Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Severity and Prognosis of Vascular Dementia in Patients with Acute Cerebral Infarction Combined with H-Type Hypertension and Its Correlation with Uric Acid Levels
Received 5 December 2024
Accepted for publication 13 June 2025
Published 24 June 2025 Volume 2025:21 Pages 1261—1270
DOI https://doi.org/10.2147/NDT.S508965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rakesh Kumar
Yang Hong,1 Min Feng2
1Graduate School of Bengbu Medical University, Bengbu, Anhui, 233030, People’s Republic of China; 2Department of Neurology, The Second Affiliated Hospital of Bengbu Medical University, Bengbu, Anhui, 233000, People’s Republic of China
Correspondence: Min Feng, Department of Neurology, The Second Affiliated Hospital of Bengbu Medical University, No. 633 Longhua Road, Huaishang District, Bengbu, Anhui, 233000, People’s Republic of China, Tel +86-13855253675, Email [email protected]
Aim: To investigate the correlation between serum uric acid levels and the severity and prognosis of acute cerebral infarction (ACI) combined with vascular dementia (VD) in patients with H-type hypertension.
Methods: A retrospective analysis was conducted on 150 patients with VD after acute ischemic stroke (AIS) admitted to the Second Affiliated Hospital of Bengbu Medical College from September 2021 to March 2023. Patients with H-type hypertension (n=84) formed the observation group, while those without (n=66) formed the control group. Uric acid levels were compared between groups. Based on cognitive function scores, patients were further classified into mild, moderate, and severe dementia subgroups, and differences in uric acid and homocysteine levels were analyzed. Prognosis was assessed in the observation group after three months of treatment using activities of daily living scores, and logistic regression was performed to identify prognostic factors.
Results: The observation group had significantly higher blood uric acid levels than the control group (P< 0.05). Within the observation group, uric acid and homocysteine levels differed significantly among dementia severity subgroups (P< 0.05) and were significantly higher than in the control group (P< 0.01). MMSE scores were negatively correlated with uric acid and homocysteine levels. Logistic regression analysis identified uric acid as an independent risk factor for prognosis.
Conclusion: Elevated serum uric acid levels in patients with ACI and H-type hypertension are associated with greater dementia severity and poorer prognosis, highlighting the importance of monitoring uric acid levels in these patients.
Keywords: acute ischemic stroke, vascular dementia, H-type hypertension, blood uric acid, blood homocysteine
Introduction
With the aging population and changes in lifestyle, the incidence of stroke in China is increasing year by year.1 Vascular dementia (VD) often occurs in stroke patients, and its clinical manifestations mainly include progressive decline in memory, attention, judgment, comprehension, and executive function, accompanied by varying degrees of mental and personality changes.2 Vascular dementia can be caused by various cerebrovascular diseases, but stroke is the most common cause. The incidence of vascular dementia within one year after stroke is about 33%, and the incidence rate can still reach 31% within five years.3 Therefore, the prevention and treatment of vascular dementia should first focus on preventing stroke and other cerebrovascular diseases.
Studies both domestically and internationally have indicated4–6 that there is a significantly increased risk of cognitive impairment in H-type hypertension patients. Cognitive function refers to the brain’s ability to process, store, extract, and execute information, which is essential for learning about the composition, performance, association, developmental direction, and basic principles of things, providing indispensable prerequisites for daily activities.7,8 The cardiovascular and cerebrovascular changes caused by elevated blood pressure, especially when hypertension coexists with hyperhomocysteinemia, can disrupt normal brain function, leading to decreased learning and functional abilities, impaired mental and memory functions, as well as deficits in language, application, cognition, and behavior, ultimately resulting in cognitive impairment.9 However, cognitive impairment in the early stages is only manifested as mild cognitive impairment (MCI), which lacks obvious symptoms in clinical practice, making it difficult for most patients to be detected.10 When cognitive functions such as memory are mildly impaired, it has not yet reached the diagnosis of dementia, but is in the transitional stage before Alzheimer’s disease, known as mild cognitive impairment.11 Therefore, it is important to identify biomarkers that can predict cognitive decline as early as possible.
Studies have found that serum uric acid (SUA) levels are elevated in patients with H-type hypertension, and hyperuricemia is positively correlated with the occurrence of H-type hypertension.12,13 Uric acid is a metabolite of nucleic acids, and the number of patients with hyperuricemia is gradually increasing,14–16 which is closely related to patients’ dietary habits, nutritional conditions, and lifestyle. Previous studies17,18 have shown that the correlation between hyperuricemia, hypertension, and hyperhomocysteinemia has a significant impact on H-type hypertension. Additionally, related research has indicated19–21 that blood uric acid levels are associated with the progression of cerebral small vessel diseases (CSVD), stroke, and other cerebrovascular diseases, and can serve as a predictive factor for cognitive impairment in CSVD. Furthermore, some studies have suggested22,23 a significant relationship between changes in blood uric acid levels and the occurrence and development of mild cognitive impairment (MCI). Therefore, we speculate that there may be a certain correlation between blood uric acid levels and mild cognitive impairment in patients with H-type hypertension.
Currently, there is limited research on the correlation between the severity and prognosis of vascular dementia in patients with H-type hypertension after acute ischemic stroke and serum uric acid levels. Therefore, this study aimed to investigate the correlation between the severity and prognosis of vascular dementia in patients with H-type hypertension after acute ischemic stroke and serum uric acid levels.
Materials and Methods
Study Design
In this retrospective analysis, 150 patients with with vascular dementia after acute ischemic stroke who visited our hospital during September 2021 to March 2023 were enrolled. Based on the occurrence of H-type hypertension, patients with concomitant H-type hypertension were divided into the observation group (84 cases), and those without H-type hypertension were divided into the control group (66 cases). The study was approved by the Ethics Committee of the Second Affiliated Hospital of Bengbu Medical University. Informed consent was obtained from all study participants. All the methods were carried out in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Inclusion Criteria
(1) Diagnosis of acute cerebral infarction (ACI) confirmed by diffusion-weighted imaging (DWI) in accordance with the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke.24 Patients included had a National Institutes of Health Stroke Scale (NIHSS) score between 0 and 15, and those with severe global aphasia, rendering MMSE evaluation impossible, were excluded. (2) Diagnosis of vascular dementia (VD) based on the criteria established by the National Institute of Neurological Disorders and Stroke and the International Society for Vascular Behavioral and Cognitive Disorders (NINDS-AIREN).25 VD evaluation was conducted 3 months after stroke onset. (3) H-type hypertension was defined as meeting both the criteria for hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg in three separate measurements under non-resting conditions, or a history of hypertension) and hyperhomocysteinemia (HHcy) (blood homocysteine level >15 µmol/L).26 (4) Patients with complete clinical data. (5) Information regarding revascularization treatment was recorded, and patients were stratified based on whether they received such treatment. (6) Activities of daily living (ADL) scores were assessed at discharge and compared with scores at the 6-month follow-up to evaluate functional recovery.
Exclusion Criteria
① individuals with pre-existing cognitive impairment; ② individuals with dementia caused by other reasons; ③ individuals with concomitant other neurological disorders; ④ individuals with other cerebrovascular diseases; ⑤ individuals with a history of depression; ⑥ individuals with impaired consciousness who cannot cooperate; ⑦ individuals with severe wasting diseases or organ dysfunction; ⑧ individuals with secondary hypertension; ⑨ individuals with incomplete or lost medical records.
Methods and Observation Indicators
(1) Collect clinical data of two groups of patients, including age, gender, education level, smoking history, alcohol consumption history, BMI, and history of diabetes. (2) All patients had peripheral venous blood drawn after an 8-hour fasting period on the second day of hospitalization, and total cholesterol, triglycerides, and glycated hemoglobin A1c were measured using the hospital’s biochemical analyzer. The differences between the factors in the two groups were compared. (3) The severity of dementia was assessed using the Mini-Mental State Examination (MMSE).27 A score of ≥27 indicates normal cognitive function, 21–26 indicates mild, 10–20 indicates moderate, and ≤9 indicates severe. (4) All patients received treatment with anticoagulants, antiplatelet agents, and neurotrophic agents. Patients with underlying diseases such as hypertension and diabetes also received symptomatic treatment for blood pressure control and glycemic control, and were advised to adopt healthy dietary and exercise habits. After 6 months, the observation group was assessed for prognosis using the Activities of Daily Living (ADL) scale. A score of >40 indicates a good prognosis, while ≤40 indicates a poorer prognosis.
Statistical Analysis
Data analysis was performed using SPSS 28.0. The continuous variables were normally distributed and expressed as (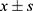 ). Independent samples t-test was used to compare differences between groups. The categorical variables were expressed as percentages (%) and analyzed using the chi-square test. One-way analysis of variance (ANOVA) was used for multiple group comparisons. Pearson’s correlation test was used for correlation analysis. Single-factor and multiple-factor logistic regression analysis were used to identify factors influencing the prognosis of the observed group of patients. A p-value less than 0.05 was considered statistically significant.
). Independent samples t-test was used to compare differences between groups. The categorical variables were expressed as percentages (%) and analyzed using the chi-square test. One-way analysis of variance (ANOVA) was used for multiple group comparisons. Pearson’s correlation test was used for correlation analysis. Single-factor and multiple-factor logistic regression analysis were used to identify factors influencing the prognosis of the observed group of patients. A p-value less than 0.05 was considered statistically significant.
Results
Clinical Data
The two groups of patients showed no statistically significant differences in age, gender, education level, smoking history, alcohol consumption history, body mass index (BMI), history of diabetes, total cholesterol, triglycerides, and glycated hemoglobin A1c (P>0.05), indicating comparability (Table 1).
 |
Table 1 General Information of Two Groups of Patients |
Comparisons the Levels of Blood Uric Acid and Blood Homocysteine in Two Groups
Compared to the control group, the observation group showed a significant increase in the levels of blood uric acid and blood homocysteine (P<0.05) (Figure 1).
 |
Figure 1 Comparing the levels of blood uric acid and blood homocysteine in two groups. (A) Blood uric acid; (B) Blood homocysteine. *(P<0.05). **(P<0.01). |
Comparisons Blood Uric Acid and Blood Homocysteine Levels in Different Subgroups of Patients with Dementia
After conducting MMSE scoring on two groups of dementia patients, they were divided into three groups: mild, moderate, and severe. This study found that there were statistically significant differences in blood uric acid and blood homocysteine levels among the three subgroups in the observation group (P<0.05). However, no significant differences were observed in blood homocysteine levels among the three subgroups in the control group (P>0.05), while the differences in blood uric acid levels were statistically significant (P<0.05) (Table 2).
 |
Table 2 Comparisons Blood Uric Acid and Blood Homocysteine Levels in Different Subgroups of Patients with Dementia |
Comparisons were Made Between the Blood Uric Acid and Blood Homocysteine Levels in the Subgroups of Dementia Patients from the Two Groups.
This study found that the levels of blood uric acid and blood homocysteine in the observation group were significantly higher than those in the control group in each subgroup of dementia (P<0.01) (Table 3).
 |
Table 3 Comparisons Were Made Between the Blood Uric Acid and Blood Homocysteine Levels in the Subgroups of Dementia Patients From the Two Groups |
Factors Affecting the Prognosis of Patients with Vascular Dementia Combined with H-type Hypertension After Acute Ischemic Stroke in a Univariate Analysis.
The observation group was divided into two groups based on ADL scores, with 59 cases in the good prognosis group and 25 cases in the poor prognosis group. Univariate analysis showed that the levels of blood uric acid and blood homocysteine were significantly higher in the poor prognosis group compared to the good prognosis group (P<0.05). However, there was no statistically significant difference between the two groups in terms of age, gender, education level, smoking history, alcohol consumption history, body mass index, history of diabetes, total cholesterol, triglycerides, and glycated hemoglobin A1c (P>0.05) (Table 4).
 |
Table 4 Factors Influencing the Prognosis of the Observation Group in Univariate Analysis |
Factors Affecting the Prognosis of Patients with Vascular Dementia Combined with H-type Hypertension After Acute Ischemic Stroke in Multivariate Logistic Regression Analysis.
The factors selected through univariate analysis were used as independent variables in logistic regression analysis. The results showed that blood uric acid and blood homocysteine were independent risk factors affecting the prognosis of patients with VD combined with H-type hypertension after AIS (P<0.05) (Table 5).
 |
Table 5 Multivariate Logistic Regression Analysis of the Influencing Factors on the Prognosis of the Observation Group |
Discussion
In this study, we found that the levels of blood uric acid were elevated in patients with vascular dementia combined with H-type hypertension after acute ischemic stroke. Additionally, the MMSE scores of different subgroups of dementia were negatively correlated with the levels of blood uric acid and homocysteine. Logistic regression analysis showed that the level of blood uric acid was an independent risk factor affecting the prognosis of the study group. We revealed that the levels of blood uric acid in patients with Vascular Dementia (VD) combined with H-type hypertension after Acute Cerebral Infarction (ACI) were significantly higher than those in patients with pure ACI and VD.
H-type hypertension, also known as hypertension combined with hyperhomocysteinemia, was first proposed by Professor Hu DY.28 Wang et al found that H-type hypertensive patients have a 5-fold higher risk of developing cardiovascular and cerebrovascular diseases compared to other types of hypertension, and a 30-fold higher risk compared to healthy individuals.29 Other studies have shown that H-type hypertensive patients are more prone to cognitive impairment compared to patients with pure hypertension.30,31 Therefore, it is important to pay special attention to patients with acute cerebral infarction combined with H-type hypertension in clinical practice and intervene as early as possible. In addition, studies have found that H-type hypertensive patients often have higher levels of uric acid.12 Uric acid participates in various oxidative-reduction reactions in the body, and its pro-oxidative effect at high concentrations in cells may be related to cognitive impairment.
The results of this study showed that the blood uric acid levels were highest in the severe dementia group and lowest in the mild dementia group, suggesting that blood uric acid may be involved in certain pathological and physiological processes in the development of vascular dementia. The possible mechanisms are as follows: (1) Uric acid has a good free radical scavenging effect at physiological concentrations and is considered one of the most important antioxidants in plasma.32 However, high concentrations of uric acid can react with various oxidants to generate free radicals and become potent pro-oxidants. Accumulation of reactive oxygen species such as hydrogen peroxide, superoxide anions, and nitric oxide in the body leads to increased oxidative stress, damage to cerebral vascular endothelial cells, impairment of neuronal structure and function in tissues related to cognition such as the hippocampus, accelerated neuronal apoptosis, and the occurrence of cognitive impairment.33 (2) Disruption of the blood-brain barrier is believed to play an important role in neuroinflammation and oxidative stress. Studies have shown that the concentration of uric acid in the cerebrospinal fluid of patients with blood-brain barrier dysfunction is partially dependent on blood uric acid levels and the balance between production and consumption in the central nervous system.34 Desideri et al35 found that uric acid can have harmful effects on brain structure and function by directly affecting the vitality of neuronal cells and their ability to establish synaptic connections, depending on the level of cell exposure to uric acid. This effect of uric acid was observed starting from a dose of 40mM, and lower concentrations of uric acid had no significant effect on cellular biology, indicating a dose-dependent effect of uric acid. In the case of hyperuricemia, blood uric acid can diffuse through the blood-brain barrier into the cerebrospinal fluid, damaging neurons and further leading to cognitive impairment. (3) Nitric oxide is mainly synthesized by vascular endothelial cells and plays an important role in regulating tissue blood flow, controlling vascular remodeling, maintaining normal blood pressure, antiplatelet aggregation, leukocyte adhesion, and protecting the endothelium.36 High concentrations of uric acid can cause eNOS enzyme uncoupling, inhibit endothelial cell proliferation and migration, as well as nitric oxide secretion, and reduce the bioavailability of nitric oxide,37,38 leading to the development of atherosclerosis and exacerbation of cerebral ischemia. (4) Uric acid has pro-inflammatory effects and can trigger leukocyte-1β-mediated inflammation through the activation of nucleotide-binding oligomerization domain-like receptor protein 3 inflammasomes. Inflammatory reactions can promote platelet aggregation and accelerate the formation of arterial and venous thrombosis. In addition, high levels of blood uric acid can activate the Toll-like receptor 4/nuclear factor-κB signaling pathway, which can reduce the dendritic length of hippocampal pyramidal neurons and impair hippocampus-dependent spatial reference memory in an inflammation-dependent manner. Tian et al39 studied the effects of uric acid concentration and exposure time on cognition and found that high uric acid diet-induced elevation of blood uric acid was significantly associated with the risk of cognitive impairment. In this study, blood uric acid levels increased with the severity of dementia, consistent with the aforementioned research results. Therefore, special attention should be paid to patients with acute ischemic stroke combined with hyperuricemia in clinical practice, and timely intervention should be implemented to prevent and delay the occurrence of vascular dementia.
The results of this study showed that the levels of blood uric acid were highest in the severe dementia group and lowest in the mild dementia group, suggesting a correlation between blood uric acid and the severity of dementia. In contrast to blood uric acid, several studies have confirmed a close relationship between blood homocysteine and cognitive impairment.40,41 Elevated homocysteine levels can promote smooth muscle cell proliferation, leading to lipid peroxidation, inhibition of endothelial cell proliferation, and decreased endothelial nitric oxide synthase activity, which can result in microinfarctions, lacunar infarcts, and cerebral white matter lesions, increasing the risk of dementia.42 This is consistent with the results of this study. Furthermore, this study found that both blood uric acid and blood homocysteine were independent risk factors for the prognosis of patients with AIS-associated VD and H-type hypertension. Increased levels of blood uric acid and blood homocysteine often indicate worsening of the patient’s condition and poor prognosis.43
Several mechanisms linking uric acid to VD severity have been proposed. Firstly, uric acid, a known antioxidant, plays a key role in neuroprotection, particularly in vascular dementia (VD). One mechanism by which it may exert its protective effects is through neutralizing free radicals and reducing oxidative stress, which contributes to neurodegeneration in VD. Oxidative stress leads to neuronal damage, endothelial dysfunction, and inflammation—critical factors in VD pathophysiology. By scavenging free radicals, uric acid could help shield neurons from oxidative damage and potentially slow cognitive decline. Additionally, uric acid may positively impact vascular health by influencing vascular tone and endothelial function, both essential for maintaining brain blood flow. In VD, endothelial dysfunction and impaired cerebral blood flow are common, promoting cognitive decline. Uric acid’s role in modulating these factors could protect neurons from further injury. Moreover, uric acid may have anti-inflammatory properties that help alleviate chronic inflammation, another key factor in VD progression. Elevated uric acid levels might therefore reduce inflammation in VD patients, improve vascular health, and possibly reverse some ischemic damage. However, the relationship between uric acid and VD is complex, and not all studies support a neuroprotective role for uric acid. High uric acid levels can also have adverse effects, such as gout or urate crystal formation, which might contribute to vascular damage and cognitive decline. Thus, while uric acid may protect neurons in certain conditions, its overall impact likely depends on factors like exposure duration, comorbidities, and individual metabolic variations. Given the dual potential for beneficial and harmful effects, it is crucial to further explore the mechanisms linking uric acid levels to VD severity. Future research should investigate how uric acid interacts with molecular pathways involved in oxidative stress, inflammation, and vascular function. Studies should also determine whether there is an optimal uric acid range for neuroprotection or if modulating uric acid levels can improve VD treatment outcomes.
The limitations of this study should be acknowledged. Firstly, the sample size was relatively small, which may limit the generalizability of the findings. Additionally, the study only utilized the MMSE score to categorize patients into mild, moderate, and severe dementia groups, which may not fully capture the complexity and heterogeneity of dementia. Furthermore, the study only examined the association between blood homocysteine levels and dementia, without considering other potential contributing factors. Notably, certain confounding variables such as NIHSS score, infarct size, and infarct territory, which may influence cognitive outcomes in acute stroke patients, were not specifically accounted for in the analysis. While we attempted to mitigate these effects through statistical adjustments, future studies with larger sample sizes and more detailed assessments of stroke severity and lesion characteristics are needed to validate and expand upon these findings.
In conclusion, the severity of vascular dementia in patients with H-type hypertension after acute ischemic stroke is related to the level of blood uric acid. There is a positive correlation between blood uric acid levels and the severity of dementia. The higher the blood uric acid level, the worse the prognosis for patients. It is speculated that blood uric acid may be a risk factor for cognitive function and prognosis in patients with vascular dementia and H-type hypertension after acute ischemic stroke, providing clinical evidence for the prevention and treatment of dementia.
Acknowledgments
An unauthorized version of the Chinese MMSE was used by the study team without permission, however this has now been rectified with PAR. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao Y, Hua X, Ren X, et al. Increasing burden of stroke in China: a systematic review and meta-analysis of prevalence, incidence, mortality, and case fatality. Int J Stroke. 2023;18(3):259–267. doi:10.1177/17474930221135983
2. Heupel-Reuter M, Denkinger M, Bauer JM, Voigt-Radloff S. Blood pressure-lowering treatment for prevention of recurrent stroke, severe vascular events and dementia in patients with stroke or transient ischemic attack in the past history. Z Gerontol Geriatr. 2019;52(2):195–197. doi:10.1007/s00391-019-01521-7
3. Dharmasaroja PA, Limwongse C, Charernboon T. Incidence and risk factors of vascular dementia in Thai stroke patients. J Stroke Cerebrovasc Dis. 2020;29(8):104878. doi:10.1016/j.jstrokecerebrovasdis.2020.104878
4. Pavlovic AM, Pekmezovic T, Trajkovic JZ, Tomic G, Cvitan E, Sternic N. Increased risk of cognitive impairment and more severe brain lesions in hypertensive compared to non-hypertensive patients with cerebral small vessel disease. J Clin Hypertens. 2018;20(9):1260–1265. doi:10.1111/jch.13357
5. Yagi S, Akaike M, Aihara K, et al. High plasma aldosterone concentration is a novel risk factor of cognitive impairment in patients with hypertension. Hypertens Res. 2011;34(1):74–78. doi:10.1038/hr.2010.179
6. Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322(6):535–545. doi:10.1001/jama.2019.10575
7. Lawson L, Mc Ardle R, Wilson S, Beswick E, Karimi R, Slight SP. Digital endpoints for assessing instrumental activities of daily living in mild cognitive impairment: systematic review. J Med Internet Res. 2023;25:e45658. doi:10.2196/45658
8. Sugimoto T, Ono R, Kimura A, et al. Impact of cognitive frailty on activities of daily living, cognitive function, and conversion to dementia among memory clinic patients with mild cognitive impairment. J Alzheimers Dis. 2020;76(3):895–903. doi:10.3233/JAD-191135
9. Kimura N, Sasaki Y, Masuda T, et al. Lifestyle factors that affect cognitive function-a longitudinal objective analysis. Front Public Health. 2023;11:1215419. doi:10.3389/fpubh.2023.1215419
10. Rookes T, Frost R, Barrado-Martin Y, et al. Type of goals set and progress towards these goals, as part of a behaviour change intervention, in people with mild cognitive impairment: a secondary analysis. Lancet. 2023;402 Suppl 1:S80. doi:10.1016/S0140-6736(23)02112-8
11. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. doi:10.1056/NEJMcp0910237
12. Han F, Yu C, Hu F, et al. Association between serum uric acid levels and peripheral artery disease in Chinese adults with hypertension. Front Endocrinol. 2023;14:1197628. doi:10.3389/fendo.2023.1197628
13. Bjornstad P, Laffel L, Lynch J, et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42(6):1120–1128. doi:10.2337/dc18-2147
14. Sellmayr M, Hernandez Petzsche MR, Ma Q, et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol. 2020;31(12):2773–2792. doi:10.1681/ASN.2020040523
15. Guo XL, Gao YY, Yang YX, et al. Amelioration effects of α-viniferin on hyperuricemia and hyperuricemia-induced kidney injury in mice. Phytomedicine. 2023;116:154868. doi:10.1016/j.phymed.2023.154868
16. Piani F, Agnoletti D, Borghi C. Advances in pharmacotherapies for hyperuricemia. Expert Opin Pharmacother. 2023;24(6):737–745. doi:10.1080/14656566.2023.2197591
17. Yu C, Wang T, Zhou W, et al. Positive association between the triglyceride-glucose index and hyperuricemia in Chinese adults with hypertension: an insight from the China H-type hypertension registry study. Int J Endocrinol. 2022;2022:4272715. doi:10.1155/2022/4272715
18. Kuwabara M, Kodama T, Ae R, et al. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens Res. 2023;46(7):1714–1726. doi:10.1038/s41440-023-01273-3
19. Li T, Liu X, Diao S, et al. H-type hypertension is a risk factor for cerebral small-vessel disease. Biomed Res Int. 2020;2020(1):6498903. doi:10.1155/2020/6498903
20. Arora T, Mantur PG, Bidri RC, Mulimani MS. Serum uric acid levels and serum lipid levels in patients with ischemic cerebrovascular accident. J Assoc Physicians India. 2018;66(7):66–68.
21. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13(8):823–833. doi:10.1016/S1474-4422(14)70026-2
22. Su C, Hu D, Wang X, et al. Association between mild cognitive impairment and serum uric acid levels among people aged 55 and above in 4 provinces of China. Wei Sheng Yan Jiu. 2021;50(1):8–14. doi:10.19813/j.cnki.weishengyanjiu.2021.01.003
23. Zhao Y, Dong X, Chen B, et al. Blood levels of circulating methionine components in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:934070. doi:10.3389/fnagi.2022.934070
24. Johnston KC, Bruno A, Pauls Q, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. 2019;322(4):326–335. doi:10.1001/jama.2019.9346
25. Coughlan G, Flanagan E, Jeffs S, et al. Diagnostic relevance of spatial orientation for vascular dementia: a case study. Dement Neuropsychol. 2018;12(1):85–91. doi:10.1590/1980-57642018dn12-010013
26. Qiao J, Zhou K, Huang C, Fu S, Xing Y, Zhang B. Comparison of serum Lp-PLA2 levels in ischemic stroke patients with H-type hypertension or non-H-type hypertension. J Clin Lab Anal. 2020;34(2):e23068. doi:10.1002/jcla.23068
27. Arevalo-Rodriguez I, Smailagic N, Roqué-Figuls M, et al. Mini-mental state examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2021;7(7):CD010783. doi:10.1002/14651858.CD010783.pub3
28. Hu DY, Xu XP. Prevention of stroke relies on valid control “H” type hypertension. Zhonghua Nei Ke Za Zhi. 2008;47(12):976–977.
29. Wang J, Xi YX, Li JQ, Zhu WW. Gender difference in association between H-type hypertension and subcortical ischemic vascular disease. Front Aging Neurosci. 2022;14:998268. doi:10.3389/fnagi.2022.998268
30. Viggiano D, Wagner CA, Martino G, et al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020;16(8):452–469. doi:10.1038/s41581-020-0266-9
31. Honarmand K, Lalli RS, Priestap F, et al. Natural history of cognitive impairment in critical illness survivors. A systematic review. Am J Respir Crit Care Med. 2020;202(2):193–201. doi:10.1164/rccm.201904-0816CI
32. Ristic B, Sikder MO, Bhutia YD, Ganapathy V. Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis. Asian J Pharm Sci. 2020;15(2):173–180. doi:10.1016/j.ajps.2019.10.002
33. Ran F, Liu F, Zhang Y, Chen L. Serum uric acid and high-sensitivity c-reactive protein as predictors of cognitive impairment in patients with cerebral infarction. Dement Geriatr Cognit Disord. 2020;49(3):235–242. doi:10.1159/000507806
34. Cao B, Li Q, Xiong L, et al. Cerebrospinal fluid uric acid levels associated with disease severity in patients with anti-N-methyl-d-aspartate receptor encephalitis. J Neuroimmunol. 2023;384:578221. doi:10.1016/j.jneuroim.2023.578221
35. Wood CA, Zhang J, Aydin D, et al. Structure and mechanism of blood-brain-barrier lipid transporter MFSD2A. Nature. 2021;596(7872):444–448. doi:10.1038/s41586-021-03782-y
36. Markus HS, de Leeuw FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. 2023;18(1):4–14. doi:10.1177/17474930221144911
37. Qin SY, Lan RY, Zeng J, et al. Effect of high-concentration uric acid on nitric oxide. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2023;45(4):666–671. doi:10.3881/j.issn.1000-503X.15049
38. Song L, Zhao Y, He X, et al. Deciphering the causal links: a Mendelian randomization study of circulating inflammatory proteins and cardiovascular diseases. Clin Mol Epidemiol. 2024;1:11. doi:10.53964/cme.2024011
39. Tian T, Liu X-R, Li T-T, et al. Detrimental effects of long-term elevated serum uric acid on cognitive function in rats. Sci Rep. 2021;11(1):6732. doi:10.1038/s41598-021-86279-y
40. Jia W, Jia Q, Pan Y, et al. Relationship between baseline haemoglobin content and poststroke cognitive impairment. J Clin Neurosci. 2022;101:212–216. doi:10.1016/j.jocn.2022.05.008
41. Chen Y, Wu J, Yang Y, Xiong M, Yu X, Lei S. Causal effects of sarcopenia-related traits on ischemic stroke: a two-sample Mendelian randomization study. Clin Mol Epidemiol. 2024;1:9. doi:10.53964/cme.2024009
42. Miwa K, Tanaka M, Okazaki S, et al. Increased total homocysteine levels predict the risk of incident dementia independent of cerebral small-vessel diseases and vascular risk factors. J Alzheimers Dis. 2016;49(2):503–513. doi:10.3233/JAD-150458
43. Niu Y, Zhang H, Li XD, et al. Uric acid is associated with worsening of diastolic function and adverse outcomes in patients with coronary slow flow. Turk Kardiyol Dern Ars. 2023;51(1):3–9. doi:10.5543/tkda.2022.32035
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

