Back to Journals » Journal of Pain Research » Volume 18
Shallow Acupuncture for Chronic Neck Pain: A Multicenter Randomized Controlled Trial Protocol with fMRI and DTI
Authors Lin J , Gu Z , Zhou P, Huang W , Ou A , Zhao Q , Xu Z
Received 15 January 2025
Accepted for publication 2 April 2025
Published 11 April 2025 Volume 2025:18 Pages 1963—1973
DOI https://doi.org/10.2147/JPR.S512989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Houman Danesh
Jiahui Lin,1 Zhilin Gu,1 Peng Zhou,2 Weikang Huang,1 Aihua Ou,1 Qi Zhao,3 Zhenhua Xu1
1The second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Department of Acupuncture, Shenzhen Bao’an Traditional Chinese Medicine Hospital, Shenzhen, People’s Republic of China; 3National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
Correspondence: Qi Zhao, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China, Email [email protected] Zhenhua Xu, The second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China, Email [email protected]
Purpose: This study aims to explore the central mechanisms of shallow acupuncture for chronic neck pain (CNP) using functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), an innovative approach not commonly applied in shallow acupuncture research.
Patients and Methods: This multi-center randomized controlled trial will recruit 252 CNP patients from three centers (84 per center). Participants will be randomly assigned to three groups: shallow acupuncture, drug control (celecoxib capsules), or waiting list group, with 84 patients per group. The primary outcomes include total effective rate, visual analogue scale (VAS), Neck Disability Index (NDI), and brain imaging results (20 randomly selected patients per group). Secondary outcomes include the Self-Rating Anxiety Scale (SAS), Short Form 36 Questionnaire (SF-36), and cervical range of motion (CROM). Data will be collected at baseline, after 2 weeks, and at 3-month follow-up. fMRI and DTI data will be collected at baseline and after 2 weeks. Analyses will include regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), functional connectivity (FC), fractional anisotropy (FA), and mean diffusivity (MD) within and between groups. An additional 20 healthy volunteers will provide baseline fMRI and DTI data for comparison.
Conclusion: This study will validate the clinical efficacy of shallow acupuncture for CNP and explore its central mechanisms using fMRI and DTI. The findings may provide neuroimaging evidence supporting the broader clinical application of shallow acupuncture in treating CNS-related diseases.
Keywords: chronic neck pain, shallow acupuncture, brain functional networks, clinical trial protocol
Introduction
Chronic neck pain (CNP) is a common chronic pain disorder characterized by persistent or recurrent neck pain, typically lasting for more than three months.1 According to epidemiological studies, the global average prevalence of CNP has reached 24.5%, with a growing trend projected for the future.2 CNP not only severely affects the quality of life of patients but also imposes a heavy burden on society and the economy, including increased healthcare costs and a decrease in workforce productivity.3
Currently, Western medicine for the treatment of CNP mainly focuses on pain relief and improving function.4 Common treatment methods include pharmacotherapy, physical therapy, and surgical interventions. Pharmacotherapy is one of the most commonly used approaches, particularly nonsteroidal anti-inflammatory drugs (NSAIDs), among which celecoxib, a COX-2 selective inhibitor, has shown clinical efficacy in the treatment of pain, especially musculoskeletal and joint pain.5,6 However, pharmacotherapy is not without limitations. Long-term use of NSAIDs may lead to gastrointestinal adverse effects, renal damage, and an increased cardiovascular risk.7
Acupuncture has significant advantages in the treatment of chronic pain.8,9 Shallow acupuncture refers to a technique in which the needle is inserted at a relatively small angle and shallow depth, penetrating only the subcutaneous tissue or superficial muscle layers.10 Unlike traditional acupuncture, which targets specific acupoints, shallow acupuncture primarily focuses on locating pathological response points on the skin surface as its needling sites. These include changes in skin color (eg, paleness or darkening), alterations in pore distribution (eg, unusually large pores in a localized area), changes in the skin’s vascular network (eg, superficial arterial and venous abnormalities), alterations in skin tension (eg, noticeable tightness upon light palpation), morphological changes (eg, palpable subcutaneous nodules or cord-like structures upon deep palpation), and sensory abnormalities (eg, tenderness, soreness, or numbness). Studies show that, like traditional acupuncture, shallow acupuncture mediates analgesia by stimulating afferent fibers such as Aα, Aβ (Groups I and II), Aδ (Group III), and C fibers (Group IV).11 With low invasiveness, fewer side effects, and significant efficacy, it has become a key treatment for chronic pain.12
Functional Magnetic Resonance Imaging (fMRI) and Diffusion Tensor Imaging (DTI) play a crucial role in revealing the central mechanisms of chronic pain.13–15 fMRI reflects neural activity by monitoring blood oxygen level changes in brain regions. On the other hand, DTI, an imaging technique that measures the microstructure of brain white matter tracts, can reveal neural pathway changes induced by chronic pain. Functional imaging reveals differences between CNP patients and healthy volunteers.16 And showed acupuncture can modulate signals in brain regions such as the thalamus, insula, caudate, claustrum, and lentiform nucleus, while relieving pain symptoms.17
Clinical evidence has indicated that shallow acupuncture is effective for CNP, potentially offering superior long-term efficacy and safety compared to conventional acupuncture.18,19 Acupuncture also demonstrates analgesic effects comparable to NSAIDs.20 However, direct comparative evidence between shallow acupuncture and analgesic medications (eg, celecoxib) remains lacking, representing an innovative aspect of our current study. Besides, current research on shallow acupuncture for chronic neuropathic pain lacks the use of fMRI and DTI to investigate underlying brain mechanisms. This study will, for the first time, combine fMRI and DTI to clarify the central mechanisms of shallow acupuncture in chronic neck pain treatment.
Materials and Methods
Study Design
This study uses a multicenter randomized controlled trial (RCT) design and will be conducted at three hospitals in China: The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou), First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Tianjin), and Shenzhen Bao’an Traditional Chinese Medicine Hospital (Shenzhen). And will comply with the Declaration of Helsinki.
In terms of ethical review, the study has been approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Approval No.: BF2024-042-01), Ethics Committee of the Shenzhen Bao’an Traditional Chinese Medicine Hospital (Approval No.: KY-2025-007-01), IRB of the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Approval No.: TYLL2024[K]No.021) and registered on the International Traditional Medicine Clinical Trial Registration Platform (ITMCTR, Registration No.: ITMCTR2024000219). Trial period: August 31, 2024 to November 30, 2025.
The study also follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines21 (Table 1) and adheres to the CONSORT statement and STRICTA guidelines for acupuncture clinical trials.22
 |
Table 1 Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Schedule of the Trial |
Study Participants
A total of 252 CNP patients will be recruited based on voluntary participation, with 84 patients recruited at each center. Additionally, 20 healthy volunteers will be recruited at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. All participants must meet the inclusion criteria and sign informed consent forms.
Inclusion Criteria
Participants must meet all of the following criteria:
Exclusion Criteria
Participants will be excluded if they meet any of the following:
Intervention Measures
Acupuncture Group
Shallow acupuncture Points:
The fundamental principles of shallow acupuncture are rooted in classical Chinese medical theory, which posits a correlation between internal disorders and their external manifestations on the body’s surface.24 While this concept shares similarities with the myofascial trigger point (MTrP) theory, it is not entirely identical, as shallow acupuncture specifically targets superficial fascial structures rather than deep muscular trigger points. Consistent with this principle, shallow acupuncture in this study focuses on superficial anatomical structures, particularly hypersensitive superficial fascial tissues (pathological response points), rather than the fixed acupoints traditionally used in conventional acupuncture therapy.19,24,25
The acupuncture points are identified at the posterior occipital region, focusing on pathological response points, which manifest as: (1) skin color changes (such as paleness or darkening), (2) changes in pore distribution (such as abnormally enlarged pores), (3) vascular changes (such as superficial, prominent veins and arteries), (4) skin tension changes (such as noticeable tightness upon light palpation), (5) morphological changes (such as palpable subcutaneous nodules or cord-like structures upon deep palpation), and (6) sensory abnormalities (such as tenderness, soreness, or numbness). These pathological response points are selected as the shallow acupuncture insertion sites.
Shallow acupuncture area on the posterior neck: The upper boundary is the superior nuchal line and the occipital protuberance; the lateral boundaries are the medial borders of the sternocleidomastoid muscles; the lower boundary is the horizontal line at the spinous process of the seventh cervical vertebra.
Shallow acupuncture technique: Disposable acupuncture needles (0.25×25 mm, Beijing Hanyi Medical Instruments Co., Ltd., China) will be used. After routine local disinfection, the needle will be inserted using either the subcutaneous or oblique insertion technique, with the needle tip directed toward the pain site at an angle of 10°–25° to the skin. The needle will be quickly inserted to the skin. The depth and angle will be adjusted based on the patient’s symptom relief during the procedure. No manipulation will be performed during needle retention, and patients will be instructed to perform slight neck and shoulder movements. All acupuncture procedures will be carried out by the same experienced clinician. The treatment will be administered every other day, with three sessions per week for a total of 2 weeks (6 treatments). Each session will involve needle retention for 15 minutes.
Drug Control Group
Participants in this group will receive oral celecoxib capsules (100 mg, Shiyao Group Ouyi Pharmaceutical Co., Ltd., China, STATE MEDICAL PERMITMENT No.H20203296). The dosage will be 100 mg twice daily for 14 consecutive days.
Waitlist Control Group
Participants in this group will enter a 2-week waiting period with no treatment. After the waiting period, they will receive the same shallow acupuncture treatment as the acupuncture group, following the same protocol.
Healthy Control Group
Healthy volunteers are recruited as a healthy control group and undergo identical fMRI and DTI scanning protocols. Their data serve as baseline references, enabling precise identification of specific functional and structural brain alterations in patients with CNP following shallow acupuncture treatment.
Outcome Measurement
At baseline time, record basic information of all participants, including age, sex, education level, occupation, disease duration, etc. Additionally, document the type and location of the pathological response points in the posterior neck region for each participant in a table.
Primary Outcome Measures
The primary outcome measures include clinical efficacy rate, Visual Analog Scale (VAS), Neck Disability Index (NDI), and brain functional imaging indicators.
Clinical Efficacy Rate: According to the Guidelines for Evaluation of traditional Chinese medicine (TCM) Diagnosis and Treatment (DB44/T 1425–2014) and General Rules for the Formulation and Revision of the Diagnosis and Efficacy Evaluation Standards of TCM Diseases and Syndromes (ZY/T 10–2024), the clinical efficacy rate is categorized as follows:
(1) Cure: Symptoms completely disappear, and the patient can resume normal life and work with no discomfort.
(2) Marked improvement: Symptoms are nearly gone, with only occasional mild neck pain that lasts for a short time and does not significantly affect daily life.
(3) Improvement: Neck pain severity, extent, frequency, and duration are reduced, though symptoms may recur.
(4) No effect: No significant change in symptoms before and after treatment, with no improvement.
VAS: The Visual Analog Scale (VAS) is a simple tool for measuring pain intensity.26 It consists of a 10 cm line with endpoints representing “no pain” and “worst possible pain.” Patients mark a point on the line to indicate their pain level, providing a quantitative measure of pain.
NDI: The Neck Disability Index (NDI) is a 10-item questionnaire that measures the impact of neck pain on daily activities.27 It assesses areas such as pain, personal care, lifting, and reading. The total score reflects the level of disability, with higher scores indicating greater impact.28
Brain functional imaging indicators: Brain Function Indicators include Functional Magnetic Resonance Imaging (fMRI) and Diffusion Tensor Imaging (DTI) analyses. fMRI analysis includes Regional Homogeneity (ReHo), Functional Connectivity (FC), and Amplitude of Low-Frequency Fluctuations (ALFF). DTI analysis includes Fractional Anisotropy (FA) and Mean Diffusivity (MD).
Secondary Outcome Measures
The secondary outcome measures include the Self-Rating Anxiety Scale (SAS), Short Form 36 Questionnaire (SF-36), and Cervical Range of Motion (CROM).
SAS: The Self-Rating Anxiety Scale (SAS) is a widely used tool to assess anxiety levels in individuals.29 It consists of 20 items, each rated on a 4-point scale, reflecting the frequency and intensity of anxiety symptoms. The total score helps gauge the severity of anxiety, with higher scores indicating greater anxiety.
SF-36: The Short Form 36 Questionnaire (SF-36) is a tool for assessing overall health and quality of life.28 It includes 36 items across eight domains, covering physical and mental health. The SF-36 is commonly used in clinical research and practice to evaluate health status and track changes over time.
CROM: The Cervical Range of Motion (CROM) is a measure of neck mobility, assessing the range of motion in the cervical spine.30 It is commonly used to evaluate neck function and flexibility in individuals with neck pain.31 In this study, we use a multi-functional angle measurement electronic device, EasyAngle (Meloq, Sweden), to measure the patient’s forward flexion, extension, left and right rotation, and left and right lateral flexion angles. Each measurement is performed three times, and the average value is taken.
Clinical assessments were performed using standardized questionnaires. Questionnaires were administered by uniformly trained third-party researchers blinded to the study aims and interventions. Participants completed questionnaires independently under neutral conditions, free from suggestive influence. Researchers were instructed not to guide or influence responses, though clarification could be provided if needed. Completed questionnaires were immediately collected and processed independently by blinded analysts.
Observation Time Points
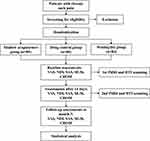
All patients will complete the VAS, NDI, SAS, SF-36, and CROM assessment before treatment, after treatment, and at the 3-month follow-up. In the acupuncture group, drug control group, and waiting treatment group recruited at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, 20 patients will be randomly selected from each group to undergo fMRI and DTI scans before and after treatment. Twenty healthy controls will complete the fMRI and DTI scans during the study period. The flow chart of the study is shown in Figure 1.
fMRI and DTI Scan Parameters
In this study, fMRI and DTI scans were conducted using a Magnetom Prisma 3.0T MRI scanner (Siemens, Germany) at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. Prior to scanning, all participants were screened for contraindications and prepared according to standard procedures. During the scan, participants were instructed to lie supine, close their eyes, relax, and remain awake. Appropriate protective measures and close monitoring during the imaging procedures were implemented to minimize such risks, ensuring participant safety and ethical compliance. The specific scanning and data processing steps are as follows:
The localization images were acquired with 53 slices, a repetition time (TR) of 520 ms, echo time (TE) of 4.92 ms, slice thickness of 2.0 mm, and a field of view (FOV) of 192 mm × 192 mm. The flip angle was set to 60°, and the voxel size was 3.0 mm × 3.0 mm × 2.0 mm, with a basic resolution of 256×256.
For structural imaging, 3D T1-weighted images of the whole brain (T1WI) were obtained using a gradient echo sequence. The scan parameters included 192 slices, TR of 1900 ms, TE of 2.26 ms, slice thickness of 1.0 mm, and a FOV of 256 mm × 256 mm. The flip angle was 9°, and the voxel size was 3.0 mm × 3.0 mm × 2.0 mm, with a resolution of 256×256.
Functional images were acquired using a T2-weighted gradient echo EPI sequence for BOLD functional imaging. The scan parameters were set to 37 slices, a TR of 2000 ms, TE of 30 ms, slice thickness of 3.0 mm, and a FOV of 224 mm × 224 mm. The flip angle was 90°, and the voxel size was 3.5 mm × 3.5 mm × 3.0 mm, with a basic resolution of 64×64.
For DTI, the scan included 25 slices with a TR of 3500 ms, TE of 95 ms, slice thickness of 4.0 mm, and a FOV of 220 mm × 220 mm. The voxel size was set to 1.7 mm × 1.7 mm × 4.0 mm, with a basic resolution of 128×128.
fMRI data were processed using the DPABI software package, based on MATLAB and Statistical Parametric Mapping (SPM) platforms. Data preprocessing included discarding the first 10 time points, head motion correction, spatial normalization to the standard MNI template, spatial smoothing using a Gaussian kernel (FWHM = 6 mm), band-pass filtering (0.01–0.08 hz), and removal of covariates (including head motion parameters, cerebrospinal fluid, and white matter signals). Group-level differences were analyzed using the General Linear Model (GLM), with multiple comparisons strictly corrected using Family-wise error (FWE) correction (corrected p < 0.05).
ReHo was calculated using Kendall’s coefficient of concordance (KCC), measuring the temporal similarity of each voxel with its nearest 26 neighboring voxels. The resulting ReHo maps quantified local synchronization of spontaneous brain activity and were standardized (Z-scored) for subsequent statistical analysis.
FC analysis involved extracting the mean time series from predefined seed regions (ROIs) and calculating Pearson’s correlation coefficients between these time series and those from all other brain voxels. Correlation coefficients were then transformed using Fisher’s Z transformation to normalize data distributions for statistical purposes.
ALFF was calculated by performing Fast Fourier Transform (FFT) on the time series of each voxel and computing the amplitude within the low-frequency band (0.01–0.08 hz). ALFF maps were standardized via Z-score transformation prior to further statistical comparisons.
DTI data were analyzed using the FMRIB Software Library (FSL) software package. Data preprocessing included eddy current correction, motion correction, registration, extraction of regions of interest (ROIs, particularly STT-related brain areas), and calculation of diffusion metrics (FA and MD). Statistical comparisons between groups utilized permutation testing provided by FSL’s Randomise tool with threshold-free cluster enhancement (TFCE), ensuring robust multiple comparisons correction (corrected p < 0.05).
To maintain statistical rigor and minimize false-positive rates, strict multiple comparisons corrections were employed across all neuroimaging analyses. Specifically, FWE correction was used for fMRI results, and TFCE permutation testing was used for DTI analyses. The statistical significance threshold was set at corrected p < 0.05 for all imaging data, ensuring the reliability, robustness, and objectivity of the study findings.
Sample Size Estimation
Based on a literature review, the total effective rate of celecoxib in treating musculoskeletal pain is approximately 75% and the effective rate of shallow acupuncture for neck pain is about 90%,25 with P1 = 75% and P2 = 90%. Using the formula:
For a one-sided test with α=0.05 and β=0.10. Substituting the values into the formula, we get n1=n2=76. Including the waiting treatment group and accounting for a 10% loss to follow-up, a total of 252 chronic neck pain patients will be enrolled. Each sub-center will recruit 84 participants, and an additional 20 healthy volunteers will be recruited at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. The total sample size will be 272. Although larger sample sizes may enhance statistical power in imaging studies, considering the complexity, cost, and recommendations from previous literature for fMRI/DTI studies, we determined a sample size of 20 participants per group, exceeding the minimum recommended size (12 per group), ensuring stability and reliability of our results.32,33
Randomization and Blinding
A block randomization method was used to randomly assign patients in a 1:1:1 ratio to the acupuncture group, drug control group, and waiting treatment group. The random sequence was generated by an independent statistician using a computer and allocation was carried out using sealed opaque envelopes. Treatment personnel were completely blinded to the study’s objectives throughout the trial.
To further ensure the implementation of blinding, data collectors were entirely independent from the treatment personnel and remained blinded to both the study’s objectives and the intervention details. All assessments and data collection were performed by researchers who were not involved in the intervention process and were unaware of the patient group assignment. Statistical analysts also remained blinded to patient group information, ensuring that the data analysis was objective and unbiased.
Although complete blinding is challenging due to the nature of shallow acupuncture intervention, we minimized potential bias by using independent random sequence generation, sealed envelope allocation, and blinded assessors throughout the study, ensuring the rigor and scientific integrity of the trial.
Statistical Analysis
The statistical analysis will be performed by independent third-party personnel to avoid bias. All data will be entered into Excel and analyzed using SPSS 26.0 software (cases with dropouts or exclusions will not be included in the analysis). Continuous variables will be expressed as means with standard deviations. If the data follow a normal distribution, paired t-tests will be used; if not, the rank-sum test will be applied. Categorical and ordinal data will be presented as proportions (%). For categorical data in a one-way ordered R×C table, the rank-sum test will be used to compare the effects among treatment groups. Ordinal data will also be analyzed using the rank-sum test. The correlation between clinical efficacy indicators and imaging parameters will be analyzed using Pearson’s correlation analysis (for normally distributed data) or Spearman’s rank correlation analysis (for non-normally distributed data). All statistical tests will be two-tailed, and a p-value < 0.05 will be considered statistically significant.
Discussion
The potential novelty of this study includes the first combined use of fMRI and DTI to reveal central neural mechanisms underlying shallow acupuncture treatment for chronic neck pain, clarifying specific brain functional and structural alteration patterns. Additionally, this study provides the first direct comparison of shallow acupuncture with analgesic medication (celecoxib), offering objective neuroimaging and clinical evidence to significantly advance acupuncture research and clinical practice.
Shallow acupuncture may exert analgesic effects by stimulating mechanoreceptors within superficial fascial tissues, thus modulating peripheral sensory input and reducing nociceptive signal transmission to the central nervous system (CNS).34 Neurophysiological responses induced by shallow acupuncture likely involve central sensory processing networks, including sensory cortical regions.35 However, current evidence clarifying the specific central mechanisms and brain network alterations of shallow acupuncture remains limited. Clarifying these mechanisms could extend clinical applications of shallow acupuncture to treating various CNS-related diseases. The clinical significance of this research lies in enhancing understanding of acupuncture’s central analgesic mechanisms, thereby potentially broadening therapeutic applications in clinical practice.
Central sensitization is one of the key factors contributing to the difficulty in curing and recurrent nature of chronic pain36 Existing research has shown that patients with chronic pain experience structural and functional remodeling of the central nervous system, manifested as changes in multiple key regions of pain transmission and regulation pathways.37 Previous studies have indicated that, under nociceptive stimuli, various brain regions, including the thalamus, primary and secondary somatosensory cortices, anterior and mid-cingulate cortices, and insula, collectively known as the “pain matrix”, show fMRI signal activation.38
The spinothalamic tract (STT) is a major ascending pain pathway (bottom-up effect), transmitting nociceptive signals from peripheral tissues to the central nervous system. Anatomically, the STT carries nociceptive information, including intensity, from the dorsal horn of the spinal cord, ascending through the lateral spinal column to the ventral posterolateral nucleus (VPL) of the thalamus, and subsequently projecting to the primary (S1) and secondary (S2) somatosensory cortices for pain perception and integration.39
Previous studies have demonstrated significant white matter microstructural alterations in the STT of patients with CNP.40 Moreover, FA and MD values correlate negatively with pain intensity and duration, suggesting that the extent of structural impairment in STT fibers closely relates to pain severity. However, the study compared mean diffusion metrics within the STT between patients with CNP and healthy controls showed no statistically significant differences were identified between groups.40
Given that shallow acupuncture stimulates peripheral receptors in superficial fascia, potentially modulating sensory transmission and decreasing nociceptive input to central networks, we hypothesize it may reduce peripheral-to-central sensitization mediated via STT pathways. These preliminary neuroimaging findings offer novel insights into the anatomical mechanisms underlying central sensitization in CNP patients, meriting further exploration.
Compared to healthy individuals, patients with chronic neck pain exhibit structural abnormalities in the brain, including smaller gray matter volumes in the anterior cingulate cortex and the parietal lobule.41 Functional abnormalities are also present in chronic neck pain patients, with signal changes observed in regions such as the anterior central gyrus, precuneus, superior temporal gyrus, superior parietal cortex, and lateral orbitofrontal cortex.42 Furthermore, in these patients, pain intensity correlates with the anterior insular cortex, the frontal operculum, and the pons.43 Combining DTI technology, reduced FA values are detected in multiple white matter regions in chronic neck pain patients, alongside increased MD and radical diffusion (RD) values. These changes are associated with the intensity of the patients’ pain.44
In recent years, an increasing number of studies have used brain functional imaging techniques to explore the central mechanisms of acupuncture in treating CNP. fMRI studies have shown that, after acupuncture treatment, the reduced ReHo values in the temporo-parietal junction of CNP patients significantly increased, and this increase was correlated with the degree of pain relief in the neck.45 Research also found that, compared to healthy controls, chronic neck, and shoulder pain patients exhibited both positive and negative FC between the periaqueductal gray (PAG) and different brain regions.46 Acupuncture was shown to normalize the FC between the PAG and other brain regions in CNP patients, indicating that acupuncture’s effects on CNP are related to the functional circuits of the PAG.47 Recent studies further suggest that acupuncture’s impact on CNP involves increased FC between the dorsal raphe (DR) and the thalamus, as well as between the median raphe nucleus (MR) and the parahippocampal gyrus, amygdala, and insula. In contrast, FC was decreased between the DR and the cingulate gyrus, and between the MR and the cingulate gyrus. Additionally, changes in DR-related circuits were found to correlate with the intensity and duration of pain.48
Neuroimaging studies suggest that NSAIDs (eg, ibuprofen) significantly decrease brain activity within pain-processing regions, particularly those involved in top-down modulation circuits (eg, insular cortex), in patients with acute pain.49,50 This indicates a potential central mechanism for NSAID-induced analgesia. However, how NSAIDs precisely modulate CNS activities in chronic pain conditions, specifically whether celecoxib similarly reverses altered insular connectivity or dysfunctional brain networks observed in chronic neck pain patients, remains unclear and warrants further investigation.
The regions of interest (ROIs) selected in this study were determined based on previous literature and preliminary findings from our pilot study. Brain regions known for pain modulation and processing, such as the thalamus, insular cortex, S1, and S2, have been extensively reported to exhibit structural and functional alterations in chronic pain conditions.45,49 Thus, we selected these specific ROIs to comprehensively investigate the effects of shallow acupuncture on brain structure and function, aiming to clarify the underlying central neural mechanisms. Nonetheless, further exploratory analysis may identify additional novel brain regions relevant to chronic pain, which could be targets for future investigation.
In conclusion, the strengths of this study include the first integration of fMRI and DTI imaging to clarify specific central neural mechanisms underlying shallow acupuncture for chronic neck pain and the inclusion of comparative groups to enhance clinical relevance. However, limitations exist, such as a relatively small sample size, short follow-up duration, and inability to fully blind the acupuncture intervention. Future studies should employ larger sample sizes, longer follow-up periods, and examine shallow acupuncture’s effectiveness across different chronic pain conditions or neurological disorders.
Funding
This study is supported by Open Project of National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion (Grant No. NCRCOP20230010).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cohen SP. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin Proc. 2015;90(2):284–299. doi:10.1016/j.mayocp.2014.09.008
2. Global. regional, and national burden of neck pain, 1990-2020, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. 2024;6(3):e142–e155. doi:10.1016/s2665-9913(23)00321-1.
3. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi:10.1016/s0140-6736(21)00393-7
4. Blanpied PR, Gross AR, Elliott JM, et al. Neck Pain: revision 2017. J Orthop Sports Phys Ther. 2017;47(7):A1–a83. doi:10.2519/jospt.2017.0302
5. Krasselt M, Baerwald C. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin Pharmacother. 2019;20(14):1689–1702. doi:10.1080/14656566.2019.1645123
6. Tive L. Celecoxib clinical profile. Rheumatology (Oxford). 2000;39(Suppl 2):21–28. discussion 57-9.doi:10.1093/rheumatology/39.suppl_2.21
7. Felson DT. Safety of nonsteroidal antiinflammatory drugs. N Engl J Med. 2016;375(26):2595–2596. doi:10.1056/NEJMe1614257
8. Vickers AJ, Vertosick EA, Lewith G, et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. 2018;19(5):455–474. doi:10.1016/j.jpain.2017.11.005
9. Kelly RB, Willis J. Acupuncture for pain. Am Fam Physician. 2019;100(2):89–96.
10. Baldry P. Superficial versus deep dry needling. Acupunct Med. 2002;20(2–3):78–81. doi:10.1136/aim.20.2-3.78
11. Kagitani F, Uchida S, Hotta H. Afferent nerve fibers and acupuncture. Auton Neurosci. 2010;157(1–2):2–8. doi:10.1016/j.autneu.2010.03.004
12. Feng XX, Huang KY, Chen L, Zhou K. Clinical efficacy of the shallow puncture and more-twirling acupuncture method in migraine treatment and its effects on serum 5-HT and β-EP levels. Technol Health Care. 2023;31(S1):533–540. doi:10.3233/thc-236047
13. Zhu D, Zhang T, Jiang X, et al. Fusing DTI and fMRI data: a survey of methods and applications. Neuroimage. 2014;102(Pt 1):184–191. doi:10.1016/j.neuroimage.2013.09.071
14. Kashanian A, Tsolaki E, Caruso J, Bari A, Pouratian N. Imaging as a pain biomarker. Neurosurg Clin N Am. 2022;33(3):345–350. doi:10.1016/j.nec.2022.02.011
15. Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am. 2011;22(2):133–9,vii. doi:10.1016/j.nec.2010.11.001
16. Cheng L, Zhang J, Xi H, et al. Abnormalities of brain structure and function in cervical spondylosis: a multi-modal voxel-based meta-analysis. Front Neurosci. 2024;18:1415411. doi:10.3389/fnins.2024.1415411
17. Ha G, Tian Z, Chen J, et al. Coordinate-based (ALE) meta-analysis of acupuncture for musculoskeletal pain. Front Neurosci. 2022;16:906875. doi:10.3389/fnins.2022.906875
18. Kligler B, Nielsen A, Kohrherr C, et al. Acupuncture therapy in a group setting for chronic pain. Pain Med. 2018;19(2):393–403. doi:10.1093/pm/pnx134
19. Gu Z, Deng M, Zhu J, Xu Z. Effect of Qianci acupuncture therapy for cervical spondylosis of neck type. Guangxi Med J. 2022;44(09):959–963.
20. Zhou J, Zeng F, Cheng S, et al. Modulation effects of different treatments on periaqueductal gray resting state functional connectivity in knee osteoarthritis knee pain patients. CNS Neurosci Ther. 2023;29(7):1965–1980. doi:10.1111/cns.14153
21. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586. doi:10.1136/bmj.e7586
22. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. J Altern Complement Med. 2010;16(10):St1–14. doi:10.1089/acm.2010.1610
23. Editor. The experts consensus on the classification, diagnosis and non-surgical treatment of cervical spondylisis (2018). Chin J Surg. 2018;56(6):401–402. doi:10.3760/cma.j.issn.0529-5815.2018.06.001.
24. Xu Z, Fu W, Liu J. The meridian diagnostic system in the internal classic and its clinical application. J Jiangxi Univ Traditional Chin Med. 2007;(02):46–48.
25. Liu C, Xie Y, Xu Z. Clinical study on different acupuncture treatments for cervical spondylosis and neck pain. China J Tradition Chinese Med Pharm. 2018;33(08):3685–3688.
26. Jones LB, Jadhakhan F, Falla D. The influence of exercise on pain, disability and quality of life in office workers with chronic neck pain: a systematic review and meta-analysis. Appl Ergon. 2024;117:104216. doi:10.1016/j.apergo.2023.104216
27. Pool JJ, Ostelo RW, Hoving JL, Bouter LM, de Vet HC. Minimal clinically important change of the neck disability index and the numerical rating scale for patients with neck pain. Spine (Phila pa 1976). 2007;32(26):3047–3051. doi:10.1097/BRS.0b013e31815cf75b
28. Carreon LY, Glassman SD, Campbell MJ, Anderson PA. Neck Disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J. 2010;10(6):469–474. doi:10.1016/j.spinee.2010.02.007
29. Dunstan DA, Scott N. Norms for Zung’s self-rating anxiety scale. BMC Psychiatry. 2020;20(1):90. doi:10.1186/s12888-019-2427-6
30. Wolan-Nieroda A, Guzik A, Mocur P, Drużbicki M, Maciejczak A. Assessment of Interrater and Intrarater Reliability of Cervical Range of Motion (CROM) Goniometer. Biomed Res Int. 2020;2020:8908035. doi:10.1155/2020/8908035
31. Fletcher JP, Bandy WD. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J Orthop Sports Phys Ther. 2008;38(10):640–645. doi:10.2519/jospt.2008.2680
32. Guo Q, Thabane L, Hall G, McKinnon M, Goeree R, Pullenayegum E. A systematic review of the reporting of sample size calculations and corresponding data components in observational functional magnetic resonance imaging studies. Neuroimage. 2014;86:172–181. doi:10.1016/j.neuroimage.2013.08.012
33. Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118(2):115–128. doi:10.1016/s0165-0270(02)00121-8
34. Fuller AM, Luiz A, Tian N, et al. Gate control of sensory neurotransmission in peripheral ganglia by proprioceptive sensory neurons. Brain. 2023;146(10):4033–4039. doi:10.1093/brain/awad182
35. Veldman MP, Maffiuletti NA, Hallett M, Zijdewind I, Hortobágyi T. Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans. Neurosci Biobehav Rev. 2014;47:22–35. doi:10.1016/j.neubiorev.2014.07.013
36. Volcheck MM, Graham SM, Fleming KC, Mohabbat AB, Luedtke CA. Central sensitization, chronic pain, and other symptoms: better understanding, better management. Cleve Clin J Med. 2023;90(4):245–254. doi:10.3949/ccjm.90a.22019
37. Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J mol Sci. 2019;20(13). doi:10.3390/ijms20133130
38. Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154(Suppl 1):S29–s43. doi:10.1016/j.pain.2013.09.001
39. Al-Chalabi M, Reddy V, Gupta S. Neuroanatomy, Spinothalamic Tract. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC; 2025. StatPearls.
40. Qiu Z, Liu T, Zeng C, Yang M, Xu X. Local abnormal white matter microstructure in the spinothalamic tract in people with chronic neck and shoulder pain. Front Neurosci. 2024;18:1485045. doi:10.3389/fnins.2024.1485045
41. Woodworth DC, Holly LT, Mayer EA, Salamon N, Ellingson BM. Alterations in cortical thickness and subcortical volume are associated with neurological symptoms and neck pain in patients with cervical spondylosis. Neurosurgery. 2019;84(3):588–598. doi:10.1093/neuros/nyy066
42. Zhang J, Wang H, Guo L. Investigating the brain functional abnormalities underlying pain hypervigilance in chronic neck and shoulder pain: a resting-state fMRI study. Neuroradiology. 2024;66(8):1353–1361. In English. doi:10.1007/s00234-024-03286-2
43. Mayr A, Jahn P, Stankewitz A, et al. Patients with chronic pain exhibit individually unique cortical signatures of pain encoding. Hum Brain Mapp. 2022;43(5):1676–1693. doi:10.1002/hbm.25750
44. Li D, Xu H, Yang Q, Zhang M, Wang Y. Cerebral white matter alterations revealed by multiple diffusion metrics in cervical spondylotic patients with pain: a TBSS study. Pain Med. 2022;23(5):895–901. doi:10.1093/pm/pnab227
45. Chen J, Wang Z, Tu Y, et al. Regional homogeneity and multivariate pattern analysis of cervical spondylosis neck pain and the modulation effect of treatment. Front Neurosci. 2018;12:900. In English. doi:10.3389/fnins.2018.00900
46. Yu CX, Li B, Xu YK, et al. Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. Neuroreport. 2017;28(12):720–725. doi:10.1097/wnr.0000000000000819
47. Xu H, Chen Y, Tao Y, et al. Modulation effect of acupuncture treatment on chronic neck and shoulder pain in female patients: evidence from periaqueductal gray-based functional connectivity. CNS Neurosci Ther. 2022;28(5):714–723. doi:10.1111/cns.13803
48. Wang X, Ni X, Ouyang X, et al. Modulatory effects of acupuncture on raphe nucleus-related brain circuits in patients with chronic neck pain: a randomized neuroimaging trial. CNS Neurosci Ther. 2024;30(3):e14335. doi:10.1111/cns.14335
49. Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp. 2015;36(6):2075–2092. doi:10.1002/hbm.22757
50. Kröger IL, May A. Central effects of acetylsalicylic acid on trigeminal-nociceptive stimuli. J Headache Pain. 2014;15(1):59. doi:10.1186/1129-2377-15-59
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.