Back to Journals » Nature and Science of Sleep » Volume 17
Sleep Duration, Sleep Habits, and Social Jetlag From 4 to 6 years Their Impacts on Myopia Among School-Aged Children: The Ma’anshan Birth Cohort Study
Authors Wang M, Tong J, Zhu D, Huang K, Wu X, Gao G, Jiang L, Yan S, Tao F, Tao S
Received 11 October 2024
Accepted for publication 4 February 2025
Published 1 March 2025 Volume 2025:17 Pages 365—378
DOI https://doi.org/10.2147/NSS.S500191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sarah L Appleton
Meng Wang,1,* Juan Tong,2,* Dongqing Zhu,1 Kun Huang,2 Xiaoyan Wu,2 Guopeng Gao,3 Liu Jiang,3 Shuangqin Yan,3 Fangbiao Tao,2 Shuman Tao4,5
1Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China; 2Department of Maternal, Child and Adolescent Health, School of Public Health/ MOE Key Laboratory of Population Health Across Life Cycle / Anhui Provincial Key Laboratory of Environment and Population Health Across the Life Course, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China; 3Ma’anshan Maternal and Child Health Care Center, Ma’anshan, Anhui, People’s Republic of China; 4Department of Ophthalmology, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230031, People’s Republic of China; 5MOE Key Laboratory of Population Health Across Life Cycle / Anhui Provincial Key Laboratory of Environment and Population Health Across the Life Course / Center for Big Data and Population Health of IHM, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuman Tao, Department of Ophthalmology, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, People’s Republic of China, Email [email protected]
Purpose: To examine the associations of sleep parameters and their trajectories at preschool age with myopia among school-aged children by using a birth cohort study design.
Patients and methods: All participants were recruited from the Ma’anshan Birth Cohort Study. Sleep duration, sleep habits, and social jetlag were collected in 4 years, 5.5 years, and 6 years. Cycloplegic refraction and ocular biometry were performed at 7 years. Key statistical analyses were performed using the latent class growth models, binary logistic regression, generalized linear models, and linear mixed models, respectively.
Results: A total of 1561 children were included in the study (mean age of 7.93 years, 52.6% boys). Social jetlag of at least 1 hour at age 4 was positively linked to an increased risk of myopia in school-age children and axial length (AL) but negatively correlated with spherical equivalent refraction (SER) (P< 0.05). Inadequate sleep duration at 5.5 years was associated with an increase in AL among school-age children (β=0.16; 95% CI: 0.07– 0.24). A pattern of increasing-declining social jetlag was positively correlated with AL/CR ratio (RR=1.55; 95%CI: 1.01– 2.37). Poor sleep habits, higher social jetlag at age 4, and the declining-increasing trajectory of social jetlag were negatively associated with SER in school-aged children (P< 0.05). Furthermore, the declining-increasing and increasing-declining trajectories of social jetlag were positively correlated with the elongation of AL.
Conclusion: The correlations between sleep parameters at preschool age and myopia in school-aged children reveal that maintaining regular sleep habits in preschool may contribute to the early prevention of myopia.
Keywords: myopia, sleep, cycloplegic spherical equivalent refraction, axial length, children
Introduction
Myopia is widely acknowledged as a significant global public health issue. As a result of increasing educational and academic pressures, early-onset myopia is becoming more prominent, particularly in younger school-aged children. The prevalence of myopia has surged markedly since the elementary school stage.1 Currently, the diagnosis of myopia relies primarily on cycloplegic refraction, which is considered as the “gold standard”.2,3 Specifically, cycloplegic spherical equivalent refraction (SER) serves as the best predictor of myopia.4 Axial length (AL), a crucial parameter in assessing ocular development, is easier to measure due to its independence from ciliary muscle regulation. The range of AL variation exhibits a relatively stable pattern with age but is also closely related to refractive development, making it a significant predictor of myopia progression.5–7 Consequently, continuous monitoring of AL can evaluate myopia progression in children and adolescents.8 Additionally, numerous cross-sectional and longitudinal studies have indicated that the ratio of axial length to corneal radius (AL/CR) can be used as a highly accurate standard for diagnosing myopia.9–11
Behavioral factors are instrumental in both the onset and progression of myopia. According to the International Myopia Institute (IMI) analysis of myopia risk factors, it is widely recognized that outdoor activities, near work are widely recognized as major contributors to acquired myopia. Moreover, sleep has emerged as a novel and potential risk factor.12 With the evolving social environment, children’s sleep duration is decreasing, and their sleep rhythms are being disrupted.13 Variations in sleep patterns and timing are often influenced by age and years of education. For instance, during infancy, sleep-wake cycles are fluctuating, alternating between brief active phases and extended sleeping hours. As individuals transition from childhood to adolescence, their sleep preferences shift from being morning-chronotype to evening-chronotype and the dim light melatonin onset gradually shifts later.14 It is well-recognized that sleep is closely associated with circadian rhythms, and various Ocular biological parameters exhibit 24-hour rhythmic fluctuations, such as AL being shortest at night and longest during the day, while the choroid thickness peaks at night and reaches its minimum during the day.15 From a biological perspective, children’s engagement in outdoor activities during the day can stimulate dopamine secretion, while retinal melatonin secretion is prevalent in dark environments at night. The regular interplay between these two hormones constitutes a crucial mechanism for regulating retinal circadian rhythms and ocular growth.16,17 However, contemporary children’s exposure to electronic devices and light environments may interfere with the synthesis of retinal dopamine and melatonin, subsequently disrupting ocular circadian rhythms and ultimately impacting refractive development.18 Furthermore, when sleep deprivation disrupts the circadian rhythm, it induces phase shifts in the diurnal patterns of axial length and the choroidal daily rhythm, which may also potentially contribute to the development of myopia.19 These observations suggest that getting sufficient nighttime sleep could potentially aid in preventing myopia. However, research has yet to confirm whether maintaining sufficient sleep and a consistent sleep rhythm during the preschool years can reduce the risk of developing myopia after attending elementary school.
In fact, poor sleep quality,20 short sleep duration,21 late bedtime22 and daytime sleepiness23 were determined to be correlated with an elevated risk of myopia, despite the preponderance of cross-sectional designs. A prospective cohort study, which tracked school-aged children over four years, uncovered a negative correlation between sleep duration and AL in school-aged children, yet this study only assessed sleep duration at a single time point.24 Another prospective cohort study analyzed 12-year trajectories of sleep behavior from childhood to adolescence. Still, the trajectories were not notably linked to alterations in refractive error, AL, or corneal radius (CR) during young adulthood.25 Remarkably, previous studies have mostly focused on older children or concentrated on research involving a single age group or time point and no study has explored the effects of sleep parameters and their trajectories on refractive development and ocular biometric parameters during the preschool period. To address and bridge this gap in prior research, our current birth cohort study focuses on emphasizing the effect of sleep parameters and their trajectories during preschool age on myopia in school-age children. More significantly, we anticipate that the findings of this study will provide a new perspective for the prevention and control of early-onset myopia in school-aged children, and we call on the entire society to recognize the importance of early sleep health in children.
Methods
Study Design and Participants
The Ma’anshan Birth Cohort Study (MABC) is an ongoing population-based prospective cohort study that was initiated between May 2013 and September 2014 in Ma’anshan City, Anhui Province, China. The enrollment procedure has been previously described in detail.26 An aggregate of 3474 pregnant women were recruited, following the exclusion of 162 adverse pregnancy outcomes and 39 twin births, 3273 singleton live births were included and engaged in annual follow-up visits. Between July 2022 and January 2024, 2101 children were successfully followed up, representing a follow-up rate of 64.19%. Of these, 1655 children underwent cycloplegic refraction and ocular biometry. After excluding 5 non-singleton live births, 28 children with cycloplegic SER greater than +2.5D, 61 children with incomplete data on sleep parameters, and 1561 children were finally incorporated into data analysis. The study complies with the Declaration of Helsinki, and the research protocol received was approved by the Ethics Committee of Anhui Medical University (No. 20131195) and all participants provided written informed consent.
Sleep Duration, Social Jetlag, and Sleep Habits
Sleep parameters were assessed by self-administered questionnaires completed by parents at the age of 4, 5.5, and 6 years. Three main sleep parameters were carefully examined in this study, which were sleep duration, social jetlag, and sleep habits.
Sleep Duration
The children’s nighttime sleep duration was obtained by asking “During the past month, how many hours of actual sleep did your baby get at night?”. According to the WHO guidelines,27 it is recommended that children aged 4–6 years should sleep for at least 10 hours per day. Therefore, sleep duration was divided into the sufficient sleep group (≥10 h/d) and the insufficient sleep group (<10 h/d).
Social Jetlag
Social jetlag was determined by calculating the sleep midpoint for weekdays and weekends. The sleep midpoint on weekdays was calculated as wake-up time minus bedtime on weekdays divided by 2, and the sleep midpoint on weekends was calculated by subtracting bedtime from wake-up time on weekends and dividing by 2. Social jetlag was then quantified as the absolute difference between the weekend and weekday sleep midpoints. For example, if a child goes to bed at 21:00 and wakes up at 7:00 on weekdays, the sleep midpoint is 2:00; on weekends, he goes to bed at 23:00 and wakes up at 9:00, the sleep midpoint is 4:00; the difference between the two midpoints is 2 hours, which means that social jetlag is 2 hours. Based on previous studies,28 social jetlag was divided into 1 hour or more and less than 1 hour.
Sleep Habits
Sleep habits were evaluated by applying the Children’s Sleep Habits Questionnaire (CSHQ), a tool meticulously developed by Owens et al29 and the Chinese version of CSHQ was introduced by Jiang et al and adopted by the National Health Commission of the People’s Republic of China as a hygienic standard (WS/T 579—2017). The retrospective questionnaire has become a prevalent method for assessing sleep behaviors in school-aged children. It consists of 33 items and contains eight key sleep domains: bedtime resistance, sleep onset delay, sleep duration, anxiety around sleep, night wakings, parasomnias, sleep-disordered breathing, and daytime sleepiness. Each item is scored on a 3-point Likert scale: rarely or never (0–1 times per week), sometimes (2–4 times per week), usually (5–7 times per week). The scoring of some items is reversed. Poor sleep habits are indicated by a total CSHQ score above 54.
Cycloplegic Refraction and Ocular Biometry
Children underwent ophthalmologic examination at 6 and 7 years, respectively, and data at 7 years of age was analyzed as primary data. First, visual acuity, intraocular pressure, and fundus examination were performed, along with a review of previous examination reports and data, to exclude any contraindications to cycloplegia. For cycloplegia, 4 drops of the compound tropicamide (comprising 0.5% tropicamide and 0.5% phenylephrine) were instilled in each eye at 10-minute intervals. After 40 minutes, an assessment was conducted to evaluate the extent of pupil dilation and the pupillary light reflex. Cycloplegia was considered fully achieved when the pupil diameter reached 6 mm or greater and the pupillary light reflex was absent. Refraction measurements were ascertained through the utilization of an autorefractor (KR-800; Topcon, Tokyo, Japan). Prior to the first use on any given day, calibration was required with a simulated eye, and each eye must undergo measurement at least three times. The maximum tolerable error for cylindrical lens degree is set at ±0.25 diopters (D). The spherical power (S), and cylindrical power (C) were recorded to calculate the cycloplegic spherical equivalent refraction (SER), where SER = S + 1/2C. Children with SER≤-0.5D in the right eye are defined as myopia. Additionally, ocular biometry, including axial length (AL) and corneal curvature radius (CR), was measured by an optical biometric instrument (SUOER SW-9000). The AL/CR ratio was subsequently calculated.
Covariates
In incorporating covariates, we comprehensively considered several factors. Firstly, we included common demographic variables associated with childhood myopia, such as age, gender, parental myopia, parental educational levels, and family monthly income. Secondly, common behavioral factors that may concurrently affect myopia and sleep, such as screen time, outdoor activity time, and secondhand smoke exposure, were also taken into account. Finally, given the specificity of this study relying on a birth cohort, some maternal and child variables related to childhood vision, such as gestational week, mode of delivery, and maternal age, were also included in the research.
A directed acyclic graph (DAG, eFigure 1) is employed to detect potential confounders. The covariates ultimately included children’s age, gender (boy or girl), parental myopia status (none, one parent, or both parents), delivery mode (vaginal or cesarean section), maternal age (≤24 years, 25–27 years, or ≥28 years), gestational age (≥37 weeks or <37 weeks), monthly household income (≤2500 RMB, 2500–4000 RMB, or >4000 RMB), children’s exposure to secondhand smoke (yes or no), parental education level (≤9 years, 9–12 years, or ≥12 years), daily outdoor activity time (≥2 hours/day or <2 hours/day), daily screen time (≥2 hours/day or <2 hours/day). All Socio-demographic information and behavioral data were collected using an electronic questionnaire, and delivery information was obtained from medical records.
Statistical Analysis
We adopted a sophisticated group-based trajectory methodology, latent class growth modeling implemented within Mplus version 8.0, to determine subgroups within our longitudinal sleep data. We tried trajectory groups ranging from one to four to determine the most fitting trajectory model. The selection of the optimal trajectory model was based on model information criteria, including the Akaike information criterion (AIC), Bayesian information criteria (BIC), sample size-adjusted Bayesian information criteria (aBIC), Lo Mendell Rubin likelihood ratio test (LMR-LRT), and Bootstrap likelihood ratio test (BLRT), with significant values below 0.05, and an entropy value exceeding 0.60. The optimal trajectories were determined to be a three-classification for sleep duration, a two-classification for sleep habits, and a three-classification for social jetlag.
Categorical variables were expressed as frequencies and percentages, whereas continuous variables were expressed as arithmetic mean and standard deviation (SD). Comparisons of myopia rates across demographic characteristics classes were using the Chi-square tests or Fisher exact tests. To assess and contrast the disparities in normally distributed continuous variables between children with myopia and non-myopic children, an independent sample t-test was used. Binary logistic regression analysis was used to examine the associations between sleep parameters and their trajectories and myopia in school-aged children after adjusting for covariates. We employed generalized linear models to analyze the correlations between sleep parameters and their trajectories with SER, AL, and AL/CR. Furthermore, a linear mixed model was undertaken to meticulously assess the correlation between sleep and myopia progression in school-aged children. All analyses were performed using SPSS software version 23.0 (SPSS Inc, Chicago, IL, USA). Missing data were populated by employing multiple imputations, and the internal reliability of the imputed data was evaluated using Cronbach’s coefficient. The data set with the highest Cronbach’s coefficient was selected for further analysis. All statistical tests were two-sided and significance was set at P<0.05.
Results
Study Participant Characteristics
The sociodemographic characteristics of children and maternal profiles are illustrated in Table 1. A total of 1561 children were encompassed in the analysis, featuring an average age of 7.93 years (SD: 0.59), among whom 52.6% were boys. The mean age of children in the myopia group is higher than that of the non-myopia group (P<0.001). The overall prevalence of myopia in this cohort was 23.5% (367/1561). Notably, 43.3% of the children had one myopic parent, while only 22.9% had neither parent myopic. Children with both parents being myopic exhibit a significantly higher rate of myopia (P<0.001). Children who spend less than 2 hours on outdoor activities and more than 2 hours on digital screens account for 59.6% and 50.4%, respectively. Nevertheless, no statistically significant difference was observed in the rate of myopia among children in different groups of outdoor activity or screen time.
 |
Table 1 Demographic Characteristics of the Participants |
Classification of Sleep Parameters Latent Variable Growth Models
Figure 1 presents the trajectories of sleep duration, sleep habits, and social jetlag from 4 to 6 years. The selection of three-class trajectories for sleep duration, two-class trajectories for sleep duration, and three-class trajectories for social jetlag was made based on their congruity with the data and theoretical interpretation (eTables 1–3). Specifically, in Figure 1A, the three-class trajectories of sleep duration represented children who reported persistent short sleep duration (69.1%), declining-increasing sleep duration (3.1%), and persistent long sleep duration (27.8%) at preschool age. In Figure 1B, the two-class trajectories of sleep habits depicted children with persistent better sleep habits (94.1%) and persistent poor sleep habits (5.9%) at preschool age. In Figure 1C, the three-class trajectories of social jetlag separately categorized children into those with persistent low-level social jetlag (82.3%), declining-increasing social jetlag (10.6%), and increasing-declining social jetlag (7.1%).
Association Between Sleep Parameters and Their Trajectories With Myopia, Myopia Progression, Cycloplegic SER, AL, or AL/CR in School-Aged Children
After adjusting for age, gender, parental myopia, mode of delivery, maternal age, gestational weeks, family residence, monthly household income, passive smoking, parental education level, daily outdoor activity time, and daily video screen time, binary logistical regression analysis revealed that social jetlag of at least 1 hour at age 4 increased the risk of myopia among school-aged children (RR=1.38, 95% CI: 1.03~1.85; P=0.031; eFigure 2). Likewise, generalized linear models revealed a negative association between social jetlag of at least 1 hour at age 4 and cycloplegic SER in school-age children (β=−0.19, 95% CI: −0.34~-0.06; P=0.006; Table 2). As shown in Table 3, Compared to the children with social jetlag of less than 1 hour, children with social jetlag of at least 1 hour at the age of 4 years were more likely to have a 0.10mm increase in AL (β=0.12, 95% CI: 0.02~0.21; P=0.023). Insufficient sleep at age 5.5 was a risk factor for AL (β=0.16, 95% CI: 0.07~0.24; P<0.001). Compared to children with persistent low social jetlag, children with increasing-declining social jetlag are more prone to experiencing AL/CR ratio >3 (RR =1.55, 95% CI: 1.01~2.37; P=0.045; Table 4). Before adjusting for covariates, there was a significant association between persistent poor sleep habits and AL/CR among school-aged children (RR =0.61, 95% CI: 0.38~0.97; P=0.036; Table 4). However, the associations were not significant after adjusting confounders.
 |
Table 2 The Generalized Linear Models of the Associations of Sleep Parameters and Their Trajectories With Cycloplegic SER Among School-Aged Children (n=1561) |
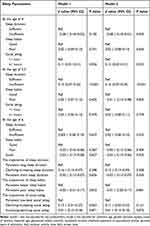 |
Table 3 The Generalized Linear Models of the Associations of Sleep Parameters and Their Trajectories With AL Among School-Aged Children (n=1561) |
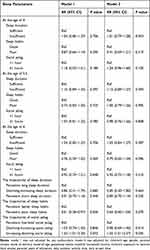 |
Table 4 The Logistic Regression Models of the Associations of Sleep Parameters and Their Trajectories With AL/CR Among School-Aged Children (n=1561) |
Association Between Sleep Parameters and Their Trajectories With Myopia Progression in School-Aged Children
eTable 4 presents the results of the linear mixed models. Poor sleep habits (β=−0.57, 95% CI: −0.94~-0.19; P=0.003) and higher social jet lag (β=−0.49, 95% CI: −0.80~-0.18; P=0.002) at the age of 4 were negatively associated with SER among school-age children. Similarly, children who exhibit a declining-increasing trajectory of social jetlag were more prone to be with lower SER (β=−0.49, 95% CI: −0.93~-0.06; P=0.027). However, children with persistent short sleep duration (β=0.38, 95% CI: 0.07~0.68; P=0.017) and persistent poor sleep habits (β=0.67, 95% CI: 0.04~1.31; P=0.038) tended to have higher SER. Furthermore, both the decreasing-increasing (β=0.59, 95% CI: 0.22~0.95; P=0.002) and increasing-declining trajectories of social jetlag (β=0.53, 95% CI: 0.07~0.98; P=0.023) were positively associated with the elongation of AL compared to persistent low-level social jetlag.
Discussion
As far as we are aware, this is the first study that delves into the associations of sleep parameters and their trajectories at preschool age with indicators of myopia based on a birth cohort study. We hypothesize that adverse sleep characteristics in preschool children will increase their risk of myopia and related ocular parameter abnormalities after attending elementary school.
Social jet lag, while frequently discussed in adults, is actually not uncommon among preschool children. Through research and observation, we typically categorize the causes of this phenomenon into three major categories. Firstly, preschool children are in a critical period of growth and development, and their circadian rhythms and sleep cycles may be more susceptible to external factors.30 Secondly, the family environment plays a crucial role. Parents may experience social jet lag due to fixed workdays and rest days, which significantly impacts preschool children. For instance, some parents may stay up late and rise late on weekend days, while others may travel on those days, both potentially affecting their children’s sleep schedules. Lastly, peer pressure is increasingly prevalent among younger children. Attending supplementary classes or interest groups on weekend days can disrupt children’s regular sleep patterns. Our study found that experiencing social jetlag of at least 1 hour during preschool years elevates the likelihood of developing myopia among school-aged children, and these children tend to have lower SER and longer AL. Children in the social jetlag increasing-decreasing group were at a higher risk of AL/CR ratio >3 during school age, compared to those with persistent low social jetlag. In addition, higher social jetlag at the age of 4 and the declining-increasing or increasing-declining trajectories of social jetlag were also discovered to be linked with myopia progression. Social jetlag, as a form of circadian rhythm disruption, mainly pertains to the misalignment between an individual’s natural biological clock on weekends and their weekday routine.31,32 In brief, individuals are required to rise early on weekdays to meet the demands of time, whereas on weekends or holidays, they tend to stay up late and rise late. This sudden shift in sleep-wake patterns can disrupt the body’s internal systems, akin to experiencing jet lag from crossing time zones, albeit induced by social and lifestyle habits rather than physical geographical changes. With the demands of academic and professional tasks, high social jetlag is becoming more prevalent. Xu et al33 found that primary school students with irregular sleep-wake patterns and social jetlag of at least 1 hour were more likely to be at a higher risk of self-reported myopia. This aligns with our findings. However, studies conducted in Shanghai, China, involving 10,142 school-aged children and research in India have not found a correlation between social jetlag and myopia.23,34 These discrepancies may stem from the fundamental differences in methodological and data between longitudinal and cross-sectional studies. Additionally, the age ranges of children in each study vary, and not all studies rely on cycloplegic refraction results for myopia diagnosis. Current research is inconclusive in definitively determining the precise impact of social jetlag on myopia, necessitating further exploration into its biological mechanism.
Sleep duration stands as one of the most frequently investigated sleep factors in relation to myopia, and its relationship with myopia remains inconclusive. In our study, children with insufficient sleep at 5.5 years old tended to have an elongation of AL. As we have previously discussed, there is a strong correlation between AL, SER, and the development of myopia. It can be observed that many studies have yielded results that are similar to those of this study. For instance, Cai et al35 discovered that the longer the total daily sleep time, the slower the growth rate of AL. A systematic review summarizing the relationship between various sleep characteristics and the risk of myopia suggests that adequate sleep duration may be a protective factor against myopia.36 Further, studies have shown that sleeping for fewer than 7 hours daily elevates the risk of myopia.37,38 An epidemiological study in Korea also showed a 0.10 D increase in SER for each additional hour of sleep.21 Moreover, a study involving 15,136 Chinese children indicated that after adjusting for confounders, those who slept less than 7 hours per night exhibited a greater risk of developing myopia in comparison to those who slept for over 9 hours per night.39 The Anyang Childhood Eye Study findings revealed a notable link between sleep duration and the progression of myopia in girls.24 Conversely, some studies failed to find an association between sleep and myopia.23,40 Additionally, the results of the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) birth cohort showed that sleep duration in infancy and the frequency of nighttime awakenings were found to have no correlation with SER in children aged 3 years.41 This may be attributed to the fact that infants’ and toddlers’ sleep patterns change as they grow older. Once they enter kindergarten, their sleep duration tends to decrease. While the correlation between sleep duration and myopia has not been consistently recognized, it is undeniable that adequate sleep duration is crucial for children’s physical development. Although our study demonstrated that adequate sleep duration at preschool age positively impacts the prevention of myopia in school-age children, further validation through extensive research is necessary.
In our study, we found that poor sleep habits at the age of 4 were associated with lower SER on repeated measures. Persistent poor sleep habit was significantly correlated with AL/CR>3 before adjusting confounders. There are similar reports suggesting that poor sleep habit play a pivotal role in the elongation of AL,42 with a high total CSHQ score being significantly associated with myopia.20 However, several studies have yielded different results. For instance, a study in Singapore showed no independent correlation between sleep characteristics in 8-year-old children and myopia one year later.43 Similarly, a study by Li et al34 using a CSHQ total score of 45.8 as a threshold found no significant association between poor sleep habits and myopia. The disparities in findings could potentially stem from variations in the sleep questionnaire used, as some studies have applied the Pittsburgh Sleep Quality Index.42 Additionally, variations in the countries and age groups studied may account for the differences in the threshold values for the CSHQ used and the findings across studies. Given the limited evidence currently available, further exploratory studies are imperative to gain a deeper understanding of the relationship between sleep habits and myopia.
Conversely, our findings reveal that persistent short sleep duration and persistent poor sleep habits were associated with higher SER, this contrasts with some of our other observations, primarily because both the two sleep metrics were categorized based on trajectory grouping. For example, Figure 1 indicates that the persistent short sleep duration is a relative measure. Moreover, while the persistent poor sleep habits group may appear to have an overall CHSQ scores of 54 or above, the small percentage of this group could also contribute to the discrepancies in our results.
The present study possesses the following strengths. Firstly, this is a study conducted based on a birth cohort. It examines two distinct growth stages, analyzes longitudinal data spanning four years, and explores the relationship between changes in preschool sleep characteristics and myopia in school-aged children, demonstrating novelty compared to prior studies. Additionally, by controlling for prenatal risk factors, birth information, genetics, and behavioral factors related to growth and development, the results of this study exhibit high credibility and can provide recommendations for myopia prevention and control from a life-course perspective. Secondly, the application of cycloplegic refraction as a criterion for categorizing myopia and the comprehensive coverage of various ocular biometric indices pertinent to myopia diagnosis are significant strengths of this study.
It cannot be denied that this study also has certain limitations. Sleep-related details were gathered from parents by questionnaires, potentially introducing recall bias from non-real-time recording; this bias could be minimized in future research by employing wrist-worn wearable measurement devices. However, the questionnaire survey has provided us with an effective means to understand the impact of environmental behavioral factors, such as parental behavior and lifestyle habits, on children’s sleep patterns. It also facilitates more in-depth exploration of the influence of these factors on children’s myopia in future research.
Conclusion
In conclusion, the findings of our study demonstrate that high social jetlag during preschool age is independently associated with myopia in school-aged children. Additionally, sleep insufficiency is linked to the elongation of the axial length during preschool age. The cultivation of family environment and habits is crucial for improving sleep quality. Certainly, having a consistent and healthy sleep pattern at preschool age, such as sufficient and stable sleep duration and low levels of social jet lag, can significantly contribute to preventing myopia. Identifying the risk factors of myopia in children from the perspective of life course can contribute to advancing prevention and control of myopia to an earlier stage and implementing precise intervention.
Data Sharing Statement
Unfortunately, the data in this study cannot be shared publicly as it is not derived from public databases and in order to protect the privacy of the study participants.
Ethics
This study complies with the Declaration of Helsinki.
Acknowledgments
The authors sincerely thank the consent of data access from The Ma’anshan Birth Cohort Study (MABC), carried out by the Institute of Anhui Medical University, and all participants involved in this study for their full support.
Author Contributions
Dr Shuman Tao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and study design: Shuman Tao and Fangbiao Tao. Acquisition, analysis, or interpretation of data: Shuman Tao, Juan Tong, Meng Wang, Dongqing Zhu, Guopeng Gao, Liu Jiang. Execution: Fangbiao Tao, Shuman Tao, Juan Tong, Kun Huang, Xiaoyan Wu, Shuangqin Yan. Drafting of the manuscript: Meng Wang. Substantially revised of the manuscript for important intellectual content: Shuman Tao. Critically reviewed the article: All authors. Statistical analysis: Meng Wang. Obtained funding: Shuman Tao and Fangbiao Tao. Administrative, technical, or material support: Fangbiao Tao and Shuangqin Yan. All authors have reached a consensus on the journal to which the article will be submitted. All authors have reviewed all versions of the article and have given their approval. All authors agree to take responsibility for the content of the article.
Funding
This study was funded by the National Natural Science Foundation of China (82273653), Major science and technology project of Anhui Provincial Science and Technology Innovation Platform (202305a12020015), the National Key Research and Development Program of China (2021YFC2702104, 2021YFC2702105). The study was also supported by Research Funds of Center for Big Data and Population Health of IHM (JKS2023011) and the Cultivation Program for Scientific and Technological Talents of the Second Affiliated Hospital of Anhui Medical University (2024PY01).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi:10.1016/j.ophtha.2016.01.006
2. Flitcroft DI, He M, Jonas JB, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–m30. doi:10.1167/iovs.18-25957
3. Morgan IG, Iribarren R, Fotouhi A, Grzybowski A. Cycloplegic refraction is the gold standard for epidemiological studies. Acta Ophthalmol. 2015;93(6):581–585. doi:10.1111/aos.12642
4. Han X, Liu C, Chen Y, He M. Myopia prediction: a systematic review. Eye. 2022;36(5):921–929. doi:10.1038/s41433-021-01805-6
5. Tao L, Wang C, Peng Y, et al. Correlation between increase of axial length and height growth in Chinese school-age children. Front Public Health. 2021;9:817882. doi:10.3389/fpubh.2021.817882
6. Richter GM, Wang M, Jiang X, et al. Ocular determinants of refractive error and its age- and sex-related variations in the Chinese American eye study. JAMA Ophthalmol. 2017;135(7):724–732. doi:10.1001/jamaophthalmol.2017.1176
7. Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi:10.1167/iovs.06-0562
8. Du R, Xie S, Igarashi-Yokoi T, et al. Continued increase of axial length and its risk factors in adults with high myopia. JAMA Ophthalmol. 2021;139(10):1096–1103. doi:10.1001/jamaophthalmol.2021.3303
9. Ojaimi E, Rose KA, Morgan IG, et al. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005;46(8):2748–2754. doi:10.1167/iovs.04-1324
10. Scheiman M, Gwiazda J, Zhang Q, et al. Longitudinal changes in corneal curvature and its relationship to axial length in the correction of myopia evaluation trial (COMET) cohort. J Optom. 2016;9(1):13–21. doi:10.1016/j.optom.2015.10.003
11. He X, Zou H, Lu L, et al. Axial length/corneal radius ratio: association with refractive state and role on myopia detection combined with visual acuity in Chinese schoolchildren. PLoS One. 2015;10(2):e0111766. doi:10.1371/journal.pone.0111766
12. Morgan IG, Wu PC, Ostrin LA, et al. IMI risk factors for myopia. Invest Ophthalmol Vis Sci. 2021;62(5):3. doi:10.1167/iovs.62.5.3
13. Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129(3):548–556. doi:10.1542/peds.2011-2039
14. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65. doi:10.1038/s41583-018-0088-y
15. Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT, Stone RA. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt. 2018;38(3):217–245. doi:10.1111/opo.12453
16. Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The circadian clock system in the mammalian retina. Bioessays. 2008;30(7):624–633. doi:10.1002/bies.20777
17. McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi:10.1016/j.preteyeres.2013.12.001
18. Bery A, Bagchi U, Bergen AA, Felder-Schmittbuhl MP. Circadian clocks, retinogenesis and ocular health in vertebrates: new molecular insights. Dev Biol. 2022;484:40–56. doi:10.1016/j.ydbio.2022.02.001
19. Saw SM, Wu HM, Hong CY, Chua WH, Chia KS, Tan D. Myopia and night lighting in children in Singapore. Br J Ophthalmol. 2001;85(5):527–528. doi:10.1136/bjo.85.5.527
20. Zhou Z, Morgan IG, Chen Q, Jin L, He M, Congdon N. Disordered sleep and myopia risk among Chinese children. PLoS One. 2015;10(3):e0121796. doi:10.1371/journal.pone.0121796
21. Jee D, Morgan IG, Kim EC. Inverse relationship between sleep duration and myopia. Acta Ophthalmol. 2016;94(3):e204–10. doi:10.1111/aos.12776
22. Qu Y, Yu J, Xia W, Cai H. Correlation of myopia with physical exercise and sleep habits among suburban adolescents. J Ophthalmol. 2020;2020:2670153. doi:10.1155/2020/2670153
23. Hussain A, Mohammad A, Tharsis A, Badakere A, Agarkar S. Association of sleep timings, duration, consistency, and chronotype with premyopia and myopia among Indian children. Eur J Ophthalmol. 2024;11206721241231335. doi:10.1177/11206721241231335
24. Wei SF, Li SM, Liu L, et al. Sleep duration, bedtime, and myopia progression in a 4-year follow-up of Chinese children: the Anyang childhood eye study. Invest Ophthalmol Vis Sci. 2020;61(3):37. doi:10.1167/iovs.61.3.37
25. Stafford-Bell N, McVeigh J, Lingham G, et al. Associations of 12-year sleep behaviour trajectories from childhood to adolescence with myopia and ocular biometry during young adulthood. Ophthalmic Physiol Opt. 2022;42(1):19–27. doi:10.1111/opo.12905
26. Zhou J, Tong J, Ru X, et al. Placental inflammatory cytokines mRNA expression and preschool children’s cognitive performance: a birth cohort study in China. BMC Med. 2023;21(1):449. doi:10.1186/s12916-023-03173-2
27. Organization WH. Guidelines on Physical Activity, Sedentary Behaviour and Sleep for Children Under 5 years of Age. Geneva: World Health Organization; 2018.
28. Tamura N, Komada Y, Inoue Y, Tanaka H. Social jetlag among Japanese adolescents: association with irritable mood, daytime sleepiness, fatigue, and poor academic performance. Chronobiol Int. 2022;39(3):311–322. doi:10.1080/07420528.2021.1996388
29. Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. doi:10.1093/sleep/23.8.1d
30. Wong SD, Wright KP Jr, Spencer RL, et al. Development of the circadian system in early life: maternal and environmental factors. J Physiol Anthropol. 2022;41(1):22. doi:10.1186/s40101-022-00294-0
31. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. doi:10.1080/07420520500545979
32. Caliandro R, Streng AA, van Kerkhof LWM, van der Horst GTJ, Chaves I. Social jetlag and related risks for human health: a timely review. Nutrients. 2021;13(12):4543. doi:10.3390/nu13124543
33. Xu S, Zong Z, Zhu Y, et al. Association between sleep-wake schedules and myopia among Chinese school-aged children and adolescents: a cross-sectional study. BMC Ophthalmol. 2023;23(1):135. doi:10.1186/s12886-023-02874-9
34. Li R, Chen Y, Zhao A, et al. Relationships between sleep duration, timing, consistency, and chronotype with myopia among school-aged children. J Ophthalmol. 2022;2022:7071801. doi:10.1155/2022/7071801
35. Cai T, Zhao L, Kong L, Du X. Complex interplay between covid-19 lockdown and myopic progression. Front Med. 2022;9:853293. doi:10.3389/fmed.2022.853293
36. Wang XX, Liu X, Lin Q, Dong P, Wei YB, Liu JJ. Association between sleep duration, sleep quality, bedtime and myopia: a systematic review and meta-analysis. Clin Exp Ophthalmol. 2023;51(7):673–684. doi:10.1111/ceo.14277
37. Wang H, Li L, Wang W, et al. Simulations to assess the performance of multifactor risk scores for predicting myopia prevalence in children and adolescents in China. Front Genet. 2022;13:861164. doi:10.3389/fgene.2022.861164
38. Saara K, Swetha S, Subhiksha R, Amirthaa M, Anuradha N. Steep increase in myopia among public school-going children in South India after COVID-19 home confinement. Ind J Ophthalmol. 2022;70(8):3040–3044. doi:10.4103/ijo.IJO_40_22
39. Xu X, Wang D, Xiao G, Yu KYT, Gong Y. Sleep less, myopia more. Theory Clin Pract Pediatrics. 2017;1(1):11–17. doi:10.25082/TCPP.2017.01.004
40. Liu XN, Naduvilath TJ, Wang J, et al. Sleeping late is a risk factor for myopia development amongst school-aged children in China. Sci Rep. 2020;10(1):17194. doi:10.1038/s41598-020-74348-7
41. Sensaki S, Sabanayagam C, Chua S, et al. Sleep duration in infants was not associated with myopia at 3 years. Asia Pac J Ophthalmol. 2018;7(2):102–108. doi:10.22608/apo.2017390
42. Yu D, Wang L, Zhou X, et al. Sleep quality is associated with axial length elongation in myopic children receiving orthokeratology: a retrospective study. Nat Sci Sleep. 2023;15:993–1001. doi:10.2147/nss.S421407
43. Li M, Tan CS, Xu L, et al. Sleep patterns and myopia among school-aged children in Singapore. Front Public Health. 2022;10:828298. doi:10.3389/fpubh.2022.828298
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Visual Acuity Prior to Cataract Surgery and Risk of Retinal Detachment – A Population-Based Study
Thylefors J, Jakobsson G, Zetterberg M, Sheikh R
Clinical Ophthalmology 2023, 17:1975-1980
Published Date: 12 July 2023
The Impact of Vergence Dysfunction on Myopia Control in Children Wearing Defocus Spectacle Lenses
Ma J, Yang X, Liu Z, Fu H, Fan S, Wang K, Li Y, Huang L, Zhao M
Clinical Ophthalmology 2024, 18:799-807
Published Date: 12 March 2024
Impact of Forms of Visual Attenuation on Short-Term Eye Changes Under Controlled Reading Visibility

Su H, Chun RKM, De Lestrange-Anginieur E
Eye and Brain 2024, 16:133-146
Published Date: 4 December 2024


