Back to Journals » Infection and Drug Resistance » Volume 18
Shifting of Distribution and Changing of Antibiotic Resistance in Gram-Positive Bacteria from Bile of Patients with Acute Cholangitis
Authors Hao Y , Li L , Du W , Lu J
Received 4 August 2024
Accepted for publication 31 January 2025
Published 27 February 2025 Volume 2025:18 Pages 1187—1197
DOI https://doi.org/10.2147/IDR.S482375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Yuqi Hao,1 Lianxin Li,2 Wenting Du,1 Jinshuai Lu1
1Department of General Internal Medicine, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, People’s Republic of China; 2Endoscopy Center, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, People’s Republic of China
Correspondence: Jinshuai Lu, Department of General Internal Medicine, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, People’s Republic of China, Email [email protected]
Background: Gram-negative bacteria are the predominant pathogens responsible for biliary infections; however, the prevalence of Gram-positive bacteria is currently increasing. Investigating the bacterial spectrum and evolving antibiotic resistance patterns of Gram-positive bacteria is crucial for optimizing the management of acute cholangitis, particularly in the context of the global rise in antibiotic resistance.
Methods: This retrospective analysis focused on Gram-positive bacteria isolated from the bile of patients undergoing biliary drainage with acute cholangitis at our hospital from January 1, 2018, to March 31, 2024. In total, 342 strains of Gram-positive bacteria were examined.
Results: The main Gram-positive bacteria detected included Enterococcus (57.23%), Staphylococcus (23.41%), and Streptococcus (13.01%). The most common species detected were Enterococcus faecium (36.42%), Enterococcus faecalis (14.16%), and Staphylococcus epidermidis (7.80%). Trend analysis revealed a decrease in the proportion of Enterococcus and an increase in Streptococcus. Additionally, the detection rate of methicillin-resistant Staphylococcus (MRS) showed a significant rise. Gram-positive bacteria exhibited high resistance to erythromycin and penicillin but remained highly susceptible to linezolid and vancomycin. Further, resistance to quinolones among Gram-positive bacteria was notably elevated.
Conclusion: The bacterial spectrum and antibiotic resistance patterns of Gram-positive bacteria in acute cholangitis have undergone significant changes. Penicillin is not recommended for the treatment of Gram-positive bacterial infections. Antibiotic resistance should be closely monitored when using quinolones. Particular attention is warranted regarding the markedly increasing antibiotic resistance of Enterococcus faecium.
Keywords: acute cholangitis, bile culture, gram-positive bacteria, distribution, antibiotic resistance
Introduction
Acute biliary infection is a common clinical diagnosis, which can lead to severe infections and even death if not treated quickly.1 Timely biliary drainage, rapid removal of causative factors, and combination with anti-infection treatment are the recommended therapeutic approaches for this disease.2 Currently, antibiotic resistance represents a significant global challenge, and the early utilization of targeted narrow-spectrum antibiotics can help prevent the emergence of resistant bacteria.3,4 In biliary infections, bile cultures demonstrate a significantly higher positivity rate than blood cultures.5,6 Therefore, obtaining bile for bacterial culture and susceptibility testing is considered the gold standard for guiding antibiotic therapy.2 Empirical anti-infection therapy is currently mainly against Gram-negative bacteria; however, studies from various regions and countries indicate a shifting bacterial spectrum in bile, with a gradual increase in the detection rate of Gram-positive bacteria.6,7 In some cases, Gram-positive bacteria have emerged as the primary pathogens of infections.6,8,9 Concurrently, antibiotic resistance among these bacteria is evolving, potentially complicating clinical management and presenting significant challenges to effective treatment strategies.
The most common Gram-positive bacteria detected in the bile of patients with acute cholangitis are Enterococcus, which mainly include Enterococcus faecium and Enterococcus faecalis. Guidelines recommend broad spectrum β-lactams and β-lactamase inhibitors for the treatment of Enterococcus infections.10 However, recent research has indicated a significant temporal increase in the resistance of Enterococcus to commonly used antibiotics, including penicillin and ampicillin, posing challenges to effective therapy. Compared with Enterococcus faecalis, Enterococcus faecium has stronger resistance to antibiotics,8 such as penicillin (85.6% vs.6.8%), ampicillin (83.8% vs2.1%), high-concentration gentamicin (41.5% vs 31%), levofloxacin (84% vs28.2%), vancomycin (3.7% vs0.1%), and linezolid (0.4% vs1.9%) and tigecycline (0% vs0%).11 Enterococcus faecium demonstrates increasing resistance to erythromycin, penicillin, ampicillin, and quinolones. In contrast, Enterococcus faecalis shows a decline in resistance to penicillin (14.8% vs 2.8%), with no significant changes observed in its resistance to other commonly used antibiotics.12
Staphylococcus is another major type of Gram-positive bacterium, with MRS representing a significant concern in antibiotic resistance. In recent years, the isolation rate of MRS has surpassed 40% in certain regions of China.13 MRS exhibits high resistance to macrolides, aminoglycosides, and quinolones, while maintaining high susceptibility to vancomycin, linezolid, teicoplanin, and tigecycline.14,15
A majority of Gram-positive bacteria are highly sensitive to linezolid (97%) and vancomycin (89.1%).16 Linezolid serves as the last line of defence against Gram-positive bacterial infections. However, with its widespread clinical use in recent years, there has been a growing number of reports documenting the emergence of resistance to linezolid among Gram-positive bacteria.17,18
Empirical therapy currently serves as the primary approach for managing acute cholangitis in clinical practice. Due to the predominant role of Gram-negative bacteria in acute cholangitis, Gram-positive bacteria often receive limited attention. Patients with suspected Gram-positive bacterial infections are frequently treated with broad-spectrum antibiotics in the absence of targeted therapy. Existing studies on Gram-positive bacteria primarily utilize samples from sputum, urine, secretions, and blood, with limited focus on bile samples. In the present study, antibiotic susceptibility data of Gram-positive bacteria isolated from the bile of patients with acute cholangitis were analysed. The distribution, temporal changes in distribution, antibiotic resistance patterns, and resistance trends of Gram-positive bacteria in bile were examined. These findings provide an evidence-based framework for optimizing anti-infective therapy in cases of cholangitis caused by Gram-positive bacteria.
Methods
Data were collected from patients at our hospital from January 1, 2018, to March 31, 2024. Inclusion criteria: (1) Conformity to the diagnostic criteria for acute cholangitis. (2) Underwent biliary drainage procedures, such as endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangial drainage (PTCD), or biliary tract incision drainage. (3) Presence of Gram-positive bacterial infections confirmed by bile cultures. Exclusion criteria: (1) Use of antibiotics for more than 3 days prior to surgery. (2) Positive bile cultures for Gram-negative bacteria or fungi (Figure 1).
 |
Figure 1 Study flowchart. |
Bile bacterial culture and identification: Bile samples were inoculated onto blood agar and McConkey agar media, and isolated strains were identified using VITEK 2 Compact (MERIEUX, France). The Kirby-Bauer paper diffusion (K-B) method was employed for antibiotic susceptibility testing. In accordance with the Tokyo Guidelines,10 recommendations from Chinese medical associations,19 and regional antibiotic usage practices, antibiotics with continuous records of susceptibility against the same bacterial species during the study period were selected for testing. The present study was conducted in compliance with the principles of the Helsinki Declaration and was approved by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region (KY2022072217). The patients and their families provided written informed consent before the biliary drainage procedure.
Data Analysis
The analysis focused on the distribution and changes of Gram-positive bacterial communities in bile cultures from January 2018 to March 2024, examining the resistance and distribution trends of the major bacterial groups. Statistical analysis was conducted using SPSS 29.0 software. Count data were reported as case numbers and percentages. Yearly comparisons were made using the Chi-square test or Fisher’s exact test. Statistical significance for comparisons between two groups was established at P < 0.05. For comparisons spanning the six-year period, adjustments were made using the Bonferroni method, setting the adjusted P-value at 0.003.
Results
This study encompassed all Gram-positive bacterial strains isolated from the bile of patients diagnosed with acute cholangitis from January 1, 2018, to March 31, 2024. A total of 346 strains of Gram-positive bacteria were detected, including 9 genera and 40 species. The dominant genera were Enterococcus, Staphylococcus and Streptococcus, which accounted for 57.22% (198/346), 23.41% (81/346), and 13.01% (45/346), respectively (Figure 2a).
 |
Figure 2 Distribution and trend of Gram-positive bacteria at the genus and species levels. (a) Distribution of bacteria at the genus level. (b) Trends in the number of genera and species of bacteria. |
The diversity of Gram-positive bacteria detected has shown an annual increase. In 2018, 10 species were identified, rising to 21 species by 2023 (Figure 2b). New species that had not been previously observed were progressively detected over the years, including Streptococcus constellatus, Bacillus licheniformis, Bacillus cereus, and Granulicatella adiacens, first identified in 2020.
The main pathogens detected were Enterococcus faecium, Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus hominis and Staphylococcus aureus, which accounted for 36.42% (126/346), 14.16% (49/346), 7.80% (27/346), 6.07% (21/346), and 5.20% (18/346), respectively (Figure 3).
 |
Figure 3 Distribution of Gram-positive bacteria at the species levels. |
Annual trend analysis of the main pathogens at the genus and species levels revealed notable changes. The detection rate of Enterococcus decreased overall, with the distribution of Enterococcus faecium remaining stable, while the proportion of Enterococcus faecalis exhibited a downward trend. The detection rate of Staphylococcus showed fluctuations but increased overall. Within this genus, the distribution of Staphylococcus epidermidis remained stable, the detection rate of Staphylococcus hominis increased, and the proportion of Staphylococcus aureus declined. Similarly, the detection rate of Streptococcus fluctuated but ultimately showed an upward trend, with a marked increase in the proportion of Streptococcus oralis (Figure 4).
 |
Figure 4 Trends of major bacteria at the genus and species levels.(a)Trends in infection rates of major bacteria at the genus level. (b)Trends of major bacteria at the species level. |
A chi-square test was conducted to analyse the annual proportions of the main genera and species. The results indicated a significant increase in the proportion of Streptococcus, particularly Streptococcus oralis (Table 1).
 |
Table 1 Distribution and P values of Gram-Positive Bacteria Detected in Bile |
Enterococcus faeciumand Enterococcus faecalis
Enterococcus faecium and Enterococcus faecalis demonstrated the highest sensitivity to linezolid (100%), followed by vancomycin (98.86%). Only a single vancomycin-resistant Enterococcus faecium strain was identified in 2020, while no vancomycin-resistant Enterococcus faecalis strains were detected. Both species exhibited the highest resistance to erythromycin (89.14%) and the second-highest resistance to rifampicin (80.00%). Enterococcus faecium showed greater resistance to antibiotics compared to Enterococcus faecalis (Table 2), particularly to penicillin (71.43% vs 4.08%) and ampicillin (60.32% vs 2.04%).
 |
Table 2 Non-Susceptibility of Enterococcus Faecium and Enterococcus Faecalis |
The resistance of Enterococcus faecium to ampicillin increased, and its resistance to penicillin, erythromycin, rifampicin and high-concentration streptomycin increased before 2023 and decreased after 2023 (Figure 5).
Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus aureus
Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus aureus showed the highest resistance to penicillin (83.33%), followed by azithromycin and erythromycin (72.73%). These species were most sensitive to linezolid and teicoplanin (100%), with vancomycin showing a sensitivity rate of 98.48% (Table 3). Throughout the study period, only a single vancomycin-resistant Staphylococcus hominis strain was identified.
 |
Table 3 Non-Susceptibility of Staphylococcus Epidermidis, Staphylococcus Hominis and Staphylococcus Aureus |
The resistance of Staphylococcus epidermidis to levofloxacin and moxifloxacin increased from 0 in 2018 to 66.67% in 2023, but its resistance to compound sulfamethoxazole decreased from 66.67% in 2018 to 33.33% in 2023. The resistance of Staphylococcus epidermidis to penicillin and oxacillin decreased in 2023 (100% vs 75%; 100% vs 33.33%)(Figure 6a).
Staphylococcus hominis showed decreased resistance to penicillin (100% vs 50%), gentamicin (50% vs 16.67%), erythromycin (100% vs 33.33%), and compound sulfamethoxazole (50% vs 16.67%), but increased resistance to oxacillin (0% vs 83.33%), levofloxacin (0% vs 50%) and moxifloxacin (0% vs 33.33%)(Figure 6b).
The resistance of Staphylococcus aureus to compound sulfamethoxazole decreased (66.67% vs 0%), but its resistance to other antibiotics remained almost unchanged (Figure 6c).
The detection rate of MRS was 71.60% (58/81), exhibiting an overall upward trend (Figure 6d). Among the Staphylococcus species, antibiotic-resistant Staphylococcus epidermidis had the highest detection rate at 85.19% (23/27), followed by Staphylococcus hominis at 61.90% (13/21), Staphylococcus aureus at 44.44% (8/18), and other Staphylococcus species at 86.67% (13/15). The detection rates of antibiotic-resistant Staphylococcus epidermidis and Staphylococcus hominis increased until 2023, followed by a decline in 2023. Conversely, Staphylococcus aureus exhibited the opposite trend, with an increase in its detection rate in 2023. Notably, among the seven Staphylococcus strains identified in 2024, six were MRS strains.
Streptococcus
Streptococcus consistently exhibited high resistance to erythromycin (75.56%), with resistance levels increasing over time. The second-highest resistance was to clindamycin (46.67%), although this resistance showed a declining trend, decreasing from 100% in 2018 to 53.33% in 2023. Resistance to penicillin rose notably, from 0% in 2018 to 53.33% in 2023 (Table 4, Figure 7). Additionally, Streptococcus strains resistant to ampicillin and ceftriaxone were first detected in 2021 and 2023, respectively.
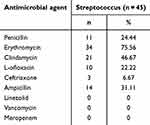 |
Table 4 Non-Susceptibility of Streptococcus |
 |
Figure 7 Trends in antimicrobial non-susceptibility of Streptococcus from 2018 to 2023. |
Discussion
Traditionally, Gram-positive bacteria are predominantly associated with respiratory and urinary infections, while biliary infections are primarily thought to originate from the intestine, making Gram-negative bacteria the main pathogens. Consequently, treatment for biliary infections has largely focused on Gram-negative bacteria. Currently available broad-spectrum antibiotics targeting Gram-positive bacteria primarily address Enterococcus. However, the findings of this study revealed a gradual decline in the proportion of Enterococcus and an increase in non-Enterococcus Gram-positive bacteria. These shifts in the composition of biliary bacteria align with similar changes observed in bacteria from urine, sputum, blood, and other clinical samples.12 This highlights the need for greater attention to antibiotic therapies specifically targeting Gram-positive bacteria in biliary infections to adapt to these evolving patterns.
Enterococcus is the dominant bacterium in the duodenum and small intestine of healthy individuals and is commonly implicated in cholangitis, which is thought to result from the retrograde migration of intestinal bacteria. Previous studies have shown that Enterococcus is the primary Gram-positive bacterium detected in the bile of cholangitis patients. However, the bacterial spectrum has evolved due to the widespread use of broad-spectrum antibiotics and rising nosocomial infection rates.20 In the present study, it was found that the proportion of Enterococcus decreased while that of Staphylococcus and Streptococcus increased. These changes are consistent with those in the bacterial spectrum of urine, sputum, secretions and other samples reported recently.
Common Gram-positive bacteria exhibit significant resistance to broad-spectrum antibiotics commonly used in clinical practice.12,13,21 Therefore, broad-spectrum β-lactams, such as piperacillin/tazobactam, should be prioritized in treatment.22,23 Additionally, attention should be given to the possibility of Gram-positive bacterial infections following the administration of broad-spectrum antibiotics. For patients who respond poorly to anti-infective therapy, repeated sampling and antibiotic susceptibility testing are essential to guide targeted antibiotic selection. In cases of multiple infections, the use of a combination of different antibiotic classes is recommended to enhance treatment efficacy and address diverse pathogen profiles effectively.
Staphylococcus epidermidis, a bacterium associated with skin colonization, is implicated in many infections, particularly those linked to medical devices.12 It shows resistance to multiple antibiotics, especially the strains found in clinical infection environments. Staphylococcus epidermidis and its resistant strains are detected at the highest rate among the Staphylococcus species, with an overall rising trend in the detection of resistant strains. Given the context of bile sample sources and related reports, this trend may be linked to the repeated use of duodenoscopes in ERCP procedures.6,24,25 The adoption of disposable duodenoscopes may become the preferred practice in future ERCP procedures.26 In addition, there is a large proportion of patients with malignant obstruction receiving PTCD. Foreign bodies in the bile duct may alter the bile microenvironment and promote the formation of biofilms.27,28 Bacteria within biofilms exhibit significant resistance to antimicrobial agents, which can contribute to persistent or recurrent bacterial infections. Therefore, it is necessary to collect bile samples for bacterial culture and routine susceptibility testing during biliary drainage procedures.
Staphylococcus hominis is rich in resistance genes and it produces biofilms to enhance antibiotic resistance.29 The present study revealed an increasing detection rate of Staphylococcus hominis and its antibiotic-resistant strains over time. This trend may be attributed to the acquisition of new antibiotic resistance factors by the commensal Staphylococcus in the infected bile duct. Additionally, the rising implantation of biliary stents, facilitated by advancements in ERCP technology in recent years, could also contribute to this increase by providing surfaces for biofilm formation and promoting bacterial colonization and resistance development.
In the present study, the detection rate of Staphylococcus aureus in bile samples was found to be low. As bile samples were collected without contact with the skin or mucosa, it is likely that Staphylococcus aureus accessed the bile via hematogenous spread in patients with systemic infections.30 The low detection rate can also be attributed to the small proportion of critically ill patients among those undergoing biliary drainage, as systemic infections and subsequent hematogenous dissemination of Staphylococcus aureus are more common in severely ill individuals. Staphylococcus aureus showed decreased resistance to compound sulfamethoxazole, and its resistance to other antibiotics remained unchanged, which may be attributed to the decreased clinical application rate. The detection rate of MRS was 44.44% in the present study, higher than global median (35%).31 This may be due to differences in sample sources, patient age, antibiotic misuse, and regional variations in MRS prevalence.
The main species in the Streptococcus isolated from bile is Streptococcus viridans, among which, Streptococcus oralis (11/45), Streptococcus salivarius (10/45), and Streptococcal pharyngitis (9/45) are opportunistic pathogens extensively distributed in the human oral cavity and upper respiratory tract. Bile is sterile in a healthy individual, and the presence of Streptococcus in bile and its increased proportion are possibly related to the extensive application of ERCP. The increased detection of Streptococcus oralis in the bile of patients with cholangitis may be for the following reasons: ① Streptococcus oralis exhibits a very high recombination rate, suggesting that this species can adapt to the fluctuating environments encountered within the human body and may invade certain pathogenic niches.32 ② Streptococcus oralis may undergo synonymous mutations, which can affect gene expression and protein folding, potentially leading to infectious diseases.33 The resistance rates of Streptococcus to ampicillin and ceftriaxone are increasing, while resistance rates to clindamycin and erythromycin are decreasing annually, likely related to the clinical drug usage rates.
In the present study, significant shifts in the composition and antibiotic resistance of Gram-positive bacteria in bile were observed. The proportion of Enterococcus decreased, mainly due to a reduced detection rate of Enterococcus faecalis, while Streptococcus species diversified, with an increased detection rate of Streptococcus oralis. The rising detection rate of MRS was primarily driven by a steady increase in MRSHo. Erythromycin and penicillin are not recommended for cholangitis patients with suspected Gram-positive bacterial infections, and resistance should be monitored when using quinolones. Despite Enterococcus faecium showing 100% and 99.71% sensitivity to linezolid and vancomycin, respectively—results consistent with existing reports23—linezolid resistance in Enterococcus faecium has increased in China.11,21 Further, the growing resistance of Enterococcus faecium impacts the resistance profiles of other strains.34 Rapid pathogen identification and early use of narrow-spectrum antibiotics are crucial for mitigating antibiotic resistance.
Abbreviations
VRE, Vancomycin-Resistant Enterococci; MRS, Methicillin-Resistant Staphylococcus; MRSE, Methicillin-Resistant Staphylococcus epidermidis; MRSHo, Methicillin-Resistant Staphylococcus hominis; MRSA, Methicillin-Resistant Staphylococcus aureus; ERCP, endoscopic retrograde cholangiopancreatography.
Acknowledgments
We would like to thank the cooperative and supportive staff of the Gastroenterology and Hepatobiliary surgery Department.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alizadeh MAH. Cholangitis: diagnosis, treatment and prognosis. J Clin Transl Hepatol. 2017;5(4):404–413. doi:10.14218/JCTH.2017.00028
2. Miura F, Okamoto K, Takada T, et al. Tokyo guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25(1):31–40. doi:10.1002/jhbp.509
3. Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019;69(7):1091–1098. doi:10.1093/cid/ciy1054
4. Uno S, Hase R, Kobayashi M, et al. Short-course antimicrobial treatment for acute cholangitis with Gram-negative bacillary bacteremia. Int J Infect Dis. 2016;55:81–85. doi:10.1016/j.ijid.2016.12.018
5. Chandra S, Klair JS, Soota K, et al. Endoscopic retrograde cholangio-pancreatography-obtained bile culture can guide antibiotic therapy in acute cholangitis. Digest Dis. 2019;37. doi:10.1159/000493579
6. Gromski MA, Gutta A, Lehman GA, et al. Microbiology of bile aspirates obtained at ERCP in patients with suspected acute cholangitis. Endoscopy. 2022;54(11):1045–1052. doi:10.1055/a-1790-1314
7. Zhang H, Cong Y, Cao L, et al. Variability of bile bacterial profiles and drug resistance in patients with choledocholithiasis combined with biliary tract infection: a retrospective study. Gastroenterol Rep. 2024;12:goae010. doi:10.1093/gastro/goae010
8. Kwon W, Jang JY, Kim EC, et al. Changing trend in bile microbiology and antibiotic susceptibilities: over 12 years of experience. Infection. 2012;41(1):93–102. doi:10.1007/s15010-012-0358-y
9. Rupp C, Bode K, Weiss KH, et al. Microbiological assessment of bile and corresponding antibiotic treatment: a strobe-compliant observational study of 1401 endoscopic retrograde cholangiographies. Medicine. 2016;95(10):e2390. doi:10.1097/MD.0000000000002390
10. Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepato-Bil-Pan Sci. 2018;25(1):3–16. doi:10.1002/jhbp.518
11. Zhou W, Zhou H, Sun Y, et al. Characterization of clinical enterococci isolates, focusing on the vancomycin-resistant enterococci in a tertiary hospital in China: based on the data from 2013 to 2018. BMC Infect Dis. 2020;20(1):356. doi:10.1186/s12879-020-05078-4
12. Zhang X, Tan L, Ouyang P, et al. Analysis of distribution and antibiotic resistance of Gram-positive bacteria isolated from a tertiary-care hospital in southern China: an 8-year retrospective study. Front Microbiol. 2023;14:1220363.
13. Luo Q, Lu P, Chen Y, et al. ESKAPE in China: epidemiology and characteristics of antibiotic resistance. Emerg Microbes Infect. 2024;13(1):2317915. doi:10.1080/22221751.2024.2317915
14. Guo Y, Ding L, Yang Y, et al. Multicenter antimicrobial resistance surveillance of clinical isolates from Major Hospitals - China, 2022. China CDC Wkly. 2023;5(52):1155–1160. doi:10.46234/ccdcw2023.217
15. Ji S, Jiang S, Wei X, et al. In-host evolution of daptomycin resistance and heteroresistance in methicillin-resistant staphylococcus aureus strains from three endocarditis patients. J Infect Dis. 2020;221(Suppl 2):S243–S252. doi:10.1093/infdis/jiz571
16. Reiter FP, Obermeier W, Jung J, et al. Prevalence, resistance rates, and risk factors of pathogens in routine bile cultures obtained during endoscopic retrograde cholangiography. Digest Dis. 2021;39. doi:10.1159/000509289
17. Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. 2018;41:76–82. doi:10.1016/j.mib.2017.11.030
18. Ma X, Zhang F, Bai B, et al. Linezolid resistance in Enterococcus faecalis associated with urinary tract infections of patients in a tertiary hospitals in China: resistance mechanisms, virulence, and risk factors. Front Public Health. 2021;9:570650. doi:10.3389/fpubh.2021.570650
19. Global Index Medicus. Guidelines for diagnosis and treatment of acute biliary tract infections(2021). Zhonghua Wai Ke Za Zhi. 2021;59(6):422–429. doi:10.3760/cma.j.cn112139-20210421-00180
20. Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352(6285):544–545. doi:10.1126/science.aad9358
21. Li Y, Zhang B, Xue F, et al. Antimicrobial susceptibility of Gram-positive organisms: Results from China antimicrobial resistance surveillance trial (CARST) program, 2021-2022. Chin J Clin Pharmacol. 2023;39(23):3509–3524. doi:10.13699/j.cnki.1001-6821.2023.23.036
22. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Resp Crit Care. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
23. Kruis T, Güse-Jaschuck S, Siegmund B, et al. Use of microbiological and patient data for choice of empirical antibiotic therapy in acute cholangitis. BMC Gastroenterol. 2020;20(1):65. doi:10.1186/s12876-020-01201-6
24. Cimen C, Bathoorn E, Loeve AJ, et al. Uncovering the spread of drug-resistant bacteria through next-generation sequencing based surveillance: transmission of extended-spectrum β-lactamase-producing Enterobacterales by a contaminated duodenoscope. Antimicrob Resist Infect Control. 2024;13(1):31. doi:10.1186/s13756-024-01386-5
25. van der Ploeg K, Klaassen CHW, Vos MC, et al. A search strategy for detecting duodenoscope-associated infections: a retrospective observational study. J Hosp Infect. 2024:14756–14762. doi:10.1016/j.jhin.2024.02.015
26. Lanka C, Bhenswala P, Lakhana M, et al. Single-use Duodenoscope: the Cleaner Standard. J Clin Gastroenterol. 2024;58(10):957–962. doi:10.1097/MCG.0000000000001994
27. Caldara M, Belgiovine C, Secchi E, et al. Environmental, microbiological, and immunological features of bacterial biofilms associated with implanted medical devices. Clin Microbiol Rev. 2022;35(2):e0022120. doi:10.1128/cmr.00221-20
28. de la Fuente-Núñez C, Reffuveille F, Fernández L, et al. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol. 2013;16(5):580–589. doi:10.1016/j.mib.2013.06.013
29. Szczuka E, Krzymińska S, Bogucka N, Kaznowski A. Multifactorial mechanisms of the pathogenesis of methicillin-resistant Staphylococcus hominis isolated from bloodstream infections. Antonie van Leeuwenhoek. 2018;111(7):1259–1265. doi:10.1007/s10482-017-1007-3
30. Ozturk-Engin D, Agalar C, Cag Y, et al. Microorganisms isolated from the bile of the patients who have undergone cholecystectomy and their antibiotic resistance pattern: multicenter prospective study. Int Microbiol. 2022;25(4):759–767. doi:10.1007/s10123-022-00251-y
31. GLASS. Global antimicrobial resistance and use surveillance system(GLASS) report 2022[EB/OL]. 2022. Available from: https://www.who.int/health-topics/antimicrobial-resistance.
32. Joyce LR, Youngblom MA, Cormaty H, et al. Comparative genomics of streptococcus oralis identifies large scale homologous recombination and a genetic variant associated with infection. mSphere. 2022;7(6):e0050922. doi:10.1128/msphere.00509-22
33. Bailey SF, Alonso Morales LA, Kassen R. Effects of synonymous mutations beyond codon bias: the evidence for adaptive synonymous substitutions from microbial evolution experiments. Genome Biol Evol. 2021;13(9). doi:10.1093/gbe/evab141
34. García-Solache M, Rice LB. The enterococcus: a model of adaptability to its environment. Clin Microbiol Rev. 2019;32(2). doi:10.1128/CMR.00058-18
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



