosis at 180-day interval, as well as ALI following sTBI were designated as the four outcome variables of interest. Before the multivariate assessments were in progression, structured RCS has been applied for substantiation of linearity relationship between serum RIPK1 levels and the foregoing outcome variables. Moreover, not only there was a statistical proof showing independent association of serum RIPK1 levels with those outcome metrics but also their associations were not significantly affected by certain factors, such as age, gender, hypertensive disorder, diabetic status, smoking hobby and alcohol consumption habit. In addition, anticipation efficiencies of serum RIPK1 for the preceding outcome variables were relatively excellent. It is mentionable that ALI may have the potential to partially mediate association of serum RIPK1 levels with death and poor prognosis at 180 days following aSAH. Overall, such evidence is strongly supportive of the inference that serum RIPK1 may be believably accepted as a prognostic predictor of good prospect in human sTBI.
RIPK1 acts a principal mediator of inflammatory processes, with the extensive involvement in various cell death modes, encompassing apoptosis and necroptosis.13–15 Expressions of RIPK1 by neurons and glial cells were experimentally evident.16 A significant elevation of RIPK expression by ischemic brain tissues was discovered in a mouse model of middle cerebral artery occlusion/reperfusion and downregulated RIPK1 expression by genetic techniques could markedly improve neurological deficits, attenuate infarction volume and alleviate neuroinflammatory responses of mice.33 Also, a consistent result was found in rats with acute ischemic brain injury, in which genetic inactivation of RIPK1 obviously lessened inflammatory reaction and reduced neuronal injury.34 Similarly, brain RIPK1 overexpression substantially promoted brain inflammation, increased neuron damage and enhanced apoptosis after traumatic brain injury in rats, and contrarily, silencing RIPK1 markedly reversed these effects.35 Moreover, the almost identical findings were demonstrated in experimental subarachnoid hemorrhage and intracerebral hemorrhage, therefore alluding to the notion that RIPK1 may function as a detrimental factor, with wide implication in neuroinflammation, neuronal apoptosis, brain edema, blood–brain barrier disruption and neurologic functional impairments.36–38 Overall, RIPK1 may be well recognized as a therapeutic target in the context of acute brain injury.
Exactly, there was an extreme upregulation of RIPK1 expressions in animal cerebral cortices suffering from experimental ischemic, traumatic or hemorrhagic insult.33–38 Hence, increased brain injury, thereby leading to release of RIPK1 from central nervous system to peripheral circulation via disrupted blood–brain barrier, may contribute to herein reported elevation of serum RIPK1 levels after humans sTBI. Admittedly, blood RIPK1 could be originated from other tissues, such as lung, heart and kidney.24,39 A factual instance was that a group of patients diseased of coronary atherosclerotic heart disease, as opposed to healthy controls, displayed profoundly incremental plasma RIPK1 levels.40 Nonetheless, sTBI actually holds strong power to induce systemic injury, which is manifested as systemic inflammatory response syndrome, ALI, cerebrocardiac syndrome and more.5–9 Thus, whether RIPK1 in peripheral blood may be derived from any tissues or cells, its elevation extent should be able to be strongly reflective of brain injury degree. In summary, the hypothesis should be solidified that blood RIPK1 may take possession of the potential as a candidate of prognostic predictors of acute brain injury diseases.
Since a clinical investigational assessment with regard to positive correlation of serum RIPK1 levels with the severity of bulbar symptoms in amyotrophic lateral sclerosis patients,41 no related reports, as far as we are concerned, have disclosed interconnection between blood RIPK levels, severity and clinical outcomes of other disorders in central nervous system. A succession of data have confirmed that GCS, pupillary enlargement and abnormal basal cisterns are the most convincing prognostic determinants of humans diagnosed with sTBI complicated with brain hernia.2,3 Accordingly, the foregoing three approaches were firmly accepted as severity metrics of sTBI in this set of patients. To the best astonishment, this subgroup of patients with bilateral pupillary enlargement possessed significantly higher serum RIPK1 levels over those with unilateral pupillary enlargement; and patients with lower GCS scores or obliterated basal cisterns also displayed a substantial elevation of these biomarker levels than remainders. Three outcome variables, namely, 180-day death, overall survival and poor prognosis, were selected as the dependent variables. Subsequently, serum RIPK1 levels, GCS scores, ALI, bilateral pupillary enlargement and obliterated basal cisterns were identified as their independent predictors in this cohort of patients with sTBI warranting decompressive craniectomy due to brain herniation. In a word, serum RIPK1 may serve as a serological marker of excellence for evaluation of sTBI severity and prognostication of clinical outcome in patients diseased of sTBI.
ALI has been firmly confirmed as one of the commonest adverse events of non-central nervous system in the setting of sTBI.42,43 Undoubtedly, ALI is a forceful determinant of poor outcomes after sTBI.44 RIPK1 was broadly distributed in lung tissues.24 Gradually increasing data have been evidential of the presumption that RIPK1, as a detrimental factor, may be involved in experimental non-traumatic ALI.25 In the current clinical investigation, serum RIPK1 levels were highly associated with ALI, independent of GCS scores, bilateral pupillary enlargement and obliterated basal cisterns. The mediation analysis was actively performed to demonstrate whether ALI may in part mediate association of serum RIPK1 levels with death and poor prognosis after sTBI. As a statistical finding, the mediation effects were actually existent, meaning that RIPK1’s linkage to acute brain injury may harbor complex intrinsic interplay with ALI. Given that the mechanism of RIPK1’s effect on experimental non-traumatic ALI may be related to its participation in inflammatory reaction.25 As for causal relationship between RIPK1, ALI and brain injury. It is hypothesized that RIPK1 from damaged brain tissue may trigger a systemic inflammatory cascade, which could then lead to ALI; or, lung- derived RIPK1 may be increased due to inflammatory signals from the injured brain, thereby resulting in ALI. As expected, investigation as regards RIPK1 as a potential therapeutic target of ALI and TBI as well as relevant mechanistic exploration will be the two noteworthy directions in future researches.
In this study, serum RIPK1 levels distinguished patients at risk of death, poor prognosis and ALI with areas under the ROC curve at 0.752, 0.761 and 0.750 separately, meaning this biomarker may occupy the medium–high predictive ability on these adverse events. Taken together, such data in conjunction with results from multivariate analyses powerfully point to the conception that serum RIPK1 levels could be measured in medical practice so as to be applied as a prognostic serological indicator for severity stratification and prognosis anticipation of sTBI, thereby improving patient outcomes and refining risk stratification methods.
Several limitations and strengths warrant to be mentioned here. The strengths include the following points. (1) Relationship between serum RIPK1 levels, traumatic severity and clinical outcomes, as far as we know, has never been analyzed, and our study explored these relations in a cohort of patients with sTBI undergoing decompressive craniectomy owing to brain hernia, and subsequently found some noteworthy results, thereby substantializing serum RIPK1 as a possible prognostic biomarker of sTBI. (2) In order to be explicitly investigatory of prognostic association with serum RIPK1 levels in this set of sTBI, all results were garnered in accordance with the multiple factorial analyses, alongside with applying the subgroup analysis and RCS assessment, thereby ensuring results strongly reliable and scientific. (3) For the sake of unveiling possible intricate interplay between ALI and serum RIPK1 levels in influencing clinical outcomes of sTBI, mediation analyses were implemented and the results of interest were therefore unraveled that ALI may partially mediate association of serum RIPK1 levels with clinical outcomes following sTBI, thereby strongly reinforcing the assumption that prognostic association may not be at state of very single pathway. Nevertheless, the limitations are still existent. (1) Evolutional trajectory of serum RIPK1 levels was not investigated after sTBI in this study, and maybe, measuring serum RIPK1 serially in future studies is informative of clinical work because longitudinal measurements would clarify whether dynamic changes in serum RIPK1 track with the progression of brain or lung injury. (2) Because the median time to admission was around 5.9 hours in the current, any additional delay beyond this point might alter the measured concentration of RIPK1 in the blood. Consequently, variations in time-to-admission could affect how accurately serum RIPK1 levels reflect the severity of brain injury. In future, the patient number would be greatly increased so that all patients could be subdivided in accordance with the time intervals, thereby leading to revelation of serum RIPK1 levels at more time-points. (3) According to statistical requirements, a sufficient sample size has been enlisted from multiple medical centers in the current clinical analysis, and however, the essential point of clinical study is that all hypotheses should warrant an extensive validation in a larger cohort study.
Conclusions
In this cohort of patients with sTBI requiring decompressive craniectomy due to brain herniation, elevated serum RIPK1 levels are associated with trauma severity, ALI, and worse 6-month outcomes; serum RIPK1 levels take possession of effective prognostic ability; using mediation analysis, ALI is proved to partially decipher the links between serum RIPK1 levels, death and poor prognosis. Therefore, RIPK1 may serve as a prognostic biomarker in sTBI.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are personal data but are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully thank all study participants, their relatives, and the staff at the recruitment centers for their invaluable contributions.
Funding
This work is financially supported by grants from the Research and Development Fund of Kangda College of Nanjing Medical University (No. KD2022KYJJZD042) and the Hangzhou Medical and Health Technology Plan Project (No. A20220239).
Disclosure
The authors declared no potential conflict of interest.
References
1. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi:10.1016/j.mcna.2019.11.001
2. Bodien YG, Barra A, Temkin NR, et al. Diagnosing level of consciousness: the limits of the Glasgow Coma Scale total score. J Neurotrauma. 2021;38(23):3295–3305. doi:10.1089/neu.2021.0199
3. Yang C, Zhang JR, Zhu G, et al. Effects of primary decompressive craniectomy on the outcomes of serious traumatic brain injury with mass lesions, and independent predictors of operation decision. World Neurosurg. 2021;148:e396–e405. doi:10.1016/j.wneu.2020.12.158
4. Wu J, Ren R, Chen T, Su LD, Tang T. Neuroimmune and neuroinflammation response for traumatic brain injury. Brain Res Bull. 2024;217:111066. doi:10.1016/j.brainresbull.2024.111066
5. Li X, Deng J, Long Y, et al. Focus on brain-lung crosstalk: preventing or treating the pathological vicious circle between the brain and the lung. Neurochem Int. 2024;178:105768. doi:10.1016/j.neuint.2024.105768
6. Jacome T, Tatum D. Systemic inflammatory response syndrome (SIRS) score independently predicts poor outcome in isolated traumatic brain injury. Neurocrit Care. 2018;28(1):110–116. doi:10.1007/s12028-017-0410-y
7. Lou M, Chen X, Wang K, Xue Y, Cui D, Xue F. Increased intracranial pressure is associated with the development of acute lung injury following severe traumatic brain injury. Clin Neurol Neurosurg. 2013;115(7):904–908. doi:10.1016/j.clineuro.2012.09.001
8. Rincon F, Ghosh S, Dey S, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012t;71(4):795–803. doi:10.1227/NEU.0b013e3182672ae5
9. Wang XC, Gao SJ, Zhuo SL, et al. Predictive factors for cerebrocardiac syndrome in patients with severe traumatic brain injury: a retrospective cohort study. Front Neurol. 2023;14:1192756. doi:10.3389/fneur.2023.1192756
10. Zou S, Xu J, Yang W, et al. A two-center prospective cohort study of serum RIP-3 as a potential biomarker in relation to severity and prognosis after severe traumatic brain injury. Neurosurg Rev. 2024;47(1):433. doi:10.1007/s10143-024-02658-9
11. Yan T, Shan H, Wang Z, et al. Temporal change of serum xanthine oxidase levels and its relation to clinical outcome of severe traumatic brain injury: a prospective cohort study. Neurosurg Rev. 2023;46(1):320. doi:10.1007/s10143-023-02233-8
12. Wang KKW, Barton DJ, McQuillan LE, et al. Parallel cerebrospinal fluid and serum temporal profile assessment of axonal injury biomarkers neurofilament-light chain and phosphorylated neurofilament-heavy chain: associations with patient outcome in moderate-severe traumatic brain injury. J Neurotrauma. 2024;41(13–14):1609–1627. doi:10.1089/neu.2023.0449
13. Du J, Wang Z. Regulation of RIPK1 phosphorylation: implications for inflammation, cell death, and therapeutic interventions. Biomedicines. 2024;12(7):1525. doi:10.3390/biomedicines12071525
14. Liu X, Tang AL, Cheng J, Gao N, Zhang G, Xiao C. RIPK1 in the inflammatory response and sepsis: recent advances, drug discovery and beyond. Front Immunol. 2023;14:1114103. doi:10.3389/fimmu.2023.1114103
15. Xu L, Zhang W, Zhuang C. Receptor-interacting protein kinase 1 (RIPK1) inhibitor: a review of the patent literature (2018-present). Expert Opin Ther Pat. 2023;33(2):101–124. doi:10.1080/13543776.2023.2195548
16. Pati S, Singh Gautam A, Dey M, Tiwari A, Kumar Singh R. Molecular and functional characteristics of receptor-interacting protein kinase 1 (RIPK1) and its therapeutic potential in Alzheimer’s disease. Drug Discov Today. 2023;28(12):103750. doi:10.1016/j.drudis.2023.103750
17. Kartik S, Pal R, Chaudhary MJ, Tiwari PC, Nath R, Kumar M. Anti-oxidative and anti-neuroinflammatory role of necrostatin-1s and docosahexaenoic acid in RIP-1-mediated neurotoxicity in MPTP-induced Parkinson’s disease model. Fundam Clin Pharmacol. 2023;37(4):794–806. doi:10.1111/fcp.12881
18. Zelic M, Pontarelli F, Woodworth L, et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 2021;35(6):109112. doi:10.1016/j.celrep.2021.109112
19. Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20(1):19–33. doi:10.1038/s41583-018-0093-1
20. Shen H, Liu C, Zhang D, et al. Role for RIP1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death Dis. 2017;8(3):e2641. doi:10.1038/cddis.2017.58
21. Lule S, Wu L, Sarro-Schwartz A, et al. Cell-specific activation of RIPK1 and MLKL after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2021;41(7):1623–1633. doi:10.1177/0271678X20973609
22. Wehn AC, Khalin I, Duering M, et al. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol Commun. 2021;9(1):138. doi:10.1186/s40478-021-01236-0
23. Su X, Wang H, Kang D, et al. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem Res. 2015;40(4):643–650. doi:10.1007/s11064-014-1510-0
24. Van Eeckhoutte HP, Donovan C, Kim RY, et al. RIPK1 kinase-dependent inflammation and cell death contribute to the pathogenesis of COPD. Eur Respir J. 2023;61(4):2201506. doi:10.1183/13993003.01506-2022
25. Yang T, Xiang CG, Wang XH, et al. RIPK1 inhibitor ameliorates pulmonary injury by modulating the function of neutrophils and vascular endothelial cells. Cell Death Discov. 2024;10(1):152. doi:10.1038/s41420-024-01921-8
26. Caroli M, Locatelli M, Campanella R, Balbi S, Martinelli F, Arienta C. Multiple intracranial lesions in head injury: clinical considerations, prognostic factors, management, and results in 95 patients. Surg Neurol. 2001;56(2):82–88. doi:10.1016/s0090-3019(01)00540-7
27. Lan Z, Richard SA, Li Q, et al. Outcomes of patients undergoing craniotomy and decompressive craniectomy for severe traumatic brain injury with brain herniation: a retrospective study. Medicine. 2020;99(43):e22742. doi:10.1097/MD.0000000000022742
28. Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20(3):225–232. doi:10.1007/BF01704707
29. Scibilia A, Gallinaro P, Todeschi J, et al. Surgical management of persistent post-traumatic trans-tentorial brain hernia. Neurochirurgie. 2022;68(1):44–51. doi:10.1016/j.neuchi.2021.06.012
30. Mao X, Miao G, Hao S, et al. Decompressive craniectomy for severe traumatic brain injury patients with fixed dilated pupils. Ther Clin Risk Manag. 2015;11:1627–1633. doi:10.2147/TCRM.S89820
31. Lyon KA, Patel NP, Zhang Y, Huang JH, Feng D. Novel hemicraniectomy technique for malignant middle cerebral artery infarction: technical note. Oper Neurosurg. 2019;17(3):273–276. doi:10.1093/ons/opy399
32. Levin HS, Boake C, Song J, et al. Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J Neurotrauma. 2001;18(6):575–584. doi:10.1089/089771501750291819
33. Zhang Y, Li M, Li X, et al. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020;11(7):565. doi:10.1038/s41419-020-02770-w
34. Stark K, Goncharov T, Varfolomeev E, et al. Genetic inactivation of RIP1 kinase activity in rats protects against ischemic brain injury. Cell Death Dis. 2021;12(4):379. doi:10.1038/s41419-021-03651-6
35. Liu J, Zhu Z, Wang L, et al. Functional suppression of Ripk1 blocks the NF-κB signaling pathway and induces neuron autophagy after traumatic brain injury. mol Cell Biochem. 2020;472(1–2):105–114. doi:10.1007/s11010-020-03789-5
36. Wu Y, Xu Y, Sun J, Dai K, Wang Z, Zhang J. Inhibiting RIPK1-driven neuroinflammation and neuronal apoptosis mitigates brain injury following experimental subarachnoid hemorrhage. Exp Neurol. 2024;374:114705. doi:10.1016/j.expneurol.2024.114705
37. Chen F, Su X, Lin Z, et al. Necrostatin-1 attenuates early brain injury after subarachnoid hemorrhage in rats by inhibiting necroptosis. Neuropsychiatr Dis Treat. 2017;13:1771–1782. doi:10.2147/NDT.S140801
38. Lule S, Wu L, McAllister LM, et al. Genetic inhibition of receptor interacting protein kinase-1 reduces cell death and improves functional outcome after intracerebral hemorrhage in mice. Stroke. 2017;48(9):2549–2556. doi:10.1161/STROKEAHA.117.017702
39. Chiou S, Al-Ani AH, Pan Y, et al. An immunohistochemical atlas of necroptotic pathway expression. EMBO Mol Med. 2024;16(7):1717–1749. doi:10.1038/s44321-024-00074-6
40. Xiao Z, Zhang Y, Kuang Y, Ma Q. Changes in plasma levels of RIPK1, RIPK3, and MLKL in patients with coronary atherosclerotic heart disease and its clinical predictive value. Zhong Nan da Xue Xue Bao Yi Xue Ban. 2020;45(9):1096–1103. doi:10.11817/j.issn.1672-7347.2020.200026
41. Wei J, Li M, Ye Z, et al. Elevated peripheral levels of receptor-interacting protein kinase 1 (RIPK1) and IL-8 as biomarkers of human amyotrophic lateral sclerosis. Signal Transduct Target Ther. 2023;8(1):451. doi:10.1038/s41392-023-01713-z
42. Atkinson JL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997;41(5):1214–1216. doi:10.1097/00006123-199711000-00052
43. Bratton SL, Davis RL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997;40(4):707–7112. doi:10.1097/00006123-199704000-00009
44. Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55(1):106–111. doi:10.1097/01.TA.0000071620.27375.BE
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.