Back to Journals » Journal of Pain Research » Volume 18
Spinal Cord Stimulation Alleviates Sensitization of Neuropathic Pain by Upregulating G Protein-Coupled Receptors to Inhibit Overexpression of Cav2.2 and Its Downstream Excitatory Neurotransmitters
Authors Liu SL , Zhang HE , Kang JY, Ji HY , Cai ZH, Qi SH, Liu SS, Zhou HC
Received 13 January 2025
Accepted for publication 28 May 2025
Published 5 June 2025 Volume 2025:18 Pages 2775—2790
DOI https://doi.org/10.2147/JPR.S514719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wendy Imlach
Si-Liang Liu,1,2 Hong-En Zhang,2,3 Ji-Yu Kang,2,3 Huai-Yu Ji,3 Zhen-Hua Cai,4 Si-Hua Qi,1 Shan-Shan Liu,3 Hua-Cheng Zhou2,3
1Department of Anesthesiology, the Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, 150001, People’s Republic of China; 2NHC Key Laboratory of Molecular Probes and Targeted Diagnosis and Therapy, Harbin Medical University, Harbin, 150001, People’s Republic of China; 3Department of Pain Management, the Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, 150001, People’s Republic of China; 4Department of Pain Management, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, 150086, People’s Republic of China
Correspondence: Hua-Cheng Zhou, Department of Pain Management, The Fourth Affiliated Hospital of Harbin Medical University, 37 Yiyuan Street, Harbin, Heilongjiang, 150001, People’s Republic of China, Email [email protected]
Purpose: Spinal cord stimulation (SCS) is an effective treatment for various forms of neuropathic pain (NP), including postherpetic neuralgia, phantom limb pain, and painful diabetic neuropathy. Currently, although there have been studies on the analgesic mechanisms associated with SCS, the roles of ion channels in the therapeutic effects of SCS are still unclear.
Methods: In this study, NP hyperalgesia was induced in a rat model using chronic constriction injury (CCI) of the sciatic nerve. Ten days after modeling, the rats were treated with SCS (50 hz, 200 μs, and 80% motor threshold) for 3 hours each day for 5 consecutive days. The role of ion channels in SCS-induced analgesia was investigated using bioinformatics, western blotting, and immunofluorescence assays.
Results: Behavioral analysis showed that SCS treatment for 5 consecutive days increased both the mechanical and thermal pain thresholds of the CCI rats. The bioinformatics results indicated that N-type calcium channels (Cav2.2) were key in the induction of NP-associated hyperalgesia, with the opioid receptor-like 1 receptor (ORL-1) functioning as an upstream inhibitor of Cav2.2. The results also showed that SCS induced analgesia through upregulation of ORL-1 to inhibit overexpression of Cav2.2 and its downstream neurotransmitters, substance P and glutamate. This analgesic effect could be reversed by both Cav2.2 agonists and ORL-1 inhibitors.
Conclusion: SCS alleviates hyperalgesia in NP through upregulation of ORL-1 to inhibit the overexpression of Cav2.2 and its downstream neurotransmitters. This may be one of the mechanisms through which SCS induces analgesia. The elucidation of the ion channel mechanism of SCS will improve the clinical application procedures of SCS.
Keywords: Neuropathic pain, Pain sensitization, Spinal cord stimulation, N-type calcium channels, Glutamate, Substance P
Introduction
Neuropathic pain (NP) results from disorders or lesions of the somatosensory nervous system. The overall prevalence of NP is approximately 7–10%.1 Pain sensitization resulting from NP inflicts both physical and psychological distress on patients. Pharmacotherapy is used as a standard treatment but is relatively ineffective, with a response rate of less than 50%. In addition, there are few medications that are effective in targeting pain sensitization.2 Spinal cord stimulation (SCS) has been shown to be effective in treating various types of NP, including postherpetic neuralgia, failed back surgery syndrome, and complex regional pain syndrome.3 Previous studies found that SCS increased the release of the inhibitory neurotransmitter GABA, endogenous opioids, and endocannabinoids in the spinal cord segments.4 SCS also activated supraspinal regions, such as the periaqueductal gray and ventrolateral medulla to exert analgesic effects. However, the function of ion channels in the analgesic process of SCS is poorly understood.
Several studies have demonstrated the involvement of voltage-gated calcium channels (VGCCs) in pain sensitization.5–7 VGCCs can be classified into two types, namely, high-voltage and low-voltage activated channels. N-type calcium channels (Cav2.2) belong to the high-voltage category and are expressed mostly in lamina I and II in the dorsal horn of the spinal cord.8 These channels are responsible for transporting Ca2+ to synaptic terminals and activating the processes of exocytosis and neurotransmitter release. Downstream molecules of the channels include excitatory amino acids, glutamate (Glu), pronociceptive neurotransmitters, and substance P (SP).9 Studies have shown that inhibition of Cav2.2 can increase the mechanical pain threshold in NP rats,10 while a study on HIV-associated pain found that Cav2.2 expression reduction was effective in alleviating nociception in mice.11 These findings suggest the potential of Cav2.2 for treating NP.
In addition, Cav2.2 channels are regulated by G protein-coupled receptors (GPCRs) involved in pain transmission.12 Opioid receptor-like 1 receptor (ORL-1) is a member of this family as well as belonging to the opioid receptor family. Similar to other G protein-coupled receptors, activation of ORL-1 recruits G proteins and promotes their breakdown to Gα and Gβγ subunits. The Gβγ subunits can block the function of Cav2.2.13
In a previous study, we found that extended pulses of high-voltage radio-frequencies attenuated NP in rats with chronic constriction injury (CCI) of the sciatic nerve through reduction of Cav2.2 expression.14 However, it remains unknown whether SCS can alleviate NP pain sensitization through modulation of Cav2.2. Here, we established a rat CCI model to determine the effects of SCS on NP alleviation through inhibition of Cav2.2 to reduce Glu and SP secretion through ORL-1 activation.
Materials and Methods
Experimental Animals
Sprague–Dawley rats (male, 280–300 g) were obtained from the Animal Experimental Center of the Second Affiliated Hospital of Harbin Medical University and were kept in a comfortable environment, with a temperature of 22–24 °C, a stable day-night cycle, and unrestricted food and water. All the experimental procedures were approved by the Animal Management Committee of The Second Affiliated Hospital of Harbin Medical University (SYDW2020-040) and in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996).
Experimental Design and Experimental Groups
Part 1: The role of Cav2.2 in pain sensitization was evaluated. The animals were allocated to three groups. Those in the Sham group underwent exposure of the right sciatic nerve without ligation, while the nerve was ligated in the CCI group and the Sham+Cav2.2 agonist group received injections of a Cav2.2 agonist into the subarachnoid space (lumbar 5–6 interspace).
Part 2: It was observed that SCS alleviated pain sensitization by reducing Cav2.2 overexpression and the release of downstream neurotransmitters. Four groups were established in this section, namely, the Sham, CCI, CCI+SCS, and CCI+SCS+Cav2.2 agonist groups. SCS was administered for five consecutive days in the CCI+SCS group. The CCI+SCS+Cav2.2 agonist group received the Cav2.2 agonist on day 3 of SCS application, after which electrical stimulation was resumed for the day.
Part 3: It was found that SCS reduced Cav2.2 overexpression through ORL-1 activation. Four groups were established in this section, namely, the Sham, CCI, CCI+SCS, and CCI+SCS+ORL-1 inhibitor groups. Animals in the CCI+SCS+ORL-1 inhibitor group received an ORL-1 inhibitor on day 3 of the SCS application, after which the rats resumed electrical stimulation.
CCI Model
The CCI model was established as described.15 Briefly, following anesthesia by sevoflurane inhalation and under sterile conditions, the right hind limb was incised (1.5-cm incision), allowing exposure of the sciatic nerve. The nerve was ligated in four places using 4–0 chromic gut at 2-mm intervals, with adjustment of the knots to permit slight twitching of the lower limb. After ligation, the muscular and cutaneous layers were closed layer by layer using surgical 4–0 absorbable sutures. Rats that did not develop hyperalgesia or experience rear paw autophagy were excluded from subsequent studies.
Electrode Implantation and SCS Procedure
On day 7 after CCI, an electrode (diameter, 0.6 mm; Beijing PINS Medical Co., Ltd., China) was implanted in the epidural space of the rat. Briefly, after anesthesia with sodium pentobarbital, the electrode was implanted in the T13 intervertebral space, with movement of the electrode head to the T10-T12 level. The electrode was fixed to the tissue surrounding the vertebral body using tissue adhesive, and after sterilization, the muscles and skin were turned off layer by layer. Two days after electrode placement, if there was no evidence of spinal cord injury, SCS was performed. Based on the findings of previous studies, the parameters used were 50 hz, 200 μs, and an 80% motor threshold (MoT). The stimulation was performed for three hours daily for five days in succession.16 The MoT represented the minimum amplitude of the current leading to discomfort in rats that were awake.
Intrathecal Injection of Drugs
For intrathecal drug administration, rats were anesthetized and the L5-L6 intervertebral space was exposed, as previously described.17 The ligamentum flavum was then punctured with a micro syringe. A successful puncture was indicated by wagging of the rat’s tail and the observation of clear cerebrospinal fluid on aspiration. As previously described, Ca2+ channel agonist-1 (CAS No.: 1402821-24-2, MedChemExpress, USA) was administered at a concentration of 50 μM in a volume of 10 μL.18 The ORL-1 inhibitor JTC-801 (CAS No.: 244218-51-7, MedChemExpress) was administered at 45 pg in a volume of 10 μL. After completion of the injection, the tissue and skin were disinfected and sutured layer by layer.18,19
Assessment of Pain Sensitization
The paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) were assessed following intrathecal drug administration.20 In a wire-mesh cage, the center of the hindfoot of the rats was stimulated with Von Frey filaments (measurement range, 0.008 to 300 g; NC12775, Yuyan Instruments Co., Ltd., Shanghai, China). Movements, such as lifting and licking the foot, indicated a positive reaction, and the value at this time was recorded. The PWL was assessed using a hot plate (Model YLS-6B, Yiyan Technology Co., Shandong, China) at 52 °C. After the rat lifted its right foot, the animal was lifted off the plate and the time spent on the plate was noted. The above process was repeated three times, with each measurement separated by 10 min. The average times were calculated and used as the PWT and PWL values for each rat.
Western Blotting
The detection of Cav2.2 protein is used as an example to explain the procedure. On day 14 following CCI, the animals were euthanized and the tissue of the ipsilateral dorsal horn of the spinal cord (L4-6 segments) was rapidly collected and frozen at −80 °C. Fifty grams of tissue were lysed in RIPA buffer with PMSF. The lysates were centrifuged, and protein contents in the supernatant were assessed by BCA assays. The proteins (30 μg per lane) were separated on 8% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked (5% skim milk, 2 h) and then treated for 24 h with a rabbit monoclonal antibody against Cav2.2 (1:1000, Cat #: ACC-002, Alomone Labs, Israel) at 4 °C, followed by a goat anti-rabbit IgG secondary antibody (2 h, room temperature), ECL development for 2 min and X-ray imaging. A rabbit anti-ORL-1 antibody (1:2000, DF2834#257, Affinity) was used for the detection of ORL-1 protein, while a rabbit anti-SP antibody (1:2000, DF7522#257, Affinity) was used for the detection of SP protein. Protein expression level representation: The ratio of protein grayscale values to GADPH grayscale values in each group. Protein band densities were analyzed by Image J with normalization to GAPDH.
Immunofluorescence
Rats were anesthetized with sevoflurane and their hearts perfused with 4% paraformaldehyde. The L4-6 spinal cord tissue was immersed in 4% paraformaldehyde for 24 h, followed by dehydration using 20 and 30% sucrose, embedding with Tissue-Tek optimal cutting temperature (OCT), and storage at −80 °C. The tissue was sectioned (14 µm sections) using a cryostat and washed thoroughly in PBS before overnight exposure to primary antibodies against Cav2.2 (1:400, Alomone Labs) at 4 °C, followed by a secondary antibody (Dylight 488 Goat Anti-Rabbit IgG, 1:400, RS23220, ImmunoWay), DAPI counterstaining (10 µg/mL, SI101-01, SEVEN), and imaging under confocal laser microscopy (Olympus, Japan).
Bioinformatics Analysis of Cav2.2
Gene Ontology (GO) Analysis
The GSE212311 dataset was obtained from the GEO database at NCBI. The dataset contained information from the lumbar 4–6 dorsal root ganglia of CCI and sham-operated rats. Data were analyzed using the DEseq2 package in R. Differentially expressed genes (DEGs) were identified using the criterion of P.adj<0.05 and the DEGs underwent GO analysis using clusterProfiler in R.
Protein-Protein Interaction (PPI) Analysis
The STRING database (Version 12.0) was utilized to construct a PPI network of the target genes, namely CACNA1B (Cav2.2) and OPRL1 (ORL-1). The species was set to “rattus norvegicus”. The top five validated core molecules of the first layer interaction were extracted and presented in the figure.
Measurement of Glutamate Concentrations
A glutamate assay kit (Catalog Number: AKAM002M, Boxbio, China) was utilized for measuring glutamate concentrations in the spinal cord, following the provided directions. Briefly, the spinal cord tissue was mixed with the kit reagents in a ratio of 1:10, homogenized, and centrifuged to obtain the supernatant. The samples were added to 96-well plates and the absorbances at 450 nm were read in a pre-warmed (> 30 min) microplate reader. The standard curve was plotted from the absorbance value of the standard. According to the absorbance value of the sample, glutamate contents in the samples were determined from the standard curve.
Statistical Analysis
Results are expressed as means ± standard deviations and were analyzed and graphed using GraphPad Prism 10.0 (GraphPad, USA). The PWT and PWL values were analyzed using two-way repeated measures ANOVA, followed by Bonferroni’s post-hoc test. Other results were compared using one-way ANOVA followed by Tukey’s post-hoc test when comparing >2 groups. P < 0.05 was considered statistically significant.
Results
The SCS electrodes were fixed on the dorsal surfaces of the rats to prevent displacement due to the rats’ activity (Figure 1A). The SCS electrodes were implanted at the thoracic spinal levels 10–12 (T10-T12) of the rats, with the position verified by X-ray imaging (Figure 1B). The experimental procedures and treatments used for the different groups are shown in Figure 1C–H.
Overexpression of Cav2.2 Is Closely Associated with Pain Sensitization Induced by CCI
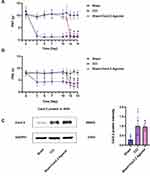
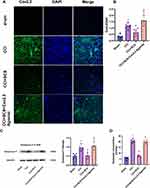
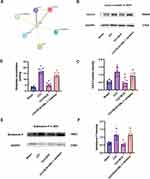
GO analysis of Cav2.2 showed enrichment in biological processes associated with ion transport, exocytosis, and positive regulation of secretion, while that in the cellular component category included distal axons, cell leading edges, and transporter complexes, and the molecular functions included channel activity and passive transmembrane transporter activity (Figure 2). The baseline PWT and PWL values in the three groups were comparable on day 3 after surgery. Relative to the Sham group, the rats in the CCI group exhibited markedly reduced PWT and PWL values, which continued to decline for 14 days. After administration of the Cav2.2 agonist, the Sham+Cav2.2 agonist group also exhibited a significant decrease in PWT and PWL relative to the Sham rats (Figure 3A and B). Western blotting indicated raised levels of Cav2.2 in the spinal cord at 14 days post-CCI surgery. Relative to the Sham group, the Sham+Cav2.2 Agonist group also showed increased Cav2.2 levels (Figure 3C). Immunofluorescence showed that Cav2.2 protein was expressed in the spinal cord dorsal horn (Figure 4A). On day 14 post-CCI surgery and after the application of the Cav2.2 agonist, there was an increase in Cav2.2 immunoreactivity (Figure 4B and C). Relative to the Sham rats, the levels of SP and Glu were raised in both the CCI and the Sham+Cav2.2 agonist groups (Figure 4D and E).
 |
Figure 2 Gene Ontology enrichment of Cav2.2. The length of the lines represents the number of genes. Abbreviations: BP, Biological Process; CC, Cellular Component; MF, Molecular Function. |
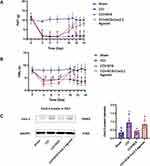
SCS Ameliorates Pain Sensitization and Reduces the Levels of Cav2.2, SP, and Glu in the Spinal Cord
Relative to the CCI group, the mechanical and thermal pain thresholds were markedly elevated in the CCI+SCS group. This improvement was observed throughout the SCS treatment and could be reversed by Cav2.2 agonists (Figure 5A and B). Relative to the CCI group, five continuous days of SCS treatment in the CCI+SCS group reduced Cav2.2 overexpression in the spinal cord. This inhibitory effect of SCS was reversed by the Cav2.2 agonists (Figure 5C). The immunofluorescence results confirmed that SCS could reduce Cav2.2 overexpression. However, relative to the CCI+SCS group, the immunofluorescence intensity of Cav2.2 in the CCI+SCS+Cav2.2 agonist group was increased (Figure 6A and B). Relative to the Sham rats, those in the CCI group had raised levels of both SP and Glu on D14. In the CCI+SCS group, SCS treatment was found to reduce SP and Glu levels relative to the CCI group. In addition, Cav2.2 agonists were found to reverse the inhibitory effects of SCS on the raised levels of SP and Glu (Figure 6C and D).
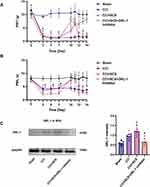
SCS Inhibits Overexpression of Cav2.2 and Its Downstream Neurotransmitters by Upregulating ORL-1
SCS treatment for five consecutive days was found to raise the thresholds of both mechanical and thermal pain in CCI rats but this effect was reversed by ORL-1 inhibitors (Figure 7A and B). The levels of ORL-1 were increased in the CCI group and were raised further after SCS administration (Figure 7C). The PPI network indicated an interaction between ORL-1 and Cav2.2 (Figure 8A). On the third day of SCS treatment (D12), administration of an ORL-1 inhibitor was used to investigate the interaction between ORL-1 and Cav2.2. The results showed downregulation of Cav2.2 in the CCI+SCS group relative to the CCI group. After ORL-1 inhibitors were used, the CCI+SCS+ORL-1 inhibitor group exhibited an upregulation of Cav2.2 expression (Figure 8B and C). In addition, SCS also reduced Glu and SP expression, and this effect was also reversed by the ORL-1 inhibitor (Figure 8D–F).
Discussion
This study investigated interactions between GPCRs and calcium channels in SCS-mediated analgesia. Specifically, it was found that SCS increased the levels of the GPCR ORL-1 which interacted with Cav2.2 calcium channels, thereby reducing the levels of Cav2.2 and its downstream excitatory neurotransmitters Glu and SP, and thus alleviating pain sensitization in an NP rat model following CCI.
Cav2.2 Is Closely Involved in Pain Sensitization Induced by CCI
Functional enrichment analyses of Cav2.2 showed associations with exocytosis and ion transport processes, indicating the importance of these pathways in NP. Opening of Cav2.2 enables calcium ion flow into the cell, resulting in the development of action potentials and the subsequent release of neurotransmitters such as Glu and SP. Thus, Cav2.2 channels are associated with nociceptive information transmission in the spinal cord.21,22 These earlier findings suggested that Cav2.2 may be linked to the effects of SCS treatment.
The study used PWT and PWL as measures of pain sensitization in CCI rats. The results showed that both values decreased from day 3 after CCI and remained low for 14 days. This is consistent with previous research results and indicates the successful establishment of the CCI model.23 Moreover, administration of Cav2.2 agonists led to marked reductions in both PWT and PWL values, indicating similar effects produced by both Cav2.2 agonists and ligation of the sciatic nerve and demonstrating that activation of Cav2.2 induced pain sensitization in healthy rats. In addition, earlier findings from our group showed that the use of Cav2.2 inhibitors (ω-conotoxins GVIA) could increase PWT and PWL values in CCI rats. To further verify the function of Cav2.2 in NP development, the levels of Cav2.2 were assessed on day 14 following CCI. This time point was selected as the SCS treatment was completed on day 14 after surgery. Western blotting results indicated increased levels of Cav2.2 on day 14, and the use of Cav2.2 agonists had a similar effect. Moreover, immunofluorescence analysis of spinal cord sections also showed that Cav2.2 was expressed specifically in the dorsal horn of the spinal cord and was markedly upregulated on day 14 after CCI. Combined with the behavioral analyses, these results suggest the significant involvement of Cav2.2 in the development of pain sensitization. These findings are consistent with those of earlier investigations showing that elevated Cav2.2 levels reduced pain thresholds in rats.24,25
The α1 subunit of Cav2.2 contains sites that interact with synaptic vesicle proteins.26 Cav2.2 is thus an ion channel associated with neuroendocrine functions, with Glu and SP functioning as downstream neurotransmitters.27 Excessive levels of Glu in the spinal cord dorsal horn increase neuronal excitability.28 SP interacts with its receptor neurokinin-1, inducing electrical activity and neuronal sensitization.29 Therefore, both Glu and SP are involved in pain sensitization. This is also one of the reasons why the Cav2.2 channels are associated with NP.
In this study, Western blotting demonstrated that SP levels in the spinal cord were raised in rats following CCI. The levels of Glu were also observed to have increased on day 14 after CCI. Similar to sciatic nerve ligation, the administration of Cav2.2 agonists raised the levels of both Glu and SP. These findings, together with those of the behavioral tests, indicated that increased levels of Cav2.2 and its downstream neurotransmitters were key to the development of pain sensitization, suggesting that inhibition of these molecules may be useful for treating NP.
SCS Alleviates Pain Sensitization by Reducing the Levels of Cav2.2 and Its Downstream Neurotransmitters
In clinical practice, SCS treatment usually lasts 24 h a day for 7–10 consecutive days. Based on previous research, we chose to stimulate for 3 h per day for 5 consecutive days, with tissue samples collected on the day after the cessation of electrical stimulation.16 These parameters could be modeled to a certain extent from the clinical application of SCS and were also within the range that experimental rats could tolerate. It was found that 5 consecutive days of SCS treatment was effective in improving the PWT and PWL values in the rats, indicating that SCS alleviated pain sensitization after CCI. These results agree with those of earlier studies.16 Moreover, the analgesic effects of SCS could be reversed by Cav2.2 agonists. SCS treatment also significantly reduced Cav2.2 in the spinal cord on day 14. This effect was reversed by administration of Cav2.2 agonists. Furthermore, immunofluorescence analysis of spinal cord sections confirmed that SCS markedly reduced Cav2.2 expression in the rat spinal cords following CCI.
These results suggested that the analgesic effect of SCS might be related to reduced Cav2.2 levels in the dorsal horn of the spinal cord. A previous study demonstrated that SCS could downregulate CSF 1 in the spinal cord as a treatment for NP,23 while other studies found that SCS could also increase opioid peptide expression to achieve therapeutic effects30 and that raising the mechanical pain threshold by SCS may be related to electric charge delivery.31 This suggests that SCS may alter the expression of analgesia-related substances through charge release, consistent with the involvement of calcium ion channels.
High-frequency SCS has been shown to reduce Glu levels in the cerebrospinal fluid, consistent with the present findings of similar effects at 50 hz SCS.32 We also found that continuous SCS decreased the levels of Glu and SP in the spinal cord. This may partly depend on the downregulation effect of SCS on Cav2.2 overexpression. Consistent with the observed neurotransmitter levels in the cerebrospinal fluid, the changes in neurotransmitter levels within the spinal cord could explain why SCS was effective in alleviating pain sensitization in CCI rats. This hypothesis complements the conventional view that SCS induces upregulation of GABA and indicates that SCS can inhibit the production of excitatory neurotransmitters. Moreover, the inhibitory effect of SCS on the overexpression of Cav2.2 downstream neurotransmitters can also explain the effect of SCS on Cav2.2 activity from another perspective. The above results confirm that SCS can induce analgesic effects by reducing the levels of Cav2.2 together with those of its downstream neurotransmitters (Glu and SP). This suggests that this may represent a mechanism underlying the effects of SCS.
SCS Upregulates ORL-1 to Inhibit Cav2.2 Overexpression
To elucidate the reason why SCS reduces Cav2.2 levels, a PPI network was constructed for the Cav2.2 protein. This indicated an interaction between ORL-1 and Cav2.2. There is a physical and functional coupling between ORL-1 and Cav2.2. ORL-1 is a GPCR that also belongs to the opioid receptor family. It has been shown that SCS induces analgesia through the modulation of opioid levels.30 It was thus hypothesized that SCS might have a regulatory effect on ORL-1. Earlier studies have also demonstrated Cav2.2 inhibition by GPCRs. Thus, ORL-1 may act as an intermediary between SCS and Cav2.2 inhibition.
ORL-1 itself may have endogenous analgesic effects. One study showed upregulation of ORL-1 in the spinal cords of CCI rats and that intrathecal injection of ORL-1 agonists induced analgesia.33 A similar phenomenon was observed in our study. Five consecutive days of SCS treatment effectively improved both the mechanical and thermal pain thresholds in CCI rats, and this effect was reversed by ORL-1 inhibitors. Western blotting showed increased ORL-1 protein levels with further elevation induced by SCS. These findings suggest that SCS induces analgesia by activating ORL-1. Interestingly, the results showed that the ORL-1 inhibitor reversed the effects of SCS on Cav2.2 overexpression. The ORL-1 inhibitor also reversed the SCS-mediated downregulation of Glu and SP. These results suggest that SCS activates ORL-1 and reduces Cav2.2 overexpression. SCS was also shown to lower the levels of Glu and SP to induce analgesia.
SCS has irreplaceable advantages in the treatment of NP. Compared to opioids, SCS is a neuromodulation therapy. There is no drug addiction associated with SCS treatment. SCS treatment also rarely causes side effects like nausea and vomiting associated with opioid drugs. In addition, GPCRs have been investigated extensively as targets for NP therapy due to their regulatory functions. Unlike conventional ion channel blockers, GPCR modulation may lead to more durable analgesic effects.34 This study found that SCS affected calcium channels through GPCRs. This also explains the long-term therapeutic effects of SCS on refractory NP. All of the above suggest that SCS shows remarkable prospects for development in NP treatment.
The mechanism through which SCS upregulates ORL-1 is currently unclear. As previously described, ORL-1 is a GPCR and thus GTP is involved in ORL-1 inhibition of Cav2.2 channels. Therefore, we speculate that SCS, which involves the release of electrical energy, may enhance ORL-1-mediated reduction of Cav2.2 expression through energy-driven mechanisms. Additionally, ORL-1 is a member of the opioid receptor family. SCS has an upregulating effect on the expression of endogenous opioid substances. This suggests that SCS may also have an upregulating effect on ORL-1. In future studies, we will explore the specific mechanism by which SCS activates ORl-1.
There are several limitations to this study. First, while the research observed the effect of SCS on Cav2.2 expression, the effect of SCS on Cav2.2 function was not explored. Second, as Cav2.2 is known to be involved in neurotransmitter secretion processes, the associations of Cav2.2 channels with SCS-induced analgesia were investigated. In the following study, we will use RNA sequencing to evaluate the functions of multiple ion channels in these processes. Third, we will continue to explore the specific mechanisms by which SCS regulates ORL-1.
Conclusions
SCS was found to upregulate ORL-1 to reduce the levels of Cav2.2 and its downstream neurotransmitters Glu and SP, inducing analgesic effects. These results suggest that this may represent a mechanism underlying the analgesic effects of SCS.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We appreciate the experimental support provided by the laboratories of the Second and Fourth Affiliated Hospitals of Harbin Medical University. We would like to express our gratitude The Beijing PINS Medical Co., Ltd. for providing the experimental equipment for this study.
Funding
This study was supported by funding from the Graduate Innovation Program of Harbin Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baskozos G, Hebert HL, Pascal MM, et al. Epidemiology of neuropathic pain: an analysis of prevalence and associated factors in UK Biobank. Pain Rep. 2023;8(2):e1066. doi:10.1097/PR9.0000000000001066
2. Yin Q, Zou T, Sun S, Yang D. Cell therapy for neuropathic pain. Front Mol Neurosci. 2023;16:1119223. doi:10.3389/fnmol.2023.1119223
3. Dones I, Levi V. Spinal Cord Stimulation for Neuropathic Pain: current Trends and Future Applications. Brain Sci. 2018;8(8):138. doi:10.3390/brainsci8080138
4. Zhang TC, Janik JJ, Grill WM. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. 2014;1569:19–31. doi:10.1016/j.brainres.2014.04.039
5. Gomez K, Duran P, Tonello R, et al. Neuropilin-1 is essential for vascular endothelial growth factor A-mediated increase of sensory neuron activity and development of pain-like behaviors. Pain. 2023;164(12):2696–2710. doi:10.1097/j.pain.0000000000002970
6. Dray A. Neuropathic pain: emerging treatments. Br J Anaesth. 2008;101(1):48–58. doi:10.1093/bja/aen107
7. Cui W, Wu H, Yu X, Song T, Xu X, Xu F. The Calcium Channel alpha2delta1 Subunit: interactional Targets in Primary Sensory Neurons and Role in Neuropathic Pain. Front Cell Neurosci. 2021;15:699731. doi:10.3389/fncel.2021.699731
8. Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316(6027):4403. doi:10.1038/316440a0
9. Hoppanova L, Lacinova L. Voltage-dependent Ca(V)3.2 and Ca(V)2.2 channels in nociceptive pathways. Pflugers Arch. 2022;474(4):421–434. doi:10.1007/s00424-022-02666-y
10. Chen K, Wang T, Li Y, et al. Rhodojaponin VI indirectly targets Cav2.2 channels via N-ethylmaleimide-sensitive fusion protein to alleviate neuropathic pain. Acta Pharm Sin B. 2023;13(3):1326–1336. doi:10.1016/j.apsb.2023.01.021
11. Luckemeyer DD, Prudente AS, de Amorim Ferreira M, et al. Critical Pronociceptive Role of Family 2 Voltage-Gated Calcium Channels in a Novel Mouse Model of HIV-Associated Sensory Neuropathy. Mol Neurobiol. 2023;60(5):2954–2968. doi:10.1007/s12035-023-03244-8
12. Currie KP. G protein modulation of CaV2 voltage-gated calcium channels. Channels. 2010;4(6):497–509. doi:10.4161/chan.4.6.12871
13. Caminski ES, Antunes FTT, Souza IA, Dallegrave E, Zamponi GW. Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders. Mol Brain. 2022;15(1):95. doi:10.1186/s13041-022-00982-z
14. Cai Z, Quan L, Chang X, Qiu Z, Zhou H. High-voltage long-duration pulsed radiofrequency attenuates neuropathic pain in CCI rats by inhibiting Cav2.2 in spinal dorsal horn and dorsal root ganglion. Brain Res. 2022;1785:147892. doi:10.1016/j.brainres.2022.147892
15. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
16. Shu B, He SQ, Guan Y. Spinal Cord Stimulation Enhances Microglial Activation in the Spinal Cord of Nerve-Injured Rats. Neurosci Bull. 2020;36(12):1441–1453. doi:10.1007/s12264-020-00568-6
17. Yang J, Xie MX, Hu L, et al. Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav Immun. 2018;71:52–65. doi:10.1016/j.bbi.2018.04.016
18. Liang M, Tarr TB, Bravo-Altamirano K, et al. Synthesis and biological evaluation of a selective N- and p/q-type calcium channel agonist. ACS Med Chem Lett. 2012;3(12):985–990. doi:10.1021/ml3002083
19. Tamai H, Sawamura S, Takeda K, Orii R, Hanaoka K. Anti-allodynic and anti-hyperalgesic effects of nociceptin receptor antagonist, JTC-801, in rats after spinal nerve injury and inflammation. Eur J Pharmacol. 2005;510(3):223–228. doi:10.1016/j.ejphar.2005.01.033
20. Huang LE, Guo SH, Thitiseranee L, Yang Y, Zhou YF, Yao YX. N-methyl D-aspartate receptor subtype 2B antagonist, Ro 25-6981, attenuates neuropathic pain by inhibiting postsynaptic density 95 expression. Sci Rep. 2018;8(1):7848. doi:10.1038/s41598-018-26209-7
21. Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18(16):6319–6330. doi:10.1523/JNEUROSCI.18-16-06319.1998
22. Weber AM, Wong FK, Tufford AR, Schlichter LC, Matveev V, Stanley EF. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat Neurosci. 2010;13(11):1348–1350. doi:10.1038/nn.2657
23. Sun C, Tao X, Wan C, et al. Spinal Cord Stimulation Alleviates Neuropathic Pain by Attenuating Microglial Activation via Reducing Colony-Stimulating Factor 1 Levels in the Spinal Cord in a Rat Model of Chronic Constriction Injury. Anesth Analg. 2022;135(1):178–190. doi:10.1213/ANE.0000000000006016
24. Pitake S, Middleton LJ, Abdus-Saboor I, Mishra SK. Inflammation Induced Sensory Nerve Growth and Pain Hypersensitivity Requires the N-Type Calcium Channel Cav2.2. Front Neurosci. 2019;13:1009. doi:10.3389/fnins.2019.01009
25. Mao M, Fan W, Zheng Y, Qi P, Xi M, Yao Y. Upregulation of N-Type Voltage-Gated Calcium Channels Induces Neuropathic Pain in Experimental Autoimmune Neuritis. Evid Based Complement Alternat Med. 2022;2022:8547095. doi:10.1155/2022/8547095
26. Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13(6):1303–1313. doi:10.1016/0896-6273(94)90417-0
27. Heinke B, Balzer E, Sandkuhler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci. 2004;19(1):103–111. doi:10.1046/j.1460-9568.2003.03083.x
28. Temmermand R, Barrett JE, Fontana ACK. Glutamatergic systems in neuropathic pain and emerging non-opioid therapies. Pharmacol Res. 2022;185:106492. doi:10.1016/j.phrs.2022.106492
29. Chen SH, Lin YW, Tseng WL, Lin WT, Lin SC, Hsueh YY. Ultrahigh frequency transcutaneous electrical nerve stimulation for neuropathic pain alleviation and neuromodulation. Neurotherapeutics. 2024;21(3):e00336. doi:10.1016/j.neurot.2024.e00336
30. Zhai FJ, Han SP, Song TJ, et al. Involvement of Opioid Peptides in the Analgesic Effect of Spinal Cord Stimulation in a Rat Model of Neuropathic Pain. Neurosci Bull. 2022;38(4):403–416. doi:10.1007/s12264-022-00844-7
31. Chen Z, Huang Q, Yang F, et al. The Impact of Electrical Charge Delivery on Inhibition of Mechanical Hypersensitivity in Nerve-Injured Rats by Sub-Sensory Threshold Spinal Cord Stimulation. Neuromodulation. 2019;22(2):163–171. doi:10.1111/ner.12910
32. Liao WT, Tseng CC, Chia WT, Lin CR. High-frequency spinal cord stimulation treatment attenuates the increase in spinal glutamate release and spinal miniature excitatory postsynaptic currents in rats with spared nerve injury-induced neuropathic pain. Brain Res Bull. 2020;164:307–313. doi:10.1016/j.brainresbull.2020.09.005
33. Wu Q, Liu L. ORL(1) Activation Mediates a Novel ORL(1) Receptor Agonist SCH221510 Analgesia in Neuropathic Pain in Rats. J Mol Neurosci. 2018;66(1):10–16. doi:10.1007/s12031-018-1140-0
34. Uniyal A, Tiwari V, Tsukamoto T, Dong X, Guan Y, Raja SN. Targeting sensory neuron GPCRs for peripheral neuropathic pain. Trends Pharmacol Sci. 2023;44(12):1009–1027. doi:10.1016/j.tips.2023.10.003
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Painful Peripheral Neuropathies of the Lower Limbs and/or Lower Extremities Treated with Spinal Cord Stimulation: A Systematic Review with Narrative Synthesis
Burkey AR, Chen J, Argoff CE, Edgar DR, Petersen EA
Journal of Pain Research 2023, 16:1607-1636
Published Date: 18 May 2023