Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Study on the Molecular Mechanism of Baicalin Phosphorylation of Tau Protein Content in a Cell Model of Intervention Cognitive Impairment
Authors Li J, Zhao C, Guan D, Liu C
Received 12 June 2024
Accepted for publication 10 February 2025
Published 19 February 2025 Volume 2025:21 Pages 309—322
DOI https://doi.org/10.2147/NDT.S482362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Juncheng Li,1 Canbin Zhao,1 Donghui Guan,2 Chunmei Liu2
1Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China
Correspondence: Chunmei Liu, Email [email protected]
Objective: This study aimed to predict the molecular mechanism of baicalein through network pharmacology, cell experiments and molecular docking. The goal was to elucidate the mechanism for the treatment of mild cognitive impairment.
Materials and Methods: The protein-protein interaction network (PPI) and Cytoscape software were used to screen the hub genes of intersection genes, while the therapeutic mechanism of baicalein was predicted through the Gene ontology (GO) and Kyoto Encyclopedia of Gene and Genomes (KEGG) enrichment analysis. Molecular docking of the core target and baicalein was conducted using AutoDock software. The neuroprotective effect of Baicalein with different concentrations on okadaic acid-treated SH-SY5Y cells was investigated in cell experiments. Scratch experiments were conducted to explore the effect of baicalein on cell migration ability, and enzyme linked immunosorbent assay was employed to measure the content of extracellular pathological proteins. Western Blot and Cellular immunofluorescence pinpointed differences in the relevant protein content.
Results: Enrichment analysis revealed significant enrichment in the PI3K-Akt signaling pathway, and molecular docking demonstrated a favorable binding energy between baicalein and AKT1. In cell experiments, baicalein presented a positive impact on mild cognitive impairment by elevating P-AKT1 and P-GSK-3β levels while reducing the overall amount of P-tau.
Conclusion: As demonstrated by the present study, the low concentration of baicalein (15μmol/L) effectively managed the mild cognitive impairment by regulating the phosphorylation of AKT1 and GSK-3β.
Keywords: baicalein, mild cognitive impairment, SH, SY5Y, PI3K, Akt signaling pathway
Introduction
Mild cognitive impairment (MCI) describes cognitive abilities below age and education-specific criteria, without reaching dementia severity. Actively treating MCI could reduce dementia incidence, but there still lacks a standardized plan. As a result, exploring drugs for MCI treatment holds promising potential.
Baicalein presents favorable properties in anti-inflammatory, antioxidant, anti-inflammatory action,1 and animal experiments have demonstrated its efficiency in improving cognitive dysfunction in mice by regulating intestinal flora and phenotypic transformation of activated microglia.2,3 Additionally, in some cell-based experiments, baicalein demonstrates a neuroprotective by modulating pathways such as the NF-κB/MAPK signaling pathway and the AMPK/Nrf2 pathway. However, the limited number of related experiments and variations in research objects necessitate further investigation to fully understand how baicalein functions in treating MCI.
Considering the extensive number of genes related to MCI and baicalin, the possible targets and signaling pathways of baicalein through network pharmacology and molecular docking were hereby predicted, and cell experiments were carried out to further explore the possible mechanism of action of baicalein, so as to provide reference for subsequent experiments.
Materials and Methods
Network Pharmacology
Collecting Targets of Baicalein and MCI
The targets of baicalein were screened using Swiss Target Prediction and Pharm Mapper, and the gene names were obtained through the UniProt. Disease-related genes were sourced from databases such as GeneCard, PharmGKB, Therapeutic Target Database, DisGeNET, and DrugBank. A Venn diagram was generated to identify the intersection genes of baicalein and MCI.
PPI
These intersection genes were introduced into STRING database, with “Homo Sapiens” selected as the protein species to get the PPI. Cytoscape 3.9.1 was used to visualize the relationship between baicalein and MCI. Additionally, the cytoHubba function in the Cytoscape software was employed to screen the hub genes.
Enrichment Analysis
GO enrichment analysis was carried out through the hub genes targets, and R software package (ggplot2, tidyverse, ggvenn, enrichplot, org.Hs.eg.db) software R 4.1.5 was installed. The KEGG enrichment analysis was performed using the installed R package.
Molecular Docking
The top one degree values of the hub genes in the PPI network were retrieved from the PDB database. The Mol2 format file of baicalein and AKT1 was downloaded from the UniProt. The molecular docking was performed in AutoDock software, and PyMol software was employed to visually represent the binding site in the molecular docking process.
Cell Experiments
Drugs and Reagents
Herein, the okadaic acid (CAS: 78111-17-8 OKA), baicalein (CAS: 491-67-8) and chloroform involved were provided by Yuanye Bio-Technology. (Shanghai, China). CCK 8 assay kit, dimethyl sulfoxide (DMSO), crystal violet and paraformaldehyde were provided by Solarbio (Beijing, China). Baicalein was dissolved in DMSO to prepare a stock solution with a concentration of 100 mmol/L, which was then stored at −20°C. OKA was dissolved in DMSO to prepare a stock solution with a concentration of 100 μmol/L, and stored at −20°C as well.
Cell Lines and Culture Conditions
SH-SY5Y cells and their complete medium were obtained from Procell Life Technology (Wuhan, China). The SH-SY5Y cells were cultured in complete medium under standard conditions: a 5% CO2 incubator at 37°C. In the model group, SH-SY5Y cells were treated with OKA at a concentration of 40 nmol/L for 6 hours to induce the desired experimental conditions.
CCK 8 Detection
SH-SY5Y cells were seeded in a TC-modified 96-well plates with a concentration of 1×105 and cultured for 24h with 200ul of in each well. After discarding the medium, the cells were incubated in complete medium containing different concentrations of baicalein (0, 5, 10, 25, 50, 100 μmol/L) and 0.5‰ DMSO for 24, 48, and 72 hours, respectively. Six wells were prepared for repeated tests. Then, 20 mL of CCK8 was added to each well, and 96-well plates were placed in a 37°C incubator for incubation for 1.5h. Microplate reader was used to measure the OD value of each well at 450nm.
SH-SY5Y cells were cultured for 24h following the method described above (2.2.2), and the medium was discarded. 100ul OKA (40 nmol/L) was added to each well for 6h of intervention, and the supernatant was then discarded. Subsequently, each well was rinsed twice with PBS 200ul. The other steps were repeated as described previously.
Scratch Experiment
Model cells were seeded at a density of 1×105 in a 6 cm diameter culture dish and treated with complete medium containing varying concentrations (0, 15, 20, 25 μmol/L) of baicalein for 24 hours. The cell fusion rate was confirmed to be greater than 95%. Subsequently, cells were scraped with 10ul pipette tip and washed twice with PBS in the dish. MEM/F12 was added for 24h of further incubation. The scratches were photographed at 0, 6, 12 and 24h, and the scratch width was analyzed using ImageJ software.
Enzyme-Linked Immunosorbent Assay
Cell modeling, as outlined in Item 2.2.3, involved adding baicalein at concentrations of 0 μmol/L, 15 μmol/L, and 25 μmol/L to the complete medium for a 24-hour incubation period. After incubation, the supernatant was collected and subjected to centrifugation at 1000×g for 20 minutes at 4°C. Following centrifugation, the supernatant was drawn, diluted tenfold, and the OD value was measured as per the provided instructions.
Western Blot
Cells were treated following Item 2.2.3. Both model and normal cells were seeded in a 6 cm diameter dish and incubated in the complete medium containing different concentrations (0, 15, 25 μmol/L) of baicalein for 24h. Total intracellular protein was then extracted. After calculating the protein concentration, 20μL protein sample was added to each well for electrophoresis. Subsequently, membrane transfer, blocking, and overnight incubation with primary antibodies were carried out. The membranes were then incubated for 1h with secondary antibodies at room temperature. ECL exposure solution was used for exposure, and the exposure intensity of the bands was analyzed.
Immunofluorescence
Cells were treated following Item 2.2.6. Cells were fixed with 4% paraformaldehyde for 20 min and then washed three times with PBS buffer. Membranes were permeabilized for 20 min (0.3% Triton X-100), followed by 3 washes with PBS. Subsequently, the cells were blocked with PBS containing 1% bovine serum albumin for 1h. After another three washes with PBS, the cells were incubated overnight at 4°C with antibodies against P-AKT1, P-GSK-3βand P-Tau. The cells were then incubated with fluorescent secondary antibodies at room temperature for 1h. DAPI was used to stain the nucleus. Finally, cells were visualized using fluorescence microscope and photographed. The fluorescence intensity of positive cells was assessed using ImageJ software.
Statistical Treatment
Experimental results were expressed as mean ± standard deviation and compared by one-way ANOVA among groups. Differences between groups were compared using the t-test. Statistical analysis and graphical representation were conducted using SPSS 26.0 and GraphPad Prism 9 software. A p-value less than 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001. Each experiment was repeated three times for reliability.
Results
Obtaining Target Genes
Herein, 87 common gene targets of baicalein and mild cognitive impairment were obtained using Venn diagram, serving as target genes, as shown in Figure 1.
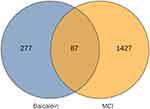 |
Figure 1 The VENN diagram of 87 genes in common with the disease and baicalin. |
PPI
The STRING database generated a PPI information map of 87 target genes for evaluating interaction relationships, involving 220 edges and 87 nodes, and the minimum required interaction score was 0.700, as shown in Figure 2. The hub genes and PPI network visualization are shown in Figure 3.
 |
Figure 2 PPI information map of 87 target genes for evaluating interaction relationships, including 220 edges and 87 nodes. |
 |
Figure 3 Network of hub genes, with the degree value represented by red and yellow in order from high to low. The highest degree value is AKT1. |
Enrichment Analysis
The enrichment analysis of GO consists of three aspects, ie, biological process (BP), cellular components (CC), and molecular function (MF). Among them, the BP mainly involved the positive regulation of kinase activity and response to peptide, etc.; the CC mainly involved the membrane rafts and neuronal cell body, etc.; and the MF was mainly adopted in protein serine/threonine/tyrosine kinase activity, endopeptidase activity, etc., as shown in Figure 4. This demonstrated the possible relationship between the neuroprotective mechanism of baicalein and protein kinase cascade transduction.
 |
Figure 4 GO analysis, with red indicating the biological process; green, cellular components; and blue, molecular function, each section showing the first 10 features. |
The KEGG enrichment analysis revealed that hub genes were predominantly enriched in neurotrophin signaling pathway and longevity regulating pathway of Cellular Processes. The Environmental Information Processing was mainly concentrated on the PI3K-Akt signaling pathway, as shown in Figure 5.
 |
Figure 5 KEGG enrichment analysis, with the color changing from dark to light, and the gene percent gradually decreasing. |
Molecular Docking
The top-ranking gene within the hub genes was selected to establish the final target-drug correspondence. Molecular docking verification was conducted, revealing a binding energy of −7.04 for the interaction between the drug molecule and the target protein. Figure 6 presents the molecular docking of AKT1 with baicalein.
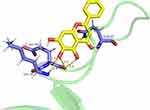 |
Figure 6 Binding site of the molecular docking of AKT1-baicalein and the binding energy of −7.04. |
The PI3K-Akt signaling pathway was considered important for nerve cell senescence and cellular inflammation, involving the phosphorylation of serine or threonine by downstream substrates, with the key gene AKT1 playing a pivotal role. Molecular docking results indicated favorable binding energy between AKT1 and baicalein. Subsequently, the role of baicalein in the PI3K-Akt signaling pathway was further explored in the cell experiments.
Baicalein in Promoting Cell Viability in the Model Group
The impact of various concentrations of baicalein on SH-SY5Y cell viability was measured (Figure 7). Specifically, cell viability in the 10 μmol/L group decreased by less than 10% compared to the control group, which, however, decreased by less than 15% in the 25 μmol/L group. Consequently, for subsequent experiments, the concentration of baicalein was kept below 25 μmol/L (n=8). The data from each group were analyzed in comparison with the control group.
 |
Figure 7 Baicalin inhibits the cell viability of SH-SY5Y cells. * means P ≤ 0.05, ** means P ≤ 0.01, *** means P ≤ 0.001. |
The impact of varying concentrations of baicalein on SH-SY5Y cell viability, in the context of OKA treatment, was also assessed (Figure 8). These findings suggested that baicalein concentrations ranging from 15 to 25 μmol/L might exert a promotive effect on cell viability in the model group.
 |
Figure 8 Baicalin promoted the cellular activity in the 20μmol/L and 25μmol/L groups, in the context of Oka treatment. |
Baicalein in Promoting Cell Migration in the Model Group
Figure 9 exhibits the observed scratch status of the cells in the model group following intervention with different concentrations of baicalein. Baicalein promoted cell migration at concentrations ranging from 0 to 25μmol/L.
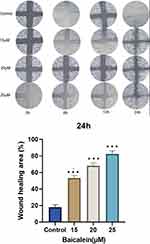 |
Figure 9 Baicalein promoting cell migration, with the promotion effect and baicalein concentration being positively correlated. |
Baicalein in Reducing the Content of Peripheral P-Tau Protein
The content of P-Tau in the medium in the control group, model group, 15 μmol/L group, 25 μmol/L group was measured after 24h (Figure 10). This indicated that the 15μmol/L group was more effective at inhibiting the release of P-Tau.
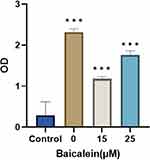 |
Figure 10 The release of P-Tau increased in the model group, and that the baicalein inhibited the release of P-Tau. |
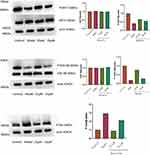
Baicalein Upregulating P-AKT1 and P-GSK-3β-Related Proteins and Downregulating the Expression of P-Tau-Related Proteins
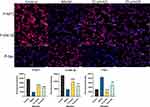
Figure 11 shows the results of Western Blot. As shown, the expression of AKT1 and GSK-3β remained basically unchanged after administration, compared with the control group. The protein expression of P-AKT1 and P-GSK-3β increased, while that of P-Tau decreased (P<0.05). Additionally, the regulation of baicalein was more obvious in the lower concentration group than in the high dose group (P<0.05). Cellular immunofluorescence photographs (Figure 12) similarly verified the conclusion. The results showed that the baicalein might upregulate the protein expression of P-AKT1 and P-GSK-3β while downregulating that of P-tau. By roughly observing the cell morphology across various groups, it was found that the cell synapses in the 15 μmol/L group were longer than those in the model group and 25 μmol/L group but shorter than those in the control group. The data from each group were analyzed in comparison with the model group. Figure 13 is the original data.
 |
Figure 13 The original data of WB. |
Discussion
In the present study, GO enrichment analysis of hub genes showed that genes were enriched in cell proliferation and neuronal cell body. KEGG enrichment analysis of hub genes indicated that the PI3K-Akt signaling pathway ranked among the top 20 enrichment results. This pathway holds significant importance in various neurodegenerative diseases, as it influences neural cell survival and synaptic plasticity.4–6
Using OKA to induce cognitive dysfunction in SH-SY5Y cells provides a relevant pathological model for studying potential therapeutic mechanisms predicted by network pharmacology. This model is chosen based on its alignment with the predicted pathways and interactions identified through network pharmacology. OKA is applied to animal and cell-type models of cognitive dysfunction.7 OKA intervention in SH-SY5Y cells can affect the PI3K/AKT signaling pathway and produce P-Tau protein, and the model is also provided with pathological features such as GSK3β activation, neuroinflammation and mitochondrial dysfunction.8 Besides, the MCI associated with P-Tau is more likely to progress to Alzheimer’s disease.9,10
Herein, CCK8 test demonstrated that baicalein promoted the mitochondrial activity of SH-SY5Y induced by OKA, and that the drug concentration was positively correlated with the promoting effect, which was consistent with the result acquired in relevant literature.11 Additionally, baicalein inhibited the mitochondrial activity of normal cells. In the scratch experiment, the migration ability of cells was found to be positively correlated with the concentration of baicalein, fully reflecting the protective effect of baicalein on the activity of nerve cells. The results were consistent with previous experiments.12 Besides, qualitative analysis of P-Tau was performed using enzyme-linked immunosorbent assay. The results demonstrated the efficacy of baicalein in inhibiting the release of P-Tau, with a more pronounced effect observed in the high concentration group compared to the low concentration group. These findings align with previous studies on the reduction of tau phosphorylation by baicalein, indicating consistency with the results of the present study.13 The article published by Alzheimer’s Association suggested that the content of extracellular P-Tau was significantly correlated with cognitive function, indicating that baicalein might improve cognitive dysfunction by reducing the content of extracellular P-Tau.14
Through the Western Blot results and immunofluorescence, the baicalein was found capable of promoting the phosphorylation of AKT1 and GSK-3β and reducing P-Tau protein content to treat cognitive impairment, which to some extent aligned with previous studies.15,16 AKT1 played a non-negligible role in cell proliferation and growth. Indeed, research has indicated the essential role of the production of p-Akt protein facilitated by Akt activation in inhibiting apoptosis. This action is associated with promoting the survival of hippocampal nerve cells.17 Activated AKT1 is an important upstream regulator of GSK-3β protein. AKT1 can inhibit GSK-3β activation by promoting phosphorylation of GSK-3β protein.18 P-Tau protein is closely related to glial activation and inflammatory response of the nervous system, thus promoting cognitive dysfunction. Additionally, intracellular P-Tau protein could better predict the outcome of cognitive dysfunction.19
However, the present study is still subject to certain shortcomings. Genes related to baicalein and cognitive dysfunction were not fully recognized, exposing the study to certain deficiencies in network pharmacology. The impact of Baicalin on cell morphology is worth delving into for further exploration.
Conclusion
Network pharmacological analysis suggested the possible role of AKT1 in the pathogenesis of MCI. Meanwhile, the results of cell experiments showed that the baicalein could promote the cell activity of SH-SY5Y after OKA intervention, enhance the ability of cell proliferation and migration, and reduce the release of P-Tau proteins. The possible action mechanism was to promote the phosphorylation of AKT1 and GSK-3β, so as to reduce the production of P-Tau. Overall, these findings contribute genetic-level study data that can advance further understanding of the treatment of cognitive function.
Data Sharing Statement
The data that support the findings of this study are openly available in STRING database (https://cn.string-db.org), GeneCard database (https://www.genecards.org), Therapeutic Target Database (https://db.idrblab.net/), DisGenNET database (https://go.drugbank.com/), DrugBank database (https://www.disgenet.org/home/), UniProt (https://www.uniprot.org/), Swiss Target Prediction (http://swisstargetprediction.ch/), and Pharm Mapper (http://lilab-ecust.cn/pharmmapper/index.html).
Ethics Approval and Consent to Participate
The cell experiments in this study did not involve ethical approval (Life Science and Medical Research Involving Human Subjects; item 1 and 2 of Article 32).
Acknowledgment
All authors agree to publish this paper to this journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflict of interest regarding the publication of this article.
References
1. Li J, Zhou Y, Du G. et al. Integration of transcriptomics and network analysis deciphers the mechanisms of baicalein in improving learning and memory impairment in senescence-accelerated mouse prone 8 (SAMP8). Eur J Pharmacol. 2019;865:172789. Epub 2019 Nov 9. PMID: 31715136. doi:10.1016/j.ejphar.2019.172789
2. Xie XM, Hao JJ, Shi JZ, et al. Baicalein ameliorates Alzheimer’s disease via orchestration of CX3CR1/NF-κB pathway in a triple transgenic mouse model. Int Immunopharmacol. 2023;118:109994. Epub 2023 Mar 14. PMID: 37098656. doi:10.1016/j.intimp.2023.109994
3. Shi J, Chen J, Xie X, et al. Baicalein-corrected gut microbiota may underlie the amelioration of memory and cognitive deficits in APP/PS1 mice. Front Pharmacol. 2023;14:1132857. doi:10.3389/fphar.2023.1132857
4. Borrie SC, Brems H, Legius E, Bagni C. Cognitive dysfunctions in intellectual disabilities: the contributions of the Ras-MAPK and PI3K-AKT-mTOR pathways. Annu Rev Genomics Hum Genet. 2017;18(1):115–142. doi:10.1146/annurev-genom-091416-035332
5. Wang L, Xu B, Sun S, Wang B. Overexpression of long non-coding RNA H19 relieves hypoxia-induced injury by down-regulating microRNA-107 in neural stem cells. Neurosci Lett. 2021;753:135855. doi:10.1016/j.neulet.2021.135855
6. Qian H, Gao F, Wu X, et al. Activation of the CD200/CD200R1 axis attenuates neuroinflammation and improves postoperative cognitive dysfunction via the PI3K/Akt/NF-κB signaling pathway in aged mice. Inflamm Res. 2023;72(12):2127–2144. doi:10.1007/s00011-023-01804-1
7. Medina M, Avila J, Villanueva N. Use of okadaic acid to identify relevant phosphoepitopes in pathology: a focus on neurodegeneration. Mar Drugs. 2013;11(5):1656–1668. doi:10.3390/md11051656
8. Del Barrio L, Martín-de-Saavedra MD, Romero A, et al. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2* nicotinic stimulation. Toxicol Sci. 2011;123(1):193–205. doi:10.1093/toxsci/kfr163
9. Hamsafar Y, Chen Q, Borowsky AD, et al. Biochemical analyses of tau and other neuronal markers in the submandibular gland and frontal cortex across stages of Alzheimer disease. Neurosci Lett. 2023;810:137330. doi:10.1016/j.neulet.2023.137330
10. Zhao A, Li Y, Deng Y. Alzheimer’s Disease Neuroimaging Initiative. TNF receptors are associated with tau pathology and conversion to Alzheimer’s dementia in subjects with mild cognitive impairment. Neurosci Lett. 2020;738:135392. doi:10.1016/j.neulet.2020.135392
11. Kong DW, Du LD, Liu RZ, et al. Baicalein attenuates rotenone-induced SH-SY5Y cell apoptosis through binding to SUR1 and activating ATP-sensitive potassium channels. Acta Pharmacol Sin. 2023;45(3):480–489. doi:10.1038/s41401-023-01187-3
12. Zheng ZV, Cheung CY, Lyu H, et al. Baicalein enhances the effect of low dose Levodopa on the gait deficits and protects dopaminergic neurons in experimental Parkinsonism. J Clin Neurosci. 2019;64:242–251. doi:10.1016/j.jocn.2019.02.005
13. Liu Y, Chang D, Liu T, Zhou X. Natural product-based bioactive agents in combination attenuate neuroinflammation in a tri-culture model. Front Pharmacol. 2023;14:1135934. doi:10.3389/fphar.2023.1135934
14. Sanchez E, Wilkinson T, Coughlan G, et al. Association of plasma biomarkers with cognition, cognitive decline, and daily function across and within neurodegenerative diseases: results from the Ontario Neurodegenerative Disease Research Initiative. Alzheimers Dement. 2023;19(S15). doi:10.1002/alz.13560
15. Wang W, Wang F, Yang YJ, et al. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol. 2011;162(6):1364–1379. doi:10.1111/j.1476-5381.2010.01143.x
16. Zhang X, Du L, Zhang W, et al. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci Rep. 2017;7(1):9968. doi:10.1038/s41598-017-07442-y
17. Wu X, Liu Y, Zhu L, et al. Cerebroprotein Hydrolysate-I Inhibits Hippocampal Neuronal Apoptosis by Activating PI3K/Akt Signaling Pathway in Vascular Dementia Mice. Neuropsychiatr Dis Treat. 2021;17:2359–2368. doi:10.2147/NDT.S311760
18. Wang H, Sui H, Zheng Y. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11(15):7481–7496. doi:10.1039/C9NR01255A
19. Yasuno F, Kimura Y, Ogata A, et al. Neuroimaging biomarkers of glial activation for predicting the annual cognitive function decline in patients with Alzheimer’s disease. Brain Behav Immun. 2023;114:214–220. doi:10.1016/j.bbi.2023.08.027
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.