Back to Journals » Journal of Pain Research » Volume 18
Spinal Cord Stimulation Explantation and Chronic Pain: A Systematic Review and Technology Recommendations
Authors Wahezi SE, Yener U , Naeimi T, Lewis JB, Yerra S, Sgobba P, Ciftci HB , Vydyanathan A , Chiu E, Cherkalin D, Darji JY, Masterson R, Lee D, Jarusriwanna A , Palee S, Ortiz NR, Caparo M, Dayon E , Fontaine C, Bikson M, Schatman ME , Pritzlaff SG , Deer TR , Hunter CW
Received 22 January 2025
Accepted for publication 11 March 2025
Published 18 March 2025 Volume 2025:18 Pages 1327—1340
DOI https://doi.org/10.2147/JPR.S514732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Sayed E Wahezi,1 Ugur Yener,1 Tahereh Naeimi,1 Joshua B Lewis,1 Sandeep Yerra,1 Philip Sgobba,2 Hatice Begum Ciftci,3 Amaresh Vydyanathan,2 Elisa Chiu,1 Denis Cherkalin,4 Jay Y Darji,5 Ryan Masterson,6 Danielle Lee,7 Atthakorn Jarusriwanna,8 Suwannika Palee,9 Nicole R Ortiz,10 Moorice Caparo,1 Eli Dayon,11 Camille Fontaine,2 Marom Bikson,12 Michael E Schatman,13 Scott G Pritzlaff,14 Timothy R Deer,15 Corey W Hunter16
1Department of Physical Medicine & Rehabilitation, Montefiore Medical Center, Bronx, NY, USA; 2Department of Anesthesiology, Montefiore Medical Center, Bronx, NY, USA; 3Physical Medicine and Rehabilitation, ROMMER International Physical Therapy and Rehabilitation Medical Center, Bursa, Turkey; 4Pain Management, New York Spine Specialist, New York, NY, USA; 5Pain Management, Regenerative Spine and Pain Institute, Plainsboro Township, NJ, USA; 6Pain Management, Old Mill District Clinic, Summit Health, Bend, OR, USA; 7Department of Neurology, Hackensack University Medical Center, Hackensack, NJ, USA; 8Department of Orthopaedics, Faculty of Medicine, Naresuan University, Phitsanulok, Thailand; 9Department of Rehabilitation Medicine, Faculty of Medicine, Naresuan University, Phitsanulok, Thailand; 10Pain Management, Sage Pain & Wellness Institute, San Diego, CA, USA; 11Department of Physical Medicine & Rehabilitation, Burke Rehabilitation Hospital, White Plains, NY, USA; 12Department of Biomedical Engineering, the City College of New York, New York, NY, USA; 13Department of Anesthesiology, Perioperative Care and Pain Medicine, Department of Population Health – Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, USA; 14Department of Anesthesiology and Pain Medicine, University of California, Davis, CA, USA; 15The Spine and Nerve Center of the Virginias, West Virginia University Hospitals, Charleston, WV, USA; 16Ainsworth Institute of Pain Management, Department of Rehabilitation & Human Performance, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Correspondence: Sayed E Wahezi, Department of Physical Medicine and Rehabilitation, Montefiore Medical Center, 1250 Waters Place, Tower #2 8th Floor, Bronx, NY, 10461, USA, Tel +1 718-920-7246, Fax +1 929-263-3950, Email [email protected]
Background: Chronic pain affects 20.5% of the US population, costing $296 billion annually in lost productivity. Spinal cord stimulation (SCS) has become a key treatment for refractory neuropathic and nociceptive pain, with increasing usage due to technological advancements. However, the durability of SCS therapy, including explantation rates, remains a concern. Understanding explantation causes is essential for improving patient selection and device effectiveness. This study aims to analyze SCS explantation rates and reasons, as well as evaluate the financial burden of these procedures on the healthcare system.
Methods: Three primary screening methods were used: manual search with keywords, MeSH term query, and reference list screening. The search covered PubMed, Cochrane, and Web of Science databases from inception to November 2024, yielding 719 articles. After applying eligibility criteria, 72 articles were identified, and 25 were selected for analysis. Data extraction was done by independent reviewers, with a second reviewer ensuring accuracy. Discrepancies were resolved by the corresponding editor.
Results: We reviewed data from 13,026 patients who underwent permanent SCS implantation between 1984 and 2024, across 25 studies. A total of 1882 patients (9.82%) underwent explantation. The most common reason was lack of efficacy and inadequate pain relief (38%), followed by lead failure (15%) and infection (14%). While SCS is generally effective, issues related to device longevity and patient satisfaction persist, with explantation rates due to technical failures and lack of efficacy being concerns.
Conclusion: SCS efficacy varies, with explantation rates reaching up to 38%, often due to inadequate pain relief. Most explantations occur within the first year, despite SCS being a safe and effective treatment. High implantation costs ($35,000 to $70,000) and revision costs ($15,000 to $25,000) raise concerns among payors. The hardware-driven model limits waveform flexibility, highlighting the need for innovation.
Keywords: chronic pain, spinal cord stimulation, explantation, implant removal, cost-effectiveness
Introduction
Chronic pain remains a challenge that affects millions (if not billions) of individuals yearly and has gained increased interest globally to address this debilitating condition.1 In the United States alone it is estimated that approximately 20.5% of the population (or 50.2 million adults) suffer from chronic pain.2 For some individuals this translates into activity modification and behavioral changes that result in both avoidance of social events and inability to work. In the US alone, reports estimate that the economic burden for patients with chronic pain costs the US $296 billion annually in lost productivity.2 While economic reports may help to demonstrate the severity of the issue on a macro level, they fail to give an accurate perspective of the individual whose quality of life is severely limited due to their chronic suffering. Studies estimate that severe depression is found in up to 85% of chronic pain patients, with approximately 20% demonstrating suicide ideation. In fact, 5% - 14% of these patients eventually attempt suicide, making chronic pain a public health concern as well.3–6
In recent decades neuromodulation has emerged as a novel technology that has shown success in the treatment of some chronic pain conditions. Spinal cord stimulation (SCS) has increased in popularity and is now a mainstay for clinicians specializing in treating refractory pain. This technology first debuted in 1967 and was initially based on the gate control theory as outlined by Melzack and Wall but is now thought to be based on activation of the dorsal horn of the dorsal column, changes in the thalamus, and potential impact on wide dynamic range neurons.7–13 Technological advancements in hardware and software have resulted in significant improvements in neuromodulation and continued technological progress points to a future with better and broader coverage for chronic pain populations. However, this has led to a deeper exploration of device success and failure, as improved technology comes with a price tag that has made some payors more careful about approving device implants.14–18 The cost of these devices has led some international payors to decline authorization due to speculative reports about lack of efficacy, and overall lack of access to advanced therapies due to the structure of the payor system.
The SCS industry offers several waveforms to treat different pain syndromes although a lack of evidence exists on how practitioners should pair waveforms to the chronic pain patient. The authors believe improved waveform selection may improve outcomes and lead to better SCS innovation. Current literature suggests that SCS explantation is closely tied to decreased efficacy. However, more data is needed to verify this supposition. If found to be true, this information can have important implications for the future of SCS, as it suggests that waveform tolerance should be considered when selecting SCS for our patients. In addition, the recent advancements in closed-loop technology which allows dose measurement of electrical delivery may add additional improvements in explant rates. While in studies, explant for efficacy has been much lower than the numbers discussed here, it will take time to see if these advances lead to long-term improvements.19,20 To support the body of evidence surrounding SCS durability we conducted a systematic review of SCS explantation rates and their associated causes. By understanding the reasons for SCS explantation across a series of manuscripts, we may gain insight into the long-term efficacy of these devices to improve our ability to enhance patient selection for this important modality.
Materials and Methods
In this systematic review, we did not target a specific population, as our primary focus is on evaluating the outcomes of SCS procedures over the past two decades. This comprehensive review encompasses a diverse array of patient populations who have undergone SCS implantation. The principal intervention of interest is the revision, reoperation, hardware removal, and explantation surgeries associated with SCS. The primary outcome of this review is to elucidate the reasons for subsequent revision or explantation surgeries following initial SCS implantation. The secondary outcome is to assess the financial burden of these procedures on the healthcare system. We aim to present findings from both the USA and globally. Studies were included if they focused on removing or revising any component of the SCS system and meeting all inclusion criteria while not meeting any exclusion criteria, detailed in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria for Eligibility |
The methodology employed in this systematic review strictly followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), ensuring adherence to standards for integrity and reproducibility (Figure 1).21
 |
Figure 1 PRISMA flow diagram. |
Search Strategy
Three primary screening methods were employed: manual search with keywords, MeSH term query (Medical Subject Headings), and screening of reference lists from relevant articles. The manual search strategy encompassed three databases, PubMed, Cochrane, Web of Science using specific keywords related to spinal cord stimulation, spinal cord stimulator, chronic pain, refractory pain, explantation, explant, neuromodulation, failure, and complications, covering literature from inception to November 2024. A comprehensive search strategy was designed utilizing key words for PubMed and this strategy was subsequently adapted for the Cochrane and Web of Science search yielding the following detailed results:
Keyword Query
PubMed Keyword Search: (472)
- Keywords “spinal cord stimulation + chronic pain” yielded 3389 results. Further refinement with “explant” resulted in 88 articles, of which 21 were selected.
- Keywords “spinal cord stimulator + explantation” initially yielded 259 results, refined to 112 articles after adding “neuromodulation”. Following deduplication, 12 articles were selected.
- Keywords “SCS + chronic pain + complication” produced 837 results, refined to 74 with the addition of “failure”. After reviewing and removing duplicates, 6 articles were selected.
- Keywords “spinal cord stimulator + explant” generated 259 results, refined to 28 with “failure”. After review and deduplication, 7 articles were selected.
Web of Science Keyword Search: (145)
- Keywords “spinal cord stimulation + chronic pain” yielded 3101 results, refined to 73 with “explant”, and 1 publication was selected.
- Keywords “spinal cord stimulator + explant” initially yielded 32 results. After review and deduplication, 3 articles were selected.
- Keywords “SCS + chronic pain + complication” produced 400 results, refined to 40 with “failure”. After review and deduplication, 3 articles were selected.
Cochrane Keyword Search: (76)
- Keywords “spinal cord stimulator + explant” generated 56 results, none of which met the inclusion criteria following the deduplication (12 included from PubMed search).
- Keywords “spinal cord stimulation” yielded 20 results, none of which were selected following the deduplication (6 included from PubMed search).
Following the manual search, a MeSH term query was devised for PubMed with five query concepts and their various combinations as shown in Appendix 1, utilizing the same keywords outlined below:
MeSH Query
Query Concept 1: “Chronic Pain” OR “refractory Pain” OR “intractable pain”
Query Concept 2: “Spinal Cord Stimulation” OR “spinal cord stimulator” OR “dorsal column stimulation” OR “electric stimulation therapy”
Query Concept 3: “Neuromodulation” OR “Transcutaneous Electric Nerve Stimulation”
Query Concept 4: “Explantation” OR “Explant” OR “Device Removal”
Query Concept 5: “Equipment failure” OR failure OR complication OR migration OR infection
Additionally, reference lists of prominent systematic and literature reviews were manually searched to identify potential articles for inclusion. Eight out of 26 articles included following the deduplication.
Study Selection
Following these three primary search methods, a total of 72 articles were identified: 53 articles from the manual keyword search, 11 articles from the MeSH term query, and 8 articles from the reference list screening of other relevant publications.
Initial screening of articles retrieved from the search strategy involved the assessment of titles and abstracts by 11 independent reviewers (UY, TN, JL, DL, RM, JD, DC, SY, AV, EC, SW), with approximately 6 articles assigned to each reviewer for the mention of spinal cord stimulation explantation. Subsequently, a thorough examination of full manuscripts was conducted for articles meeting the eligibility criteria (Table 1). Articles with unclear reasons for explanation or uncertain eligibility criteria were referred to the corresponding editor for further evaluation and resolution. Some studies included multiple articles reporting results from the same patient cohort at different follow-up periods. In such cases, only the publication with the longest follow-up period and most comprehensive data was selected for inclusion.
After this rigorous screening and evaluation process, a total of 31 articles were determined to meet the criteria for inclusion in our study. Six out of 31 articles were excluded for redundant data after accuracy review.
Data Extraction
Following the screening and eligibility processes, 25 articles were selected for further analysis (Table 2).22–46 Data extraction was carried out by 13 independent reviewers (BC, MC, NO, CF, SP, AP, UY, TN, DL, EC, JD, DC, SY), with a second reviewer ensuring accuracy. Discrepancies in decisions were referred to the corresponding editor for resolution.
 |
Table 2 Studies Included in the Review |
The information extracted from the selected articles included author, year of publication, authors’ institution, title of the article, type of study, number of study participants, participant demographics, study location, characteristics of the study (including study design, timeline, follow-up time, and cohort size), reason for explantation, number of replacements with the same vendor and different vendor, and key results. The reasons for explantation were detailed as follows: Lack of efficacy/loss of stimulation, Infection, lead high impedances, lead failure/migration, pain at IPG (Implantable Pulse Generator) site, IPG failure/migration, IPG charging problem, resolution of pain, repeat spine surgery, MRI requirement, and other reasons.
In some publications, the sum of explantations or revisions reported exceeded the number of individual patients due to multiple reoperations observed in certain patients. Furthermore, some studies detailed reasons for explantation where the total count of reasons surpassed the number of explantation procedures observed, with a note indicating multiple adverse events contributing to a single explantation.
Results
This review summarizes the findings from twenty-five key studies examining failure rates, and complications associated with SCS procedures. These studies collectively cover data from 1984 to 2023 and involve single-center and multicenter approaches across the USA, Canada, and Europe.
Study Characteristics
- Study Types and Locations: The studies include retrospective single-center and multicenter analyses and a prospective clinical trial. Sixteen studies were conducted in the USA, with two studies extending globally, and other involving centers in Canada, France, UK, Germany, Belgium, and the Netherlands (Table 3).
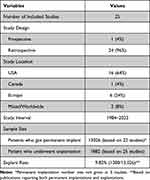
Table 3 Study Summary
- Patient Population: The total number of patients who underwent SCS procedures across the studies ranges from 18 to 2737, with a mean age between 43–67.8 (Table 2).
Key Findings
Explantation Rates
Explantation rate was as low as 1.8% in Bendel et al study (2017)42 and as high as 38% in Langford’s study (2021)34 (Table 2).
Hagedorn et al (2021)23 reported an explantation rate for 10 kHz-SCS devices of approximately 10%, primarily due to inadequate pain relief and surgical complications. Similarly, Rauk et al (2023),24 reported a 7.6% explantation rate primarily due to inadequate pain relief. In Patel’s 2019 study14 the main reasons for SCS removal were lack of efficacy and loss of stimulation. Van Buyten et al (2017)25 observed a 19% rate of therapy-related explants (8% per year), mostly related to inadequate pain relief. Of 577 SCS implants in Rosenow’s study (2006),26 7.9% were removed and 35.6% were revised. Chapman in 202230 studied 249 patients and 2% of them underwent explantation secondary to inadequate pain relief. In Hines’ study in 202131 with 31 patients, 26% had explantation surgery. Among 199 implants in Moeschler’s study (2015),32 33 devices were explanted, representing a 17% explantation rate.
Removal Reasons and Complications
The literature reports various reasons for the removal of spinal cord stimulators, with the most common being lack of efficacy and inadequate pain relief. Other frequently cited reasons include infection, lead migration or failure, pain at the IPG site, and IPG failure or migration, as detailed in Table 4. While most studies did not extensively discuss postoperative complications, some did report infection rates and general complications. For instance, Patel et al (2019)22 noted that 129 patients required reoperation due to various complications, with 15 experiencing postoperative complications. Kleiber et al35 documented postoperative complications in 74 out of 212 patients (34.9%). Additionally, Maldonado-Naranjo et al43 reported postoperative adverse events in 8 out of 382 patients (2.1%).
 |
Table 4 Reasons for SCS Explantation |
Revised or Re-Implanted SCS
Some studies discussed the explantation of SCS systems but also detailed their associated revision procedures. In particular, revisions were highlighted due to battery failure or charging issues, which led to IPG changes, and IPG relocations prompted by site pain or discomfort. Moreover, revisions involving electrode replacements due to hardware (lead, wire, IPG) failures were nearly as prevalent as explantations and, in some publications, even more common. In the study by Mekhail et al27 50 cases required revision due to connection failures, compared to 32 cases of explantation. Similarly, Wolter et al28 documented that two patients required an IPG change due to battery discharge (after 4 and 9 years, respectively), and one patient needed an IPG relocation. Furthermore, Papadopoulos et al37 reported six cases of SCS replacement due to malfunctioning, along with 31 cases of SCS replacement or repositioning due to inadequate pain coverage. Eight studies discussing revision surgeries have explicitly reported the number of permanent implant and revision procedures. Based on these studies, a total of 1784 patients with permanent implants underwent 591 (33.1%) revision surgeries (Table 5).
 |
Table 5 Studies Reporting Revision Surgeries |
Duration Between SCS Implantation and Explantation
Only 8 out of 25 studies provided the average duration until the explantation procedure, with the reported duration varying from 9.3 months to 54 months (Table 2). Some studies additionally noted explantation rates by year, with the majority of procedures occurring in the first and second years following initial implantation. Hagedorn et al (2021)23 reported on the timing and rates of explantation, finding that most explants (40/76; 52.6%) occurred during the first year after implantation. By the second year, the rate of explants decreased to 23.7% (18/76), and this further declined to 15.8% (12/76) in the third year and 7.9% (6/76) in the fourth year.
Device and Vendor Replacement
The vast majority of patients who underwent replacement surgery were replaced with the same vendor SCS system. Notably, replacements with different vendors were minimal, suggesting brand loyalty or limited alternatives.
However, Hunter et al (2020)29 investigated SCS waveform rotation in reversing lack of efficacy. In one cohort, patients underwent surgical revision of their existing SCS platforms to enable the transmission of the D-Burst waveform, either by modifying the IPG with adaptors or by replacing both the IPG and leads. Another cohort required only reprogramming to initiate the transmission of D-Burst (D-Burst On). He demonstrated that waveform modification salvaged SCS as therapy for the patients in his study.
Safety
The prospective study by Rauck et al (2023)24 demonstrated the long-term safety of SCS systems, with adverse events primarily related to technical failures rather than biological reactions. Other studies echoed these findings, emphasizing that while technical failures are prevalent, serious adverse events are relatively rare.22–46
Overall, these studies indicate that while SCS is generally effective, there are significant challenges related to device longevity and patient satisfaction primarily due to diminished efficacy of the original SCS waveform. Explantation rates due to lack of efficacy are the most common reason for explantation. Device related challenges are relatively uncommon but remain an important reason for device failure.
Discussion
Spinal cord stimulation is a widely used technology to treat recalcitrant chronic pain which has demonstrated efficacy in some of the most challenging clinical scenarios. There is robust evidence supporting its use in the management of several different pain disorders. However, the long-term efficacy of the procedure has been recently debated. The issue to consider is whether the cost of the device is worthwhile considering the overall success of therapy and potential reduction in health care utilization. The cost of an SCS implant can range between $35,000-$70,000 and repeat surgeries to regain efficacy can cost another $15-25K per patient. Therefore, the viability of the SCS industry may rest on the ability to achieve long-term outcomes. Here, the authors reviewed the literature and confirmed habituation as the main reason for SCS failure in order to spur pain neuromodulation innovation and revitalize the industry.
In this systematic review examining the post-operative outcomes of SCS, explantation rates varied between 1.8 to 38% among various studies. Inadequate pain relief was consistently highlighted as the primary cause in nearly every study reviewed. Many patients underwent removal of their device within the first one to two years with a reported range of 9 to 54 months to explant. More than half of explants occurred within the first year. Explantation rates declined each consecutive year afterward, suggesting that patient selection or physician performance may also play a role. Despite the rate of reoperation, SCS is a safe procedure. The most common cause of SCS revision surgeries other than lack of efficacy included device migration, pain at the IPG site, and system replacement. However, these revision surgeries may suggest SCS efficacy because the patient would have had the device removed otherwise. Infection and hardware rejection were uncommon.
The current industry model is heavily hardware dependent, permitting the use of waveforms only compatible with the implanted devices, limiting patient treatment options. Waveform innovation directed toward spinal cord gray matter neurons and synaptic molecules may improve durability and outcomes. Therefore, continued investment into waveform development by independent and corporate partners is needed. Most SCS companies will attempt to reprogram patients, offering different energy options, sometimes blending their proprietary waveforms, to salvage patient satisfaction. Currently, most device companies can only offer a limited number of therapeutic options due to the restrictions of the hardware and software, as well as regulatory hurdles. Responders to SCS therapy are identified during the trial phase when they are waveform-naive but may later develop tolerance to the trialed therapy. Currently, most physicians do not have the option of selecting different waveforms from different vendors during the trial, so they do not know if they are choosing the optimal waveform for their patient. A typical SCS trial duration is 3–10 days for most practitioners in the United States and may not offer enough time for a patient to appreciate the efficacy of the trialed system. There is growing evidence that different waveforms may be preferred for specific pain conditions. In our review Hunter et al29 implanted a burst waveform delivery system for patients who failed ultra-high frequency and did demonstrate improvement in pain, suggesting that waveform modification can improve symptoms. Waveform switching/cycling has also been documented as superior over singular therapy by other authors.47–49 We submit that more research is needed to evaluate the effect of multiple and combined waveform therapy to determine if it improves outcomes and decreases revision surgery. Therefore, there is utility in creating practice guidelines which direct waveform selection based on individualized patient criteria such as medical history, anatomy, physiology, psychology, and activity level. A future innovation may be the use of artificial intelligence toward this endeavor.
The durability of automated closed-loop and surround inhibition paradigms are on the horizon. However, they were not evaluated as part of this systematic analysis because they are newer generation products with limited long-term evidence. Preliminary data suggests that they may not be subject to habituation to the same degree as older generation products.19,50 Current closed-loop technology modulates tonic waveforms via Evoked Compound Action Potentials (ECAPs) to deliver consistent stimulation to the axons of the dorsal columns; however, an innovation in this space may be to create constant delivery to the other cell lines in the spinal cord such as wide dynamic neurons and oligodendrocytes which are thought to produce clinical effect as well.51,52
Comorbid depression, anxiety, sleep deprivation, post-traumatic stress, and sleeping disorders are known to have a limited response to SCS and can skew durability data. The authors submit that better physician education around chronic pain psychology is required to target these patients for implant appropriateness. Though psychiatric evaluations are the standard of care for selecting patients for SCS trial appropriateness, they have not demonstrated to change SCS explantation rates. Patel et al reported association between early SCS explantation and the presence of depression and anxiety, emphasizing the impact of psychological factors on SCS outcomes. A potential innovation to temper fluctuating maladaptive mood and behavioral challenges in patients with SCS may be to integrate bidirectional wearable devices such as a watch or phone which monitor physiology as a function of mood and subsequently execute a command to calm a patient who is having a pain or emotional crisis. Additionally, SCS devices may be configured to work with other neuromodulation devices, such as hypoglossal, vagal stimulators, or deep brain stimulation to modify sleep or mood. Seamless integration of SCS neuromodulation with other devices known to affect behavior may also improve SCS patient satisfaction.
Several articles included in this study mentioned post-operative complications, but did not specifically share details surrounding these complications.22,24,35,39,43 It will remain important that future SCS studies include more widespread post-operative outcomes as this awareness will spur innovation from understanding.
Despite the challenges reported in this paper the superiority of neuromodulation as salvage therapy over other treatments in chronic pain patients has not been widely criticized. Millions of patients worldwide are benefactors of this treatment modality; however, the durability is debated and is highlighted by our review. Unfortunately, there is growing literature focusing on SCS limitations primarily due to the rate of explanation and revision. Insurance carriers have used this information to deny or reduce payment, and there is concern amongst SCS implanters in the community that this may lead to the demise of the field.53–55
The authors submit that for there to be an enlightenment of SCS a revised industry thought model may be needed to propel innovation. Continued dedication to waveform design, closed-loop sub perception innovation, improved physician education for SCS patient selection, guidelines for pairing technology with pathology, and integration of neuromodulation devices to limit catastrophizing may be ways to improve the patient experience. The ideas here represent ideas for future investigation but require an abundance of research to actualize their conception.
Limitations to this study are present. Firstly, the initial screening involved a review of journals and abstracts from independent reviewers. Opinion and bias are inevitably inherent, but these were minimized by consistently referring to the eligibility criteria outlined in Table 1. Secondly, some patients underwent multiple reoperations and revisions following the initial SCS implantation. This makes the analysis of post-operative complications, such as infection rates, more complex.
All in all, the discussion regarding spinal cord stimulator removals and revisions is multifaceted. Although there remains work to be done in perfecting this treatment modality, our team is hopeful that changes made to spinal cord stimulators will offer improved pain relief to millions of patients globally.
Conclusion
SCS is an effective treatment for a range of chronic pain conditions, but the therapy requires better durability data to withstand criticism from payers if we hope to preserve this treatment for future generations. The recent expansion of SCS therapy waveforms provides patients with several options for pain relief, but the industry still lacks the ability to deliver all available energy formats to every patient with an SCS device, limiting the potential for maximizing outcomes. Our results suggest that tolerance is the most common reason for SCS explantation, which aligns with the conclusions of other reports. However, the industry has yet to develop a solution to this problem. One potential solution may be a shift toward an application-based software commercial model. The integration of artificial intelligence and physiological monitoring could also lead to more patient-specific energy delivery, maximizing patient benefit.
Abbreviations
SCS, Spinal Cord Stimulation; IPG, Implantable Pulse Generator; LoE, Lack of Efficacy; LoS, Loss of Stimulation; ECAPs, Evoked Compound Action Potentials.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr. Wahezi receives research funding from Boston Scientific, Abbott, and Vertos. He is also a consultant for Boston Scientific. He reports patents 11,964,153, 12,138,454 and 12,138,455 issued. Dr. Deer is a consultant for Abbott, SpineThera, Biotronik, Saluda, and Boston Scientific (relevant). He also consults with SPR, Nervonik, Painteq, Cornorloc, Spinal Simplicity and Aurora. He reports a pending patent for Abbott. Dr. Pritzlaff is a consultant for SPR Therapeutics, Nalu Medical, Bioventus, royalties from Wolters Kluwer and receives educational grants from Medtronic, Abbott, Biotronik, and Nevro. Dr. Schatman is a senior medical advisor for Apurano Pharma. Dr. Hunter is a consultant for Abbott, Saluda, Biotronik, and Mainstay. All other authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN. 2019;160(1):19. doi:10.1097/j.pain.0000000000001384
2. Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. PAIN. 2022;163(2):e328. doi:10.1097/j.pain.0000000000002291
3. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433
4. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
5. Williams L, Jones W, Shen J, Robinson R, Weinberger M, Kroenke K. Prevalence and impact of depression and pain in neurology outpatients. J Neurol Neurosurg Psychiatry. 2003;74(11):1587–1589. doi:10.1136/jnnp.74.11.1587
6. Tang NKY, Crane C. Suicidality in chronic pain: a review of the prevalence, risk factors and psychological links. Psychol Med. 2006;36(5):575–586.
7. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979.
8. Kumar K, Abbas M, Rizvi S. The use of spinal cord stimulation in pain management. Pain Manag. 2012;2(2):125–134. doi:10.2217/pmt.11.83
9. Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg. 1970;32(5):560–564. doi:10.3171/jns.1970.32.5.0560
10. Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol. 2012;107:87–119.
11. Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2(3):150–164. doi:10.1046/j.1525-1403.1999.00150.x
12. Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4(4):402–408. doi:10.1038/86054
13. Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24(2):122–126. doi:10.1016/S0166-2236(00)01714-8
14. Resnic FS, Matheny ME. Medical devices in the real world. N Engl J Med. 2018;378(7):595–597. doi:10.1056/NEJMp1712001
15. Salazar JW, Redberg RF. Leading the call for reform of medical device safety surveillance. JAMA Intern Med. 2020;180(2):179–180. doi:10.1001/jamainternmed.2019.5170
16. Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14(11):1631–1649. doi:10.1111/pme.12146
17. Taylor RJ, Taylor RS. Spinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysis. Int J Technol Assess Health Care. 2005;21(3):351–358. doi:10.1017/S0266462305050464
18. Manca A, Kumar K, Taylor RS, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12(8):1047–1058. doi:10.1016/j.ejpain.2008.01.014
19. Levy R, Deer TR, Poree L, et al. Multicenter, randomized, double-blind study protocol using human spinal cord recording comparing safety, efficacy, and neurophysiological responses between patients being treated with evoked compound action potential-controlled closed-loop spinal cord stimulation or open-loop spinal cord stimulation (the evoke study). Neuromodulation. 2019;22(3):317–326. doi:10.1111/ner.12932
20. Brooker C, Russo M, Cousins MJ, et al. ECAP-controlled closed-loop spinal cord stimulation efficacy and opioid reduction over 24-months: final results of the prospective, multicenter, open-label avalon study. Pain Pract. 2021;21(6):680–691. doi:10.1111/papr.13008
21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [published correction appears in Int J Surg. 2010;8(8):658]. Int J Surg. 2010;8(5):336–341. doi:10.1016/j.ijsu.2010.02.007
22. Patel SK, Gozal YM, Saleh MS, Gibson JL, Karsy M, Mandybur GT. Spinal cord stimulation failure: evaluation of factors underlying hardware explantation. J Neurosurg Spine. 2019;32(1):133–138. doi:10.3171/2019.6.SPINE181099
23. Hagedorn JM, Lam CM, D’Souza RS, et al. Explantation of 10 kHz spinal cord stimulation devices: a retrospective review of 744 patients followed for at least 12 months. Neuromodulation. 2021;24(3):499–506. doi:10.1111/ner.13359
24. Rauck RL, Loudermilk E, Thomson SJ, et al. Long-term safety of spinal cord stimulation systems in a prospective, global registry of patients with chronic pain. Pain Manag. 2023;13(2):115–127. doi:10.2217/pmt-2022-0091
25. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642–649. doi:10.1111/ner.12642
26. Rosenow JM, Stanton-Hicks M, Rezai AR, Henderson JM. Failure modes of spinal cord stimulation hardware. J Neurosurg Spine. 2006;5(3):183–190. doi:10.3171/spi.2006.5.3.183
27. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148–153. doi:10.1111/j.1533-2500.2010.00407.x
28. Wolter T, Kieselbach K. Cervical spinal cord stimulation: an analysis of 23 patients with long-term follow-up. Pain Physician. 2012;15(3):203–212. doi:10.36076/ppj.2012/15/203
29. Hunter CW, Carlson J, Yang A, et al. BURST(able): a retrospective, multicenter study examining the impact of spinal cord stimulation with burst on pain and opioid consumption in the setting of salvage treatment and “upgrade”. Pain Physician. 2020;23(6):E643–E658.
30. Chapman KB, Yang A, Mogilner AY, et al. Dorsal root ganglion stimulation device explantation: a multicenter pooled data analysis. Pain Pract. 2022;22(5):522–531. doi:10.1111/papr.13113
31. Hines K, Swaminathan V, Thalheimer S, Kogan M, Wu C, Sharan A. Single-center retrospective analysis of device-related complications related to dorsal root ganglion stimulation for pain relief in 31 patients. Neuromodulation. 2022;25(7):1040–1044. doi:10.1111/ner.13498
32. Moeschler SM, Sanders RA, Hooten WM, Hoelzer BC. Spinal cord stimulator explantation for magnetic resonance imaging: a case series. Neuromodulation. 2015;18(4):285–288. doi:10.1111/ner.12254
33. Dupré DA, Tomycz N, Whiting D, Oh M. Spinal cord stimulator explantation: motives for removal of surgically placed paddle systems. Pain Pract. 2018;18(4):500–504. doi:10.1111/papr.12639
34. Langford B, Hunt C, Lerman A, Mauck WD. Analyzing spinal cord stimulator explants in refractory angina pectoris patients. Pain Med. 2021;22(7):1699–1701. doi:10.1093/pm/pnaa456
35. Kleiber JC, Marlier B, Bannwarth M, Theret E, Peruzzi P, Litre F. Is spinal cord stimulation safe? A review of 13 years of implantations and complications. Rev Neurol. 2016;172(11):689–695. doi:10.1016/j.neurol.2016.09.003
36. Stauss T, El Majdoub F, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol. 2019;6(3):496–507. doi:10.1002/acn3.720
37. Papadopoulos DV, Suk MS, Andreychik D, Nikolaou V, Haak M. Rates and causes of reoperations following spinal cord stimulation within a 2-12 year period. Global Spine J. 2023;15(2):467–473. doi:10.1177/21925682231194466
38. Nissen M, Ikäheimo TM, Huttunen J, Leinonen V, von Und Zu Fraunberg M. Long-term outcome of spinal cord stimulation in failed back surgery syndrome: 20 years of experience with 224 consecutive patients. Neurosurgery. 2019;84(5):1011–1018. doi:10.1093/neuros/nyy194
39. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603–609. doi:10.1111/ner.12312
40. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543–552. doi:10.1111/ner.12634
41. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481–496. doi:10.1227/01.NEU.0000192162.99567.96
42. Bendel MA, O’Brien T, Hoelzer BC, et al. Spinal cord stimulator related infections: findings from a multicenter retrospective analysis of 2737 implants. Neuromodulation. 2017;20(6):553–557. doi:10.1111/ner.12636
43. Maldonado-Naranjo AL, Frizon LA, Sabharwal NC, et al. Rate of complications following spinal cord stimulation paddle electrode removal. Neuromodulation. 2018;21(5):513–519. doi:10.1111/ner.12643
44. Thomson SJ, Kruglov D, Duarte RV. A spinal cord stimulation service review from a single centre using a single manufacturer over a 7.5 year follow-up period. Neuromodulation. 2017;20(6):589–599. doi:10.1111/ner.12587
45. Simopoulos T, Aner M, Sharma S, Ghosh P, Gill JS. Explantation of percutaneous spinal cord stimulator devices: a retrospective descriptive analysis of a single-center 15-year experience. Pain Med. 2019;20(7):1355–1361. doi:10.1093/pm/pny245
46. Al-Kaisy A, Royds J, Al-Kaisy O, et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg Anesth Pain Med. 2020;45(11):883–890. doi:10.1136/rapm-2020-101681
47. Metzger CS, Hammond MB, Pyles ST, et al. Pain relief outcomes using an SCS device capable of delivering combination therapy with advanced waveforms and field shapes. Expert Rev Med Devices. 2020;17(9):951–957. doi:10.1080/17434440.2020.1812383
48. Wallace MS, North JM, Phillips GM, et al. Combination therapy with simultaneous delivery of spinal cord stimulation modalities: COMBO randomized controlled trial. Pain Manag. 2023;13(3):171–184. doi:10.2217/pmt-2022-0101
49. Kapural L, Patterson DG, Li S, et al. Multiphase spinal cord stimulation in participants with chronic back or leg pain: results of the BENEFIT-02 randomized clinical trial. Neuromodulation. 2023;26(7):1400–1411. doi:10.1016/j.neurom.2023.05.006
50. Anitescu M, Loudermilk E, Trainor D, et al. Clinical impact of a novel fast-acting sub-perception SCS therapy engaging surround inhibition (FAST Prospective Study). Neuromodulation. 2023;26(4 Suppl):S20. doi:10.1016/j.neurom.2023.04.033
51. Wahezi SE, Caparo MA, Malhotra R, et al. Current waveforms in spinal cord stimulation and their impact on the future of neuromodulation: a scoping review. Neuromodulation. 2024;27(1):47–58. doi:10.1016/j.neurom.2023.11.002
52. Sharma M, Bhaskar V, Yang L, et al. Novel evoked synaptic activity potentials (ESAPs) elicited by spinal cord stimulation. eNeuro. 2023;10(5):ENEURO.0429–22.2023. doi:10.1523/ENEURO.0429-22.2023
53. Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51(1):106–116. doi:10.1097/00006123-200207000-00016
54. Manchikanti L, Pampati V, Vangala BP, et al. Spinal cord stimulation trends of utilization and expenditures in fee-for-service (FFS) medicare population from 2009 to 2018. Pain Physician. 2021;24(5):293–308.
55. Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5(3):191–203. doi:10.3171/spi.2006.5.3.191
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Interventional Pain Procedures: A Narrative Review Focusing On Safety and Complications. PART 2 Interventional Procedures For Back Pain
Lo Bianco G, Tinnirello A, Papa A, Marchesini M, Day M, Palumbo GJ, Terranova G, Di Dato MT, Thomson SJ, Schatman ME
Journal of Pain Research 2023, 16:761-772
Published Date: 9 March 2023
Real World Clinical Utility of Neurophysiological Measurement Utilizing Closed-Loop Spinal Cord Stimulation in a Chronic Pain Population: The ECAP Study Protocol
Leitner A, Hanson E, Soliday N, Staats P, Levy R, Pope J, Kallewaard JW, Doleys D, Li S, Weisbein J, Amirdelfan K, Poree L
Journal of Pain Research 2023, 16:2497-2507
Published Date: 21 July 2023
Spinal Cord Stimulation for Intractable Visceral Pain Originating from the Pelvic and Abdominal Region: A Narrative Review on a Possible New Indication for Patients with Therapy-Resistant Pain
Bieze M, van Haaps AP, Kapural L, Li S, Ferguson K, de Vries R, Schatman ME, Mijatovic V, Kallewaard JW
Journal of Pain Research 2024, 17:691-736
Published Date: 19 February 2024
Distinct Functional Connectivity Patterns for Intermittent Vs Constant Neuropathic Pain Phenotypes in Persistent Spinal Pain Syndrome Type 2 Patients
Pahapill PA, Arocho-Quinones EV, Chen G, Swearingen B, Tomas CW, Koch KM, Nencka AS
Journal of Pain Research 2024, 17:1453-1460
Published Date: 12 April 2024
A Systematic Guideline by the ASPN Workgroup on the Evidence, Education, and Treatment Algorithm for Painful Diabetic Neuropathy: SWEET
Sayed D, Deer TR, Hagedorn JM, Sayed A, D'Souza RS, Lam CM, Khatri N, Hussaini Z, Pritzlaff SG, Abdullah NM, Tieppo Francio V, Falowski SM, Ibrahim YM, Malinowski MN, Budwany RR, Strand NH, Sochacki KM, Shah A, Dunn TM, Nasseri M, Lee DW, Kapural L, Bedder MD, Petersen EA, Amirdelfan K, Schatman ME, Grider JS
Journal of Pain Research 2024, 17:1461-1501
Published Date: 13 April 2024

