Back to Journals » International Journal of Nanomedicine » Volume 19
Sintilimab Combined with Nanoparticle Albumin-Bound Paclitaxel-Based Chemotherapy in Severe Locally Advanced or Metastatic Squamous NSCLC Showed Good Efficacy and Safety: A Pilot Retrospective Analysis
Authors Zhong Y, Mao Y, Fu X, Huang H
Received 28 June 2024
Accepted for publication 23 October 2024
Published 7 November 2024 Volume 2024:19 Pages 11433—11444
DOI https://doi.org/10.2147/IJN.S484765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Yan Shen
Yonghong Zhong,1,* Yanxiong Mao,2,* Xiaofang Fu,1 Huaqiong Huang2
1Department of Respiratory and Critical Care Medicine, Linping Campus, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huaqiong Huang, Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 jiefang Road, Hangzhou, Zhejiang, 311100, People’s Republic of China, Tel +86 15005818703, Email [email protected]
Introduction: Squamous non-small cell lung carcinoma (sqNSCLC) is associated with a poorer prognosis and limited treatment options. Sintilizumab combined with chemotherapy is used as first-line treatment for advanced sqNSCLC. However, the efficacy and safety of sintilimab combined with nanoparticle albumin-bound paclitaxel-based chemotherapy for severe squamous NSCLC remain to be unknown in clinical studies.
Methods: Patients with confirmed unresectable stage III/IV sqNSCLC were retrospectively collected between July 1st, 2019, and December 31st. According to performance status (PS) scores, these patients received first-line sintilimab plus nab-PTX-based chemotherapy were divided into severe (PS=2) and non-severe groups (PS=0– 1). The treatment regimen was repeated every 3 weeks for a maximum of six cycles, or until unacceptable toxicity occurred. The primary endpoint of this study was to assess progression free survival (PFS), with secondary endpoints including the objective response rate (ORR), adverse events (AEs) and disease control rate (DCR).
Results: Among 367 patients with unresectable stage III/IV sqNSCLC, 28 male patients, with a median age of 65.5 years, received first-line sintilimab plus nab-PTX-based chemotherapy. These patients were divided into a severe group (11 patients) and a non-severe group (17 patients). The severe group had a significantly higher incidence of chronic obstructive pulmonary disease (COPD) compared to the non-severe group (54.5% vs 11.8%, p = 0.03). The two groups had a similar median number of treatment cycles and safety profiles. Although the severe group showed higher ORR (63.6% vs 47.1%) and DCR (100% vs 76.5%) than the non-severe group, these differences were not statistically significant. Median PFS and Kaplan-Meier curves were also comparable between the groups.
Conclusion: Sintilimab combined with nab-PTX-based chemotherapy was effective and well tolerated in a small sample of severe lung squamous cell carcinoma population. This combination may offer a potential treatment option for these patients.
Keywords: chemotherapy, immunotherapy, severe lung cancer, squamous non-small cell lung carcinoma
Introduction
Lung squamous cell carcinoma, a subtype of non-small cell lung carcinoma (NSCLC), has a poorer prognosis compared to other non-squamous subtypes.1 The 1- and 5-year survival rates for this condition are 14.6% and 1.6%, respectively.1–3 Treatment options for patients with squamous NSCLC (sqNSCLC) are limited. Immunotherapy, particularly those targeting programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), offers a new treatment approach for sqNSCLC.4–7 Combining immunotherapy with chemotherapy has shown a synergistic effect in NSCLC. Major NSCLC guidelines recommend the combination of PD-1/PD-L1 inhibitors and chemotherapy as a preferred treatment option for locally advanced or metastatic sqNSCLC without driver gene mutations, irrespective of PD-L1 expression.3,8,9
Severe lung cancer, a concept introduced by a global consensus group in 2021, refers to a condition where patients exhibit an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2–4 at certain stages due to various acute or chronic co-morbidities, the tumor itself, and/or treatment-related adverse events (AEs).10 Despite this, there is a high likelihood of survival and/or improvement in the PS score through supportive care and anti-tumor treatment based on dynamic and precise testing. Unlike end-stage lung cancer, severe lung cancer allows for the possibility of survival through individualized treatment.11,12 Patients with severe lung cancer typically have poor PS scores, leading to low tolerance to anti-tumor treatments. Consequently, treatments for these patients should have a relatively rapid onset to better address disease progression. Therefore, tailored, effective, and safe anti-tumor treatments are necessary, along with robust life support measures. For patients with severe locally advanced or metastatic sqNSCLC, which often lack driver gene mutations, combining immunotherapy with chemotherapy may represent a potential anti-tumor treatment approach.
Sintilimab is a fully human IgG4 monoclonal antibody designed to block the binding of PD-1. Preclinical data suggests that it has a higher binding affinity compared to nivolumab and pembrolizumab.7,13 In the ORIENT-12 study, combining sintilimab with gemcitabine/platinum (GP) treatment resulted in a significant prolongation of progression-free survival (PFS) compared to GP alone as first-line treatment for locally advanced or metastatic sqNSCLC (5.5 months vs 4.9 months).14 Another real-world study showed that combining sintilimab with paclitaxel/nanoparticle albumin-bound paclitaxel (nab-PTX) and platinum achieved a PFS of 13.9 months as first-line treatment for locally advanced or metastatic sqNSCLC.15 Currently, sintilimab in combination with GP has been approved for the first-line treatment of sqNSCLC in China.
Nab-PTX is a nanoparticulate formulation of paclitaxel bound to human serum albumin.16–18 Unlike solvent paclitaxel (sb-PTX), nab-PTX offers several advantages that make it a preferred chemotherapy agent for use in severe lung cancer.16 Firstly, nab-PTX does not contain polyoxyethylene castor oil, the lipid-based solvent found in sb-PTX, which has been linked to allergic reactions. Consequently, nab-PTX does not necessitate corticosteroid pre-medication to prevent hypersensitivity reactions. Secondly, nab-PTX delivers significantly higher doses of paclitaxel over a shorter infusion time (0.5 hours vs 3 hours for sb-PTX).19 Thirdly, numerous studies have demonstrated the efficacy and safety of nab-PTX in treating NSCLC, particularly in older patients.17,18,20,21 Subgroup analysis in patients aged ≥ 70 years in a Phase III trial showed a significant improvement in median overall survival with nab-PTX, with better tolerability of adverse events compared to sb-PTX.20
Hence, the combination of sintilimab and nab-PTX-based chemotherapy holds promise as a potential regimen for treating sqNSCLC. Nonetheless, the efficacy and safety of this combination in patients with severe locally advanced or metastatic sqNSCLC remain largely unexplored. Therefore, the aim of this study was to conduct a preliminary investigation into the efficacy and safety of sintilimab and nab-PTX-based chemotherapy as a first-line treatment for patients with severe locally advanced or metastatic sqNSCLC.
Methods
Ethical Approval
This retrospective study was conducted at the Second Affiliated Hospital of Zhejiang University School of Medicine in China. Ethical approval was obtained from the Ethics Committee of the hospital (Approval number: 2021–0608). Given that the study was non-interventional and retrospective, it posed minimal risk to participants. Therefore, the Ethics Committee waived the requirement for informed consent. All procedures adhered to the ethical principles outlined in the Declaration of Helsinki, ensuring the privacy and confidentiality of patient data.
Patients
The medical records of consecutive patients diagnosed with locally advanced or metastatic sqNSCLC between July 1st, 2019, and December 31st, 2021, were retrospectively reviewed. Eligible patients were identified based on the following criteria: (1) histological confirmation of nonresectable stage IIIB and IIIC or stage IV sqNSCLC according to the 8th edition of the TNM classification for lung cancer; (2) receipt of sintilimab and nab-PTX-based chemotherapy as first-line treatment. Patients included in the study did not harbor sensitive epidermal growth factor receptor mutations or anaplastic lymphoma kinase rearrangements.
Severe Lung Cancer
Severe lung cancer was defined as previously described,10 meeting the following criteria: (A) Eastern Cooperative Oncology Group performance status (ECOG PS) of 2–4; (B) presence of at least one of the following: (a) diagnosis of heart failure by cardiologists, (b) confirmation of chronic obstructive pulmonary disease (COPD) through spirometry, (c) diagnosis of interstitial lung disease (ILD) based on clinical features and findings from pretreatment chest high-resolution computed tomography (HRCT), (d) obesity with a body mass index (BMI) > 28, (e) extensive pleural effusion leading to lung collapse, (f) substantial pericardial effusion requiring pericardiocentesis, (g) significant tracheal or bronchial proximal stenosis, (h) venous thromboembolism (VTE), including deep vein thrombosis in the lower extremities and pulmonary embolism.
Treatment
Sintilimab at a dose of 200 mg was administered intravenously (IV) on day 1 of each treatment cycle, along with nab-PTX (130 mg/m2 IV on day 1 and 8, or 260 mg/m2 IV on day 1), and either nedaplatin (100 mg/m2 IV on day 1) or carboplatin (area under the concentration–time curve, 5 mg/mL/min IV on day 1). The ratio of each component in this product is Paclitaxel: albumin = 1:7–1:11. In cases of severe AEs, dose adjustments or interruptions were implemented at the discretion of the treating physicians. The treatment regimen was repeated every 3 weeks for a maximum of six cycles, or until unacceptable toxicity occurred, with the option for maintenance therapy with sintilimab left to the discretion of the physicians.
Evaluation of Response and AEs
Treatment response was assessed according to the response evaluation criteria in solid tumors version 1.1. Tumor size was evaluated using chest CT scans and B-scan ultrasonography of abdominal organs and lymph nodes every two treatment cycles. AEs were assessed based on medical records using the Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Analysis
The primary endpoint of this study was to assess PFS, with secondary endpoints including the objective response rate (ORR) and AEs. PFS was determined using the Kaplan–Meier method, calculated from the initiation of chemotherapy until disease progression, death from any cause, or the last follow-up date, whichever came first. ORR was defined as proportion of patients with best overall response of complete response or partial response based on applicable criteria. Data analysis was conducted using IBM SPSS Statistics 20. Continuous variables are expressed as mean with standard deviation (SD) or median with interquartile range (IQR), based on data distribution. Comparisons between variables were made using unpaired Student’s t-test, Welch t-test, or Wilcoxon rank sum test with continuity correction, depending on data normality and variance homogeneity. Categorical data are presented as absolute values and percentages and analyzed using the chi-square test or Fisher’s exact test as appropriate. Statistical significance was defined as p < 0.05.
Results
Patient Characteristics
Out of the 1316 patients diagnosed with sqNSCLC between July 1st, 2019, and December 31st, 2021, at the study hospital, 367 had unresectable stage III/IV disease. Among them, 28 patients received first-line treatment with sintilimab and nab-PTX-based chemotherapy and were included in the analysis (Figure 1). Among these patients, 11 met the criteria for severe lung cancer and were categorized into the severe group. COPD and airway stenosis were the most common reasons for severe lung cancer (Table 1). The remaining 17 patients were classified into the non-severe group.
 |
Table 1 Causes of Severe Lung Cancer |
 |
Figure 1 Flow chart of study population. NSCLC: non-small cell lung carcinoma. |
The baseline characteristics of the patients were documented (Table 2). The median age was 65.5 (range: 57.0 to 72.2) years for the severe group and 64.0 (range: 61.0 to 72.0) years for the non-severe group. All patients in both groups were male. Both groups exhibited similar BMI and smoking status, although there was a tendency for more former or current smokers in the severe group. Significantly more patients in the severe group had COPD compared to the non-severe group (54.5% vs 11.8%, p = 0.03). Additionally, the severe group had significantly lower FEV1/FVC ratios than the non-severe group (57.9% vs 72.0%, p = 0.03), which corresponded to the higher prevalence of COPD in the severe group. Other lung function test results were similar between the two groups.
 |
Table 2 Baseline Demographics |
The distribution of stage III and IV diseases was similar between the two groups (Table 3). The PD-L1 expression status was not evaluated in 63.6% of patients in the severe group and 88.2% of patients in the non-severe group, respectively. Among the assessed patients, all had positive PD-L1 expression. Blood test results were comparable between the two groups. Platinum was included in the chemotherapy regimens for all but one patient. Carboplatin was the most frequently used platinum agent in both groups, followed by nedaplatin.
 |
Table 3 Baseline Disease Characteristics |
Efficacy
The median number of treatment cycles was comparable between the two groups, with 5.0 (4.0, 6.0) cycles for the severe group and 4.0 (3.0, 5.0) cycles for the non-severe group (Table 4). Although the severe group exhibited higher ORR and disease control rate (DCR) compared to the non-severe group (ORR: 63.6% vs 47.1%; DCR: 100% vs 76.5%), these differences did not reach statistical significance.
 |
Table 4 Response to Treatment |
At the time of data cutoff, disease progression was observed in 19 patients (66.7%). The overall median PFS was 8.7 months (95% CI, 7.4–9.9 months) (Figure 2). The median PFS values were similar between the two groups, with 13.3 months (95% CI, 0.1–26.5 months) for the severe group and 8.7 months (95% CI, 5.0–12.3 months) for the non-severe group. Kaplan–Meier curves indicated no significant difference between the two groups (log rank: p = 0.583) (Figure 3).
 |
Figure 2 Kaplan-Meier survival analysis of study population.Disease progression was observed in 19 patients (66.7%). The overall median PFS was 8.7 months (95% CI, 7.4–9.9 months). |
Tumor size was evaluated using chest CT scans and B-scan ultrasonography of abdominal organs and lymph nodes every two treatment cycles in our study. Two patient’s CT images were retrieved from clinical stations and compared before treatments and after 2 cycles treatments.
Patient 1, a 59-year-old male, was diagnosed with right upper lung squamous cell carcinoma T4N2bM0 stage IIIB complicated with superior vena cava obstruction with a PS score of 2. He was treated with sintilimab + carboplatin + nab-PTX for 2 cycles on February 5, 2020 and February 26, 2020, respectively. Chest CT enhancement indicated PR on March 5, 2020 and PS score was 1 (Figure 4).
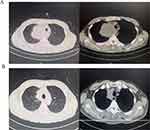 |
Figure 4 CT images compared before treatments and after 2 cycles treatments of patient 1. (A) Before treatment (on February 5 2020); (B) After treatment (on March 25 2020). |
Patient 2, male, was diagnosed with right lower lung squamous cell carcinoma T4N2M0 stage IIIB with PS score of 1. He was treated with sintilimab + carboplatin + nab-PTX for 2 cycles on July 11, 2020 and August 4, 2020, respectively. Chest CT enhancement assessment PR was reviewed on August 25, 2020 (Figure 5).
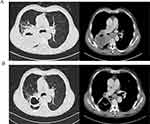 |
Figure 5 CT images compared before treatments and after 2 cycles treatments of patient 2. (A) Before treatment (on July 2 2020); (B) After treatment (on August 25 2020). |
Safety Profile
The predominant AEs in both groups were hematological, such as leukopenia, neutropenia, and anemia. Liver dysfunction, rash, and renal insufficiency were the most common non-hematological AEs (Table 5). In the severe group, a total of 19 AEs, with 2 AEs graded as 3 or higher (1 case of rash and 1 case of Thrombocytopenia), were recorded. Conversely, the non-severe group experienced a total of 47 AEs, including 13 graded as 3 or higher (3 cases of leukopenia, 4 cases of neutropenia, 1 case of anemia, 1 case of thrombocytopenia, 1 case of arrhythmia, 1 case of renal insufficiency and 2 cases of tuberculosis) (Table 5). Notably, two patients in the non-severe group contracted tuberculosis (TB), leading to discontinuation of anti-tumor treatment.
 |
Table 5 Treatment-Related Adverse Events |
Discussions
To our understanding, this was the first such study to explore the preliminary efficacy and safety of sintilimab combined with nab-PTX-based chemotherapy as an initial treatment for 11 cases of severe locally advanced or metastatic sqNSCLC. Employing real-world data, our study suggested that this regimen could be a viable, well-tolerated, and efficacious option for these patients instead of best supportive treatment. Given the challenges of conducting prospective studies in this patient population, real-world evidence such as this study provided valuable insights for clinical management. Therefore, sintilimab and nab-PTX-based chemotherapy might offer a promising therapeutic approach for some patients with severe locally advanced or metastatic sqNSCLC.
Severe lung cancer represents a novel concept intricately linked with elevated PS scores.10 Clinical trials typically focus on patients with PS scores ranging from 0 to 1, often excluding those with higher scores of 2 to 4.5,6,14 Consequently, there is a lack of robust evidence guiding the management of this particular population, with supportive care frequently recommended for those with PS scores of 3 to 4 according to prominent lung cancer guidelines.3,8,9 However, it’s noteworthy that PS scores are stage-specific and can be reversed with effective treatment. In real-world scenarios, approximately a quarter of patients present with PS scores of 3 to 4, and it’s observed that a subset of these patients may experience survival benefits or improvements in PS scores following supportive care and effective anti-tumor treatment.11,12,22 Hence, the concept of severe lung cancer has emerged to delineate this subgroup of patients.
For individuals with locally advanced or metastatic sqNSCLC lacking driver gene mutations, the combination of immunotherapy and chemotherapy has become the standard of care.3,9 Despite the common occurrence of immunotherapy-related toxicities, it is generally better tolerated than chemotherapy, rendering it feasible for patients with severe lung cancer.23 Among chemotherapy agents, nab-PTX stands out due to its notable tolerance and efficacy.16 Nab-PTX is a solvent-free formulation of paclitaxel bound to albumin. Compared to conventional paclitaxel, nab-PTX carries a reduced risk of hypersensitivity reactions and blood toxicity associated with organic solvents. Moreover, nab-PTX exhibits enhanced antitumor activity owing to increased intratumoral concentrations. In a phase III trial, patients with sqNSCLC treated with nab-PTX/carboplatin demonstrated a significantly higher ORR compared to those receiving paclitaxel/carboplatin (41% vs 24%).21 Additionally, in patients who were over 70 years of age, median overall survival (OS) was notably prolonged with nab-PTX/carboplatin compared to paclitaxel/carboplatin (19.9 vs 10.4 months). Thus, the remarkable tolerability and efficacy of nab-PTX position it as an ideal chemotherapy option for individuals with severe lung cancer.
The study found that sintilimab and nab-PTX-based chemotherapy demonstrated good efficacy in patients with severe lung cancer. The PFS, which was the primary endpoint, was found to be comparable to findings from previous studies. Specifically, the median PFS was 13.3 months for the severe group and 8.7 months for the non-severe group in our study. These results align with a real-world study investigating sintilimab plus paclitaxel/nab-PTX and platinum as first-line treatment for locally advanced or metastatic sqNSCLC, which reported a median PFS of 13.9 months.15 Furthermore, our observed PFS rates were consistent with those reported in pivotal trials such as KEYNOTE-407 (median PFS, 6.4 months), IMpower131 (median PFS, 6.3 months), RATIONALE 307 (median PFS, 7.6 months), and CameL-Sq (median PFS, 8.5 months).5,6,24,25
Sintilimab and nab-PTX-based chemotherapy demonstrated favorable tolerability in patients with severe lung cancer, as evidenced by a low incidence of grade 3 or higher AEs. Most AEs were either resolved or improved and were manageable. Interestingly, the severe group exhibited a lower frequency of AEs of any grade and grade 3 or higher AEs compared to the non-severe group. Common AEs observed in both groups included leukopenia, neutropenia, and anemia, which aligns with findings from previous studies.24,25 These hematological AEs were attributed to the chemotherapy regimen. Notably, two patients in the non-severe group, who had no prior history of TB, developed active TB during treatment. TB reactivation associated with PD-1/PD-L1 inhibitors has been reported previously, highlighting a significant risk to patients.26 In our study, the occurrence of active TB necessitated discontinuation of anti-tumor treatment. However, some studies have indicated that combining PD-1/PD-L1 inhibitors with anti-TB therapy is well-tolerated and does not result in significant unexpected toxic effects.27,28
Through the comparison of pre- and post-treatment imaging findings in these two patients, it is evident that the combination of Sintilimab and nab-PTX-based chemotherapy demonstrates significant efficacy in the treatment of squamous non-small cell lung cancer (SqNSCLC). This therapeutic regimen not only substantially reduces tumor size but also alleviates related symptoms and improves patients’ quality of life. These imaging changes provide strong evidence for the effectiveness of this combined therapy in SqNSCLC. Furthermore, these results underscore the importance of further investigating the potential of this treatment regimen to bring clinical benefits to a larger patient population.
Several limitations were noted in this study. Firstly, its retrospective nature and small sample size restricted the statistical power of the analysis. Therefore, larger prospective cohort studies are warranted to validate these findings comprehensively.
Another limitation was the variability in chemotherapy agent dosages, which were adjusted at the discretion of attending physicians rather than following a standardized protocol. Additionally, there was a lack of data on PD-L1 expression and tumor mutation burden for most of the patients, precluding evaluation of these factors as potential biomarkers.
Conclusions
The findings of this study suggested that sintilimab combined with nab-PTX-based chemotherapy show good efficacy and safety in patients with some severe locally advanced or metastatic sqNSCLC. This regimen might be an alternative treatment option and need more larger sample sizes research to support this conclusion in further.
Highlights
1. This was the first study to investigate the combination of sintilimab with nanoparticle albumin-bound paclitaxel (nab-PTX)/platinum in treating severe squamous NSCLC.
2. The combination of sintilimab with nab-PTX/platinum demonstrated good efficacy and safety in a small sample of severe lung squamous cell carcinoma population.
Abbreviations
NSCLC, non-small cell lung carcinoma; sqNSCLC, squamous non-small cell lung carcinoma; nab-PTX, nanoparticle albumin-bound paclitaxel-based; sb-PTX, solvent paclitaxel; PS, performance status; COPD, chronic obstructive pulmonary disease; ORR, objective response rate; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; OS, Overall survival; AEs, adverse events; GP, gemcitabine/platinum; PFS, progression-free survival; ILD, interstitial lung disease; HRCT, high resolution computed tomography; BMI, body mass index; VTE, venous thromboembolism; IV, intravenously; SD, stand deviation; IQR, interquartile range; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; PD-L1, programmed cell death-ligand 1; CRP, C-reactive protein; TB, tuberculosis.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due patients’ individual privacy could be compromised, but are available from the corresponding author on reasonable request.
Ethical Approval
This retrospective study was conducted at the Second Affiliated Hospital of Zhejiang University School of Medicine in China. Ethical approval was obtained from the Ethics Committee of Linping Campus, The Second Affiliated Hospital, Zhejiang University School of Medicine (Approval number: 2021-0608). Given that the study was non-interventional and retrospective, it posed minimal risk to participants. Therefore, the Ethics Committee waived the requirement for informed consent. All procedures adhered to the ethical principles outlined in the Declaration of Helsinki, ensuring the privacy and confidentiality of patient data.
Funding
This study was supported by Natural Science Foundation of Zhejiang Province [NO. LY22H010004].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the surveillance, epidemiology and end results program. Clin Epidemiol. 2011;3:139–148. doi:10.2147/CLEP.S17191 Epub 2011 Apr 28. PMID: 21607015; PMCID: PMC3096514.
2. Socinski MA, Obasaju C, Gandara D, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13(2):165–183. doi:10.1016/j.jtho.2017.11.111 Epub 2017 Nov 23. PMID: 29175116.
3. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. doi:10.6004/jnccn.2022.0025 PMID: 35545176.
4. Chen RL, Zhou JX, Cao Y, et al. The efficacy of PD-1/PD-L1 inhibitors in advanced squamous-cell lung cancer: a meta-analysis of 3112 patients. Immunotherapy. 2019;11(17):1481–1490. doi:10.2217/imt-2019-0101 Epub 2019 Nov 12. PMID: 31713453.
5. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–1360. doi:10.1016/j.jtho.2020.03.028 Epub 2020 Apr 14. PMID: 32302702.
6. Paz-Ares L, Luft A, Vicente D, et al. KEYNOTE-407 investigators. pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865 Epub 2018 Sep 25. PMID: 30280635.
7. Jiang H, Zheng Y, Qian J, et al. Efficacy and safety of sintilimab in combination with chemotherapy in previously untreated advanced or metastatic nonsquamous or squamous NSCLC: two cohorts of an open-label, phase 1b study. Cancer Immunol Immunother. 2021;70(3):857–868. doi:10.1007/s00262-020-02738-x Epub 2020 Oct 17. PMID: 33070260; PMCID: PMC7907015.
8. Planchard D, Popat S, Kerr K, et al. ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi:10.1093/annonc/mdy275 PMID: 30285222.
9. Singh N, Temin S, Baker SJ, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO living guideline. J Clin Oncol. 2022;40(28):3323–3343. doi:10.1200/JCO.22.00825 PMID: 35816668.
10. Zhou C, Li S, Liu J, et al. International consensus on severe lung cancer-The first edition. Transl Lung Cancer Res. 2021;10(6):2633–2666. doi:10.21037/tlcr-21-467 PMID: 34295668; PMCID: PMC8264326.
11. Kancharla H, Gundu N, Pathak N, et al. Cytotoxic chemotherapy in advanced non-small cell lung cancer with poor performance status: a retrospective analysis from routine clinical practice. Curr Probl Cancer. 2020;44(3):100550. doi:10.1016/j.currproblcancer.2020.100550 Epub 2020 Jan 20. PMID: 31987521.
12. Lewis MA, Hendrickson AW, Moynihan TJ. Oncologic emergencies: pathophysiology, presentation, diagnosis, and treatment. CA Cancer J Clin. 2011;61(5):287–314. doi:10.3322/caac.20124 PMID: 21858793.
13. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol. 2020;10:594558. doi:10.3389/fonc.2020.594558 PMID: 33324564; PMCID: PMC7726413.
14. Zhou C, Wu L, Fan Y, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol. 2021;16(9):1501–1511. doi:10.1016/j.jtho.2021.04.011 Epub 2021 May 25. PMID: 34048947.
15. Lin X, Deng H, Li S, et al. Sintilimab with chemotherapy as first-line treatment for locally advanced or metastatic squamous non-small-cell lung cancer: a real-world data study. J Cancer Res Clin Oncol. 2023;149(2):757–764. doi:10.1007/s00432-021-03903-0 Epub 2022 Feb 10. PMID: 35146575.
16. Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. doi:10.2147/DDDT.S88023 PMID: 26244011; PMCID: PMC4521678.
17. Fang Y, Wang L, Xia GH, Shi MQ. Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(17):7453–7457. doi:10.7314/apjcp.2014.15.17.7453 PMID: 25227858.
18. Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17(8):1263–1268. doi:10.1093/annonc/mdl104 Epub 2006 Jun 1. PMID: 16740598.
19. Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. doi:10.1158/1078-0432.CCR-05-1634 Dosage error in article text. PMID: 16489089.
20. Liu Y, Dong Y, Zhu H, Jing W, Guo H, Yu J. Nanoparticle albumin-bound paclitaxel in elder patients with advanced squamous non-small-cell lung cancer: a retrospective study. Cancer Med. 2020;9(4):1365–1373. doi:10.1002/cam4.2791 Epub 2019 Dec 26. PMID: 31876976; PMCID: PMC7013054.
21. Socinski MA, Manikhas GM, Stroyakovsky DL, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(6):852–861. doi:10.1097/jto.0b013e3181d5e39e PMID: 20521351.
22. Maclay JD, Farley JM, McCowan C, Tweed C, Milroy R. Obtaining tissue diagnosis in lung cancer patients with poor performance status and its influence on treatment and survival. Respir Med. 2017;124:30–35. doi:10.1016/j.rmed.2017.01.002 Epub 2017 Jan 13. PMID: 28284318.
23. Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–289. doi:10.6004/jnccn.2019.0013 PMID: 30865922.
24. Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–717. doi:10.1001/jamaoncol.2021.0366 PMID: 33792623; PMCID: PMC8017481.
25. Ren S, Chen J, Xu X, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol. 2022;17(4):544–557. doi:10.1016/j.jtho.2021.11.018 Epub 2021 Dec 16. PMID: 34923163.
26. Zaemes J, Kim C. Immune checkpoint inhibitor use and tuberculosis: a systematic review of the literature. Eur J Cancer. 2020;132:168–175. doi:10.1016/j.ejca.2020.03.015 Epub 2020 May 3. PMID: 32375103.
27. Shi J, Li J, Wang Q, et al. The safety and efficacy of immunotherapy with anti-programmed cell death 1 monoclonal antibody for lung cancer complicated with Mycobacterium tuberculosis infection. Transl Lung Cancer Res. 2021;10(10):3929–3942. doi:10.21037/tlcr-21-524 PMID: 34858782; PMCID: PMC8577979.
28. Su S, Ye MF, Cai XT, et al. Assessment of anti-PD-(L)1 for patients with coexisting malignant tumor and tuberculosis classified by active, latent, and obsolete stage. BMC Med. 2021;19(1):322. doi:10.1186/s12916-021-02194-z PMID: 34923987; PMCID: PMC8686368.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Pathologic Complete Response Prediction to Neoadjuvant Immunotherapy Combined with Chemotherapy in Resectable Locally Advanced Esophageal Squamous Cell Carcinoma: Real-World Evidence from Integrative Inflammatory and Nutritional Scores
Feng J, Wang L, Yang X, Chen Q, Cheng X
Journal of Inflammation Research 2022, 15:3783-3796
Published Date: 6 July 2022
Nanoparticles for Chemoimmunotherapy Against Triple-Negative Breast Cancer
Liu S, Li J, Gu L, Wu K, Xing H
International Journal of Nanomedicine 2022, 17:5209-5227
Published Date: 7 November 2022
Pembrolizumab in Lymphopenic Metastatic Breast Cancer Patients Treated with Metronomic Cyclophosphamide: A Clinical and Translational Prospective Study
Mery B, Ménétrier-Caux C, Montané L, Heudel PE, Ray-Coquard I, Bachelot T, Derbel O, Augereau P, Treilleux I, Berthet J, Nkodia A, Bardin-Dit-Courageot C, Attignon V, Ferrari A, Garin G, Perol D, Caux C, Dubois B, Trédan O
Breast Cancer: Targets and Therapy 2023, 15:311-325
Published Date: 27 April 2023
Systemic Treatment-Decision Algorithms in Muscle-Invasive Bladder Cancer: Clinical Complexities and Navigating for Improved Outcomes
Giles M, Crabb SJ
Research and Reports in Urology 2023, 15:321-331
Published Date: 7 July 2023
Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives
Wang J, Wu SG
Breast Cancer: Targets and Therapy 2023, 15:721-730
Published Date: 20 October 2023


