Back to Journals » International Journal of Nanomedicine » Volume 19
Stem Cell-Derived Exosomes: Natural Intercellular Messengers with Versatile Mechanisms for the Treatment of Diabetic Retinopathy
Authors Song Y, Yin C , Kong N
Received 26 May 2024
Accepted for publication 5 October 2024
Published 24 October 2024 Volume 2024:19 Pages 10767—10784
DOI https://doi.org/10.2147/IJN.S475234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Zhang
Yameng Song,1,2 Caiyun Yin,2 Ning Kong1
1Department of Regenerative Medicine, School of Pharmaceutical Sciences, Jilin University, Changchun, People’s Republic of China; 2National Health Commission (NHC) Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun, People’s Republic of China
Correspondence: Ning Kong, Email [email protected]
Abstract: Diabetic retinopathy is one of the complications of diabetes mellitus that occurs in the early stages. It is a disease that has a serious impact, and may lead to blindness when the disease progresses to advanced stages. Currently, treatments for diabetic retinopathy are mainly limited to its advanced stages of the disease, being restricted to a single therapeutic mechanism. Stem cells hold the promise of regenerative therapy and have the potential to comprehensively improve diabetic retinopathy. However, direct stem cell therapy carries some risk of carcinogenesis. Exosomes secreted by stem cells have shown a similar overall improvement in disease as stem cells. Exosomes can carry a number of biologically active materials from donor cells to recipient cells or distant organs, regulating intercellular signaling. Exosomes have shown remarkable efficacy in alleviating oxidative stress, inhibiting inflammatory responses, suppressing angiogenesis, reducing apoptosis and protecting neural tissues. Currently, the experimental literature using stem cell exosomes in the treatment of diabetic retinopathy tends to converge on the above experimental results. With this in mind, we have chosen to explore exosomes in depth from a subtle molecular perspective. We will elaborate on this perspective in this paper and propose to advocate exosome therapy as one promising approach for the treatment of diabetic retinopathy to ameliorate the lesions through multiple mechanisms.
Keywords: stem cell, exosomes, diabetic retinopathy, versatile mechanisms, treatment
Introduction
According to data from the 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas, an estimated 537 million adults aged 20–79 currently live with diabetes worldwide. This number is projected to reach 783 million by 2045, signifying a major global public health challenge.1 Diabetes, fundamentally a metabolic disorder, is typically characterized by hyperglycemia (HG). While the disease itself is not inherently painful, the persistent hyperglycemic state over time can lead to a multitude of complications. Research indicates that diabetes primarily manifests as macrovascular and microvascular diseases, as well as specific neuropathies. These complications significantly impact patients’ quality of life and can even reduce life expectancy in some cases.2 Among these complications, diabetic retinopathy (DR) is regarded as one of the most prevalent manifestations in the progression of diabetes. The presence of DR indicates that the microcirculation has already been compromised by the diabetic milieu, making it a reliable biomarker of the detrimental effects of diabetes on an individual.3,4 It is a leading cause of moderate to severe vision loss globally.5 Between 1990 and 2020, DR was the only cause of blindness with an increasing global age-standardized prevalence. As the number of individuals with diabetes continues to rise and life expectancy increases, the burden of DR is expected to grow, leading to more cases of vision impairment and even blindness, thereby further diminishing the quality of life for affected individuals.6,7 This pressing issue underscores the importance of emphasizing the prevention and treatment of DR. Despite the array of available treatment modalities, whether surgical or pharmaceutical, their limitations are substantial, offering only symptomatic relief without providing a comprehensive cure.8–10 Exploring new treatment methods is crucial.
Stem cell regenerative therapy holds promise as a feasible method for multi-mechanism treatment of DR.11 Stem cells are capable of infinite renewal, producing at least one highly differentiated offspring.12 Many stem cell therapies are rapidly advancing, including those for treating acquired immune deficiency syndrome, restoring vision from blindness, reversing spinal cord injury, and treating other disorders affecting motor neurons and demyelinating cells.13
Mesenchymal stem cells (MSCs), a broad class of cells derived from tissues such as bone marrow, umlibical cord blood, adipose tissue, and dental pulp, have demonstrated remarkable potential and significant impact on retinal regeneration and repair after injury. For example, they have been shown to improve the regeneration of retinal ganglion cells (RGCs) to alleviate retinal neurodegeneration.14 Clinical research on stem cells is currently permitted and thriving, with trials such as NCT01736059 using bone-marrow CD34+ stem cells to treat retinopathy and NCT03403699 aiming to fix vasodegenerative vessels in DR with human induced pluripotent stem cells.
However, despite the alluring appeal of regenerative therapy, there are still some practical challenges that remain unresolved. Although stem cells hold significant potential for treating DR, their large diameter poses a risk of pulmonary accumulation following intravenous injection, leading to infusion toxicity. Additionally, the antigens carried by allogeneic stem cells may trigger an immune response.15 Moreover, the effects of stem cell treatments may occasionally produce adverse effects, including the risk of tumorigenesis. Fortunately, while stem cell research has stalled, we have discovered that exosomes secreted by stem cells seem to have an unexpected effect.16,17
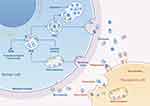
Previous research has shown that exosomes in MSC culture medium possess similar repair abilities to MSCs and are not associated with cancer development.18,19 Exosomes are widely distributed in body fluids, and almost all cells secrete them, which can accurately deliver some signaling molecules to specific organ cells via cell surface receptors for intercellular communication. Exosomes are 30 to 200 nanometers in diameter, have a single membrane, share the same topology as cells, and are enriched in specific nucleic acids, lipids, proteins, and glycoconjugates. They are particularly rich in non-coding RNAs, including micro ribonucleic acids (miRNAs) and transfer RNAs. Coincidentally, miRNA has been found to play a significant role in the pathogenesis of DR, which we will elaborate on later in the section on the molecular mechanisms of DR production. Exosomes can transfer extracellular RNA to other cells and tissues in a functional form, as evidenced by a cascade of reactions in which RNA transcribed by one cell is released from the cell of origin, subsequently taken up by a recipient cell, and expressed therein (Figure 1). The cargo capacity of exosomes is finite, encapsulating a specific subset of RNAs that reflects the distinct subpopulations of the donor cell. The functional integrity of these RNAs is maintained in exosomes, and information is transmitted between cells through exosome binding to cell surface receptors and membrane fusion.20 MiRNAs, serving as pivotal signaling molecules, exert profound effects on cellular functionality through regulatory mechanisms operating at both transcriptional and epigenetic tiers.21,22 Exosomes possess several advantages over MSCs. Most of the risks can be avoided by using exosomes with low immunogenicity, high stability, and ease of storage for treatment compared to direct use of MSCs. This could be a new strategy as an alternative to stem cells.23
Diabetic Retinopathy
Pathological Manifestations and Pathogenesis
DR is commonly regarded as a vascular disease and is categorized into two main stages based on the progression of vascular lesions: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).24 The former is characterized by the appearance of exudates, hemorrhages, dilated veins, and microaneurysms, while the latter is characterized by neovascularization. NPDR is an early stage of DR characterized by increased vascular permeability and partial occlusion of capillaries. At this stage, the patient may not have clinically significant symptoms, but fundus examination already shows abnormalities, such as exudates, hemorrhages, and microaneurysms. PDR represents the advanced stage of DR, in which the patient’s vision may be severely impaired when the neovascularized abnormalities encroach on the vitreous body and cause hemorrhages or lead to retinal detachment. Diabetic macular edema (DME) can occur at any stage of DR, resulting in distorted visual images, and it is the most common cause of vision loss in patients with DR. Due to the breakdown of the blood-retinal barrier (BRB), fluid accumulates, causing swelling and thickening of the macula.25
With the deepening of research on DR, an increasing number of scholars recognize that nerve damage is also an important part of its development.26,27 Much experimental and clinical evidence suggests that both neurodegeneration and inflammation are key contributors to diabetic retinal damage in the early stages of DR.28 These mechanisms are intricate and complex, and for the time being, the academically accepted view is that metabolic factors such as high blood glucose and elevated blood pressure and lipids trigger a series of physiologic changes that ultimately lead to retinal pathology.29,30
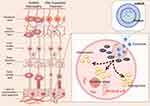
The composition of retinal tissue is complex. Currently, the most widely accepted model for DR is based on the concept of the neurovascular unit (NVU).31 The NVU is broadly conceptualized as consisting of blood vessels, glia, and neurons. Blood vessels involve endothelial cells and pericytes, neuroglia mainly include Müller cells (MCs), astrocytes and microglia, and retinal neurons involve ganglion cells, bipolar cells and horizontal cells. In a healthy retina, there is functional coupling and interdependence between the cells of the NVU, and they work closely with each other to ensure normal metabolic activity of the retinal tissue.32 Persistent HG disrupts the metabolic homeostasis of the NVU, leading to overproduction of reactive oxygen species (ROS), inflammatory mediators, and levels of various stressors, resulting in cellular damage33 and eventual retinal dysfunction. Stressors include advanced glycation end products (AGEs), inflammatory factors, ROS, polyol pathways, and protein kinase C (PKC).34,35 Elevated levels of these stressors can lead to vascular damage as well as nerve damage, and potentially increased expression of vascular endothelial growth factor (VEGF) within the ocular vasculature. This ultimately produces disruption of the retinal barrier. Throughout this process, a decline in factors such as neurotrophic factors can also lead to nerve cell degeneration, death, and ultimately vision loss.26
Major Molecular Mechanisms Associated
MicroRNAs
MiRNAs are a class of post-transcriptional inhibitory RNAs with numerous biological functions, which have been shown to play a role in the progression of many diseases, making them widely valued therapeutic targets.36 They are essentially non-coding RNAs, usually consisting of 19 to 25 nucleotides. A large body of literature in ophthalmology and molecular biology suggests that miRNAs play a crucial role in the development of DR, such as inducing oxidative stress, triggering inflammation, bringing about apoptosis, neoangiogenesis, and neurodegeneration. Many DR-targeted therapies targeting specific miRNAs have emerged, and all of them have shown promising results.37
MiRNAs are categorized into different families depending on their mature sequences. In a family, the miRNAs share the same sequence and also have their own specific sequences. There are about 300 human miRNAs belonging to 177 different miRNA families. MiRNAs in DR may be related to their widespread expression, and changes in expression levels guide disease progression. Studies have found that patients with DR show abnormal miRNA expression in serum and plasma, and some studies have shown that miRNAs in peripheral blood endothelial progenitor cells and circulating cellular exosomes show varying degrees of abnormal expression. Numerous studies have confirmed the role of miRNAs in the emergence of oxidative stress in DR.38 In response to oxidative stress in DR, miRNAs are generated through several steps, including increased polyol pathway flux, formation of AGEs, increased AGE receptor expression in cells, PKC activation and hexosamine activation. In addition, miRNAs interact with various inflammatory mediators39–42 which play a significant role in multiple signaling responses during the development of DR, particularly by inducing inflammatory signaling pathways that mediate interactions between cells and important role the extracellular matrix. Aberrant miRNA expression in the nervous system can also have deleterious effects, including retinal neuronal apoptosis and reactive gliosis. For example, overexpression of miRNA-14543 and miRNA-27b44 can alleviate oxidative stress induced by HG, while miRNA-383,45 miRNA-34a,46 and miRNA-30a47 can promote DR progression. Furthermore, miRNA-49548 and miRNA-34a46 damage the retina of diabetic patients by damaging retinal nerve cells or endothelial cells.
Wnt Signaling
The Wnt pathway is a signaling pathway that plays an important role in the development and homeostasis of organisms, and it is conserved across species, suggesting that it is important in the communication of information in organisms. It functions through the transcriptional coactivator β-catenin, and there is also an atypical Wnt signaling pathway that does not depend on β-catenin but signals through calcium ions.49 These two pathways are considered to be an integrated signaling network, and they are not separated from each other, but have an interactive relationship. Numerous studies have shown that mutated or aberrantly expressed Wnt signaling pathways are associated with a number of common diseases across a broad spectrum, including diseases of the cardiovascular system and cancer.
Some researchers have observed upregulation of Wnt signaling pathway components in DR, which demonstrates that Wnt signaling also plays a role in the progression of DR.50 After deep exploration, it was concluded that dysregulation of Wnt signaling may be related to oxidative stress imbalance, pathological angiogenesis and inflammation.51 Specifically, Wnt signaling can indirectly affect or regulate ROS production, and this regulation can control the level of oxidative stress in vivo. In addition, a large body of evidence suggests that Wnt/β-catenin signaling plays a critical role in angiogenesis during retinal development and in the establishment and maintenance of BRB function in adulthood.52,53
However, the role of Wnt/β-catenin signaling in the pathogenesis of retinal vascular diseases has not yet been clarified, and more studies are still needed. Although the studies are still incomplete and we do not yet understand its specific role, it is certain that the Wnt signaling pathway does play a crucial role in this.53 Therefore, it could also be a therapeutic target for the treatment of diabetes and other retinal diseases.
Current and Emerging Therapies of DR
Despite the growing understanding of DR, the actual approved application of treatments is still limited to those targeting its advanced vascularization, typically occurring after the emergence of PDR or DME.54,55 Current therapeutic options for DR primarily include pharmacotherapy, laser photocoagulation, and surgical interventions.5 Prior to the advent of anti-vascular endothelial growth factor (anti-VEGF) therapies, intravitreal corticosteroids were widely used. Although corticosteroids can effectively reduce inflammation at the affected site and influence the production of vascular and inflammatory cytokines, they are also associated with a heightened risk of increased intraocular pressure (IOP) and glaucoma, as well as an elevated likelihood of cataract formation.5 Intravitreal injection of anti-VEGF agents works by inhibiting VEGF to reduce the formation of retinal neovascularization. However, in routine clinical practice, the necessity for repeated intravitreal injections imposes significant financial burdens and psychological stress on patients.56 Panretinal photocoagulation (PRP) remains a first-line treatment for preventing vision loss in DR patients; by coagulating non-perfused retinal areas, PRP can reduce the formation of retinal neovascularization and lower the levels of vascular growth factors and VEGF in retinal tissues.57,58 Nevertheless, the destructive nature of laser therapy can lead to a range of adverse effects, including vision impairment and visual field defects. Vitrectomy is employed for patients with advanced DR, particularly those with vitreous hemorrhage and tractional or rhegmatogenous retinal detachment, to prevent further vision loss and visual field defects. Despite continuous advancements towards minimally invasive surgical techniques, it remains invasive, and some patients still experience suboptimal postoperative recovery. Many researchers are exploring the preoperative intravitreal injection of anti-VEGF agents as a novel strategy to mitigate postoperative complications. Despite ongoing advancements in treatment modalities, inherent risks and potential complications persist. Furthermore, most patients suffer irreversible visual impairment by the time they receive these treatments.59 Therefore, there is an urgent need to develop new therapeutic approaches.
Due to the complex nature of DR, multiple (non-mutually exclusive) theories exist to explain its etiology and progression.60 Arguably, successful therapies must take into account these multiple mechanisms rather than focusing on one pathway or molecule. However, most research and preclinical therapies target only one specific mechanism or signaling pathway. For example, anti-VEGF drug therapies control the progression of DR by targeting proliferating endothelial cells.61 As these targeted drugs are limited, the question arises: are there effective therapeutic options that can address multiple mechanisms? Exosomes, as intercellular signaling molecules, have been shown to contain large amounts of miRNAs, suggesting that exosomes could potentially be used to correct disordered miRNA signaling in DR patients.62,63
Treatment of Exosomes in Retinal Diseases
We conducted a review of the past five years of research on the use of exosomes for treating retinal diseases. Numerous studies have shown that the delivery of exosomes into the eye can significantly improve various retinal disease models.64–66 The efficacy of exosomes has been demonstrated in treating the following diseases: retinal ischemia, age-related macular degeneration (AMD), autoimmune uveitis (AU), glaucoma, traumatic optic neuropathy, corneal disease, retinopathy of prematurity, and uveal melanoma.
AMD is a retinal disease occurring in older adults and is categorized into dry AMD and wet AMD based on the characteristics of the lesion. Dry macular degeneration is marked by the drusen underneath the retinal pigment epithelium (RPE) and is accompanied by atrophic changes in the retina, also known as atrophic AMD. Wet AMD, or neovascular AMD, is characterized by choroidal neovascularization (CNV) and associated hemorrhages in the choroid and retina. Wet AMD causes more severe vision loss than dry AMD, primarily due to the neovascularization. Research has demonstrated that exosomes possess anti-angiogenic properties, which help to decrease retinal vascular leakage and inhibit CNV.65 Some researchers have experimentally shown that MSC-derived exosomes can modulate the Nrf2/Keap1 signaling pathway to reduce oxidative damage to retinal pigment epithelial cells, ultimately effectively decreasing the incidence of dry AMD.67 Furthermore, research involving intravitreal injection of exosomes derived from retinal Müller glial cells into mice with retinopathy exhibited notable retinoprotective effects. These effects included alleviation of retinal ischemic symptoms, attenuation of VEGF expression, and inhibition of retinal cell apoptosis.68 These findings indicate that exosomes hold promise as a potential therapeutic approach for AMD.69
Uveitis refers to inflammation of the uvea, including the choroid. It encompasses various subtypes based on the location of inflammation. As the disease progresses, it has been observed that the RPE modulates the immune response through the release of exosomes endowed with immunosuppressive characteristics, consequently contributing to inflammation.70 Whereas, recent research has explored the role of exosomes in uveitis and found that MSC-derived exosomes also possess notable immunomodulatory properties. These exosomes exhibit the ability to resist lesions, potentially facilitating the repair of injured or diseased tissue and organ structures. Researchers conducted experiments involving periocular injection of exosomes derived from human mesenchymal stem cells (hMSCs) into an AU rat model, and the results showed a reduction in intraocular leukocyte infiltration and alleviation of uveitis.71 As crucial pathogenic factors in the development of experimental autoimmune uveitis (EAU), Th1 and Th17 cells were observed to significantly decrease in number in mice treated with MSC exosomes compared to the control group, as demonstrated by flow cytometry analysis of cervical draining lymph nodes. This suggests that MSC exosomes may inhibit the progression of EAU by suppressing Th cells.72 These findings underscore the potential of exosomes from hMSCs in the treatment of AU.73
Glaucoma manifests in two primary subtypes: open-angle glaucoma and closed-angle glaucoma, which can be futher divided into primary and secondary categories. Glaucoma is characterized pathologically by optic nerve atrophy, leading to clinical manifestations such as visual field defects and vision loss. Ischemia of the optic nerve and pathologically elevated IOP stand as primary risk factors, with the level of elevated IOP closely associated with the optic nerve’s tolerance to pressure-induced damage. This relationship is intricately linked to the occurrence and progression of optic nerve atrophy and visual field defects in glaucoma.74 Notably, selective RGC death is a typical manifestation of glaucoma. Therefore, to effectively prevent RGC death and dysfunction in response to its pathological changes, treatments must be able to address the damage caused by pathologic IOP elevation and inadequate optic nerve blood supply. A study transplanted bone marrow mesenchymal stem cell (BMSC) exosomes into the vitreous of three different animal models of glaucoma.75 In all three models, BMSC exosomes significantly promoted the survival of RGCs and prevented their functional decline, which precisely prevents the selective death of glaucomatous RGCs.76 This suggests that exosomes could be an appropriate strategy for glaucoma cases where IOP-lowering therapy is not available and as an adjunctive therapy for the full range of glaucoma types. To determine whether the ideal outcomes they observed also apply to humans, certain scholars artificially induced damage to retinal cells differentiated from human embryonic stem cell lines and assessed the effects of exosomes ex vivo human retinal tissue cultures. Although ex vivo systems cannot fully replicate glaucoma, they provide some evidence suggesting that the efficacy of exosomes observed in animal models may apply to humans. This underscores the need for further research employing human tissue to genuinely understand and elucidate this point.
Optic nerve crush serves as a model for traumatic optic neuropathy. This model appears to result in selective death of certain RGC subtypes and has some research value.77 It has been suggested75 that exosomes extracted from BMSC were transplanted into the rat vitreous after optic nerve extrusion. By preloading the exosomes with fluorescent markers, the exosomes delivered their contents into the RGCs, providing significant neuroprotection. In contrast, fibroblast exosomes, which served as a control group, did not produce any therapeutic effect. In addition, a role for BMSC exosomes in maintaining RGC function was also observed by electroretinogram measurements. These findings indicate that exosomes operate through various mechanisms.
The therapeutic potential of stem cell-derived exosomes in treating these retinal diseases underscores their promise in this field. These successful instances also lay the groundwork for further investigation. In the following section, we will delve into the role of stem cell-derived exosomes in DR, a prominent condition within retinal diseases.
Treatment of Exosomes in DR
In recent years, the exploration of stem cell-derived exosomes has garnered significant attention, particularly in relation to their implications for DR. A burgeoning body of literature has emerged, underscoring the potential therapeutic role of exosomes in this context. Exosomes derived from stem cells retain the therapeutic properties of stem cell regenerative therapy while reducing immunogenic responses. Due to their smaller size and simpler structure compared to cells, exosomes are easier to isolate and preserve.23 Additionally, their nanoscale size allows them to effectively target specific tissues after administration, exhibiting excellent targeting ability without accumulating in the pulmonary microvasculature, thereby reducing the risk of pulmonary embolism.78 Studies have shown that stem cell-derived exosomes can protect molecules from degradation and enhance cellular uptake through endocytosis.79 As a non-invasive therapeutic approach, exosomes demonstrate a strong ability to cross biological barriers. Presently available experimental literature on the use of exosomes derived from stem cells for treating DR converges on analogous experimental outcomes (Table 1). Our enthusiasm is piqued by the prospect that exosomes may offer a promising avenue for the treatment of DR through a multitude of mechanisms. A pivotal aspect driving research interest in exosomes lies in their broad spectrum of therapeutic effects that can treat DR through various mechanisms. Notably, studies in Table 1 have shown that the exosomes secreted by stem cells have remarkable effects in alleviating oxidative stress, suppressing inflammatory responses, anti-angiogenesis, reducing cell apoptosis, and protecting neural tissues (Figure 2). We will elaborate on this viewpoint through a review of recent pertinent studies.
 |
Table 1 Studies Related to the Role of Exosomes in Diabetic Retinopathy |
Stem Cell-Derived Exosomes Impair Oxidative Stress of DR
Oxidative stress, characterized by disturbed ROS production and impaired antioxidant defense mechanisms, is a key factor in the pathogenesis of DR.89 It is thought to arise from HG-induced metabolic disturbances, with its typical manifestations of metabolic abnormalities associated with oxidative damage to the diabetic retina: the accrual of AGEs, activation of PKC, enhanced flux through the polyol pathway, and activation of the hexosamine pathway. These pathways potentiate ROS generation, triggering oxidative stress and activating cascading reactions intricately intertwined with the progression of DR. All these abnormal metabolic alterations promote ROS production leading to retinopathy, and ROS themselves serve to amplify these metabolic abnormalities in turn. The two form a vicious circle that leads to the gradual development of irreversible retinopathy. In addition, these metabolic pathways are interconnected with all other pathways through ROS or other active substances, and they are intertwined in a web that complicates the pathogenesis.90 Elevated ROS activate poly ADP ribose polymerase, which inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. The polyol pathway is then activated, increasing intracellular AGE formation through interaction with intracellular methylglyoxal, triggering PKC, which in turn triggers NF-κB, and activating the hexosamine pathway. Fructose-6-phosphate amidotransferase (FAT) in the hexosamine pathway is associated with transforming growth factor-β (TGF-β) expression and PKC activity.89 Excessive glucose metabolism produces sorbitol via the polyol pathway, which is eventually metabolized to fructose. The by-products of the polyol pathway act as potent glycosylating agents to promote AGE formation, and elevated AGE levels exacerbate oxidative stress and activate PKC pathways. In addition, HG mediates epigenetic modifications that inhibit antioxidant defenses, further upsetting the balance between ROS production and clearance. Excessive ROS accumulation leads to a series of damaging responses such as mitochondrial damage, apoptosis, inflammation, lipid peroxidation, and ultimately to alterations in retinal structure and function. The interplay between these mechanisms has been widely demonstrated, while the abnormalities associated with them have provided several potential therapeutic targets for the development of safe and effective treatments for DR.38
Many of the experiments mentioned in Table 1 were conducted to explore signaling molecules in oxidative stress-related pathways. For example, STAT1, the target gene of miR-17-3p, has been shown to be associated with the promotion of oxidative stress and ROS generation.59 In addition, another study found activation of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activities, along with a decrease in malondialdehyde (MDA) levels after upregulation of miR-133b-3p or downregulation of FBN1- or BMSC-derived exosomes, while a control assay found reversal of the effects of these factors by overexpression of FBN1 or by use of a miR-133b-3p mimic.83 In HG-treated MCs, BMSC-derived exosomes or exosomes collected from miR-486-3p mimic-transfected BMSCs augmented SOD and CAT activities and elevated GSH level.84 Toll-like receptor 4 (TLR4), a target of miR-486-3p, was chosen as the target in the control assay of this study, and the effects of increased SOD and CAT activity and elevated GSH levels were found to be counteracted by enhancing its expression level.84 As mentioned earlier, the Wnt/β-catenin signaling pathway provides a key clue to the functional development of the retina. Further studies revealed that Wnt3a stimulated the Wnt/β-catenin pathway and increased NADPH oxidase 2 (Nox2) and Nox4 mRNA levels and Nox1 concentration in the retina of diabetic rats, while decreasing SOD1 and SOD2 mRNA expression and SOD activity. However, concomitant administration of MSC exosomes and Wnt3a to diabetic rats reversed this effect.86
Stem Cell Derived-Exosomes Impair Inflammation in DR
Currently, intravitreal anti-VEGF therapy stands as the primary treatment for DME, offering benefits such as edema reduction and visual acuity improvement.91 However, most patients exhibit poor responses to these treatments, suggesting the involvement of factors beyond VEGF in DME pathogenesis. Accumulating evidence underscores the pivotal role of low-grade inflammation in the pathogenesis and progression of DR.
Under HG, the expression of inflammatory factors increases, with their levels positively correlated with DR severity.92 In addition, these inflammatory factors are associated with leukocyte recruitment and activation. Researchers have observed upregulation of inflammatory factors in the retina of diabetic animals. Inflammatory phenomena such as leukocyte aggregation and glial cell activation were also seen, which can lead to lesions such as retinal ischemia, hypoxia, and endothelial cell damage, as well as occlusion of blood vessels. These inflammatory factors, together with VEGF and others, lead to vascular injury, neuroinflammation, and pathologic angiogenesis in the DR. In addition, some retinal cells are activated to secrete a number of inflammatory mediators, which can exacerbate apoptosis and the appearance and progression of vascular damage. There are novel therapies that target these inflammatory molecules or related signaling pathways to inhibit retinal inflammation and prevent DR.93
In the studies mentioned above, exosomes have been shown to not only attenuate oxidative stress but also mitigate inflammatory responses. It was found that upregulation of miR-17-3p suppressed TNF-α, IL-1β, IL-6, MDA, VEGF, and ROS levels while increasing SOD and GSH-Px activities. While downregulation of miR-17-3p had the opposite effect on these parameters.59 Its effects could be antagonized by STAT1 overexpression in control experiments. Inflammatory factors are elevated in the DR model. In one study, scholars observed upregulation of these mediators in HG-treated MCs, and restoration of miR-486-3p or depletion of TLR4 effectively suppressed their levels.84 Increasing TLR4 reversed the levels suppressed by miR-486-3p restoration. In addition, BMSC-derived exosomes or exosomes of miR-486-3p mimic-transfected BMSCs decreased the levels of TNF-α, IL-1β, and IL-6. In conclusion, the repair of miR-486-3p, TLR4 depletion, or BMSC-derived exosomes attenuated the inflammatory response in HG-treated MC. MSC exosomes were isolated from the culture medium of human umbilical cord mesenchymal stem cells (hUCMSCs) and miRNA-126 was transferred into them after isolation. MSC exosomes or MSC exosomes overexpressing miR-126 were injected intravenously into diabetic rats and co-cultured in vitro with human retinal endothelial cells (HRECs) affected by high glucose. The results showed that there was a significant inflammatory response in diabetic rats or HRECs affected by high glucose, as evidenced by elevated levels of caspase-1, IL-1β, and IL-18. However, MSC exosomes were effective in reversing this inflammatory response. Compared with control MSC exosomes, MSC exosomes overexpressing miR-126 were more successful in inhibiting the HMGB1 signaling pathway, which not only inhibited the activity of NLRP3 inflammasomes in the experimental HRECs, but also achieved the effect of attenuating the inflammatory response in diabetic rats.82 Nesrine Ebrahim’s study also showed that exosomes inhibited the Wnt/β-catenin pathway in diabetic retinas, thereby suppressing retinal inflammation.86 In their study, they conducted a very rigorous controlled trial, grouped the possible influencing factors and tested their effects separately, proving the role of Wnt signaling pathway in DR and the therapeutic efficacy of stem cell exosomes from different perspectives. It was observed that a significant upregulation appeared with the induction of diabetes, including mRNA levels of c-Myc, cyclin D1, TNF-α and ICAM-1. In contrast, the expression of these inflammatory markers was significantly reduced in the treatment group compared with the diabetic control group. Injections of MSC-derived exosomes also produced similar effects to those of the treatment group, with the aforementioned inflammatory factors showing varying degrees of reduction, respectively. In contrast, the levels of inflammatory factors were elevated in the retinal tissues of Wnt3a-injected diabetic rats compared with the diabetic group, suggesting that Wnt signaling can have an enhancing effect on retinal inflammation. This phenomenon could be modified by MSC-derived exosomes, as evidenced by a significant upregulation of these inflammatory markers, respectively, compared with the Wnt3a-injected group. Administration of Wnt3a to diabetic rats exacerbated retinal inflammation as evidenced by elevated levels of mRNA expressing inflammatory factors.
Stem Cell Derived-Exosomes Inhibit Cell Apoptosis and Promotes Proliferation of DR
As shown in Table 1, exosomes derived from stem cells do have a significant inhibitory effect on cell apoptosis, whether targeting precursor cells in the retina, vascular endothelial cells, nerve cells, or stromal cells. Treatment of retinal degeneration induced by streptozotocin in diabetic rabbits with exosomes derived from ASCs resulted in the organization of retinal cell composition into distinct layers after 12 weeks, closely resembling normal retinal layers.80 Intravitreal exosome injection demonstrated clear retinal layer organization similar to that of healthy retinas, while intravenous injection led to irregular ganglion cell layers and increased retinal thickness. Interestingly, exosomes derived from BMSCs, containing miR-483-5p, reversed HG-induced apoptosis in ARPE-19 cells by targeting IGF-1R.81 Similarly, exosomes from hUMSCs containing miRNA-17-3p inhibited apoptosis and promoted cell proliferation.59 Notably, experimental animals treated with these exosomes exhibited increased body weight, whereas being underweight is a common clinical symptom among diabetic patients. Furthermore, exosomes containing miRNA-486-3p were found to inhibit apoptosis of MCs under HG conditions.84 These facts demonstrate that exosomes play a prominent role in inhibiting apoptosis in DR tissues.
Stem Cell Derived-Exosomes and Retinal Neurons
Only a few of the articles mentioned in Table 1 deal with the neuroprotective effects of exosomes, focusing on their roles in alleviating neuroinflammation, inhibiting apoptosis, and secreting human brain-derived neurotrophic factor (BDNF), respectively. Retinal ischemic injury results in neuroinflammation, which in turn manifests as necrosis and apoptosis of neuronal cells, culminating in a massive loss of RGCs, impairment of the BRB, and neurodegeneration. Experimental results by Biji Mathew et al suggest that exosome treatment after retinal ischemia improves retinal function by reducing apoptosis and neuroinflammation.87 MSC-EVs were observed within retinal neurons, indicating their neuroprotective potential.
BDNF plays a protective role in RGCs, and in the retinas of patients with DR, the levels of BDNF significantly decrease.94,95 The downregulation of these neuroprotective factors in the retinal tissue of DR patients is a significant factor in retinal neurodegeneration. Notably, human umbilical cord mesenchymal stem cell derived exosomes (hUCMSC-Exos) have been shown effectively reduced retinal neuron apoptosis by activating the BDNF-TrkB pathway under HG conditions. In this study, ELISA assays confirmed high BDNF concentrations in hUCMSC-Exos., which significantly reduced retinal neuron apoptosis and improved neuron survival rates. Immunofluorescence analysis revealed increased TrkB fluorescence intensity in neurons following treatment with hUCMSC-Exos.88
MCs are the predominant neuroglia in the human retina and play an important role in the structural and metabolic functions of the retina.96 MCs are present in every layer of the retina, and they interact with all types of neurons. As typical glial cells, they can provide a supportive role for neurons. In addition, they induce the synthesis of tight junction proteins, which form tight junctions between cells, thus contributing to the inner retinal barrier. MCs have been shown to protect the retina from free radicals, so it is hypothesized that they may have significant neuroprotective effects, which is why their importance is repeatedly emphasized. Surprisingly, microRNAs contained in exosomes secreted by stem cells also play a good role in protecting MCs. In one study, the expression of miRNA-486-3p was low in HG-treated MCs, while the expression of TLR4 and NF-κB was high.84 Experiments using miRNA-containing BMSC-derived exosomes for treatment revealed that oxidative stress and inflammatory responses in MCs were alleviated, while apoptosis of MC cells was significantly inhibited and proliferation was observed, which is certainly good news for neuroprotection.
Stem Cell Derived-Exosomes and Angiogenesis
Vascular lesions have attracted attention earlier compared to neuropathy. In addition to the presence of neuroprotective effects, MCs have been shown to modulate the BRB by controlling blood flow to the retina and regulating angiogenesis. Studies have shown that MCs can enhance vascular endothelial barrier function by secreting many different factors, including neurotrophic factors and a subset of proangiogenic factors. Under pathological conditions, MCs activate multiple signaling pathways, leading to increased expression of proangiogenic factors.97 Retinal microvasculature is critical for maintaining visual function in the neural retina.98 Microvessel formation, maturation, and stabilization then require pericyte-endothelial cell interactions, and many retinal vascular diseases interfere with pericyte-endothelial cell interactions thereby leading to aberrant microvascular behavior. During embryonic development, signaling pathways such as VEGF and TGF-β can play important regulatory roles in angiogenesis and vasculogenesis. They can also play a role in remodeling blood vessels under pathological conditions. When signaling is abnormal or metabolic problems occur, they can disrupt intercellular signaling, leading to BRB abnormalities and a number of microvascular-related pathologies.
The definition of anti-angiogenic is specific. Stem cell-derived exosomes containing miRNA-222,80 miRNA-133b-3p,83 miR-486-3p,84 and SNHG785 have demonstrated anti-angiogenesis effects in DR. For example, microRNA-133b-3p, derived from BMSC exosomes, can inhibit angiogenesis in DR by suppressing the downstream molecule FBN1. BMSC exosomes have also been shown to hinder angiogenesis in DR mice by inhibiting the TLR4/NF-κB axis and upregulating miR-486-3p. Overexpression of SNHG7 in human retinal microvascular endothelial cells (HRMECs) inhibited HG-induced endothelial-to-mesenchymal transition and tube formation via the miR-34a-5p/XBP1 axis. In addition, Nesrine Ebrahim’s experiments demonstrated that exosomes downregulating retinal angiogenic markers blocked the Wnt/β-catenin pathway in DR.86
By reviewing recent studies, it is evident that in certain animal models, stem cell-derived exosomes have demonstrated significant roles in reducing retinal damage by alleviating oxidative stress and inflammatory responses. Additionally, they have shown potential in slowing disease progression by inhibiting neovascularization, ultimately improving visual outcomes. This highlights the role of stem cell-derived exosomes in regulating key pathways involved in disease progression.
It is noteworthy that early clinical trials focusing on stem cell-derived exosomes in DR are actively underway. The trial registered as NCT06198543 centers on the alterations in exosomes associated with DR, aiming to explore their therapeutic potential. Additionally, trials NCT06188013 and NCT03264976 investigate the possibility of utilizing exosomes as biomarkers for DR, with further exploration into their underlying molecular mechanisms and therapeutic targets. It is anticipated that future clinical trials will expand the use of exosomes in the treatment of DR, thereby advancing the clinical application of this emerging therapeutic approach.
From Bench to Clinic
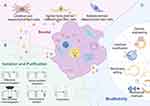
Exosomes are undoubtedly favorable candidates for effective activation of multiple therapeutic pathways, but there is still a long way to go from the emergence of exosomes to practical applications, and we still have numerous questions remain unanswered, for instance, concerns about their potential toxicity, whether the production materials are not readily available, whether the properties of exosomes can be unified, and so on. Relying solely on animal studies to describe their characteristics is insufficient. We still need a lot of clinical trials to ensure that the perfect effects produced by exosomes in laboratory animals can be replicated in humans. Specialized research in this area remains limited, and there are many issues to consider during the injection, dosing, and large-scale production of exosomes (Figure 3).
For diabetic patients, retinopathy is commonly present. If exosomes are to be widely applied in these patients, the issue of large-scale production must be carefully considered. Currently, the production of exosomes primarily relies on stem cells, followed by further processing and optimization. First and foremost, stem cells are excellent candidates for large-scale production, as their proliferative capacity is evident.99 As shown in Table 1, most of the exosomes used for DR are derived from umbilical cord mesenchymal stem cells (UCMSCs) and human bone marrow mesenchymal stem cells (hBMSCs), followed by adipose mesenchymal stem cells (ASCs). These cells are all commonly used in stem cell therapy. These stem cells have shown good therapeutic effects in the experimental stage, but if they are really put into the process of clinical application, they still need to face many problems such as stem cell heterogeneity. For example, relevant studies indicate that the heterogeneity of hUCMSCs arises from different individuals, cloning processes, and other factors.100 If this is to be done well, the preservation of cord blood, the selection of cord blood source, and the number of passages of clones should all be taken into account. For UCMSCs, the preservation of umbilical cord blood is a problem. Autologous stem cells, such as BMSCs, are likely to be more suitable for individuals than allogeneic stem cells. Hopefully there will be a more complete plan in the future. In addition to the source of stem cells, exosome production yields not a single product but rather various subpopulations with different expressed molecules. Therefore, characterization and analysis of exosomes are essential during research. Moving forward, a more comprehensive plan is needed, along with further research into the unique properties of exosomes from different stem cell sources, to provide a basis for the standardization of therapeutic exosomes.
In addition, the isolation and purification of exosomes is also a matter. To date, there is no technique to isolate absolutely pure exosomes.101 Most isolation protocols obtain exosomes by simply removing large extracellular vesicles by size, without the cellular origin or the contents of the exosomes being analyzed and isolated in detail.102 There are four main methods for isolation and purification of exosomes that are currently the most widely used and effective. Differential ultracentrifugation is the most commonly used method for isolation of exosomes, but the operation process of this method is time-consuming and it is easy to produce contamination, which cannot guarantee the purity of exosomes.103 Density gradient separation continues the differential ultracentrifugation method with some optimization. This technique effectively removes non-exosome contamination and improves the purity of exosome purification by one grade.104 Size exclusion chromatography is ideal for isolating exosomes from plasma. Although the yield of this technique is low, contamination is insignificant and still has some merit.105 In recent years, a novel separation technique called high specificity immunoaffinity capture has begun to be applied to isolate exosomes. This technique utilizes the immunoaffinity between the antigen on the exosome membrane and its antibody for separation.106 As for precipitation, it is simpler compared to the three previous methods. First, the biological sample is mixed with a polymer and then exosome precipitation is achieved by centrifugation at a lower speed.107
Modifiability is also important for such drugs. Coincidentally, our exosomes are naturally endowed with this advantage, which would provide a solid foundation for their clinical modification and application.108,109 First, multiple engineering approaches can be utilized to sort exosomes and also to target the regulation of the secretion kinetics of these exosomes.110 Second, several strategies can also be utilized to translocate drugs into exosomes so that they can be safely delivered to the target site,111,112 which promises to enable targeted drug engagement with the site of transfer. In addition, enhancing the secretory process of exosomes from cellular sources and improving their intrinsic properties through external molecular modifications have also emerged as viable strategies. Achieving these goals often requires a combination of nanomolecular and molecular biology techniques, resulting in engineered exosomes with high purity and specificity characteristics.113 Notably, modified exosomes exhibit superior stability, enhanced drug bearing and delivery capabilities, greater bioaffinity, and better targeting capabilities that can be achieved with some adjustments.114 Thus, they are excellent platforms for the natural delivery of therapeutic drugs. These customized structures are known as artificial exosomes or biomimetic exosomes.115 Delving deeper into the field of engineering strategies, engineering has categorized them into three groups, including bottom-up, top-down, and hybrid. These strategies depend on different specific purpose of the modification. To briefly introduce these approaches, the bottom-up paradigm116 is the systematic construction of complex enhancement structures from a single molecule through progressive assembly methods, from small accumulations into final products, which commonly include physical methods, chemical modifications, membrane editing techniques, and genetic engineering. In contrast, top-down approaches break down larger, structurally complex cellular entities into smaller components, thereby assembling small vesicles with compact dimensions, and are commonly used, including co-incubation and chemical foaming and cell membrane coating nanotechnology.117 Notably, the abundance of specific nucleic acid molecules within exosomes is quite low, meaning a large quantity of exosomes is required for the active components to exert their biological effects in recipient cells. Therefore, increasing the payload of these active components has become an effective solution, and the modification and optimization of exosomes are increasingly becoming a new trend.118
Fortunately, it has been shown that exosomes do not show toxic effects in the retina. There are also some studies that have explored this in detail, for example, it has been found that the retention time of exosomes varies in different retinal cells, especially in RGCs in vivo, where the retention time is the longest at 14 days.119 These results provide important data for the design of preclinical studies on the therapeutic effects of intravitreal injection of exosomes.
Through our review and discussion, the potential of stem cell-derived exosomes in the treatment of DR is evident. However, numerous issues and challenges remain. For instance, there is a lack of standardized processes for exosome production and optimal storage methods, as well as insufficient exploration of administration routes and specific dosing for exosome therapies. The safety and efficacy of exosome-mediated drug delivery methods are still underdeveloped, and there are relatively few clinical trials underway. Nevertheless, we are confident that advancements in technologies such as nanotechnology and gene editing will lead to the development of effective and safe exosome-based therapeutic strategies.
The future of exosomes is undoubtedly promising, as it is difficult to find such a perfect way to comprehensively improve a disease from the perspective of multiple molecular mechanisms, especially the type of DR that is difficult to prevent and control. It is believed that through the improvement of experiments and clinical trials (Figure 3), exosomes will become a gospel for patients with DR.
Conclusion
Stem cell-derived exosomes exhibit significant therapeutic potential in the treatment of DR through a variety of molecular mechanisms. Despite the promising prospects of these treatments, several challenges impede their clinical application. These challenges encompass the standardization of production and purification processes, optimization of dosage and delivery methods, and comprehensive evaluation of long-term safety and efficacy. Moreover, the regulatory and ethical complexities associated with exosome therapy further complicate its path to clinical implementation. To address these challenges and fully harness the potential of stem cell-derived exosomes in DR treatment, future research should focus on a thorough understanding of their underlying mechanisms, the development of innovative targeted delivery systems, and the advancement of personalized treatment approaches. Multicenter collaborations and the establishment of standardized protocols will be crucial for ensuring the reproducibility and reliability of research findings. Additionally, long-term clinical studies and a robust regulatory framework are essential to guarantee the safe and effective integration of stem cell-derived exosomes into clinical practice. By overcoming these obstacles and exploring new research avenues, stem cell-derived exosomes hold the promise of becoming a potent and effective tool in the management of DR, offering hope for improved outcomes in this challenging condition.
Abbreviations
IDF, International Diabetes Federation; DR, diabetic retinopathy; HG, hyperglycemia; MSC, mesenchymal stem cell; miRNA, micro ribonucleic acids; NVU, neurovascular unit; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; anti-VEGF, anti-vascular endothelial growth factor; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; BRB, blood-retinal barrier; DME, diabetic macular edema; CNV, choroidal neovascularization; AU, autoimmune uveitis; AMD, age-related macular degeneration; IOP, intraocular pressure; MC, Müller cell; RPE, Retinal pigment epithelium; RGC, retinal ganglion cells; ASC, adipose mesenchymal stem cell; BMSC, bone marrow mesenchymal stem cells; GAPDH, glyceraldehydes-3-phosphate dehydrogenase; TNF, tumor necrosis factor; IL, interleukin; SOD, activate superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; UCMSC, umbilical cord mesenchymal stem cell; hUCMSC, human umbilical cord mesenchymal stem cell; hUCMSC-Exos, human umbilical cord mesenchymal stem cell derived exosomes; hBMSC, human bone marrow mesenchymal stem cells; BDNF, brain-derived neurotrophic factor; AGEs, advanced glycation end products; PKC, protein kinase C; PRP, panretinal photocoagulation; EAU, experimental autoimmune uveitis; hMSCs, human mesenchymal stem cells; TGF-β, transforming growth factor-β; TLR4, Toll-like receptor 4; HRECs, human retinal endothelial cells; Nox, NADPH oxidase; HRMECs, human retinal microvascular endothelial cells.
Acknowledgments
We would like to thank the Science and Technology Development Plan of Jilin Province (No.20210204070YY).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–598. doi:10.1038/s41591-022-01707-4
3. Kropp M, Golubnitschaja O, Mazurakova A, et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—risks and mitigation. Epma Journal. 2023;14(1):21–42. doi:10.1007/s13167-023-00314-8
4. Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62(4):211–217. doi:10.1159/000499541
5. Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with diabetic retinopathy. N Engl J Med. 2020;382(17):1629–1637. doi:10.1056/NEJMra1909637
6. Steinmetz JD, Bourne RRA, Briant PS. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e144–e160. doi:10.1016/s2214-109x(20)30489-7
7. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/s2214-109x(17)30393-5
8. Thulliez M, Angoulvant D, Pisella PJ, Bejan-Angoulvant T. Overview of systematic reviews and meta-analyses on systemic adverse events associated with intravitreal anti-vascular endothelial growth factor medication use. JAMA Ophthalmol. 2018;136(5):557–566. doi:10.1001/jamaophthalmol.2018.0002
9. Zafar S, Walder A, Virani S, et al. Systemic adverse events among patients with diabetes treated with intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 2023;141(7):658–666. doi:10.1001/jamaophthalmol.2023.2098
10. Nakashima H, Iwama Y, Tanioka K, Emi K. Paracentral acute middle maculopathy following vitrectomy for proliferative diabetic retinopathy: incidence, risk factors, and clinical characteristics. Ophthalmology. 2018;125(12):1929–1936. doi:10.1016/j.ophtha.2018.07.006
11. Paris F, Pizzuti V, Marrazzo P, Pession A, Alviano F, Bonsi L. Perinatal stem cell therapy to treat type 1 diabetes mellitus: a never-say-die story of differentiation and immunomodulation. Int J Mol Sci. 2022;23(23). doi:10.3390/ijms232314597
12. Kimbrel EA, Lanza R. Next-generation stem cells—ushering in a new era of cell-based therapies. Nat Rev Drug Discov. 2020;19(7):463–479. doi:10.1038/s41573-020-0064-x
13. Aly RM. Current state of stem cell-based therapies: an overview. Stem Cell Invest. 2020;7:8. doi:10.21037/sci-2020-001
14. Wang Y, Tang Z, Gu P. Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials. Cell Death Dis. 2020;11(9):793. doi:10.1038/s41419-020-02955-3
15. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Natu Rev Bioeng. 2023;1(9):608–609. doi:10.1038/s44222-023-00064-2
16. Chu D-T, Nguyen TT, Tien NLB, et al. Recent progress of stem cell therapy in cancer treatment: molecular mechanisms and potential applications. Cells. 2020;9(3):563. doi:10.3390/cells9030563
17. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell‐derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi:10.1111/cas.14563
18. Yunusova NV, Dandarova EE, Svarovsky DA, et al. Production and internalization of extracellular vesicules in normal and under conditions of hyperglycemia and insulin resistance. Biomed Khim. 2021;67(6):465–474. Produktsiia i internalizatsiia vnekletochnykh vezikul v norme i v usloviiakh giperglikemii i insulinorezistentnosti. doi:10.18097/pbmc20216706465
19. Yang M, Chen J, Chen L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front Endocrinol. 2022;13:1027686. doi:10.3389/fendo.2022.1027686
20. Ratajczak MZ, Ratajczak J. Leukemogenesis occurs in a microenvironment enriched by extracellular microvesicles/exosomes: recent discoveries and questions to be answered. Leukemia. 2024;38(4):692–698. doi:10.1038/s41375-024-02188-9
21. Dragomir MP, Knutsen E, Calin GA. SnapShot: unconventional miRNA Functions. Cell. 2018;174(4):1038–1038.e1. doi:10.1016/j.cell.2018.07.040
22. Dragomir MP, Knutsen E, Calin GA. Classical and noncanonical functions of miRNAs in cancers. Trends Genet. 2022;38(4):379–394. doi:10.1016/j.tig.2021.10.002
23. Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9(1):17. doi:10.1038/s41392-023-01704-0
24. Yang Z, Tan T-E, Shao Y, Wong TY, Li X. Classification of diabetic retinopathy: past, present and future. Front Endocrinol. 2022;13. doi:10.3389/fendo.2022.1079217
25. Singh H, Singh R, Singh A, et al. Role of oxidative stress in diabetes-induced complications and their management with antioxidants. Arch Physiol Biochem. 2023:1–26. doi:10.1080/13813455.2023.2243651
26. Oshitari T. Neurovascular impairment and therapeutic strategies in diabetic retinopathy. Int J Environ Res Public Health. 2021;19(1):439. doi:10.3390/ijerph19010439
27. Montesano G, Ometto G, Higgins BE, et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62(3):35. doi:10.1167/iovs.62.3.35
28. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6). doi:10.3390/ijms19061816
29. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/s0140-6736(09)62124-3
30. Sachdeva MM. Retinal neurodegeneration in diabetes: an emerging concept in diabetic retinopathy. Curr Diab Rep. 2021;21(12):65. doi:10.1007/s11892-021-01428-x
31. Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14). doi:10.1172/jci.insight.93751
32. Oshitari T. Diabetic retinopathy: neurovascular disease requiring neuroprotective and regenerative therapies. Neural Regen Res. 2022;17(4):795–796. doi:10.4103/1673-5374.322457
33. Oshitari T. The pathogenesis and therapeutic approaches of diabetic neuropathy in the retina. Int J Mol Sci. 2021;22(16):9050. doi:10.3390/ijms22169050
34. Rohilla M, Bansal S, Bansal S, et al. Discussing pathologic mechanisms of Diabetic retinopathy & therapeutic potentials of curcumin and β-glucogallin in the management of Diabetic retinopathy. Biomed Pharmacother. 2023;169:115881. doi:10.1016/j.biopha.2023.115881
35. Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. 2022;12(4):542. doi:10.3390/biom12040542
36. Kassambara A, Jourdan M, Bruyer A, et al. Global miRNA expression analysis identifies novel key regulators of plasma cell differentiation and malignant plasma cell. Nucleic Acids Res. 2017;45(10):5639–5652. doi:10.1093/nar/gkx327
37. Zhao X, Ling F, Zhang GW, Yu N, Yang J, Xin XY. The correlation between MicroRNAs and diabetic retinopathy. Front Immunol. 2022;13:941982. doi:10.3389/fimmu.2022.941982
38. Satari M, Aghadavod E, Mobini M, Asemi Z. Association between miRNAs expression and signaling pathways of oxidative stress in diabetic retinopathy. J Cell Physiol. 2019;234(6):8522–8532. doi:10.1002/jcp.27801
39. Murinello S, Usui Y, Sakimoto S, et al. miR-30a-5p inhibition promotes interaction of Fas(+) endothelial cells and FasL(+) microglia to decrease pathological neovascularization and promote physiological angiogenesis. Glia. 2019;67(2):332–344. doi:10.1002/glia.23543
40. Ye EA, Liu L, Jiang Y, et al. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J Neuroinflammation. 2016;13(1):305. doi:10.1186/s12974-016-0771-8
41. Lv YN, Ou-Yang AJ, Fu LS. MicroRNA-27a negatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cell Mol Neurobiol. 2017;37(2):195–210. doi:10.1007/s10571-016-0361-4
42. Chen M, Obasanmi G, Armstrong D, et al. STAT3 activation in circulating myeloid-derived cells contributes to retinal microvascular dysfunction in diabetes. J Neuroinflammation. 2019;16(1):138. doi:10.1186/s12974-019-1533-1
43. Hui Y, Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-κB signaling. Life Sci. 2018;207:212–218. doi:10.1016/j.lfs.2018.06.005
44. Li J, Hui L, Kang Q, Li R. Down-regulation of microRNA-27b promotes retinal pigment epithelial cell proliferation and migration by targeting Nox2. Pathol Res Pract. 2018;214(7):925–933. doi:10.1016/j.prp.2018.05.025
45. Jiang Y, Sang Y, Qiu Q. microRNA-383 mediates high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells by repressing peroxiredoxin 3. Am J Transl Res. 2017;9(5):2374–2383.
46. Thounaojam MC, Jadeja RN, Warren M, et al. MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants. 2019;8(9):328. doi:10.3390/antiox8090328
47. Dong N, Wang Y. MiR-30a regulates S100A12-induced retinal microglial activation and inflammation by targeting NLRP3. Curr Eye Res. 2019;44(11):1236–1243. doi:10.1080/02713683.2019.1632350
48. Zhang X, Yang Y, Feng Z. Suppression of microRNA-495 alleviates high-glucose-induced retinal ganglion cell apoptosis by regulating Notch/PTEN/Akt signaling. Biomed Pharmacother. 2018;106:923–929. doi:10.1016/j.biopha.2018.07.018
49. Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: a comprehensive review. Cell Biol. Int. 2022;46(6):863–877. doi:10.1002/cbin.11797
50. Wang Z, Liu CH, Huang S, Chen J. Wnt Signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–133. doi:10.1016/j.preteyeres.2018.11.008
51. Chen Q, Ma JX. Canonical Wnt signaling in diabetic retinopathy. Vision Res. 2017;139:47–58. doi:10.1016/j.visres.2017.02.007
52. Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife. 2019;8. doi:10.7554/eLife.43257
53. Nguyen H, Lee S-J, Li Y. Selective activation of the wnt-signaling pathway as a novel therapy for the treatment of diabetic retinopathy and other retinal vascular diseases. Pharmaceutics. 2022;14(11):2476. doi:10.3390/pharmaceutics14112476
54. Mansour SE, Browning DJ, Wong K, Flynn HW Jr, Bhavsar AR. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;Volume 14:653–678. doi:10.2147/OPTH.S236637
55. Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retinal Eye Res. 2016;51:156–186. doi:10.1016/j.preteyeres.2015.08.001
56. Arrigo A, Aragona E, Bandello F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann Med. 2022;54(1):1089–1111. doi:10.1080/07853890.2022.2064541
57. Everett LA, Paulus YM. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diabetes Rep. 2021;21(9):1–12. doi:10.1007/s11892-021-01403-6
58. Gozawa M, Takamura Y, Miyake S, et al. Photocoagulation of the retinal nonperfusion area prevents the expression of the vascular endothelial growth factor in an animal model. Invest Ophthalmol Visual Sci. 2017;58(13):5646–5653. doi:10.1167/iovs.17-22739
59. Li W, L-y J, Cui Y-B, Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int Immunopharmacol. 2021;90:107010. doi:10.1016/j.intimp.2020.107010
60. Anfuso CD, Giurdanella G, Longo A, et al. Antioxidant activity of cyanidin-3-O-glucoside and verbascoside in an in vitro model of diabetic retinopathy. Front Biosci. 2022;27(11):308. doi:10.31083/j.fbl2711308
61. Meng C, Gu C, He S, et al. Pyroptosis in the retinal neurovascular unit: new insights into diabetic retinopathy. Front Immunol. 2021;12:763092. doi:10.3389/fimmu.2021.763092
62. Anand S, Trounce IA, Gangoda L. Role of extracellular vesicles in mitochondrial eye diseases. IUBMB Life. 2022;74(12):1264–1272. doi:10.1002/iub.2687
63. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signaling. 2021;19(1):47. doi:10.1186/s12964-021-00730-1
64. Massoumi H, Amin S, Soleimani M, et al. Extracellular-vesicle-based therapeutics in neuro-ophthalmic disorders. Int J Mol Sci. 2023;24(10). doi:10.3390/ijms24109006
65. Liu J, Jiang F, Jiang Y, et al. Roles of exosomes in ocular diseases. Int j Nanomed. 2020;15:10519–10538. doi:10.2147/ijn.s277190
66. Mead B, Tomarev S. Extracellular vesicle therapy for retinal diseases. Prog Retinal Eye Res. 2020;79:100849. doi:10.1016/j.preteyeres.2020.100849
67. Tang Y, Kang Y, Zhang X, Cheng C. Mesenchymal stem cell exosomes as nanotherapeutics for dry age-related macular degeneration. J Control Release. 2023;357:356–370. doi:10.1016/j.jconrel.2023.04.003
68. Zhao Z, Sun W, Guo Z, Zhang J, Yu H, Liu B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020;254:116900. doi:10.1016/j.lfs.2019.116900
69. Gu F, Jiang J, Sun P. Recent advances of exosomes in age-related macular degeneration. Front Pharmacol. 2023;14:1204351. doi:10.3389/fphar.2023.1204351
70. Li N, Zhao L, Wei Y, Ea VL, Nian H, Wei R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res Ther. 2019;10(1):278. doi:10.1186/s13287-019-1372-0
71. Bai L, Shao H, Wang H, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7(1):4323. doi:10.1038/s41598-017-04559-y
72. Shigemoto-Kuroda T, Oh JY, Kim DK, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8(5):1214–1225. doi:10.1016/j.stemcr.2017.04.008
73. Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi:10.3389/fimmu.2021.749192
74. Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retinal Eye Res. 2021;83:100916. doi:10.1016/j.preteyeres.2020.100916
75. Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6(4):1273–1285. doi:10.1002/sctm.16-0428
76. Mead B, Ahmed Z, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in a genetic DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2018;59(13):5473–5480. doi:10.1167/iovs.18-25310
77. Tran NM, Shekhar K, Whitney IE, et al. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron. 2019;104(6):1039–1055.e12. doi:10.1016/j.neuron.2019.11.006
78. Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14(1):66. doi:10.1186/s13287-023-03287-7
79. Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 2018;26(7):1610–1623.
80. Safwat A, Sabry D, Ragiae A, Amer E, Mahmoud RH, Shamardan RM. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J Circ Biomark. 2018;7:1849454418807827. doi:10.1177/1849454418807827
81. Cao D, Zhou L, Hu R. Exosomes derived from BMSCs alleviates high glucose-induced diabetic retinopathy via carrying miR-483-5p. J Biochem Mol Toxicol. 2024;38(1):e23616. doi:10.1002/jbt.23616
82. Zhang W, Wang Y, Kong Y. Exosomes derived from mesenchymal stem cells modulate mir-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60(1):294–303. doi:10.1167/iovs.18-25617
83. Liang G, Qin Z, Luo Y, et al. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Genet Ther. 2022;29(12):710–719. doi:10.1038/s41434-021-00310-5
84. Li W, Jin L, Cui Y, Nie A, Xie N, Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J Endocrinol Invest. 2021;44(6):1193–1207. doi:10.1007/s40618-020-01405-3
85. Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272:119232. doi:10.1016/j.lfs.2021.119232
86. Ebrahim N, El-Halim HEA, Helal OK, et al. Effect of bone marrow mesenchymal stem cells-derived exosomes on diabetes-induced retinal injury: implication of Wnt/ b-catenin signaling pathway. Biomed Pharmacother. 2022;154:113554. doi:10.1016/j.biopha.2022.113554
87. Mathew B, Ravindran S, Liu X, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146–160. doi:10.1016/j.biomaterials.2019.01.016
88. Gao X, He GH, Zhang XT, Chen S. Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway. Int J Ophthalmol. 2021;14(11):1683–1689. doi:10.18240/ijo.2021.11.06
89. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37. doi:10.1016/j.redox.2020.101799
90. Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Diabetic complications and oxidative stress: a 20-year voyage back in time and back to the future. Antioxidants. 2021;10(5):727. doi:10.3390/antiox10050727
91. Forrester JV, Kuffova L, Delibegovic M. The Role of Inflammation in Diabetic Retinopathy. Front Immunol. 2020;11:583687. doi:10.3389/fimmu.2020.583687
92. Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med. 2020;30(3):030502. doi:10.11613/bm.2020.030502
93. Tang L, Xu GT, Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023;18(5):976–982. doi:10.4103/1673-5374.355743
94. Boia R, Ruzafa N, Aires ID, et al. Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges ahead. Int J Mol Sci. 2020;21(7):2262. doi:10.3390/ijms21072262
95. Moosaie F, Mohammadi S, Saghazadeh A, Dehghani Firouzabadi F, Rezaei N. Brain-derived neurotrophic factor in diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2023;18(2):e0268816. doi:10.1371/journal.pone.0268816
96. Kobat SG, Turgut B. Importance of müller cells. Beyoglu Eye J. 2020;5(2):59–63. doi:10.14744/bej.2020.28290
97. Li X, Liu J, Hoh J, Liu J. Müller cells in pathological retinal angiogenesis. Transl Res. 2019;207:96–106. doi:10.1016/j.trsl.2018.12.006
98. Huang H. Pericyte-endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21(19):7413. doi:10.3390/ijms21197413
99. Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32(2):113–120. doi:10.4103/tcmj.tcmj_182_19
100. Lyons FG, Mattei TA. Sources, Identification, and clinical implications of heterogeneity in human umbilical cord stem cells. Adv Exp Med Biol. 2019;1169:243–256. doi:10.1007/978-3-030-24108-7_13
101. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
102. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi:10.1016/j.cell.2016.01.043
103. Lucchetti D, Fattorossi A, Sgambato A. Extracellular vesicles in oncology: progress and pitfalls in the methods of isolation and analysis. Biotechnol J. 2019;14(1):e1700716. doi:10.1002/biot.201700716
104. Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684. doi:10.7150/thno.41580
105. An M, Wu J, Zhu J, Lubman DM. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018;17(10):3599–3605. doi:10.1021/acs.jproteome.8b00479
106. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
107. Soares Martins T, Catita J, Martins Rosa I, O ABd CES, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13(6):e0198820. doi:10.1371/journal.pone.0198820
108. Deng M, Wu S, Huang P, Liu Y, Li C, Zheng J. Engineered exosomes-based theranostic strategy for tumor metastasis and recurrence. Asian J Pharm Sci. 2023;18(6):100870. doi:10.1016/j.ajps.2023.100870
109. Xu X, Xu L, Wen C, Xia J, Zhang Y, Liang Y. Programming assembly of biomimetic exosomes: an emerging theranostic nanomedicine platform. Mater Today Bio. 2023;22:100760. doi:10.1016/j.mtbio.2023.100760
110. Gilligan KE, Dwyer RM. Engineering exosomes for cancer therapy. Int J Mol Sci. 2017;18(6). doi:10.3390/ijms18061122
111. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
112. Liu Q, Li D, Pan X, Liang Y. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J Nanobiotechnology. 2023;21(1):334. doi:10.1186/s12951-023-02081-0
113. Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnology. 2019;17(1):85. doi:10.1186/s12951-019-0517-8
114. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi:10.1016/j.actbio.2019.05.054
115. Zhang H, Wang Y, Li M, et al. A self-guidance biological hybrid drug delivery system driven by anaerobes to inhibit the proliferation and metastasis of colon cancer. Asian J Pharm Sci. 2022;17(6):892–907. doi:10.1016/j.ajps.2022.09.003
116. Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19(1):242. doi:10.1186/s12951-021-00986-2
117. Zhang X, Zhang H, Gu J, et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33(14):e2005709. doi:10.1002/adma.202005709
118. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signaling. 2022;20(1):145. doi:10.1186/s12964-022-00959-4
119. Mathew B, Torres LA, Gamboa Acha L, et al. Uptake and distribution of administered bone marrow mesenchymal stem cell extracellular vesicles in retina. Cells. 2021;10(4):730. doi:10.3390/cells10040730
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.