Back to Journals » International Journal of Nanomedicine » Volume 20
Strategies to Enhance Nanocrystal Formulations for Overcoming Physiological Barriers Across Diverse Routes of Administration
Authors Yanamadala Y, Muthumula CMR, Khare S , Gokulan K
Received 2 September 2024
Accepted for publication 30 November 2024
Published 9 January 2025 Volume 2025:20 Pages 367—402
DOI https://doi.org/10.2147/IJN.S494224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Zhang
Yaswanthi Yanamadala,* Chandra Mohan Reddy Muthumula,* Sangeeta Khare, Kuppan Gokulan
Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, Jefferson, AR, 72079, USA
*These authors contributed equally to this work
Correspondence: Kuppan Gokulan, Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, 3900 NCTR Road, Jefferson, AR, 72079, USA, Email [email protected]
Abstract: Poor aqueous solubility and bioavailability limit the translation of new drug candidates into clinical applications. Nanocrystal formulations offer a promising approach for improving the dissolution rate and saturation solubility. These formulations are applicable for various routes of administration, with each presenting unique opportunities and challenges posed by the physiological barriers. The development of nanocrystal formulation requires comprehensive understanding of these barriers and the biological environment, along with strategic modulation of particle size, surface properties, and charge to facilitate improved bioavailability to the target site. This review focuses on applications of nanocrystals for diverse administration routes and strategies in overcoming anatomical and physiological delivery barriers. The orally administered nanocrystals benefit from increased solubility, prolonged gastrointestinal retention, and enhanced permeation. However, the nanocrystals, due to their small size and high surface area, are susceptible to aggregation in the presence of gastric fluids and are more prone to enzymatic degradation compared to the macrocrystalline form. Although nanocrystal formulations are composed of pure API, the application of excipients like stabilizers reduces the aggregation and improves formulation stability, solubility, and bioavailability. Some excipients can facilitate sustained drug release. Emerging research in nanocrystals include their application in blood-brain barrier transport, intranasal delivery, stimuli responsiveness, multifunctionality, and diagnostic purposes. However, the challenges related to toxicity, scale-up, and clinical translation still need further attention. Overall, nanocrystal engineering serves as a versatile platform for expanding the therapeutic potential of insoluble drugs and enabling dose reduction for existing drugs, which can minimize toxicity and improve bioavailability at lower dosages.
Keywords: nanocrystals, nanotechnology, nanosuspension, drug delivery, route of administration, physiological barriers, brain delivery, oral, bioavailability
Introduction
The pharmaceutical industry has witnessed major changes over the past few decades with the discovery of new therapeutic candidates, development of new formulation strategies, and vector molecules. Despite many advancements, 70% of new therapeutic drugs have dropped out of the clinical pipeline before reaching the formulation stage, owing to poor aqueous solubility.1 To overcome these issues, the addition of cosolvents, surfactants, inclusion compounds, solid dispersion, amorphization, and complexation strategies have been implemented. However, these approaches come with limitations that can drastically impact the therapeutic efficiency and possible solvent-related adverse effects.
To overcome the challenges such as solubility and formulation issues, and to achieve targeted therapy nanotechnology offers promising strategies. Nanocrystals are nanocrystalline forms of pure active pharmaceutical ingredients (API) in the nanometer range (1–100) that can be produced by top-down, bottom-up, or a combination of the two techniques.2,3 The atoms in the nanocrystals are arranged in a regular, repeating lattice structure and their small size allow them to bridge the properties of both bulk and individual molecules. Due to the small size and high surface area, they exhibit unique physicochemical properties like improved bioavailability compared to the original API. According to the Noyes-Whitney theory, smaller particles with a higher surface-to-volume ratio boost the dissolution rate of therapeutic compounds by increasing their saturation solubility.4 Reduction from the micrometer to nanometer scale through nanonization techniques improves drug saturation solubility because of the increased dissolution pressure of strongly curved small nanocrystals.3,5 Nanocrystal formulations offer unique advantages compared to other nano formulations, as they eliminate the need for carriers or excipients, reduce complexity and toxicity, and offer predictable pharmacokinetics. These characteristics, along with their ability to interact with the biological membranes and cellular environments in distinct ways from the micron sized drugs make them a desirable option for targeted delivery and controlled release.
Nanocrystals were initially formulated to increase the solubility of poorly soluble BCS class II drugs, which possess poor solubility but good permeability through biological membranes. In addition to improving the solubility, they also enhance the drug-loading capacity, adhesion to cell membranes, absorption, and stability, offering range of clinical applications.6 Nanocrystals stand out from other colloidal drug delivery methods because of their potential to offer almost 100% API, which increases the probability of attaining an improved therapeutic concentration with desired pharmacological action.7 The versatility of nanocrystals lies in their ability to be formulated such that they can be administered through multiple routes, including oral, parenteral, cutaneous, ophthalmic, and pulmonary (Figure 1).8
 |
Figure 1 Routes of nano crystalized drug administration ocular, oral, nasal, dermal, intravenous, and intramuscular routes. |
Nanocrystals also offer the feasibility of surface modification for target-specific therapies for cancer and immune diseases.9,10 Several studies have shown that modification of NCs alters the physicochemical properties of compounds to further improve formulation stability, dispersion, pharmacokinetics, and bioavailability.11,12 The modifications of nanocrystals can also be made in terms of the route of administration.2,13 The route of administration plays a key role in determining the fate of these particles, as interactions with different physiological barriers and conditions in the biological environment determine the sequence of events and the therapeutic efficiency of the drug.4 This review focuses on updating the formulation and modification of nanocrystals to bypass physiological barriers based on (that are encountered) route of administration (Figure 1).
Route of Administration
Nanocrystal Fabrication
Drug formulation depends on the solubility, stability, formulation-methods, and route of drug administration. Figure 2 highlights that influence the safety, efficacy, formulation, and absorption of the nanocrystal drugs. Crystalline solids are self-assembled ordered structures, whereas amorphous solids pack molecules randomly. Amorphous formulations are widely used in pharmaceuticals owing to their high dissolution rates. Nevertheless, they often need to be stabilised with polymeric additives/excipients, which raises safety issues because of their intrinsic physical instability. This can be achieved by employing nanocrystal-formulation methods.4,14
 |
Figure 2 Factors influencing effective drug delivery of nanocrystals. |
Nanocrystals can be manufactured using top-down or bottom-up approaches, or a combination of these two approaches, commonly referred to as the nano-edge technique. Additionally, a novel approach has been suggested for the preparation of nanocrystals by utilising the microfluidic pathway to reduce the production costs with the efficiency and swiftness it offers.3,15 Some of the techniques employed in nanocrystal production include precipitation, ball milling, spray drying, jet milling, solvent based methods, antisolvent (supercritical antisolvent), and micronization.16
Top-down approaches, such as high-pressure homogenisation (HPH) and media milling, involve reducing the bulk particle size to the nanosized range using high-energy methods. Bottom-up approaches in nanocrystal formulations involve the use of precipitation techniques to obtain nanosized drug particles, specifically via precipitation from a supersaturated drug solution. The drug was dissolved in a suitable solvent and precipitated by adding a nonsolvent, resulting in the formation of nanocrystals.17 Additionally, various combination strategies and supercritical fluid technologies, such as gas antisolvent recrystallisation (GAS), aerosol solvent extraction systems (ASES), atomised rapid injection for solvent extraction (ARISE), rapid expansion of supercritical solution (RESS), and depressurisation of an expanded liquid organic solution (DELOS), have been utilised in the formulation of nanocrystals.4 Furthermore, to achieve uniform and monodisperse nanocrystals, hot-injection or heat-up methods can be employed.18
Although nanocrystals offer a wide range of advantages, their formulation requires the inclusion of a small volume of excipients to improve their stability. Minimal amounts of stabilizers are commonly used to prevent Ostwald ripening caused by supersaturation. Stabilizers are typically selected based on their versatile benefits such as reduced protein binding, alterations in solubility, minimal drug interactions, and low toxicity.19,20
Nanocrystal Fabrication Challenges with Respect to Physiological Barriers
The formulation of nanocrystals presents various challenges, especially when addressing the physiological barriers to drug administration (Table 1). The human body is equipped with various barriers that protect it from the external environment while maintaining the integrity and proper functioning of the organs. These barriers include the blood-brain barrier (BBB), gastrointestinal (GI) barrier, and cellular barriers within tissues, as well as mechanical (skin), biological (microbiome), and chemical (enzymes) barriers. Nanocrystal are showing to be promising to cross various biological barriers, however their capacity to cross highly selective barriers such as BBB still throws challenges.
 |
Table 1 Comprehensive Assessment of Route of Administration for Nanocrystal Drugs: Advantages, Disadvantages, Delivery Challenges and Potential of Nanocrystals |
The transportation of drugs and diagnostic probes to the brain is highly restricted by the BBB because of tight junctions between endothelial cells. The endothelial cells that line the BBB undergo modifications to restrict the passage of these particles into the brain, resulting in instability of nanocrystals.21 The surface modification of nanocrystals can facilitate their transport through the BBB by exploiting specialised transportation pathways, such as receptor-mediated transcytosis (RMT), adsorptive mediated transcytosis (AMT), and/or carrier-mediated transcytosis (CMT).22,23 The BBB mostly allows permeation of positively charged lipophilic molecules with a molecular weight of less than 500 Da.24
Orally administered nanocrystals face challenges due to the harsh environment of the GI-tract, such as enzymatic and microbial, mechanical forces, mucosal layer, and pH changes (acid/base).25 Additionally, the GI barrier is an epithelial barrier with tight junctions, goblet cells, and microfold cells that restrict the movement of therapeutic molecules.26 The GI barrier serves as a limiting factor for the absorption of nanocrystals and their payload into systemic circulation. Strategies have been developed to improve the stability of nanocrystals and prevent abrupt drug release, including the application of protective coatings or stabilising excipients to prevent agglomeration. Furthermore, changing the viscosity of the formulation and incorporating surfactants can further enhance drug stability and prolong the physiological interaction time of nanocrystals, thereby improving therapeutic efficiency and facilitating sustained drug release in the GI-tract.19
Nanocrystals encounter various cellular barriers, including those in the pulmonary epithelium and skin, nasal, and ocular tissues. These barriers comprise numerous layers of cells that restrict the movement of nanocrystals, resulting in reduced efficacy. The modification of nanocrystal surfaces can help increase their interactions with cellular entities and promote cellular absorption and distribution. Cellular barriers exhibit significant variability depending on the cell type and spatial distribution in specific organs. The ability of nanocrystals to efficiently transport deeper into target cells is significantly influenced by their formulation parameters, size, and shape. Drug particles larger than 500 nm are engulfed by phagocytic cells, limiting their ability to reach the target cells.3
The above-mentioned physiological barriers can be effectively circumvented via nanocrystal formulation, which can be accomplished by optimising particle properties, surface modifications, and employing protective measures. Nanocrystals have the potential to enhance the efficiency of drug administration, increase therapeutic outcomes, and facilitate the targeted and controlled release of drugs across various tissues and organs. The subsequent sections provide a comprehensive analysis of physiological barriers related to the route of administration.
Oral Route of Administration
The oral route of administration is widely preferred because of its safety, non-invasiveness, practicality, rapid onset of action, and high patient tolerance. The dissolution rate of drugs plays a vital role in determining the absorption rate of orally administered drugs.4 However, poorly soluble drugs are not readily absorbed in the GI-tract, which reduces their bioavailability.27 Furthermore, many drugs with poor solubility often require repeated dose with higher concentrations owing to their limited bioavailability, resulting in gastric lesions.3 Hence, understanding these bottlenecks and the internal mechanism of oral delivery is important to overcome the challenges presented by this route of administration and develop strategies to improve drug delivery and bioavailability with reduced toxicity. Nanonization of drugs is a viable approach to overcome these challenges as it improves the dissolution rate and solubility. The increased surface area of smaller nanocrystals can accelerate the rapid absorption and metabolism of drugs owing to their specific interactions in the GI-tract than parent drugs.28,29 While formulating NC drugs few factors need to be considered that include gastric environment, gastrointestinal barriers, absorption mechanisms, and drug morphology.26,30
Absorption: Barriers and Mechanisms
Luminal environment: The pH of the digestive system varies from 1 to 8, with stomach pH 1.2–3, duodenal pH 5.5, jejunal pH 6, ileal pH 7.2–8, and colon pH 6.4–7.26 Gradient changes in pH as the drug moves along the GI-tract may cause spatial conformational modifications that affect ionisation and chemical structure, resulting in decreased bioactivity. These pH gradient changes have been shown to significantly impacts proteins and peptide drugs, which also cause deamination/oxidation of drugs by disrupting chemical bonds, result changes in charge distribution and local hydrophilicity.31 GI-tract enzymes are involved in the absorption process, including salivary enzymes stomach enzymes, intestinal enzymes, brush border enzymes, membrane-bound enzymes, pancreatic enzymes, cytosolic enzymes, and exopeptidases. Some enzymes may cause enzymatic degradation by cleaving peptide bonds and affecting structural stability that include elastase, trypsin, and chymotrypsin.
Gastric barriers: The GI-tract also poses physical barriers such as mucus and epithelial barriers. Mucus primarily comprises 95% water with the remaining constituents being proteins, lipids, and electrolytes. Mucus is continuously secreted by the foveolar and goblet cells, and its thickness varies along the digestive tract. Mucus is glycoprotein with overall negative charge that traps the positively charged molecules. Additionally, the mucus layer enhances viscosity and gelation of the surrounding environment, thereby reducing drug absorption.32 The drug molecules bind to mucin, resulting in increased viscoelasticity. Second, the steric barrier prevents the entry of larger particles into the mucin mesh, which is formed by a mucin nonglycosylated network. Nanocrystals with < 200 nm size coated with mucus inert materials such as PEG or zwitterions have demonstrated increased penetration through mucus.33 Epithelial cells that are essential for the absorption of drugs from the GI-tract to the systemic circulation restrict their uptake by tight junctions or trigger lysosomal degradation or basolateral exocytosis. Muco-inert lipid-based nanocarriers, pegylation have demonstrated improved mucus permeability and potential to overcome the epithelial barrier.26,33
Morphology and size: The size and morphology of a drug are the two major factors that primarily affect the bioavailability of orally administered drugs. For example, rod-shaped nanocrystals have shown significant advantages over spherical ones, because of larger surface area, which enabled enhanced cellular exposure (Figure 3A).3,29 The rod-shaped nanocrystals are shown to overcome the mucus entrapment compared to the spherical molecules. In addition to the size and shape/structure is also plays an essential role in enhancing permeability. Studies have shown that biodegradable and tuneable nanoneedle structure showed high efficacy in achieving nano-therapeutics with limited toxicity.34,35 Consequently, increased cellular exposure promoted enhanced cellular absorption and facilitated epithelial transport. Similarly, reducing the particle size can enhance the efficacy of oral drug delivery. Nanocrystal drug formulations exhibit a twenty-fold increase in bioavailability than pure API, as well as a five-fold increase than micron-sized drug formulations.3 Oral administration of Zileuton nanocrystals with a size range of 700 nm has shown a greater maximum concentration (Cmax) in the plasma of rats than pure API or micron-sized drug formulations.36
Absorption mechanisms: Several studies have shown that most nanocrystals are primarily absorbed in the jejunum compared with the ileum, indicating that a unique absorption mechanism is involved in drug molecule translocation.37 Additionally, most of these nanocrystals are entrapped in mucus, allowing for the sustained release of drug molecules. The paracellular pathway is a potential route for nanocrystal uptake, but the strict diametric constriction of openings between cells by glycocalyx filaments makes it difficult for larger nanocrystals to pass through, even after mucus mesh permeation.3 Studies have suggested using permeation enhancers or surfactants enable the opening of these junctions by shortening the glycocalyx filaments; however, there is little evidence to support this claim.38 Currently various nano based technology has been developed for drug delivery for numerous cancer therapy, which has several advantages than traditional drug formulation. Micro and nanomotor is one among them, which is a small device that effectively increases the therapeutic efficiency. This device efficiently changes different form of energy into mechanical motion that empowers the PK properties, biodistribution, stability, solubility of poorly soluble drugs with controlled release mechanism that subsequently decreases the cellular cytotoxicity.5 Additionally, this mechanical movement capable of increasing tissue permeability, result increased cellular uptake that facilitates the accumulation of drug in the target site. Nanocrystals can be directly taken up by cells via the clathrin-mediated, caveolin-mediated, pinocytosis (actin-dependent), phagocytosis (actin-dependent), and non-caveolin and clathrin-mediated pathways.4,39 Earlier, Khun et al showed that nanoparticles were taken up by a combination of mechanisms, rather than relying on a single mechanism.39 Owing to the small pore size of the vascular epithelium, nanocrystals may be available for lymphatic transport rather than venous transport after endocytosis. It has also been reported that M cells transport nanocrystals via an active transepithelial vesicular transport system; however, the efficacy of this process remains debatable.4,40
Strategies to Overcome Limitations of Orally Administered Nanocrystals
Although nanocrystal formulation of drugs seems promising, there are still a few limitations that need to be addressed with the oral route of administration. The major challenge is the instability of nanocrystals in the GI-tract environment. Numerous approaches have been used to address this issue, including coating nanocrystals with a protective layer, carrier system to improve stability and prevent aggregation, production procedures and techniques can also improve drug efficacy.41 Vitamin K antagonists are commonly used as pharmaceutical agents owing to their affordability and effectiveness. In one study, the use of wet media milling followed by spray drying and lyophilisation increased the solubility, stability, and dissolution of the ziprasidone nanocrystal oral formulation42 The vitamin K antagonist (VKA) can inhibit the activity of vitamin K epoxide reductase and blocks the recycling of vitamin k epoxide (VKO).43
Stabilizers
The stabilizers employed in nanocrystal formulations can help to overcome the barriers, preventing Ostwald ripening and agglomeration in mucosal layers. The optimal concentration of stabilizer is crucial to improve the bioavailability of the nanocrystals. Crptotanshinone nanocrystals showed 2.87-fold improved pharmacokinetics compared to coarse drug when ideal concentration of stabilizer poloxamer 407 was used.44 Figure 3A illustrates corona formation around pure nanocrystals, and reduced aggregation and improved permeability with surface modification with stabilizers, surfactants, and encapsulation. The stabilizers, permeation enhancers and cryoprotectant play an important role in stabilizing the nanoformulation during the freeze drying. Specifically, due to the steric hindrance the nanoparticles were prevented the formation of aggregates. The stabilizers also involved in opening of tight junctions by reversibly, which in turn increase the permeability and bioavailability through paracellular transport. The incorporation of stabilizers and permeation enhancers increases the bioavailability and efficacy in the nanocrystal therapeutics. Additionally, it requires low dose of therapeutics which can be correlated less toxicity in liver, kidney, and other organelles.3,45,46 If the nanoparticles are larger in size and more chances of detecting nanoparticles in the kidney. If the nanoparticle size and pore of glomerular filtration unit pore sizes are same the chance of nanoparticles clearance rate is approximately 50%.47 In vivo study revealed that nanotherapeutics treated mice showed significantly decreased level of ALT and AST enzyme than micron sized treated animals. The immunohistochemistry result further supported less hepatotoxicity than non-nano formulated drug stabilizers have been recognised for their impact on the absorption mechanism of nanocrystals, such as endocytosis, as well as on transport mechanisms like intracellular and transmembrane transport.3,48 When used alone, charged stabilizers, such as sodium deoxycholate, have been found to exacerbate agglomeration and hinder the onset of absorption.3 Agglomeration can have either positive or negative effects, depending on the therapeutic effect of the drug and the location of deposition. In the stomach, agglomerated particles are most likely entrapped in the mucus tissue. Failure to expel these particles, which occurs because of hourly renewal of gastric mucus, may lead to an extended duration of drug retention within the physiological system. The incorporation of stabilizers such as polyvinylpyrrolidone (PVP) or a combination of hydroxypropyl methylcellulose (HPMC), polyvinyl alcohol (PVA), and ethyl cellulose in nanocrystal formulations has shown enhanced oral bioavailability.
Permeation enhancers in nanocrystal formulations are another strategy for improving the bioavailability of drugs, which reversibly modify the intestinal cells to improve drug permeability.49 Several permeation enhancers can be employed in nanocrystal formulations including surfactants, fatty acids, chelating agents, and bile salts.50 Surfactants are common permeation enhancers that reduce the surface tension between the drug and intestinal epithelium.51 Studies have shown that sodium lauryl sulphate (SLS) significantly improves the solubility of poorly soluble drugs. The incorporation of the surfactant and vitamin E, TPGS, and Pluronic F-68 in the nanocrystal formulation increased the drug load, disintegration, and dissolution rate. These factors are also influenced by the type of surfactant, concentration employed, and inter- and intra-molecular drug interactions.52 Bile salts are naturally occurring organic surfactants secreted by the liver and it can also be used as precursors in the synthesis of steroids and amphiphilic molecules with specific functionalities.53 These compounds interact with phospholipids in various cells to increase permeation.
The fatty acid derived permeation enhancers, such as oleic and linoleic acids, permeate by modifying the intestinal epithelium and increasing drug bioavailability.54 The incorporation of oleic acid along with other co-surfactants improves the oral bioavailability of poorly soluble tetrandrine drug.55,56 Similarly, chelating agents, such as ethylenediaminetetraacetic acid (EDTA), are also used in nanocrystal formulations as permeation enhancers. They increase permeability by chelating divalent cations present in the intestinal epithelium. A previous study showed a 4.8-fold increase in oral bioavailability with the addition of EDTA to a nanocrystal formulation.57
Other techniques, such as pH modification, enzyme inhibition, and nanoparticle coating, can also alter the bioavailability of nanocrystal drugs.58 Addition of compatible acids or bases is a common technique for pH adjustment. Enzyme inhibitors help drugs to prevent degradation by digestive enzymes. Coating nanocrystals with a polymer or lipid layer also protects against enzymatic degradation and increases intestinal absorption by enhancing the epithelial cell uptake. Furthermore, oral nanocrystal films are ideal for patients with oral pathologies, swallowing difficulties and for children.59
Application of Nanocrystals in Oral Drug Delivery
Nanocrystal formulations for the oral administration of several drugs, including fenofibrate, itraconazole, and curcumin, have shown improved bioavailability compared with API.8–10 The FDA approved oral nanocrystal drugs and their formulations are included in Table 2. Fenofibrate, a medication that exhibits low solubility in water, has demonstrated enhanced solubility in both fasted and fed conditions following nanocrystal formulation (Figure 3B).4,60 Fast and fed states significantly influence the drug absorption and stability. Figure 3B shows that compared to coarse drug formulation nanocrystals are less affected by these states. The fenofibrate nanocrystals into oral strip films resulted in a 1.4-fold enhancement in bioavailability and sustained presence within the system for approximately 24 hours.59 Rapamune, the first oral nanocrystal approved by the US Food and Drug Administration (FDA), consists of sirolimus nanocrystals combined with an excipient mixture suitable for compression into readily consumable tablets. Compared to the API sirolimus solution, the bioavailability of sirolimus nanocrystal tablets increased by 21%.4,50 Saquinavir, a protease inhibitor used for treatment of HIV exhibits poor solubility; however, nanocrystal formulation of approximately 200 nm in rod shape has shown significant improved dissolution and cellular uptake compared to the coarse crystals. Despite their rod shape, the enhanced cellular uptake and transport across membranes was attributed to the size of the nanocrystals as particles smaller than 200 nm are preferentially internalized through the clathrin-coated pits.61 Similarly, nanocrystal formulation of Olmesartan medoxomil stabilized with TPGS and Pluronic F-68 showed increased shelf life and improved solubility. These nanocrystals have irregular shape, and this could have contributed to the rapid dissolution.62 Numerous studies have demonstrated the efficacy of nanocrystal formulations in enhancing oral bioavailability. In addition, nanocrystal formulations have other advantages such as enhanced drug-targeting ability, reduced toxicity, and prolonged retention time. According to one study, camptothecin nanocrystal formulations showed reduced toxicity in animal models and enhanced drug accumulation in tumour tissues.63 Similarly, cyclosporine nanocrystal formulations improved drug targeting to the liver and reduced systemic toxicity in rats.64
 |
Table 2 FDA Approved Nanocrystal Formulated Drugs and It is Ingredients and Route of Administration |
Parenteral Route of Administration
Parental administration is the practice of injecting therapeutic doses directly into the vein (intravenous [IV]), muscle (intramuscular [IM]), or subcutaneously, as opposed to the most common oral route. This method of delivery is especially helpful in situations where oral administration is impractical or ineffective, such as during emergencies, when treating drugs with low oral bioavailability, or compounds that can quickly metabolise in the digestive system, as it enables a steady flow of the medication into the bloodstream under regulated conditions.81,82 Furthermore, drugs administered via the parental route can bypass first-pass metabolism in GI-tract and interactions with ingested foods. Moreover, parental administration, such as IV injection, provides a prompt response, lowers dosage requirements, and ensures complete absorption.4
In recent years, there has been growing interest in the use of parenteral delivery for nanocrystal compositions, because of its ability to handle the toxicity and quickly to reach target sites. Nanocrystal formulations require minimal utilisation of excipients and solvents that are employed in insoluble pharmaceutical compounds. Additionally, owing to their small size and large surface area, nanocrystals are especially suited for this mode of delivery. Nanosuspensions can be manufactured as a viable option for parenteral delivery of nanocrystals. It has the potential to enhance drug retention time and absorption, and reduce the dosage required to achieve a therapeutic outcome. The enhanced therapeutic outcomes can be attributed to the rapid dissolution and absorption of the drug facilitated by the small particle size of the nanocrystals. Compared to other administration methods, IV administration of nanosuspensions demonstrated 100% bioavailability. Danazol microemulsion had 5.1% bioavailability, but when formulated as a nanosuspension, the bioavailability was 82.3%. However, it showed 100% bioavailability when given intravenously.83 Most water-insoluble drugs are found in the CNS and oncology domains, and with the increased incidence of these diseases, there is a great need to develop nanosuspensions to increase the therapeutic profile for such conditions.84
Absorption: Barriers and Mechanisms
The IV route of administration bypassed by intestinal absorption and metabolic enzymes. However, several factors should be considered when formulating nanocrystals for parenteral administration. Upon administration, nanocrystals infiltrate the bloodstream, where they may not experience harsh environments during oral administration but are subjected to changes in viscosity and pH. The viscosity is an essential parameter for drug permeability, bioavailability, and efficacy. For an oral drug dissolution gastric content also plays an essential role that is greatly influenced by intake of food. The intestinal gastric contents exhibit viscosity between 1.4 to 6.4 mPa.S (with a shear rate of 100s-1) at the fasting state, which is several lower than after consuming meals.85 In majority of the nano formulation the drug concentration ranges vary between 2% to 30%. If the drug concentration exceeds above 30%, result aggregation and forming a larger crystal. For example, azelaic acid is used as a topical to treat acne. Azelaic parent drug, and nanocrystal formulated drug Poloxamer used up to between 10 to 20% to prepare the hydrogel and no impact on viscosity. If the Poloxamer increased above 20% that resulted in increased viscosity.86 Additionally, nanocrystal formulations must be meticulously designed to avoid acquisition by Kupffer cells in the liver and the endothelial reticulum system (RES) for rapid absorption. Intravenously injected nanocrystals undergo molecular dispersion because of their high dissolution rate and solubility. Alternatively, they may remain intact and form colloidal particles in circulation, which are largely disseminated by mononuclear phagocyte system (MPS) cells.87 MPS cells later phagocytose these colloidal particles which may be degraded by phagolysosomes or deposited in the liver, lungs, or spleen. The deposition or dissolution of nanocrystals is affected by their size, with nanocrystals larger than 500 nm accumulating in the liver and those smaller than 100 nm poorly captured by immune cells.3,4 Larger particles are often bound or surface-deposited on plasma proteins, resulting in aggregation or opsonisation by macrophages. Macrophages to reduce the toxicity act along with Kupffer cells by depositing these particles in the liver.88 The nanocrystals deposited in the liver and spleen slowly released the contrast mechanism exhibited by the nanocrystals.4
Smaller nanocrystals (<100 nm) dissolved in the bloodstream behaved more like a solution. They may enter cells via paracellular transport, clathrin-mediated, non-clathrin-mediated endocytosis, and pinocytosis.88 Despite the advantages of NCs, the use of these formulations for parenteral delivery is difficult. One of the main difficulties is the possibility of particle aggregation owing to viscosity changes or enzymes (proteases), which can result in the formation of larger particles that are less effective in targeting tissues or cells.89 There are insufficient studies to determine the in vivo fate of smaller nanocrystals after they enter the bloodstream.
Strategies to Overcome Limitations of Parentally Administered Nanocrystals
In addition to these barriers, various other factors require careful consideration to improve drug stability and bioavailability, while minimising the adverse effects of the formulation. It is important to consider parameters such as viscosity, pH, osmolality, and sterility when formulating nanocrystal drugs. Hypertonic formulations can induce erythrocyte shrinkage resulting in pain. Administering smaller volumes of hypertonic solutions or using tonic agents can help overcome this limitation.90 Similarly, maintaining a narrow pH range within the physiological conditions can prevent aggregation in biological fluids. Buffering agents are used to maintain the optimal pH of the formulation, which is crucial for preserving the drug stability. Numerous drugs require the use of cyclodextrin complexes, salt forms, pH modifications, and cosolvents to improve their solubility.88 However, the use of a salt form that is only compatible with ionizable drugs may lead to precipitation owing to pH fluctuations. Additionally, larger quantities of cyclodextrins for IV administration can increase viscosity and potentially cause nephrotoxicity.90 Ethanol cosolvents can induce haemotoxicity, whereas PEG can trigger hypersensitivity responses.91 To overcome these limitations, suitable processing techniques can be employed by carefully formulating nanocrystals with ideal excipients or incorporating surface modifications.
Stabilizers: The development of parenteral nanocrystal formulations can be challenging because the number of approved excipients for this route is limited. Some excipients that may be incorporated into formulations include stabilizers, tonicity adjusters, and buffering agents. The amount and type of stabilizer and surfactant play a crucial role in nanosuspension formulation, as they affect not only the stability of the formulation but also the in vivo efficacy. Irrespective of method employed in formulations, an ideal stabilizer is essential for maintaining the stability and particle-size of nanocrystal formulations.92 Few nanocrystal drugs can be formulated without stabilizers; however, they require vigorous mixing, and these formulations are available in powder form.93 The stabilizer concentrations of IV drugs can vary between 1:20 and 20:1. However, the concentration of excipients should not exceed the critical micelle concentration, at higher concentrations micelle can cause toxicity in IV.90 Some surfactants have been reported to affect viability and stimulate cytokine production by macrophages, whereas neither the drug nor the size of the drug had any effect.94 To prevent aggregation, sedimentation, and crystalline transformation, an appropriate stabilizer with an ideal interaction with the drug can be used.
Particles >500 nm when delivered intravenously are not easily internalized by cells, resulting in 90% of the drug being removed by macrophages within 5 min. When 480 nm nevirapine nanocrystals were administered IV, they showed 40% deposition in the liver and 37% in the spleen, which can be attributed to the particle size of the drug.95 Opsonization by macrophages is a common occurrence following the IV delivery of nanocrystal drugs. However, this could be prevented by modifying the drug surface with PEG. Another constraint of nanocrystal IV formulations is their circulation and retention times, which can also be addressed by PEG modifications. These modifications not only enhance the stability of the formulation, but also overcome the previously mentioned limitations. Furthermore, vitamin-E TPGS surface changes could be used to decrease P-gp efflux inhibition and target ligand shedding. Similarly, sodium deoxycholate surfactants can be incorporated into IV nanocrystal formulations to enhance paracellular transport.
Application of Nanocrystals in Parental Drug Delivery
Parenteral nanocrystals have been formulated for several drugs, including paclitaxel, danazol, and celecoxib, parenteral nanocrystals have been formulated (Table 2 outlines some of FDA approved parental formulations).96–98 For instance, a paclitaxel nanocrystal formulation demonstrated a four-fold increase in anticancer activity and a two-fold increase in tumour size deposition than the API formulation.99 Similar results were observed with danazol nanocrystals, which showed an improved half-life and three-fold increase in bioavailability.100 In another study, an itraconazole parenteral nanocrystal formulation showed superior bioavailability compared with the marketed oral formulation.101 Similarly, paclitaxel parenteral nanocrystals showed greater antitumour activity than micron-sized formulation, which could be attributed to their superior solubility and improved pharmacokinetic properties.102
Targeted delivery through parenteral nanocrystal formulation: A study on parenteral administration of curcumin nanocrystals formulation caused less toxicity than a commercial formulation, specifically, due to the enhanced pharmacokinetic properties and targeted distribution of nanocrystals.89 Nanocrystals have demonstrated an extended residence time inside the liver and lungs, leading to extended therapeutic benefits, while requiring minimal dosage.7 In cancer cell-targeted therapy, nanocrystals can be subjected to modifications involving the application of cationic lipid coatings such as DOTAP, Immunoglobulin G, and protamine.103 These coatings enable immune cell identification and facilitate the deposition of the nanocrystals on cancer cells. Moreover, most cancer cells express folate receptors. Consequently, these nanocrystals can be altered by incorporating folic acid or Pluronic F68 adsorption, thereby enabling targeted therapy.104,105 Specifically, docetaxel nanocrystals modified for folate receptor targeting have demonstrated a three-fold increase in tumour accumulation and a longer half-life. However, it is important to note that these modified nanocrystals have also exhibited increased cytotoxicity.20,99,105
Topical Administration
The application of a drug/formulation directly onto skin surfaces is known as topical administration. It is a non-invasive route of administration and is preferred for drugs that require localised effects. The topical distribution offers the advantage of reducing potential systemic side effects commonly associated with alternative administration; specifically, to protein, peptide, and hormone administration. The oral administration of these compounds is highly limited by their reduced bioavailability, metabolic stability, and inadequate membrane permeability. In addition, drugs are easily broken down by proteolytic enzymes in the stomach.106 In such instances, topical administration is considered advantageous because it overcomes the first-pass mechanisms. Moreover, the topical administration can minimise the need for repeated-dose and improve patient compliance. Although topical administration offers several benefits, the skin primarily allows the permeation of drugs that are non-polar, lipophilic, and have a molecular weight below 500 Da.107–109 These limitations can be overcome by nanocrystal formulations, which have shown promising results for topical distribution because of their ability to penetrate the protective layer of the skin and reach the desired site of action.110 However, it is crucial to understand the morphology and process of drug absorption through the epidermal barrier. Water-insoluble pharmaceutical compounds have distinct advantages in terms of their ability to permeate the epidermal layer of skin.
Absorption: Barriers and Mechanisms
Most pharmaceutical substances can permeate the skin by utilising either the trans-epidermal or intercellular pathways. The skin epidermis contains two layers: stratum corneum, and horny hydrophobic layer formed by dead cells.111 The stratum corneum is allows the permeation of hydrophobic molecules and regresses hydrophilic moieties. The drug that accumulates in the stratum corneum can enter the epidermis through passive diffusion or penetrate the dermis or subcutaneous layer through resorption, depending on the drug interactions and formulation.108,112,113 Drug molecules that permeate the dermis can easily enter the systemic circulation because of their extensive vascularisation. Alternatively, drugs can enter the skin via the appendageal pathway, which involves hydrophilic microchannels that facilitate passive permeation. In contrast, lipophilic molecules penetrate the skin by traversing the lipid matrix located between the intercellular spaces surrounding corneocytes.114 Topically administered nanocrystals can penetrate skin structures, including sebaceous and sweat glands, through hair follicles. The epidermis is the most viable pathway for penetration, allowing access to Langerhans cells, keratinocytes, and melanocytes.113 Nanocrystalline drugs have greater surface areas than parent drugs, thereby facilitating increased contact with biological membranes and conferring mucoadhesive characteristics. They also improve transdermal delivery through various mechanisms, resulting in an increased concentration gradient across the epidermis and passive diffusion to the deeper layers. In addition, nanocrystals can precisely target hair follicles, facilitate the formation of a diffusional corona, and adhere to the epidermis, thereby enhancing their delivery capabilities.111 The mechanism of absorption of nanocrystals of the same drug may differ from that of the API form. In contrast to API drugs, dexamethasone nanocrystals are absorbed through the stratum corneum.115
Nanocrystals <100 nm are absorbed through energy-independent mechanisms or endocytosis, whereas the remaining nanocrystals interact with or translocate through the cells. Some cells are phagocytosed by macrophages, Peyer’s patch cells, and Langerhans cells. Within Langerhans cells, if not broken down by phagolysosomes, smaller particles are transported to cellular compartments or diffuse into surrounding tissues.111
Strategies to Overcome Limitations of Parenteral Nanocrystal Formulations
The enhanced solubility of drug nanocrystals contributes to the preservation of the concentration gradient between the supersaturated suspension of drug nanocrystals and the intended cellular target.116 Smaller nanocrystals permeate deeper layers of the skin than larger nanocrystals.111,117 Furthermore, an increased concentration of nanoparticles increases the rate of permeation of the skin. Maintaining the pH of the formulation is another crucial factor in the nanocrystal formulation for topical use. To prevent skin irritation, it is advisable to formulate the formulation within the pH range of 5–10. Similarly, wounds, cuts, scars, psoriasis, and acne on the skin also limit the use of topical drugs as they cause irritation at the site. In addition, the structure of the skin in these areas was distinct from that of regular skin. Hence, it is important to consider dermatological conditions when developing formulations for affected areas of the skin. These regions exhibit ready access to the systemic circulation or an increased presence of enzymes that can metabolise the drug, thereby reducing its half-life. Surface coating of a drug can help in enzymatic degradation and increase its half-life.
The application of nanocrystal drugs to the skin allows bypassing of liver metabolism. However, the skin also contains enzymes such as p450 enzymes, sulfatases, N-acetyl transferases, epoxide hydrolase, and glucuronyl transferases, which account for approximately 5–10% of hepatic activity. To overcome this problem, increasing the drug concentration or residence time in the skin can help maintain a concentration gradient at the site of action. Therefore, dermal strips containing nanocrystals have demonstrated advantages over conventional drug formulations.108 Anti-microbial dermal patches of resveratrol nanocrystals have shown a five-fold increase in efficacy compared with coarse drug formulations.118 Research is being conducted on the controlled-release properties of nanocrystal dermal patches. Additionally, the skin contains sex hormone receptors. Therefore, modifying the surface of nanocrystal formulations and using receptor-specific drugs can potentially enhance their efficacy.108
Stabilizers: Similar to other drug administration methods, dermal formulations require the inclusion of stabilizers, surfactants, and polymers. These excipients serve to avoid drug aggregation, which may occur due to temperature fluctuations and mechanical forces employed during the application. Stabilizers play a crucial role in diminishing the surface tension at the interface, hence facilitating electrostatic repulsions or steric repulsions by adsorbed polymers on to the surface.117,119,120 Curcumin nanocrystals administered with the stabilizers glycerol, urea, and polyglycol enhanced passive diffusion, whereas curcumin administered with ethanol decreased passive diffusion but prevented stratum corneum swelling.80 Diosmin is a flavonoid with limited solubility that is used to treat diabetic ulcers. It also has anticancer, anti-ulcer, and anti-inflammatory properties. For the treatment of diabetic ulcers, diosmin nanocrystals can be formulated with stabilizers, such as hydroxypropyl methylcellulose or microcrystalline cellulose, as transdermal patches, resulting in enhanced therapeutic efficacy and sustained release of diosmin.121,122
Permeation enhancers: The common limitation of topical administration is the permeation of drug moieties into the deeper tissues. However, permeation enhancers can be employed in formulations that reversibly alter the epidermal lipid matrix and modify the corneocyte proteins. Depending on the type of permeation enhancer employed, a drug is either pulled by increasing its solubility or drawn into the skin by increasing its thermodynamic activity. Alternatively, intracellular keratin modifications may alter the solvent properties of the stratum corneum to increase permeability.111 These modifications aid in the permeation of drugs into deeper tissues, and these excipients can also enhance the dissociation and partition coefficients of the drug.108,114 Some surfactants used in nanocrystal formulations can also help permeate drugs through the transdermal route by disrupting the protein and lipid domains.123 The selection of permeation enhancers must be carefully considered to prevent toxic reaction.111 Nanocrystals measuring approximately 160 nm demonstrated increased permeation into deeper skin layers, whereas medium-sized nanocrystals showed an enhanced retention time. Consequently, formulations can be tailored by combining these two nanocrystals according to specific requirements.124
Formulation techniques: The nanocrystals can be formulated as creams, ointments, gels, sprays and lotions based on the drug, area of application, viscosity, solubility and skin permeability of the drug.125 To increase stability and skin penetration, nanocrystals can also be coated with polymers.126 The ideal attributes for a compound are as follows: (i) a log P value (octanol-water partition coefficient) between 1 and 3, (ii) an aqueous solubility exceeding 1 mg/mL, (iii) a low melting point below 200 °C, (iv) a molecular weight below 500 Da, and (v) fewer than five hydrogen bond donors and 10 hydrogen bond acceptors.111 Employing optimised fabrication techniques involving protective coatings or encapsulation methods can minimise these challenges.
Applications of Nanocrystals in Topical Administration
Dermal administration is a viable alternative to the oral administration of cortisone and dexamethasone nanocrystals. Apremilast, an oral drug used to treat psoriasis, has low solubility and lipophilicity, resulting in reduced bioavailability and increased systemic toxicity. Nanocrystal formulation of the apremilast drug was exhibited a 2.5-fold increase in diffusion into the stratum corneum than traditional drug.120 The topical administration has also been explored for the treatment of various medical disorders, including rheumatoid arthritis and diabetes. Furthermore, the topical application of nitro-glycerine ointment has demonstrated efficacy in mitigating the occurrence of angina.127
Nanocrystal formulations have been widely studied for transdermal drug delivery applications. Li et al investigated transdermal delivery of curcumin using nanocrystals. Compared to traditional formulations, nanocrystals showed improved skin permeability and prolonged drug release.128 Nanocrystal formulations for topical administration have been developed for various drugs including tretinoin, ketoprofen, and ibuprofen. The ketoprofen formulation in nanocrystals demonstrated a five-fold boost in anti-inflammatory action and a two-fold increase in penetration compared with coarse drug formulation.129–132 In another study, topical administration of ketoprofen showed a ten-fold increase in bioavailability; however, when the same drug was formulated as a controlled-release nanocrystal colloidal dispersion, it showed further enhanced bioavailability.133,134 These results highlight the potential of nanocrystal formulations for topical administration. Another crucial use of topical administration is in the investigation of nanocrystals for mucosal delivery. Additionally, investigations are underway to explore the potential of nanocrystal colour dyes for extended colour retention when topically applied to hair.3,135
In conclusion, owing to their capacity to permeate the epidermal barrier and mucosal surfaces, nanocrystal formulations have demonstrated tremendous potential for topical drug delivery. Dermal administration is an appealing alternative for various applications because of its multiple benefits, including localised drug administration and prolonged release. However, more research is required to assess the safety and effectiveness of topical nanocrystal compositions in clinical settings. Furthermore, new approaches to nanocrystal formulations can increase the permeability of hormones and lipophilic molecules to the skin.
Ocular Administration
Ocular drug administration is the preferred route for the treatment of various eye disease. The administration of drugs via this route can be invasive, (ocular injections and implants), or non-invasive (eye drops). Most ocular drug formulations that are applied topically encounter challenges in achieving optimal dosages because of the intricate barriers present in the eye. Nanocrystal formulations for ocular administration can overcome this problem by enhancing the permeability, bioavailability, retention time, and safety of ocular drugs.
Absorption: Barriers and Mechanism
The ocular route of administration is a non-invasive approach that, despite its ease of application, presents substantial hurdles owing to the unique anatomical and physiological properties of the ocular system. Approximately 5% of the supplied dose permeated into the anterior chamber. The obstacles of ocular route are, including the limited volume that can be administered owing to the capacity of the conjunctival sac (approximately 7 µL); nasolacrimal drainage (rapid clearance); tonicity; pH; ocular barriers; blinking and lacrimal reflexes; irritation; and the tolerability of a formulation instilled into the eye.121 The cornea, which serves as a protective barrier that limits drug penetration into the eye, is a primary physiological barrier to ocular drug delivery. There is an exterior hydrophobic coating on the cornea, also called tear film, which makes it difficult for hydrophilic drugs to penetrate. The tear film is composed of various molecules, such as albumin, globulin, lysozymes, and tear fluid. Tear fluid constantly cleanses and replenishes the eye, resulting in the limited bioavailability of drugs.136
Additionally, this route also poses anatomical barriers that includes corneal barrier, conjunctival barrier, blood-ocular barrier, and drug efflux transporters, as illustrated in Figure 4. The corneal epithelium contains tight junctions formed by adhesion proteins, which restrict the movement of large particles. Mucin on the corneal epithelium helps maintain a lower surface tension between the tear film and epithelium.137 Anterior infections are treated with drugs that can pass only through the corneal barrier that subsequently reach the aqueous humour and then enter the anterior uvea, where they confront the conjunctival barrier, which is characterised by a dense network of vasculature. The blood-aqueous and blood-retinal barriers restrict the passage of most drug molecules into systemic circulation (Figure 4). Subsequently, these molecules penetrate the sclera, which exhibits a higher permeability to macromolecules than the cornea; however, they have a lower permeability than the conjunctiva. Furthermore, efflux transporters present in the cornea and blood-retinal barrier restrict the movement of drugs in ocular spaces. Corneal permeability is influenced by pH, lipophilicity, charge, and the degree of ionisation.136 Desmosomes are leaky junctions that allow the penetration of hydrophilic substances into the cornea.138 These issues can be addressed by nanocrystals that offer a greater concentration gradient, solubility, and bioavailability at lower dosages.3 Drug absorption is facilitated by the ability of nanocrystal formulations to improve drug solubility and increase the drug concentration at the corneal surface.
 |
Figure 4 Anatomical structure of the eye and ocular administration routes for nanocrystal delivery. |
Strategies to Overcome the Limitation of Ocular Delivery
Nanocrystal formulations for ocular delivery also encounter challenges concerning of stability, biocompatibility, targeted delivery, and overcoming of ocular barriers. It can be solved by incorporating excipients that do not cause eye irritation. Furthermore, the blood-aqueous and blood-retinal barriers, along with the cornea and tear film, impair the efficient delivery of medicines to ocular tissues. However, by applying a variety of targeting techniques, nanocrystal formulations can help drugs to pass through these barriers more easily. Additionally, enhancing drug accumulation at the target site through surface modification of nanocrystals with ligands or antibodies tailored to ocular tissues can improve therapeutic results. Nanocrystalline delivery systems with specific moieties have been researched for the treatment of retinal diseases such as age-related macular degeneration.136,139
Stabilizer: The stability of nanocrystal ocular formulations is a major concern because of aggregation caused by changes in pH and osmolality upon entering the eye. This can be minimized by employing appropriate stabilizers for ocular formulations. Hydroxypropyl methylcellulose is a commonly used stabilizer in ocular nanocrystal formulations because of its chemical (non-irritant) and physical (size) properties. Tuomela et al showed that the use of hydroxypropyl methylcellulose in brinzolamide nanocrystal formulations to treat glaucoma retained the nanoparticle size and reduced aggregation of the drug, as compared to without a stabilizer.19,140 Some non-irritant polymers commonly employed in ocular nanocrystal formulations include PVP, Poloxamers, and PVA.3
Permeation enhancers: Ideally, enhancers for ocular delivery would offer rapid and reversible effects. Although a few options are available, such as cyclodextrins, cell-penetrating peptides, and surfactants, they often induce irritation and damage, even at lower concentrations. However, substances like oleic acid, medium-chain triglycerides, and mono- and diglycerides have demonstrated enhanced permeability, safety, and stability in formulations.141
Triamcinolone acetonide is an anti-inflammatory corticosteroid that shows potential as a first-line therapy for ocular pathologies, due to low solubility it has been precluded. Administration of a nanocrystal formulation of triamcinolone acetonide resulted in a reduction in inflammation with no harmful effects on the ocular tissues.142 Nevertheless, the stability of the formulation was limited to 120 days, which can be extended through the utilisation of previously illustrated stabilizers. The incorporation of mannitol during the antisolvent precipitation method in the production of tedizolid phosphate nanocrystals effectively prevents crystal growth and enhances stability during freeze-drying.143
Acetazolamide, a BCS class IV medicine, is commonly used to treat glaucoma. However, systemic administration is preferred because of its limited absorption, which causes numerous adverse effects. Marwa et al, demonstrated that antisolvent precipitation using sonication technology, together with stabilizers (PVA and lecithin), resulted in the preparation of a 200 nm acetazolamide nanocrystal nanosuspension.144 This formulation exhibited improved drug-loading ability. The surfaces of the nanocrystals were modified using hyaluronic acid salt and poly δ-glutamic acid to specifically target CD44 receptors, which are abundant in the ocular tissue and enhance the stability of the nanocrystals. This formulation demonstrated enhanced dispersion properties, increased saturation solubility, sustained drug release, and improved tolerance for ocular applications. To enhance the stability of acetazolamide nanocrystals in the solid state, it is necessary to employ a spray-drying process using leucine and mannitol stabilizers.144
Applications of Nanocrystals in Ocular Administration
Nanocrystals offer numerous advantages for drug delivery to the eye, including improved ocular safety, increased retention, corneal permeability, bioavailability, dual drug release profiles, and increased tolerability.142 Table 3 outlines some of ocular nanocrystal formulations, their role in overcoming physiological and formulation challenges and observed therapeutic benefits. Earlier research has demonstrated that poorly water-soluble cyclosporine can be formulated using nanocrystals to increase corneal penetration and therapeutic efficacy.145 Formulated nanocrystals exhibited both immediate and sustained drug release profiles, with immediate release owing to increased saturation solubility and dissolution. Prolonged drug release is possible owing to their high surface area, which facilitates their interaction with biological membranes. The mucoadhesive properties of NCs increase their retention time in the cul-de-sac region, resulting in a prolonged drug action. Administration of acetazolamide nanocrystal drugs in combination with sodium hyaluronate resulted in enhanced corneal penetration and prolonged release for up to eight hours. This effect can be attributed to the abundance of hyaluronan receptors within the cornea.144 Similarly, the poorly water-soluble itraconazole, when formulated as nanocrystals, showed an improved inhibitory zone. When formulated with hydroxypropyl methylcellulose, Pluronic F68, and F127 to produce a thermosensitive ocular gel, itraconazole nanocrystals exhibit an increase in residence time.146 Increasing the viscosity of nanosuspensions or incorporating nanocrystals into in situ gelling systems can further prolong the drug release profile.147
 |
Table 3 Nanocrystal Drug Formulation Classified by Route of Administration with Challenges Addressed and Observed Bioavailability Changes |
The obstacles caused by the tear film, which can quickly dilute and eliminate topically administered drugs, can also be overcome by nanocrystals. Owing to the small size of nanocrystals, drugs are more stable, remain on the ocular surface for longer periods of time, and absorb more quickly. This has been proven in experiments where voriconazole and other ophthalmic drugs in nanocrystal formulations showed enhanced ocular bioavailability than traditional formulations.182 Moreover, an even smaller fraction of these treatments successfully reaches the posterior parts of the eye, where chronic disorders typically emerge. Therefore, administration of frequent and higher drug concentrations, intravitreal delivery, or systemic administration are typically favoured, all of which present their own limitations. Low concentrations of NCs have been shown to effectively penetrate and reach deeper tissue regions. The controlled-release properties of nanocrystal formulations offer additional benefits for ocular administration. Nanocrystal-based intraocular implants for the treatment of chronic diseases such as glaucoma have been developed using controlled release capabilities.183
In general, nanocrystal formulations have great potential for overcoming physiological obstacles in ocular drug delivery than conventional methods. Their ability to increase drug solubility, bioavailability, targeted administration, and controlled release is an important strategy for improving the therapeutic outcomes in ocular diseases. Additional studies are required to optimise the formulation characteristics, comprehend the long-term safety profile, and assess the therapeutic efficacy of nanocrystal-based ocular treatment.
Pulmonary Administration
The administration of nanocrystals drugs via the pulmonary route offers several advantages that include a wide surface area, low enzymatic activity, thin barriers, high vascularisation, and capacity to circumvent hepatic portal drainage.184 Additionally, it allows for great molecular dispersion as drugs are rapidly transported to the systemic circulation. Pulmonary administration of therapeutics has been investigated as a non-invasive mode of administration for systemic drug distribution. This route has direct access to the external environment, and the risk of infection, especially in immunocompromised individuals, is very common. Hence, nanocrystal formulations must withstand the conditions offered by pulmonary passages.
Absorption: Barriers and Mechanisms
During the formulation of drugs for pulmonary administration, it is important to consider many parameters, such as the clearance mechanisms of the lungs (mucociliary elimination and alveolar macrophages that clear larger molecules), mucus entrapment, and the rate at which the drug dissolves. Drug disposition is influenced by the competitive interplay between mucociliary clearance systems and drug absorption.3 Insoluble pharmaceutical compounds that adhere to the mucus layer prior to absorption are sequestered within the mucus and subsequently removed by macrophage-mediated or ciliary clearance mechanisms.147 These concerns can be effectively modified by nanocrystal formulations that facilitate the rapid absorption of many therapeutic substances, particularly those that are insoluble in nature.
Nanocrystal formulations have demonstrated improved mucus penetration and decreased macrophage clearance than micron-sized drug formulations. This mesh-like structure is formed by mucin monomers and cysteine bridges, across which drugs must traverse to access the bloodstream. Failure to do so results in the deposition of drugs in the pharynx, which are subsequently expelled by coughing or ingested by the stomach. The aerodynamics of the lungs plays an important role in drug inhalation. For effective deposition within deep lung tissues, it is crucial for particles to possess an optimal size to prevent deposition in the upper respiratory tract by inertial contact. Small particles are deposited within the airways through Brownian diffusion. Alternatively, these particles should be sufficiently large enough to prevent their expulsion during the process of exhalation. Rod-shaped nanocrystals have demonstrated superior mucus penetration and enhanced contact with alveoli compared with spherical nanocrystals.185 In artificial cystic fibrosis models, rod-shaped PEG-coated C109 nanocrystals demonstrated effective mucus penetration, which facilitated diffusion and enhanced the therapeutic efficacy.186
Strategies to Overcome Limitations of Pulmonary Nanocrystals
The formulation of nanocrystals for pulmonary administration has effectively addressed the challenges encountered with traditional pulmonary drugs, particularly in achieving adequate bioavailability. However, issues related to the stability and retention time of nanocrystals within the pulmonary system persist owing to the innate pulmonary defence mechanisms. The implementation of surface modifications, ideal engineering techniques, mucus penetration strategies, and controlled-release formulations can help to overcome the above stated limitations. Selecting the ideal form of the formulation can also increase the stability of the nanocrystals. The pulmonary route of administration offers the advantage of delivering drugs in dry powder form. Ciprofloxacin nanocrystals administered as a dried powder showed bioavailability similar to that of aerosol formulations and spray-dried ciprofloxacin nanocrystals had a more uniform particle size distribution than freeze-dried nanocrystals.93
The risk of pulmonary toxicity and inflammation caused by exposure to foreign particles, particularly nanocrystals, is a major obstacle. It has been used in various formulations for numerous years, and a recent study showed that exposure to cellulose nanocrystals through the pulmonary route is detrimental and comparable to that of carbon nanotubes. The results showed variability between the sexes.187,188 Additionally, drug deposition and dispersion in the lungs are influenced by many variables including individual lung features, disease conditions, and variations in inhalation procedures. All these factors influence therapeutic outcomes. Therefore, for successful translation to clinical applications, it is essential to carefully analyse and evaluate the safety and effectiveness of nanocrystal formulations for pulmonary delivery.154,189,190
Stabilizers: The incorporation of stabilizers may enhance the stability of the formulation and prevent the aggregation of nanocrystals, a phenomenon frequently observed in the pulmonary environment because of mucus and lung secretion. Nanocrystals can also be combined with surfactants to improve drug deposition and retention in lungs. However, the concentration and type of stabilizer used in the formulation should be carefully chosen, considering that the lungs contain a pulmonary surfactant consisting of 10% protein and 90% lipids.185 The use of Tween 80 stabilizers in wet media milling and spray drying resulted in the production of curcumin nanocrystals. When administered as an aerosol, these nanocrystals demonstrate enhanced deposition in the lungs, improved bioavailability, and prolonged half-life.191 Similarly, paclitaxel nanocrystals formulated with the sole excipient TPGS surfactant demonstrated a greater therapeutic effect in Taxol-resistant pulmonary cancers, reversing drug resistance. Administration of dried powder, which offers bioavailability like that of aerosols, is a distinct advantage over the pulmonary route. Furthermore, some powder formulations have been developed without the need for stabilizers.93
Applications of Nanocrystals in Pulmonary Administration
Nanocrystal formulations have demonstrated the ability for extended release following pulmonary treatment, which represents a significant benefit of nanocrystals compared with other nanotechnologies.185 Additionally, nanocrystal medicines may be promising alternatives to combat multidrug resistance.190 Reducing particle size improves the ability of drug particles to reach the alveoli of lungs.192 Deep lung penetration made possible by nanocrystals can enhance the absorption and bioavailability of the drug. Furthermore, they enhance drug distribution to the lungs and reduce systemic side effects when administered either as aerosols or dry powders. Budesonide, salmeterol, and rifampicin, among other pharmaceutical compounds are some nanocrystal formulations for pulmonary delivery (Table 3 for details).193,194 For instance, a budesonide nanocrystal formulation demonstrated a two-fold increase in anti-inflammatory action and a six-fold increase in lung deposition compared to a standard formulation.194 Similar results were observed when comparing a salmeterol nanocrystal formulation to an API formulation, which showed a two-fold increase in lung deposition and longer bronchodilation.193 Rifampicin’s lung deposition and antibacterial activity were improved in a study by Beck-Broichsitter et al compared to microparticle formulations.185 Several nanocrystal formulations have been developed for use in inhalation devices. For example, the paclitaxel nanocrystal-nebulised formulation showed better antitumour activity and decreased systemic toxicity than the API drug formulation.195
Brain Delivery
Nanocrystals represent a promising approach for drug delivery to the brain by overcoming the complex BBB. They have the potential to reach the brain parenchyma owing to their small size and surface properties. Although oral and systemic nanocrystals have been developed to target the brain, nonspecific binding limits drug availability. Recent advancements in nanocrystal formulations for targeted delivery have led to significant advancements in therapeutic strategies.
Absorption: Barriers and Mechanisms
The BBB is a physiological barrier comprised primarily of endothelial cells, glial cells, and pericytes, along with cerebrospinal fluid, and arachnoid epithelium to protect the brain. Specialised endothelial cells that line the BBB can modify most drugs and are selectively permeable. This prevents most therapeutics, including nanoparticles from entering the brain.23,196 Tight junctions, transendothelial transport systems, enzymes, and leukocyte permeation regulate physical, transport, enzymatic, and immune functions of the BBB. The presence of ATP-binding transmembrane ABC proteins influences drug delivery. Lipophilic molecules with low molecular weights (MW) typically pass through the BBB, with permeation decreasing 100-fold when the drug MW increases from 300 Da to 450 Da.3 The ability of NCs to traverse the BBB depends significantly on their size, however, only 2–3% of the small molecules can effectively permeate the brain. Studies have shown that smaller nanocrystals cross the BBB faster than larger nanocrystals, because the paracellular transport channels that exist between the BBB’s endothelial cells197 (Figure 5). Additionally, the surface area-to-volume ratio of smaller nanocrystals is higher, which improves their interaction with the BBB and their absorption by the brain.100,198
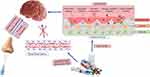 |
Figure 5 Pathways of nanocrystal drugs to the brain. Nose to brain delivery and systemic delivery. |
Another significant aspect that may affects the transport of nanocrystals to the brain is the surface charge. Negatively charged endothelial cells make up the BBB, which can resist negatively charged nanocrystals and prevent them from entering the brain.81 Theoretically, it is suggested that positively charged nanocrystals may cross the BBB faster than negatively charged nanocrystals, due to the electrostatic interactions between positively charged nanocrystals and negatively charged endothelial cells of the BBB, which promote their absorption by the brain.101 Positively charged nanocrystals can be used in the clinical setting; however, this can potentially result in toxicity and inflammation.102,199 Despite advancements in imaging techniques and tools, the exact mechanism of nanocrystal transport into the brain remains unclear. However, nanocrystals are believed to be transported via either endocytosis or passive diffusion. Despite the potential use of nanocrystal formulations for other delivery routes, bypassing the BBB and accessing the brain remains a challenge for several drugs. Nanocrystals improve the solubility, dissolution rate and pharmacokinetics of most of the drugs, however these advantageous are often insufficient to achieve BBB permeation. Parental or orally administered brain targeting drugs may aggregate and loose effectiveness at the BBB interface. The optimal formulation necessitates ideal surface modifications, additives or targeting ligands to facilitate transport. However, various alternative invasive and non-invasive methods are available for bypassing the BBB.
Invasive methods: Invasive methods, including intracerebral injection and implantation of drug-eluting devices, can be used to deliver NCs to the brain to improve the permeability of drug compared to macromolecules. Although these methods circumvent the blood-brain barrier (BBB) and provide direct access to the brain they carry a high risk of infection and tissue damage, and may not be appropriate for all individuals, they may be useful in some circumstances. However, nanocrystals showed better permeability in the brain after intraperitoneal and tail vein injections (in vivo studies). Intraperitoneal administration of Px-18 nanocrystals after cerebral ischemia/reperfusion showed the ability to alleviate delayed neuronal death caused by ischemia. Px-18 nanocrystals were formulated as nanosuspensions by high-pressure homogenisation.200 However, these trials should be expanded to the clinical setting.
Intranasal administration (Non-invasive method): The BBB can be bypassed by intracarotid infusion, hyperthermia techniques, transmucosal drug delivery, and intranasal administration.201 The intranasal administration of nanocrystals provides a non-invasive method for direct access to the brain from the external environment (Figure 5). Figure 5 illustrates brain accessibility achieved through olfactory administration of nanocrystals which otherwise would be limited by BBB. The nose-to-brain route offers quick and easy administration, reduced side effects, overcomes first-pass hepatic metabolism issues, BBB passage, and quick effects of fast-acting drugs.23 Drugs can travel directly to the brain from the olfactory area of the nasal cavity.23,201 This route also provides access to the spinal cord, lymphatic system, and cerebrospinal fluid.202 Nanocrystal formulations offer notable benefits when administered intranasally, as they enable the deposition of higher drug concentrations and enhance drug absorption, which facilitates rapid deposition in the brain parenchyma, prior to elimination through mucosal clearance or ingestion into the pharynx.203,204 Thus, increasing therapeutic efficiency effectively treats diseases such as Alzheimer’s disease, Parkinson’s disease, and brain tumours. Although intranasally administered nanocrystals hold promise for the treatment of various neurological conditions, there are considerable challenges in getting these formulations into systemic circulation and the human brain.
Intranasal administration often requires the administration of lower doses and offers a rapid onset of action. However, mucociliary clearance is a crucial factor to consider for intranasal drug administration, as it limits drug passage and absorption and decreases the bioavailability of the drug. Innovative strategies, such as using absorption promoters, increasing formulation viscosity, and designing in situ gelling systems, aim to increase drug residence time in the nasal cavity. Incorporating NCs into in situ gelling systems composed of deacetylated gelling gum (DGG) has shown a promising targeting efficiency of 400%.3,180 Viscosity also plays a key role in improving the stability and bioavailability of the formulations. Paeoniflorin NCs, used in the treatment of Parkinson’s disease, have shown enhanced neuroprotection and bioavailability than parent drugs. In-situ gelling systems are widely used because of their ease of administration and drug residence time. Various stabilizers such as TPGS have been utilised in these systems.205
Strategies to Overcome the Limitations of Nanocrystals to Brain
Despite the great potential of nanotechnology and nanocrystals for drug delivery to the brain, toxicity and targeted delivery remain major limitations. Optimising the size and morphology of nanocrystals improves BBB permeability. Rod-shaped nanoparticles have shown higher specificity and seven-fold increase in deposition in the brain.180 Controlled-release strategies can also minimise the off-target effects of the nanocrystals. However, not all nanocrystals have demonstrated effective BBB permeability and bioavailability. For instance, 20 (S) protopanaxadiol even at 91nm particle size has not shown nanocrystals showed enhanced solubility but not significant brain targeting. Incorporation of TPGS stabilizer (physical mixture) enhanced Cmax and AUC to 4.75 and 1.81 times while other additives have not shown any significant changes.206 Hence, modification of the nanocrystals is essential for stabilizing and improving the bioavailability.
Surface modifications: The toxicity of nanocrystals to the brain is a significant concern and requires careful consideration. Surface-modified nanocrystals are a viable strategy for delivering drugs to the brain more effectively, while reducing their toxicity. PEGylation of nanocrystals has been shown to improve circulation time and deposition in the brain owing to their cationic nature.89 They also minimise immune response and reticuloendothelial clearance, thereby reducing the immunogenicity and toxicity of nanocrystal drugs.199 Similarly, nevirapine nanocrystals modified with serum albumin were able to cross the BBB in less than 30 min and maintain adequate levels for up to 24 hours.95 Additionally, surface modification with stabilizers such as polysorbate 20, 40, 60, and 80 has been shown to improve the transport of dalargin to the brain; however, polysorbate 80 shows enhanced delivery to the brain.207 Similarly, another surfactant, SDS, increases the brain uptake of atovaquone nanocrystals when administered orally.208 Studies have shown that SDS and Tween80 coated polymorphic poly L-Lactide (PLLA) and perfluorodecyl acrylate (PFDL) nanoparticles was detected significant amount in brain parenchymal tissue and did not induce glial cell activation nor neuroinflammatory changes.209 Given the challenges of BBB permeation, employing these strategies could improve the bioavailability and drug efficacy.
Targeted delivery: Targeted delivery is a major limitation of nanocrystals in brain delivery which can be attributed to the distribution of drugs to and within the brain. It can be stated that less than 1% of the systemic drug reaches the brain. This could be addressed using targeting ligands that specifically bind to receptors expressed in the BBB and the brain. Targeted ligands utilise receptor-mediated transcytosis to permeate the BBB.210,211 For instance, transferrin receptors overexpressed in BBB endothelial cells can be targeted by transferrin.106,212 Therefore, transferrin conjugation to nanocrystal surfaces can increase its accumulation in the brain and enhance therapeutic potency. Similarly, L57 receptors are abundantly expressed in endothelial cells, and the surface modification of nanocrystals with ligands can help bind these receptors. Various peptide and nanocarriers have been engineered to incorporate these ligands, resulting in improved permeability and targeted penetration across the BBB.22 Modifications of nanocrystals or conjugation with peptides or proteins that can bind to receptors on endothelial cells and encourage internalisation can also be considered viable alternatives to improve permeability and targeted approaches.106 The implementation of nanocrystal modification techniques helps mitigate the non-specific dispersion of nanocrystals, while facilitating enhanced specific delivery to the intended target.213 Several nanocrystal formulations including antipsychotic, anticancer, and neuroprotective agents have been developed to enhance drug delivery to the brain.196 A study showed that folic acid-conjugated cellulose nanocrystals showed target-specific binding to brain tumour cells.104
To enhance specificity, targeted delivery, sustained release, and stability of nanocrystal polymers or liposome encapsulation have also been explored. Paclitaxel-loaded nanocrystals encapsulated in liposomes have shown better outcomes in glioma models in vivo.214,215 In a similar study, paclitaxel nanocrystals were modified with apo-transferrin and hyaluronic acid for targeted delivery.176 It has also been demonstrated that polymeric nanoparticles improve brain transport and distribution of nanocrystals. For instance, siRNA-loaded nanocrystals were delivered to the brain via chitosan nanoparticles, which suppressed target genes in a mouse model of Alzheimer’s disease.179 Several other non-toxic carriers, such as cell-penetrating peptides (CPP), receptor-mediated peptides (RMT), and cell-mediated delivery, have been investigated for nanocrystal conjugation and brain administration.22,201
Applications of Nanocrystals in Brain Delivery
Nanocrystals in brain delivery have shown potential for effectively crossing the BBB and have many applications. However, the full potential of the applicability of nanocrystals remains largely unexplored, highlighting the need for further research on a range of drugs, especially to optimize targeting strategies. Oral administration of nanocrystal drugs like schisantherin, 20 (S)-protopanaxadiol, puerarin has shown improved oral bioavailability and enhanced brain delivery.206,216,217 Similarly, Oral or IV administration of atovaquone coated with apoE peptides enhanced the BBB permeability in murine models.218 IV administration of curcumin nanosuspension coated with tween 80 enhanced brain tissue availability and distribution.219 A simple approach of stabilizing with Tween 80 enhanced the formulation stability and bioactivity. Another study showed combination of TPGS and tween 80 with apoE adsorption on the baicalin nanocrystals resulted in 6.67 times higher deposition in the brain when administered IV, compared to coarse drug formulation.177 Oral and systemically administered nanocrystals overcome size exclusion, with gradual dissolution along their path to the brain.214 The size exclusion phenomenon of nanocrystals helps them overcome biological barriers and reach their intended target. The development of novel nanocrystals with improved pharmacokinetics, safety profiles, and targeting abilities for nanocrystal-based brain drug delivery may be an ideal strategy for the treatment of neuronal disorders. One area of research that is gaining interest is the stimuli-responsive nanocrystals, that deliver the drugs in response to stimuli like changes in temperature, pH and osmolality. Hydrogels are viable option for achieving sustained drug release, improving solubility, and enhancing bioavailability. Magnolol nanocrystals incorporated into thermosensitive PNIPAM gel upon intranasal administration showed improved solubility, prolonged residence time and brain targeting in Parkinson disease models (Table 3).220 Intranasal administration of armodafinil nanocrystal hydrogels showed improved solubility, bioavailability and efficient brain targeting.221 Buccoadhesive chitosan films of aripiprazole nanocrystals were developed to provide rapid drug delivery in schizophrenia patients.222 Several CNS targeting nanocrystal formulations were formulated including risperidone, ziprasidone, donepezil, secretory phospholipase A2 inhibitor, and calpain inhibitor nanocrystals.223,224 Another area of study is the development of targeted nanocrystal formulations for precise delivery of drugs to specific tissues or cells. These nanocrystals improve the efficiency and safety of drug administration by localising to the targeted site of action. For example, targeted nanocrystals have been used for the delivery of diagnostic compounds and anticancer drugs to tumour cells. This method improves the visualization of the brain and tumour vasculature, resulting in better treatment efficacy and reduced toxicity.194,225 Nanocrystals show great potential for imaging applications owing to their unique features. Recent developments in imaging technology have facilitated the real-time monitoring of drug delivery and pharmacokinetics through the utilisation of nanocrystal formulations, enabling their visualization in vivo. Gold nanocrystals have been employed as contrast agents in computed tomography (CT) imaging to facilitate visualization of drug distribution and accumulation in vivo.226,227 The fluorescent amplification of thulium-based cubic-phase downshifting nanocrystals (α-TmNPs) facilitates non-invasive real-time multifunctional imaging of cerebrovascular vasomotion and neutrophil behaviour in ischaemic stroke models.228 Similarly, rare-earth Er-based nanocrystals were engineered to enhance 1550 nm luminescence by down-conversion through Ce3+ doping and inert shell coating optimisation. Non-invasive NIR-IIb imaging can help monitor and visualize cerebrovascular abnormalities for the diagnosis and treatment of cerebral diseases. The surface of Er-based nanocrystals was modified with PMH and PEG for biocompatibility and to diminish fluorescence quenching by water.229
Another exciting area of research with the potential for nanocrystals is targeting nanocrystals for gene therapy. Nanocrystals have been conjugated to minicircle DNA to knock down genes in the liver and for MRI visualization. These multifunctional nanocrystals have shown promising results, with improved transfection ability and sensitivity for visualization.230 Similarly, in another study siRNA-conjugated nanocrystals have shown targeted delivery for the treatment of cancer and infections.231
Surface Modification and Stabilization
Surface modification and stabilisation play a significant role in the development of nanocrystal formulations. The addition of functional groups or polymers to the surface of the particles can enhance the physicochemical features of nanocrystals, including solubility, stability, and specificity.232 For instance, adding PEG to the surface of nanocrystals can increase their biocompatibility and decrease their clearance by the RES.233 The specificity of nanocrystals to targeted regions can also be improved by surface modification with targeted ligands such as antibodies or peptides.234,235
Surfactants attach to the nanocrystal surface and alter the chemistry and characteristics of the surface to increase solubility, bioavailability, and stability and prevent aggregation.19 Furthermore, some surfactants have enhanced drug-carrier interactions and drug-loading ability as well as improved dissolution rate and stability. The incorporation of a small concentration of TPGS surfactant in paclitaxel nanocrystals for lung cancer treatment improved the stability, drug-loading capacity, sustained release, and inhibited P-gp function.190 Hence, the selection of an ideal stabilizer is crucial for nanocrystal formulation. TPGS, polysorbate, and poloxamer stabilizers can also open the tight junctions in the intestine by acting as P-gp inhibitors.19
To protect nanocrystals and avoid aggregation, polymers, such as polyvinylpyrrolidone (PVP) and hydroxypropyl methylcellulose (HPMC), are frequently used as surface stabilizers. The incorporation of surfactants into the formulation can alter the charge of the nanocrystals, depending on the surfactant charge and structure. The addition of non-ionic, anionic, and cationic surfactants to the CNCs did not alter the size or structure of the nanocrystals. However, it improved the drug-loading capacity of the hydrophobic drug paclitaxel to the CNCs.236 The selection of surfactants also depends on their method of administration. Topical administration of hydrophobic surfactants such as oleic acid is known to induce skin irritation, whereas hydrophilic surfactants such as sodium lauryl sulphate show minimal irritation.19,108 Higher concentrations of ionic surfactants improve the loading capacity of insoluble drugs, whereas increasing the concentration of non-ionic solvents decreases the loading capacity. Non-ionic surfactants are preferable in nanocrystal formulations for drug loading because of their lower critical micelle concentrations.236 These surfactants can attach to the nanocrystal surface and lower the surface tension, which enhances stability and solubility.20,237 The stabilizers used in the formulation must meet three basic requirements: i) stable attachment to the surface of the drug, ii) a high percentage of surface coverage on the nanocrystals, and iii) hydrophilic/lipophilic balance of the structure.19
The choice and concentration of an ideal surface modifier are critical in nanocrystal formulations. Inorganic coatings such as silica or gold can help improve drug loading and release, and provide a stable surface for nanocrystals.238 Similarly, organic coatings such as lipids or polymers can provide nanocrystals with a more biocompatible surface and promote cellular uptake.20 The incorporation of chelating agents and stabilizers prevents the oxidisation and reduction of drug molecules.28 Cryoprotectants can also be used as stabilizers in the production process, which shields nanocrystals during freezing and storage and prevents aggregation and loss of therapeutic action.239 Surfactants alter the surface of nanocrystals to ensure their stability and facilitate efficient delivery of drugs to target organs. The choice of stabilizer depends on several factors, including the route of administration and scaled-up production procedures. The stabilizers incorporated in the formulation must sustain shear forces and temperature fluctuations in the production process while protecting the drug from harsh environments. A combination of stabilizers can be used in nanocrystal formulations, and the periodic addition of these stabilizers helps achieve the desired particle size and reduce the viscosity of the formulation.19 Additional care should be taken when selecting stabilizers to minimise interference with the crystalline structure of the drug. The successful selection of a stabilizer or excipient depends on the choice of drug, production procedure, route of administration, etc., and no single excipient works for all pharmaceutical formulations.
Future Directions and Challenges
Nanocrystal formulations are emerging as promising solutions for the delivery and formulation of poorly soluble drugs, specifically BCS Class-II and IV drugs. However, the full potential of the nanocrystals has not been fully established because of the paucity of clinical studies, regulatory guidelines, and safety profiles of these drugs. Experimental evidence is required to primarily focus on determining the safety, effectiveness, and conducting clinical studies. Nanocrystals may exhibit increased toxicity and immunogenicity compared with conventional API owing to their altered physicochemical properties and reduced size.240 Owing to their distinct behaviour and course of action, nanocrystal formulations require FDA approval, even if the same drug is commercially available.
Regulatory standards can vary depending on the route of administration and intended application. Several challenges with respect to nanocrystals for the desired administrative routes and pathological conditions are discussed in this paper. Hence, optimising nanocrystals to the desired route and addressing the challenges associated with the production and scale-up processes are required. In addition to toxicity, the biocompatibility and stability of nanocrystals have also challenged the development of nanocrystal formulations. As discussed in the previous sections, the incorporation of biocompatible excipients and surface modifications tailored to specific routes and conditions can improve the stability, toxicity, and biocompatibility of biological fluids.19 However, for nanocrystal formulations to be successful in pharmaceutical settings, standard production stability, optimal particle size, cost-effectiveness, and scalable production techniques are ideal. Despite these challenges, researchers and manufacturers have focused on the potential benefits of the nanocrystal formulations.
Furthermore, nanocrystal research has primarily focused on achieving the target specificity and deposition. Several studies have demonstrated the ability of nanocrystals to reach the target site with minimal surface modifications and changes in morphology and size. Multifunctional nanocrystals that exhibit both therapeutic efficiency and target specificity have also been developed.241 Similarly, ongoing research is exploring the conjugation abilities of nanocrystals with other drugs and carrier molecules to improve therapeutic efficiency.65 To demonstrate the efficiency of nanocrystal formulation, safety in diverse disease conditions and novel combination techniques must be developed and evaluated in clinical studies.
Conclusion
Nanocrystal formulations have shown promise in overcoming the physiological barriers encountered while administrating traditional (non-nanocrystalline) drug delivery, which restrict the optimal bioavailability and efficacy. Owing to their unique physicochemical characteristics, nanocrystals are well-suited for enhancing drug solubility, improving bioavailability, and enabling sustained release. Furthermore, extensive research is being conducted to confirm the biocompatibility and reduce toxicity of nanocrystals. Nanocrystal-based formulations have been developed and assessed for various delivery methods and under various disease conditions. However, the efficacy and safety of these formulations rely on various factors, including the physicochemical properties of the nanocrystals and the formulation ingredients, excipients, and surfactants. A major challenge of nanocrystals comes from aggregation and non-specificity. While solutions have been proposed in this review to address these issues, further research is necessary to optimize their clinical potential. Future studies should focus on integrating advanced targeting strategies and long-term safety of nanocrystal formulations. In conclusion, nanocrystal formulations enable the administration of drugs that were previously insoluble or moderately soluble. Specifically, drugs belong to BCS-II and IV drugs suitable candidates for nanocrystal formulations for various routes of administration.
Disclaimer
This review reflects the views of the authors and does not necessarily reflect those of the US Food and Drug Administration. Any mention of a commercial product is for clarification only, it is not an endorsement for the use of it.
Acknowledgments
The authors thank Soumana Daddy-Gaoh, Dr. Ahn YoungBeom (Division of Microbiology, NCTR), and Goutam Palui (Nanocore, NCTR) for reviewing this document and for providing valuable comments and suggestions. We would also like to thank the Division of Microbiology at NCTR.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–430. doi:10.1016/j.jconrel.2012.03.013
2. Haddad R, Alrabadi N, Altaani B, Li T. Paclitaxel drug delivery systems: focus on nanocrystals’ surface modifications. Polymers. 2022;14(4):658. doi:10.3390/polym14040658
3. Zingale E, Bonaccorso A, Carbone C, Musumeci T, Pignatello R. Drug nanocrystals: focus on brain delivery from therapeutic to diagnostic applications. Pharmaceutics. 2022;14(4):691. doi:10.3390/pharmaceutics14040691
4. Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66. doi:10.1016/j.jconrel.2014.03.030
5. Wang ZH, Chu M, Yin N, et al. Biological Chemotaxis-Guided Self-Thermophoretic Nanoplatform Augments Colorectal Cancer Therapy Through Autonomous Mucus Penetration. Science Advances. 2022. 826. doi: 10.1126/sciadv.abn3917
6. Lu Y, Qi J, Dong X, Zhao W, Wu W. The in vivo fate of nanocrystals. Drug Discovery Today. 2017;22(4):744–750. doi:10.1016/j.drudis.2017.01.003
7. Ganta S, Paxton JW, Baguley BC, Garg S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int J Pharm. 2009;367(1):179–186. doi:10.1016/j.ijpharm.2008.09.022
8. McGuckin MB, Wang J, Ghanma R, et al. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J Control Release. 2022;345:334–353. doi:10.1016/j.jconrel.2022.03.012
9. Meng Z, Wang H, Fang X, et al. Surface decoration via physical interaction of cupric diethyldithiocarbamate nanocrystals and its impact on biodistribution and tumor targeting. ACS Appl Mater Interfaces. 2021;13(31):36894–36908. doi:10.1021/acsami.1c09346
10. Eyley S, Thielemans W. Surface modification of cellulose nanocrystals. Nanoscale. 2014;6(14):7764–7779. doi:10.1039/C4NR01756K
11. Bangar SP, Harussani MM, Ilyas RA, et al. Surface modifications of cellulose nanocrystals: processes, properties, and applications. Food Hydrocoll. 2022;130:107689. doi:10.1016/j.foodhyd.2022.107689
12. Xiong W, Sang W, Linghu KG, et al. Dual-functional Brij-S20-modified nanocrystal formulation enhances the intestinal transport and oral bioavailability of berberine. Int J Nanomed. 2018;13:3781–3793. published Online First: 20180628. doi:10.2147/ijn.S163763
13. Lu L, Xu Q, Wang J, Wu S, Luo Z, Lu W. Drug nanocrystals for active tumor-targeted drug delivery. Pharmaceutics. 2022;14(4). doi:10.3390/pharmaceutics14040797
14. Salazar J, Ghanem A, Müller RH, Möschwitzer JP. Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur J Pharm Biopharm. 2012;81(1):82–90. doi:10.1016/j.ejpb.2011.12.015
15. de Waard H, Hinrichs WLJ, Frijlink HW. A novel bottom–up process to produce drug nanocrystals: controlled crystallization during freeze-drying. J Control Release. 2008;128(2):179–183. doi:10.1016/j.jconrel.2008.03.002
16. Couillaud BM, Espeau P, Mignet N, Corvis Y. State of the art of pharmaceutical solid forms: from crystal property issues to nanocrystals formulation. ChemMedChem. 2019;14(1):8–23. doi:10.1002/cmdc.201800612
17. Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–141. doi:10.1016/j.ijpharm.2013.01.019
18. Kwon SG, Hyeon T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small. 2011;7(19):2685–2702. published Online First: 20110801. doi:10.1002/smll.201002022
19. Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2):16. published Online First: 20160520. doi:10.3390/pharmaceutics8020016
20. Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–1579. doi:10.1111/j.2042-7158.2010.01022.x
21. Stalmans S, Bracke N, Wynendaele E, et al. Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLoS One. 2015;10(10):e0139652. published Online First: 20151014. doi:10.1371/journal.pone.0139652
22. Rodrigues JP, Prajapati N, DeCoster MA, Poh S, Murray TA. Efficient LRP1-mediated uptake and low cytotoxicity of peptide L57 in vitro shows its promise as CNS drug delivery vector. J Pharm Sci. 2021;110(2):824–832. published Online First: 20201014. doi:10.1016/j.xphs.2020.09.019
23. Yanamadala Y, Saleh MY, Williams AA, Lvov Y, Murray TA. Clay nanotubes loaded with diazepam or xylazine permeate the brain through intranasal administration in mice. Int J Mol Sci. 2023;24(11):9648. doi:10.3390/ijms24119648
24. Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Italian J Pediatr. 2018;44(2):131. doi:10.1186/s13052-018-0563-0
25. Rowlands BJ, Soong CV, Gardiner KR. The gastrointestinal tract as a barrier in sepsis. Br Med Bul. 1999;55(1):196–211. doi:10.1258/0007142991902213
26. Liu S, Wen X, Zhang X, Mao S. Oral delivery of biomacromolecules by overcoming biological barriers in the gastrointestinal tract: an update. Expert Opin Drug Delivery. 2023;1–15. doi:10.1080/17425247.2023.2231343
27. Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549. published Online First: 20140320. doi:10.1155/2014/180549
28. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. published Online First: 20090908. doi:10.1016/j.colsurfb.2009.09.001
29. Puntes VF, Krishnan KM, Alivisatos AP. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 2001;291(5511):2115–2117. doi:10.1126/science.1058495
30. Klepach A, Tran H, Ahmad Mohammed F, ElSayed MEH. Characterization and impact of peptide physicochemical properties on oral and subcutaneous delivery. Adv Drug Deliv Rev. 2022;186(114322):114322. published Online First: 20220506. doi:10.1016/j.addr.2022.114322
31. Jiang L, Sun Y, Lu A, Wang X, Shi Y. Ionic liquids: promising approach for oral drug delivery. Pharm Res. 2022;39(10):2353–2365. doi:10.1007/s11095-022-03260-8
32. Elsayed A, Al-Remawi M, Jaber N, Abu-Salah KM. Advances in buccal and oral delivery of insulin. Int J Pharm. 2023;633:122623. doi:10.1016/j.ijpharm.2023.122623
33. Haddadzadegan S, Dorkoosh F, Bernkop-Schnürch A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Delivery Rev. 2022;182:114097. doi:10.1016/j.addr.2021.114097
34. Chen X, Zhang W. Diamond nanostructures for drug delivery, bioimaging, and biosensing. Chem Soc Rev. 2017;46(3):734–760. doi:10.1039/c6cs00109b
35. Chiappini C, De Rosa E, Martinez JO, et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat Mater. 2015;14(5):532–539. published Online First: 20150330. doi:10.1038/nmat4249
36. Khare S, Jog R, Bright A, Burgess DJ, Chakder SK, Gokulan K. Evaluation of mucosal immune profile associated with Zileuton nanocrystal-formulated BCS-II drug upon oral administration in Sprague Dawley rats. Nanotoxicology. 2023;17(10):583–603. doi:10.1080/17435390.2023.2289940
37. Tian Z, Mai Y, Meng T, Ma S, Gou G, Yang J. Nanocrystals for improving oral bioavailability of drugs: intestinal transport mechanisms and influencing factors. AAPS Pharm Sci Tech. 2021;22(5):179. doi:10.1208/s12249-021-02041-7
38. Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Effects of absorption enhancers on the transport of model compounds in Caco-2 cell monolayers: assessment by confocal laser scanning microscopy. J Pharmaceut Sci. 1997;86(7):779–785. doi:10.1021/js960529n
39. Kuhn DA, Vanhecke D, Michen B, et al. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol. 2014;5:1625–1636. published Online First: 20140924. doi:10.3762/bjnano.5.174
40. Des Rieux A, Fievez V, Théate I, Mast J, Préat V, Schneider Y-J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30(5):380–391. doi:10.1016/j.ejps.2006.12.006
41. Zhou Y, Du J, Wang L, Wang Y. Nanocrystals technology for improving bioavailability of poorly soluble drugs: a mini-review. J Nanosci Nanotechnol. 2017;17(1):18–28. doi:10.1166/jnn.2017.13108
42. Koradia KD, Sheth NR, Koradia HD, Dabhi MR. Ziprasidone nanocrystals by wet media milling followed by spray drying and lyophilization: formulation and process parameter optimization. J Drug Delivery Sci Technol. 2018;43:73–84. doi:10.1016/j.jddst.2017.09.011
43. Holmes MV, Hunt BJ, Shearer MJ. The role of dietary vitamin K in the management of oral vitamin K antagonists. Blood Rev. 2012;26(1):1–14. published Online First: 20110913. doi:10.1016/j.blre.2011.07.002
44. Zhao W, Ruan B, Sun X, Yu Z. Preparation and optimization of surface stabilized cryptotanshinone nanocrystals with enhanced bioavailability. Front Pharmacol. 2023;14:1122071. doi:10.3389/fphar.2023.1122071
45. Sharma S, Verma A, Pandey G, Mittapelly N, Mishra PR. Investigating the role of Pluronic-g-Cationic polyelectrolyte as functional stabilizer for nanocrystals: impact on paclitaxel oral bioavailability and tumor growth. Acta Biomater. 2015;26:169–183. doi:10.1016/j.actbio.2015.08.005
46. Ndlovu ST, Ullah N, Khan S, et al. Domperidone nanocrystals with boosted oral bioavailability: fabrication, evaluation and molecular insight into the polymer-domperidone nanocrystal interaction. Drug Delivery Transl Res. 2019;9(1):284–297. doi:10.1007/s13346-018-00596-w
47. Nolte TM, Lu B. Size and dose of nanoparticles modulate between toxic and medicinal effect on kidney. OpenNano. 2024;16. doi:10.1016/j.onano.2024.100200
48. Tian Z, Zhao Y, Mai Y, et al. Nanocrystals with different stabilizers overcome the mucus and epithelial barriers for oral delivery of multicomponent Bufadienolides. Int J Pharm. 2022;616:121522. published Online First: 20220129. doi:10.1016/j.ijpharm.2022.121522
49. Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. Aaps j. 2009;11(4):740–746. published Online First: 20091030. doi:10.1208/s12248-009-9144-x
50. Kesisoglou F, Panmai S, Wu Y. Nanosizing — oral formulation development and biopharmaceutical evaluation. Adv Drug Delivery Rev. 2007;59(7):631–644. doi:10.1016/j.addr.2007.05.003
51. Kontogiannis O, Selianitis D, Lagopati N, Pippa N, Pispas S, Gazouli M. Surfactant and block copolymer nanostructures: from design and development to nanomedicine preclinical studieS. Pharmaceutics. 2023;15(2):501. doi:10.3390/pharmaceutics15020501
52. Vuddanda PR, Montenegro-Nicolini M, Morales JO, Velaga S. Effect of surfactants and drug load on physico-mechanical and dissolution properties of nanocrystalline tadalafil-loaded oral films. Eur J Pharm Sci. 2017;109:372–380. doi:10.1016/j.ejps.2017.08.019
53. Di Gregorio MC, Travaglini L, Del Giudice A, Cautela J, Pavel NV, Galantini L. Bile salts: natural surfactants and precursors of a broad family of complex amphiphiles. Langmuir. 2019;35(21):6803–6821. doi:10.1021/acs.langmuir.8b02657
54. Konno H, Taylor LS. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J Pharm Sci. 2006;95(12):2692–2705. doi:10.1002/jps.20697
55. Kim HJ, Yoon KA, Hahn M, Park ES, Chi SC. Preparation and in vitro evaluation of self-microemulsifying drug delivery systems containing idebenone. Drug Dev Ind Pharm. 2000;26(5):523–529. doi:10.1081/ddc-100101263
56. Liu C, Lv L, Guo W, et al. Self-nanoemulsifying drug delivery system of tetrandrine for improved bioavailability: physicochemical characterization and pharmacokinetic study. Biomed Res Int. 2018;2018:6763057. published Online First: 20180927. doi:10.1155/2018/6763057
57. Sheshala R, Khan N, Darwis Y. Formulation and optimization of orally disintegrating tablets of sumatriptan succinate. Chem Pharm Bull. 2011;59(8):920–928. doi:10.1248/cpb.59.920
58. J-n Y, Zhu Y, Wang L, et al. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin. 2010;31(6):759–764. doi:10.1038/aps.2010.55
59. Kevadiya BD, Barvaliya M, Zhang L, et al. Fenofibrate nanocrystals embedded in oral strip-films for bioavailability enhancement. Bioengineering. 2018;5(1). published Online First: 20180213. doi:10.3390/bioengineering5010016
60. Junghanns JU, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295–309. doi:10.2147/ijn.s595
61. He Y, D-n X, Q-x L, J-s T, Gan Y, Wang C. Enhancement of cellular uptake, transport and oral absorption of protease inhibitor saquinavir by nanocrystal formulation. Acta Pharmacol Sin. 2015;36(9):1151–1160. doi:10.1038/aps.2015.53
62. Jain S, Patel K, Arora S, Reddy VA, Dora CP. Formulation, optimization, and in vitro-in vivo evaluation of olmesartan medoxomil nanocrystals. Drug Deliv Transl Res. 2017;7(2):292–303. doi:10.1007/s13346-016-0355-2
63. Gao L, Zhang D, Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res. 2008;10(5):845–862. doi:10.1007/s11051-008-9357-4
64. Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16(7–8):354–360. published Online First: 20100303. doi:10.1016/j.drudis.2010.02.009
65. Li T, Cipolla D, Rades T, Boyd BJ. Drug nanocrystallisation within liposomes. J Control Release. 2018;288:96–110. doi:10.1016/j.jconrel.2018.09.001
66. Aoyagi N, Ogata H, Kaniwa N, et al. Bioavailability of griseofulvin from tablets in beagle dogs and correlation with dissolution rate and bioavailability in humans. J Pharm Sci. 1982;71(10):1169–1172. doi:10.1002/jps.2600711023
67. Kahan BD, Koch SM. Current immunosuppressant regimens: considerations for critical care. Curr Opin Crit Care. 2001;7(4):242–250. doi:10.1097/00075198-200108000-00006
68. Balch RJ, Trescot A. Extended-release morphine sulfate in treatment of severe acute and chronic pain. J Pain Res. 2010;3:191–200. published Online First: 20100921. doi:10.2147/JPR.S6529
69. Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23(10):1281–1299. doi:10.1592/phco.23.12.1281.32697
70. Shayani S, Palmer JM, Stiller T, et al. Aprepitant (Emend) significantly increases sirolimus levels in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012;47(2):291–293. published Online First: 20110307. doi:10.1038/bmt.2011.42
71. Shegokar R, Muller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399(1–2):129–139. published Online First: 20100730. doi:10.1016/j.ijpharm.2010.07.044
72. Tziomalos K, Athyros VG. Fenofibrate: a novel formulation (Triglide) in the treatment of lipid disorders: a review. Int J Nanomed. 2006;1(2):129–147. doi:10.2147/nano.2006.1.2.129
73. Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomed. 2009;4:185–192. published Online First: 20090910. doi:10.2147/ijn.s6308
74. C VP. Chapter 4—Nanobased Nano Drug Delivery: A Comprehensive Review. Amsterdam, The Netherlands: Elsevier; 2019.
75. Pharmacological management of persistent pain in older persons. Pain Med. 2009;10(6):1062–1083. doi:10.1111/j.1526-4637.2009.00699.x
76. Chue PC, Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12(12):1383–1397. doi:10.1586/ern.12.137
77. Chang C-LH. Improving of cognition and quality of life in schizophrenia with one-month and three-month paliperidone palmitate treatment. Res Sq. 2021;2021:1–16.
78. Preda A, Shapiro BB. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin Drug Saf. 2020;19(12):1529–1538. published Online First: 20201016. doi:10.1080/14740338.2020.1832990
79. Blair HAJD. Perspectives, T. Paliperidone palmitate intramuscular 6-monthly formulation in schizophrenia: a profile of its use. Drug Ther Perspect. 2022;28:335–342. doi:10.1007/s40267-022-00931-9
80. Pelikh O, Keck CM. Hair Follicle Targeting and Dermal Drug Delivery with Curcumin Drug Nanocrystals-Essential Influence of Excipients. Nanomaterials. 2020;10(11):2323. published Online First: 20201123. doi:10.3390/nano10112323
81. Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19. doi:10.1016/s0169-409x(00)00118-6
82. Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi:10.1023/a:1016212804288
83. Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23. doi:10.1016/j.ajps.2014.08.005
84. Wong J, Brugger A, Khare A, et al. Suspensions for intravenous (IV) injection: a review of development, preclinical and clinical aspects. Adv Drug Delivery Rev. 2008;60(8):939–954. doi:10.1016/j.addr.2007.11.008
85. Vinarov Z, Abdallah M, Agundez JAG, et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: an UNGAP review. Eur J Pharm Sci. 2021;162:105812. published Online First: 20210320. doi:10.1016/j.ejps.2021.105812
86. Tomic I, Miocic S, Pepic I, Simic D, Filipovic-Grcic J. Efficacy and safety of azelaic acid nanocrystal-loaded in situ hydrogel in the treatment of acne vulgaris. Pharmaceutics. 2021;13(4). published Online First: 20210416. doi:10.3390/pharmaceutics13040567
87. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852.
88. Ahire E, Thakkar S, Darshanwad M, Misra M. Parenteral nanosuspensions: a brief review from solubility enhancement to more novel and specific applications. Acta Pharmaceutica Sinica B. 2018;8(5):733–755. doi:10.1016/j.apsb.2018.07.011
89. Bi C, Miao XQ, Chow SF, et al. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine. 2017;13(3):943–953. doi:10.1016/j.nano.2016.11.004
90. Patel D, Zode SS, Bansal AK. Formulation aspects of intravenous nanosuspensions. Int J Pharm. 2020;586:119555. doi:10.1016/j.ijpharm.2020.119555
91. Reed KW, Yalkowsky SH. Lysis of human red blood cells in the presence of various cosolvents. II. The effect of differing NaCl concentrations. J Parenter Sci Technol. 1986;40(3):88–94.
92. Van Eerdenbrugh B, Vermant J, Martens JA, et al. A screening study of surface stabilization during the production of drug nanocrystals. J Pharmaceut Sci. 2009;98(6):2091–2103. doi:10.1002/jps.21563
93. Xu L-M, Zhang Q-X, Zhou Y, Zhao H, Wang J-X, Chen J-F. Engineering drug ultrafine particles of beclomethasone dipropionate for dry powder inhalation. Int J Pharm. 2012;436(1):1–9. doi:10.1016/j.ijpharm.2012.06.038
94. Schöler N, Olbrich C, Tabatt K, Müller RH, Hahn H, Liesenfeld O. Surfactant, but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophages. Int J Pharm. 2001;221(1):57–67. doi:10.1016/S0378-5173(01)00660-3
95. Shegokar R, Singh KK. Surface modified nevirapine nanosuspensions for viral reservoir targeting: in vitro and in vivo evaluation. Int J Pharm. 2011;421(2):341–352. doi:10.1016/j.ijpharm.2011.09.041
96. Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S1–7. doi:10.12980/apjtb.4.2014c980
97. Gupta A, Aggarwal G, Singla S, Arora R. Transfersomes: a novel vesicular carrier for enhanced transdermal delivery of sertraline: development, characterization, and performance evaluation. Sci Pharm. 2012;80(4):1061–1080. published Online First: 20120831. doi:10.3797/scipharm.1208-02
98. Subongkot T. Development and mechanistic study of a microemulsion containing vitamin E TPGS for the enhancement of oral absorption of celecoxib. Int J Nanomed. 2019;14:3087–3102. published Online First: 20190430. doi:10.2147/ijn.S201449
99. Tao W, Zeng X, Liu T, et al. Docetaxel-loaded nanoparticles based on star-shaped mannitol-core PLGA-TPGS diblock copolymer for breast cancer therapy. Acta Biomater. 2013;9(11):8910–8920. published Online First: 20130628. doi:10.1016/j.actbio.2013.06.034
100. Li J, Cai C, Li J, et al. Chitosan-Based Nanomaterials for Drug Delivery. Molecules. 2018;23(10). published Online First: 20181016. doi:10.3390/molecules23102661
101. Wang J, Mao W, Lock LL, et al. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano. 2015;9(7):7195–7206. published Online First: 20150715. doi:10.1021/acsnano.5b02017
102. Atia MM, Abdel-Tawab HS, Mostafa AM, Mobarak SA. Nanocurcumin and curcumin prevent N, N’-methylenebisacrylamide-induced liver damage and promotion of hepatic cancer cell growth. Sci Rep. 2022;12(1):8319. doi:10.1038/s41598-022-12406-y
103. Lee SE, Bairstow SF, Werling JO, et al. Paclitaxel nanosuspensions for targeted chemotherapy – nanosuspension preparation, characterization, and use. Pharmaceutical Development and Technology. 2014;19(4):438–453. doi:10.3109/10837450.2013.789911
104. Dong S, Cho HJ, Lee YW, Roman M. Synthesis and Cellular Uptake of Folic Acid-Conjugated Cellulose Nanocrystals for Cancer Targeting. Biomacromolecules. 2014;15(5):1560–1567. doi:10.1021/bm401593n
105. Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6(2):106–113. published Online First: 20160111. doi:10.1016/j.apsb.2015.11.005
106. Ghilzai NMK. Therapeutic peptides and proteins: formulation, processing, and delivery systems. Am J Pharm Educ. 2006;70(4):94.
107. Chaulagain B, Jain A, Tiwari A, Verma A, Jain SK. Passive delivery of protein drugs through transdermal route. Artif Cells Nanomed Biotechnol. 2018;46(sup1):472–487. published Online First: 20180129. doi:10.1080/21691401.2018.1430695
108. Ostróżka-Cieślik A. the potential of pharmaceutical hydrogels in the formulation of topical administration hormone drugs. Polymers. 2022;14(16):3307. published Online First: 20220814. doi:10.3390/polym14163307
109. Mortazavi SM, Moghimi HR. Skin permeability, a dismissed necessity for anti-wrinkle peptide performance. Int J Cosmet Sci. 2022;44(2):232–248. published Online First: 20220428. doi:10.1111/ics.12770
110. Sun L, Xiang H, Ge C, et al. A nanocrystals-based topical drug delivery system with improved dermal penetration and enhanced treatment of skin diseases. J Biomed Nanotechnol. 2021;17(12):2319–2337. doi:10.1166/jbn.2021.3202
111. Parmar PK, Wadhawan J, Bansal AK. Pharmaceutical nanocrystals: a promising approach for improved topical drug delivery. Drug Discov Today. 2021;26(10):2329–2349. published Online First: 20210713. doi:10.1016/j.drudis.2021.07.010
112. Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–470. published Online First: 20151022. doi:10.3390/pharmaceutics7040438
113. Benson HAE, Grice JE, Mohammed Y, Namjoshi S, Roberts MS. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr Drug Deliv. 2019;16(5):444–460. doi:10.2174/1567201816666190201143457
114. Pant N, Wairkar S. Topical nanocrystals of bioflavonoids: a new technology platform for skin ailments. Int J Pharm. 2022;619:121707. published Online First: 20220329. doi:10.1016/j.ijpharm.2022.121707
115. Lohan SB, Saeidpour S, Colombo M, et al. Nanocrystals for improved drug delivery of dexamethasone in skin investigated by EPR spectroscopy. Pharmaceutics. 2020;12(5):400. published Online First: 20200427. doi:10.3390/pharmaceutics12050400
116. Patel V, Sharma OP, Mehta T. Nanocrystal: a novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin Drug Deliv. 2018;15(4):351–368. published Online First: 20180301. doi:10.1080/17425247.2018.1444025
117. Pelikh O, Stahr PL, Huang J, et al. Nanocrystals for improved dermal drug delivery. Eur J Pharm Biopharm. 2018;128:170–178. published Online First: 20180420. doi:10.1016/j.ejpb.2018.04.020
118. Karakucuk A, Tort S. Preparation, characterization and antimicrobial activity evaluation of electrospun PCL nanofiber composites of resveratrol nanocrystals. Pharm Dev Technol. 2020;25(10):1216–1225. published Online First: 20200810. doi:10.1080/10837450.2020.1805761
119. Kaur A, Parmar PK, Jadhav S, Bansal AK. Chapter 12 - advances in nanocrystals as drug delivery systems. In: Kesharwani P, Singh KK, editors. Nanoparticle Therapeutics. Academic Press; 2022:413–454.
120. Parmar PK, Bansal AK. Novel nanocrystal-based formulations of apremilast for improved topical delivery. Drug Deliv Transl Res. 2021;11(3):966–983. doi:10.1007/s13346-020-00809-1
121. Kalhapure RS, Palekar S, Patel K, Monpara J. Nanocrystals for controlled delivery: state of the art and approved drug products. Expert Opin Drug Deliv. 2022;19(10):1303–1316. published Online First: 20220808. doi:10.1080/17425247.2022.2110579
122. Atia NM, Hazzah HA, Gaafar PME, Abdallah OY. Diosmin nanocrystal-loaded wafers for treatment of diabetic ulcer: in vitro and in vivo evaluation. J Pharm Sci. 2019;108(5):1857–1871. published Online First: 20181230. doi:10.1016/j.xphs.2018.12.019
123. Williams AC, Barry BW. Penetration enhancers. Adv Drug Delivery Rev. 2012;64:128–137. doi:10.1016/j.addr.2012.09.032
124. Liu X, Shen B, Shen C, Zhong R, Wang X, Yuan H. Nanoparticle-loaded gels for topical delivery of nitrofurazone: effect of particle size on skin permeation and retention. J Drug Delivery Sci Technol. 2018;45:367–372. doi:10.1016/j.jddst.2018.04.005
125. Chourasia MK, Kang L, Chan SY. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011;1(1):60–67. published Online First: 20111013. doi:10.1016/j.rinphs.2011.10.002
126. Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv. 2014;5(3):337–365. doi:10.4155/tde.14.5
127. Scheuplein RJ. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol. 1967;48(1):79–88. doi:10.1038/jid.1967.11
128. Imam SS, Ahad A, Aqil M, Akhtar M, Sultana Y, Ali A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: in-vitro characterization, and in-vivo appraisal. Mater Sci Eng C Mater Biol Appl. 2017;75:1198–1205. published Online First: 20170227. doi:10.1016/j.msec.2017.02.149
129. Halfter N, Espinosa-Cano E, Pontes-Quero GM, et al. Ketoprofen-based polymer-drug nanoparticles provide anti-inflammatory properties to HA/Collagen Hydrogels. J Funct Biomater. 2023;14(3):160. published Online First: 20230317. doi:10.3390/jfb14030160
130. Rençber S, Karavana SY, Özyazici M. Bioavailability File: KETOPROFEN. FABAD J Pharm Sci. 2012. doi:10.2147/IJN.S36883
131. Nagai N, Iwamae A, Tanimoto S, Yoshioka C, Ito Y. Pharmacokinetics and antiinflammatory effect of a novel gel system containing ketoprofen solid nanoparticles. Biol Pharm Bull. 2015;38(12):1918–1924. doi:10.1248/bpb.b15-00567
132. Lai F, Schlich M, Pireddu R, Corrias F, Fadda AM, Sinico C. Production of nanosuspensions as a tool to improve drug bioavailability: focus on topical delivery. Curr Pharm Des. 2015;21(42):6089–6103. doi:10.2174/1381612821666151027152350
133. Haroutiunian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–549. doi:10.1111/j.1526-4637.2010.00809.x
134. Vergote GJ, Vervaet C, Van Driessche I, et al. An oral controlled release matrix pellet formulation containing nanocrystalline ketoprofen. Int J Pharm. 2001;219(1–2):81–87. doi:10.1016/s0378-5173(01)00628-7
135. Santos AC, Pereira I, Reis S, Veiga F, Saleh M, Lvov Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin Drug Delivery. 2019;16(11):1169–1182. doi:10.1080/17425247.2019.1665020
136. Sharma OP, Patel V, Mehta T. Nanocrystal for ocular drug delivery: hope or hype. Drug Deliv Transl Res. 2016;6(4):399–413. doi:10.1007/s13346-016-0292-0
137. X-j Y, Wang Y, Fu-Shin XY. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Visual Sci. 2000;41(13):4093–4100.
138. Grass GM, Robinson JR. Mechanisms of corneal drug penetration I: in vivo and in vitro kinetics. J Pharmaceut Sci. 1988;77(1):3–14. doi:10.1002/jps.2600770103
139. Yang B, Li G, Liu J, et al. Nanotechnology for age-related macular degeneration. Pharmaceutics. 2021;13(12):2035. doi:10.3390/pharmaceutics13122035
140. Tuomela A, Liu P, Puranen J, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1):34–41. doi:10.1016/j.ijpharm.2014.03.048
141. El-Gendy MA, Mansour M, El-Assal MIA, Ishak RAH, Mortada ND. Delineating penetration enhancer-enriched liquid crystalline nanostructures as novel platforms for improved ophthalmic delivery. Int J Pharm. 2020;582:119313. doi:10.1016/j.ijpharm.2020.119313
142. Formica ML, Awde Alfonso HG, Paredes AJ, et al. Development of triamcinolone acetonide nanocrystals for ocular administration. Pharmaceutics. 2023;15(2):683. doi:10.3390/pharmaceutics15020683
143. Kalam MA, Iqbal M, Alshememry A, Alkholief M, Alshamsan A. Fabrication and characterization of tedizolid phosphate nanocrystals for topical ocular application: improved solubilization and in vitro drug release. Pharmaceutics. 2022;14(7):1328. published Online First: 20220623. doi:10.3390/pharmaceutics14071328
144. Donia M, Osman R, Awad GAS, Mortada N. Polypeptide and glycosaminoglycan polysaccharide as stabilizing polymers in nanocrystals for a safe ocular hypotensive effect. International Journal of Biological Macromolecules. 2020;162:1699–1710. doi:10.1016/j.ijbiomac.2020.07.306
145. Terreni E, Zucchetti E, Tampucci S, Burgalassi S, Monti D, Chetoni P. Combination of nanomicellar technology and in situ gelling polymer as ocular drug delivery system (ODDS) for Cyclosporine-A. Pharmaceutics. 2021;13(2):192. published Online First: 20210201. doi:10.3390/pharmaceutics13020192
146. Permana AD, Utami RN, Layadi P, et al. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int J Pharm. 2021;602:120623. published Online First: 20210421. doi:10.1016/j.ijpharm.2021.120623
147. Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discovery Today. 2018;23(3):534–547. doi:10.1016/j.drudis.2018.01.016
148. Markham A. Cabotegravir Plus Rilpivirine: First Approval. Drugs. 2020;80(9):915–922. doi:10.1007/s40265-020-01326-8
149. Sun W, Gao J, Fan R, et al. The effect of particle size on the absorption of cyclosporin A nanosuspensions. Int j Nanomed. 2022;17:1741–1755. doi:10.2147/IJN.S357541
150. Cheng X, Han X, Si J, et al. Cationic curcumin nanocrystals liposomes for improved oral bioavailability: formulation development, optimization. In Vitro and in vivo Eval Pharm. 2024;16(9):1155.
151. Sun J, Wang F, Sui Y, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int j Nanomed. 2012;7(null):5733–5744. doi:10.2147/IJN.S34365
152. Ma Y, Yang X, Chen G, Zhang Y, Zhang H, Zhang W. Effect of particle size on the oral absorption of isoliquiritigenin nanocrystals. Braz J Pharm Sci. 2022;58:e201186. doi:10.1590/s2175-97902022e201186
153. Bhairam M, Pandey RK, Shukla SS, Gidwani B. Preparation, optimization, and evaluation of dolutegravir nanosuspension: in vitro and in vivo characterization. J Pharm Innovation. 2023;18(4):1798–1811. doi:10.1007/s12247-023-09756-z
154. Yang W, Peters JI, Williams RO. Inhaled nanoparticles--a current review. Int J Pharm. 2008;356(1–2):239–247. published Online First: 20080216. doi:10.1016/j.ijpharm.2008.02.011
155. Bakhaidar RB, Naveen NR, Basim P, et al. Response surface methodology (RSM) powered formulation development, optimization and evaluation of thiolated based mucoadhesive nanocrystals for local delivery of simvastatin. Polymers. 2022;14(23):5184. doi:10.3390/polym14235184
156. Choi J-S, Park J-S. Development of docetaxel nanocrystals surface modified with transferrin for tumor targeting. Drug Des Devel Ther. 2016;11:17–26. doi:10.2147/DDDT.S122984
157. Hill A, Geißler S, Weigandt M, Mäder K. Controlled delivery of nanosuspensions from osmotic pumps: zero order and non-zero order kinetics. J Control Release. 2012;158(3):403–412. doi:10.1016/j.jconrel.2011.12.005
158. Peters K, Leitzke S, Diederichs JE, et al. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother. 2000;45(1):77–83. doi:10.1093/jac/45.1.77
159. Sigfridsson K, Skantze P, Skantze U, et al. Nanocrystal formulations of a poorly soluble drug. 2. Evaluation of nanocrystal liver uptake and distribution after intravenous administration to mice. Int J Pharm. 2017;524(1):248–256. doi:10.1016/j.ijpharm.2017.03.062
160. Wang Y, Ma Y, Ma Y, et al. Formulation and pharmacokinetics evaluation of puerarin nanocrystals for intravenous delivery. J Nanosci Nanotechnol. 2012;12(8):6176–6184. doi:10.1166/jnn.2012.6436
161. Permana AD, Paredes AJ, Volpe-Zanutto F, Anjani QK, Utomo E, Donnelly RF. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur J Pharm Biopharm. 2020;154:50–61. doi:10.1016/j.ejpb.2020.06.025
162. Kotian V, Koland M, Mutalik S. Nanocrystal-based topical gels for improving wound healing efficacy of curcumin. Crystals. 2022;12(11):1565. doi:10.3390/cryst12111565
163. Yu Q, Wu X, Zhu Q, et al. Enhanced transdermal delivery of meloxicam by nanocrystals: preparation, in vitro and in vivo evaluation. Asian J Pharm Sci. 2018;13(6):518–526. doi:10.1016/j.ajps.2017.10.004
164. Vadher B, Shah S, Dudhat K, Dhaval M, Sing S, Prajapati BG. Ketoconazole nanocrystals fortified gel for improved transdermal applications. Nanofabrication. 2024;9. doi:10.37819/nanofab.9.1885
165. Men Z, Su T, Tang Z, Liang J, Shen T. Tacrolimus nanocrystals microneedle patch for plaque psoriasis. Int J Pharm. 2022;627:122207. doi:10.1016/j.ijpharm.2022.122207
166. Mandal B, Alexander KS, Riga AT. Sulfacetamide loaded Eudragit RL100 nanosuspension with potential for ocular delivery. J Pharm Pharm Sci. 2010;13(4):510–523. doi:10.18433/J3SW2T
167. Bucolo C, Maltese A, Maugeri F, Busà B, Puglisi G, Pignatello R. Eudragit RL100 nanoparticle system for the ophthalmic delivery of cloricromene. J Pharm Pharmacol. 2004;56(7):841–846. doi:10.1211/0022357023835
168. Singh J, Sharma RB, Mehan N, Beniwal SK. Itraconazole-loaded nanocrystals development and characterization for the treatment of ophthalmic fungal infection. Lat Am J Pharm. 2023;42(3):839–847.
169. Romero GB, Keck CM, Müller RH, Bou-Chacra NA. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215–222. doi:10.1016/j.ejpb.2016.07.005
170. Seto Y, Ueno K, Suzuki H, Sato H, Onoue S. Development of novel lutein nanocrystal formulation with improved oral bioavailability and ocular distribution. Journal of Functional Foods. 2019;61:103499. doi:10.1016/j.jff.2019.103499
171. Casula L, Lai F, Pini E, et al. Pulmonary delivery of curcumin and beclomethasone dipropionate in a multicomponent nanosuspension for the treatment of bronchial asthma. Pharmaceutics. 2021;13(8):1300. doi:10.3390/pharmaceutics13081300
172. Liu T, Han M, Tian F, Cun D, Rantanen J, Yang M. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym. 2018;181:1143–1152. doi:10.1016/j.carbpol.2017.11.018
173. Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420(1):180–188. doi:10.1016/j.ijpharm.2011.08.023
174. Zheng H, Li J, Leung SSY. Inhalable polysorbates stabilized nintedanib nanocrystals to facilitate pulmonary nebulization and alveolar macrophage evasion. Biomater Adv. 2025;166:214084. doi:10.1016/j.bioadv.2024.214084
175. Kumar M, Jha A, Bharti K, et al. Lipid-coated nanocrystals of paclitaxel as dry powder for inhalation: characterization, in-vitro performance, and pharmacokinetic assessment. Colloids Surf B. 2024;237:113865. doi:10.1016/j.colsurfb.2024.113865
176. Sohn JS, Yoon D-S, Sohn JY, Park J-S, Choi J-S. Development and evaluation of targeting ligands surface modified paclitaxel nanocrystals. Mater Sci Eng C. 2017;72:228–237. doi:10.1016/j.msec.2016.11.065
177. Liu Y, Ma Y, Xu J, et al. Apolipoproteins adsorption and brain-targeting evaluation of baicalin nanocrystals modified by combination of Tween80 and TPGS. Colloids Surf B. 2017;160:619–627. doi:10.1016/j.colsurfb.2017.10.009
178. Schöler N, Krause K, Kayser O, et al. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother. 2001;45(6):1771–1779. doi:10.1128/aac.45.6.1771-1779.2001
179. Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Delivery Transl Res. 2017;7(4):497–506. doi:10.1007/s13346-017-0368-5
180. Hao J, Zhao J, Zhang S, et al. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf B Biointerfaces. 2016;147:376–386. published Online First: 20160820. doi:10.1016/j.colsurfb.2016.08.011
181. Dalvi A, Ravi PR, Uppuluri CT. Design and evaluation of rufinamide nanocrystals loaded thermoresponsive nasal in situ gelling system for improved drug distribution to brain. Front Pharmacol. 2022;13:943772. published Online First: 20221004. doi:10.3389/fphar.2022.943772
182. Qin T, Dai Z, Xu X, et al. Nanosuspension as an efficient carrier for improved ocular permeation of voriconazole. Curr Pharm Biotechnol. 2021;22(2):245–253. doi:10.2174/1389201021999200820154918
183. Cao Y, Samy KE, Bernards DA, Desai TA. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov Today. 2019;24(8):1694–1700. published Online First: 20190604. doi:10.1016/j.drudis.2019.05.031
184. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–599. doi:10.1046/j.1365-2125.2003.01892.x
185. Yue P, Zhou W, Huang G, et al. Nanocrystals based pulmonary inhalation delivery system: advance and challenge. Drug Deliv. 2022;29(1):637–651. doi:10.1080/10717544.2022.2039809
186. Costabile G, Provenzano R, Azzalin A, et al. PEGylated mucus-penetrating nanocrystals for lung delivery of a new FtsZ inhibitor against Burkholderia cenocepacia infection. Nanomed Nanotechnol Biol Med. 2020;23:102113. doi:10.1016/j.nano.2019.102113
187. Yanamala N, Farcas MT, Hatfield MK, et al. In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nanomaterial of the future. ACS Sustainable Chem Eng. 2014;2(7):1691–1698. doi:10.1021/sc500153k
188. Shvedova AA, Kisin ER, Yanamala N, et al. Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part Fibre Toxicol. 2016;13(1):28. published Online First: 20160608. doi:10.1186/s12989-016-0140-x
189. Rawal T, Kremer L, Halloum I, Butani S. Dry-powder inhaler formulation of rifampicin: an improved targeted delivery system for alveolar tuberculosis. J Aerosol Med Pulm Drug Deliv. 2017;30(6):388–398. published Online First: 20170516. doi:10.1089/jamp.2017.1379
190. Liu Y, Huang L, Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm. 2010;7(3):863–869. doi:10.1021/mp100012s
191. Hu L, Kong D, Hu Q, Gao N, Pang S. Evaluation of high-performance curcumin nanocrystals for pulmonary drug delivery both in vitro and in vivo. Nanoscale Res Lett. 2015;10(1):381. doi:10.1186/s11671-015-1085-y
192. Zheng JY, Bosch HW. Sterile filtration of nanocrystal™ drug formulations. Drug Dev Ind Pharm. 1997;23(11):1087–1093. doi:10.3109/03639049709150497
193. Arora R, Aggarwal G, Harikumar SL, Kaur K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv Pharm. 2014;2014:468456. doi:10.1155/2014/468456
194. Selvamuthukumar S, Velmurugan R. Nanostructured Lipid Carriers: a potential drug carrier for cancer chemotherapy. Lipids Health Dis. 2012;11(1):159. doi:10.1186/1476-511X-11-159
195. Li W, Li Z, Wei L, Zheng A. Evaluation of paclitaxel nanocrystals in vitro and in vivo. Drug Res. 2018;68(4):205–212. published Online First: 20171130. doi:10.1055/s-0043-119461
196. Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10(4):269. published Online First: 20181211. doi:10.3390/pharmaceutics10040269
197. Reddy S, Tatiparti K, Sau S, Iyer AK. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov Today. 2021;26(8):1944–1952. published Online First: 20210416. doi:10.1016/j.drudis.2021.04.008
198. Lombardo SM, Schneider M, Türeli AE, Günday Türeli N. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol. 2020;11:866–883. doi:10.3762/bjnano.11.72
199. Gu L, Fang RH, Sailor MJ, Park J-H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano. 2012;6(6):4947–4954. doi:10.1021/nn300456z
200. Wang Q, Sun AY, Pardeike J, Müller RH, Simonyi A, Sun GY. Neuroprotective effects of a nanocrystal formulation of sPLA(2) inhibitor PX-18 in cerebral ischemia/reperfusion in gerbils. Brain Res. 2009;1285:188–195. published Online First: 20090613. doi:10.1016/j.brainres.2009.06.022
201. Pandey PK, Sharma AK, Gupta U. Blood brain barrier: an overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers. 2016;4(1):e1129476. published Online First: 20151215. doi:10.1080/21688370.2015.1129476
202. Bonaccorso A, Gigliobianco MR, Pellitteri R, et al. Optimization of curcumin nanocrystals as promising strategy for nose-to-brain delivery application. Pharmaceutics. 2020;12(5). doi:10.3390/pharmaceutics12050476
203. Moradi F, Dashti N. Targeting neuroinflammation by intranasal delivery of nanoparticles in neurological diseases: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(2):133–148. published Online First: 20220104. doi:10.1007/s00210-021-02196-x
204. Islam SU, Shehzad A, Ahmed MB, Lee YS. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;25(8):1929. published Online First: 20200421. doi:10.3390/molecules25081929
205. Wu C, Li B, Zhang Y, et al. Intranasal delivery of paeoniflorin nanocrystals for brain targeting. Asian J Pharm Sci. 2020;15(3):326–335. doi:10.1016/j.ajps.2019.11.002
206. Chen C, Wang L, Cao F, et al. Formulation of 20(S)-protopanaxadiol nanocrystals to improve oral bioavailability and brain delivery. Int J Pharm. 2016;497(1):239–247. doi:10.1016/j.ijpharm.2015.12.014
207. Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood–brain barrier using surfactant-coated nanoparticles. J Control Release. 1997;49(1):81–87. doi:10.1016/S0168-3659(97)00061-8
208. Shubar HM, Lachenmaier S, Heimesaat MM, et al. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood–brain barriers. J Drug Targeting. 2011;19(2):114–124. doi:10.3109/10611861003733995
209. Bechinger P, Serrano Sponton L, Grutzner V, et al. In-vivo time course of organ uptake and blood-brain-barrier permeation of poly(L-lactide) and poly(perfluorodecyl acrylate) nanoparticles with different surface properties in unharmed and brain-traumatized rats. Front Neurol. 2023;14:994877. published Online First: 20230206. doi:10.3389/fneur.2023.994877
210. Chai Z, Ran D, Lu L, et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano. 2019;13(5):5591–5601. doi:10.1021/acsnano.9b00661
211. Roman M, Dong S, Hirani A, Lee YW. Cellulose nanocrystals for drug delivery. Polysaccharide Materials: Performance by Design: American Chemical Society. 2009;2009:81–91.
212. Leitner DF, Connor JR. Functional roles of transferrin in the brain. Biochim Biophys Acta Gen Subj. 2012;1820(3):393–402. doi:10.1016/j.bbagen.2011.10.016
213. Mule S, Khairnar P, Shukla R. Recent advances in nanocrystals heralding greater potential in brain delivery. Part Part Syst Charact. 2022;39(9):2200087. doi:10.1002/ppsc.202200087
214. Müller RH, Gohla S, Keck CM. State of the art of nanocrystals – special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9. doi:10.1016/j.ejpb.2011.01.007
215. Sun X, Pang Z, Ye H, et al. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33(3):916–924. doi:10.1016/j.biomaterials.2011.10.035
216. Chen T, Li C, Li Y, Yi X, Lee SM-Y, Zheng Y. Oral delivery of a nanocrystal formulation of schisantherin a with improved bioavailability and brain delivery for the treatment of Parkinson’s disease. Mol Pharmaceut. 2016;13(11):3864–3875. doi:10.1021/acs.molpharmaceut.6b00644
217. Xiong S, Liu W, Li D, et al. Oral delivery of puerarin nanocrystals to improve brain accumulation and anti-parkinsonian efficacy. Mol Pharm. 2019;16(4):1444–1455. published Online First: 20190306. doi:10.1021/acs.molpharmaceut.8b01012
218. Shubar HM, Dunay IR, Lachenmaier S, et al. The role of apolipoprotein E in uptake of atovaquone into the brain in murine acute and reactivated toxoplasmosis. J Drug Targeting. 2009;17(4):257–267. doi:10.1080/10611860902718680
219. Dibaei M, Rouini M-R, Sheikholeslami B, Gholami M, Dinarvand R. The effect of surface treatment on the brain delivery of curcumin nanosuspension: in vitro and in vivo studies. Int j Nanomed. 2019;14(null):5477–5490. doi:10.2147/IJN.S199624
220. Tan Y, Liu Y, Liu Y, et al. Rational design of thermosensitive hydrogel to deliver nanocrystals with intranasal administration for brain targeting in parkinson’s disease. Research. 2021;2021. doi:10.34133/2021/9812523
221. Zhu S, Zhang S, Pang L, et al. Effects of armodafinil nanocrystal nasal hydrogel on recovery of cognitive function in sleep-deprived rats. Int J Pharm. 2021;597:120343. doi:10.1016/j.ijpharm.2021.120343
222. Al-Dhubiab BE. Aripiprazole nanocrystal impregnated buccoadhesive films for schizophrenia. J Nanosci Nanotechnol. 2017;17(4):2345–2352. doi:10.1166/jnn.2017.12588
223. Gol D, Thakkar S, Misra M. Nanocrystal-based drug delivery system of risperidone: lyophilization and characterization. Drug Dev Ind Pharm. 2018;44(9):1458–1466. doi:10.1080/03639045.2018.1460377
224. Tashan E, Karakucuk A, Celebi N. Development of nanocrystal ziprasidone orally disintegrating tablets: optimization by using design of experiment and in vitro evaluation. AAPS Pharm Sci Tech. 2020;21(3):115. doi:10.1208/s12249-020-01653-9
225. Lugoloobi I, Maniriho H, Jia L, Namulinda T, Shi X, Zhao Y. Cellulose nanocrystals in cancer diagnostics and treatment. J Control Release. 2021;336:207–232. doi:10.1016/j.jconrel.2021.06.004
226. Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35(3):209–217. doi:10.1039/B514191E
227. Tingting B, Lu P, Zhang K, et al. Gold/Silver bimetallic nanocrystals: controllable synthesis and biomedical applications. J Biomed Nanotechnol. 2017;13:1178–1209. doi:10.1166/jbn.2017.2423
228. Yang Y, Chen Y, Pei P, et al. Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging. Nature Nanotechnol. 2023;18(10):1195–1204. doi:10.1038/s41565-023-01422-2
229. Zhong Y, Ma Z, Zhu S, et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat Commun. 2017;8(1):737. doi:10.1038/s41467-017-00917-6
230. Gao L, Xie L, Long X, et al. Efficacy of MRI visible iron oxide nanoparticles in delivering minicircle DNA into liver via intrabiliary infusion. Biomaterials. 2013;34(14):3688–3696. doi:10.1016/j.biomaterials.2013.01.094
231. Draz MS, Fang BA, Zhang P, et al. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4(9):872–892. published Online First: 20140611. doi:10.7150/thno.9404
232. Choi HW, Lee HJ, Kim KJ, Kim H-M, Lee SC. Surface modification of hydroxyapatite nanocrystals by grafting polymers containing phosphonic acid groups. J Colloid Interface Sci. 2006;304(1):277–281. doi:10.1016/j.jcis.2006.05.069
233. Gómez-Vallejo V, Puigivila M, Plaza-García S, et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale. 2018;10(29):14153–14164. doi:10.1039/C8NR03084G
234. Wörle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–1268. doi:10.1021/nl060177c
235. Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Delivery Rev. 2010;62(3):284–304. doi:10.1016/j.addr.2009.11.002
236. Putro JN, Ismadji S, Gunarto C, et al. The effect of surfactants modification on nanocrystalline cellulose for paclitaxel loading and release study. J Mol Liq. 2019;282:407–414. doi:10.1016/j.molliq.2019.03.037
237. Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2(1):12–17. doi:10.4103/2230-973x.96921
238. Schroedter A, Weller H, Eritja R, Ford WE, Wessels JM. Biofunctionalization of Silica-Coated CdTe and Gold Nanocrystals. Nano Lett. 2002;2(12):1363–1367. doi:10.1021/nl025779k
239. Agarwal V, Bajpai M. Design, fabrication and characterization of esomeprazole nanocrystals for enhancing the dissolution rate and stability. Recent Pat Nanotechnol. 2021;15(2):165–179. doi:10.2174/1872210514666201016150915
240. Ventura C, Pinto F, Lourenço AF, Ferreira PJT, Louro H, Silva MJ. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose. 2020;27(10):5509–5544. doi:10.1007/s10570-020-03176-9
241. Joseph E, Singhvi G. Chapter 4 - Multifunctional nanocrystals for cancer therapy: a potential nanocarrier. In: Grumezescu AM, editor. Nanomaterials for Drug Delivery and Therapy. William Andrew Publishing; 2019:91–116.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


