Back to Journals » International Journal of Nanomedicine » Volume 19
Surface Engineering of Magnetic Iron Oxide Nanoparticles for Breast Cancer Diagnostics and Drug Delivery
Authors Xie M, Meng F, Wang P, Díaz-García AM , Parkhats M, Santos-Oliveira R, Asim MH , Bostan N, Gu H, Yang L, Li Q, Yang Z, Lai H, Cai Y
Received 10 May 2024
Accepted for publication 6 August 2024
Published 17 August 2024 Volume 2024:19 Pages 8437—8461
DOI https://doi.org/10.2147/IJN.S477652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Mengjie Xie,1,* Fansu Meng,2,* Panpan Wang,3,* Alicia Marcelina Díaz-García,4 Marina Parkhats,5 Ralph Santos-Oliveira,6 Mulazim Hussain Asim,7 Nazish Bostan,8 Honghui Gu,9 Lina Yang,9 Qi Li,9 Zhenjiang Yang,9 Haibiao Lai,2 Yu Cai1
1State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University International Cooperative Laboratory of Traditional Chinese Medicine Modernization and Innovative Drug Development of Ministry of Education (MOE) of China / Guangdong Key Laboratory of Traditional Chinese Medicine Informatization / International Science and Technology Cooperation Base of Guangdong Province/School of Pharmacy, Jinan University, Guangzhou, Guangdong, 510632, People’s Republic of China; 2Zhongshan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Traditional Chinese Medicine, Zhongshan, Guangdong, 528400, People’s Republic of China; 3The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, 510632, People’s Republic of China; 4Bioinorganic Laboratory, Faculty of Chemistry, University of Havana, Havana, 1400, Cuba; 5B. I. Stepanov Institute of Physics, National Academy of Sciences of Belarus, Minsk, 220072, Belarus; 6Brazilian Nuclear Energy Commission, Nuclear Engineering Institute, Laboratory of Nanoradiopharmacy and Synthesis of New Radiopharmaceuticals, Rio de Janeiro, RJ, 21941906, Brazil; 7College of Pharmacy, University of Sargodha, Punjab, 40100, Pakistan; 8Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan; 9Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, Guangdong, 518033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Cai; Haibiao Lai, Email [email protected]; [email protected]
Abstract: Data published in 2020 by the International Agency for Research on Cancer (IARC) of the World Health Organization show that breast cancer (BC) has become the most common cancer globally, affecting more than 2 million women each year. The complex tumor microenvironment, drug resistance, metastasis, and poor prognosis constitute the primary challenges in the current diagnosis and treatment of BC. Magnetic iron oxide nanoparticles (MIONPs) have emerged as a promising nanoplatform for diagnostic tumor imaging as well as therapeutic drug-targeted delivery due to their unique physicochemical properties. The extensive surface engineering has given rise to multifunctionalized MIONPs. In this review, the latest advancements in surface modification strategies of MIONPs over the past five years are summarized and categorized as constrast agents and drug delivery platforms. Additionally, the remaining challenges and future prospects of MIONPs-based targeted delivery are discussed.
Keywords: Nanopreparations, Organic Nanomaterials, Inorganic Nanomaterials, Surface Engineering, Targeted Delivery
Graphical Abstract:

Introduction
Breast cancer (BC) ranks among the top 10 cancers affecting women worldwide. In 2020, the incidence of BC (11.4%) surpassed that of lung cancer (11.1%), establishing it as the most prevalent form of cancer globally. According to the American Cancer Society, new cases of BC in women are projected to account for 31% of all cancer patients by 2023, making it the most prevalent cancer type. Furthermore, BC is identified as the second leading cause of cancer-related deaths among women. During the 1980s and 1990s, BC incidence and mortality reached peak levels.1 Over the past decade, significant advancements such as the widespread adoption of breast X-ray screening, magnetic resonance imaging (MRI), and neoadjuvant therapies (including tumor plasticity techniques, adjuvant chemotherapy options, immunotherapy approaches, targeted therapy interventions, and endocrine therapy applications) have markedly reduced BC incidence and mortality rates.2,3 Despite these improvements, global BC incidence and mortality rates continue to rise, alongside regional disparities. For instance, systematic disparities in treatment outcomes and prognosis persist, particularly among black women in less developed regions, who experience higher incidences and mortality rates associated with triple-negative breast cancer (TNBC) compared to other racial groups.4
According to the tumor scope, the development of BC is primarily categorized into five stages: stage 0: represents carcinoma in situ or non-invasive BC; stage I: indicates a maximum tumor diameter of less than or equal to 2 cm; stage II: corresponds to a maximum tumor diameter of 2–5 cm; stage III: signifies a maximum tumor diameter exceeding 5 cm; and stage IV: denotes BC invasion and metastasis. The treatment of BC depends on individualized clinical characteristics, necessitating the development of effective strategies based on diagnostic results, staging, biological subgroups, genetic factors, genomics characteristics, and individual patient conditions and preferences.5 Studies have demonstrated that the incidence of BC subtypes is influenced by race and age. HER2-positive BC shows a higher incidence compared to other subtypes while maintaining consistent ratios among racial groups.4 Hormone therapy or monoclonal antibodies targeting HER2 are commonly employed for BC treatment,6 whereas TNBC relies on traditional cytotoxic chemotherapy due to the absence of targeted proteins such as estrogen receptors and progesterone receptors. TNBC is characterized by greater aggressiveness compared to other BC subtypes, with an increased recurrence rate and mortality observed during early treatment phases, along with shorter survival times in advanced stages. Early screening, diagnosis, and precision treatment are pivotal in the fight against BC. Extensive data underscores the necessity for developing novel drug-targeting strategies to address the existing challenges in diagnosing and treating BC.
Magnetic iron oxide nanoparticles (MIONPs) targeted delivery system is one of the challenging researches in the field of BC diagnosis and treatment. MIONPs are generally defined as advanced multifunctional nanomaterials consisting of iron oxide nanoparticles and surface modifications that render them biocompatible, biodegradable, and biosafety. The core of MIONPs typically consists of magnetite (Fe3O4) or maghemite (γ-Fe2O3), with diameters ranging from 1 to 100 nm. Surface modification allows small-sized MIONPs to evade uptake by the reticuloendothelial system (RES), thereby prolonging their circulation time within the body. Furthermore, they can disperse and penetrate the dense internal structure of tumors. However, under normal physiological conditions, they cannot traverse capillary cell gaps. Commonly used preparation methods for MIONPs7 include thermal decomposition, co-precipitation, flow injection synthesis, microemulsion, sol-gel, hydrothermal methods, and sonochemistry. Among these methods, the thermal decomposition method is considered the industry standard for producing large-scale MIONPs with controllable particle size and uniform dispersion. However, Fe3O4 particles prepared through this method exhibit poor water solubility, necessitating further modification with hydrophilic materials to enhance solubility. The coprecipitation method suffers from limitations in accurately controlling particle size and dispersion. The flow injection synthesis method relies on the regulation of capillary reactor process parameters,8 making the cost of large-scale production a significant consideration. The crystal composition of iron oxide nanoparticles prepared by the sol-gel method9 is heavily affected by the annealing temperature and the initial mixture ratio. Although the technology is relatively simple, product stability is a potential challenge.
Various synthetic approaches and surface modification materials influence the physicochemical properties of MIONPs as well as their subsequent biological applications. Factors such as size distribution,10 nanocrystalline shape,11,12 nanoparticle arrangement pattern13 and magnetic parameters14 are known to affect tumor targeting efficiency, retention performance, and magnetic heating capability. These interactions occur due to the high permeability and strong retention effect (EPR effect) observed in breast tumor tissue, as well as unique physiological characteristics15 such as vascular system leakage and lymphatic drainage dysfunction associated with tumor tissues.
MIONPs and the Diagnosis and Treatment of BC
MRI is a crucial non-invasive technique for tissue and molecular imaging in the diagnosis of BC. The magnetic resonance signal is an image obtained by spatially arranging the resonance signal of protons in human tissues by gradient magnetic fields. Essentially, it is a density map of protons in human tissues. The transverse magnetization reflected by the magnetic resonance pixel value is not only related to the number of protons, but also to the characteristics of their relaxation process, and the “relaxation time” is described by the time constants “T1” and “T2”. The relaxation time of normal tissue and diseased tissue usually shows only slight differences and limited contrast. The significance of contrast agents (CAs) lies in their ability to enhance the MR signal by influencing the proton relaxation rate, thereby enhancing the sensitivity and accuracy of MRI. Clinical CAs are categorized into T1-weighted positive CAs and T2-weighted negative CAs. This review focuses on MIONPs T2 CAs with a size exceeding 10 nm. In recent years, there has been growing interest in the research and discussion of ultra-small MIONPs (0–5 nm) as T1 CAs.16
MIONPs significantly shorten the transverse T2 relaxation time of protons and exhibit a more obvious dark contrast in T2-weighted MRI. Moreover, owing to the substantial magnetic moment of MIONPs, an enhanced signal-to-noise ratio can be attained at low concentrations, thereby augmenting the resolution of soft tissue MRI. When an external magnetic field is applied to tumor tissue, MIONPs actively or passively accumulate at the tumor site, showing high sensitivity to the tumor. MIONPs are recognized for their excellent capabilities in enhancing MRI contrast17 biosafety18 and favorable pharmacokinetic properties,19 showing substantial potential in the construction of molecular imaging probes for BC.
Clinical Application of Iron Oxide Nanoparticles
Multiple formulations have received FDA approval as clinical diagnostic T2 CAs, driven by the potential of both intracellular and extracellular research and development. Feridex IV® (Ferumoxides) has been promoted in the field of MRI for liver tumor imaging. In addition, there are lymph node metastasis imaging agents Combidex® (Ferumoxtran-10), gastrointestinal contrast agents Gastromark® (Ferumoxsil), and iron supplements including Feraheme® (Ferumoxytol) are also available.
Feridex IV®
Feridex IV®20 is a sterile aqueous colloid composed of superparamagnetic iron oxide and dextran, with a particle size of 80–150nm. Following intravenous administration, it is phagocytosed by the RES in the liver, thereby creating a distinct contrast between normal and cancerous liver tissues, and facilitating lesion localization. Early studies utilizing long/short double-echo acquisition of T2-weighted MRI with ferumoxides enhancement not only yield high-quality images but also enable differentiation of metastases from benign lesions in noncirrhotic livers.21 In the context of cell therapy, where there is significant interest in the development of cell tracking and repair strategies, it has been demonstrated that in vivo labeling of monocytes with iron increases by twofold when a mixture of ferumoxides and sodium ferric gluconate is used instead of ferumoxides alone. Further relevant animal experiments are expected to establish this approach as an effective means for tracking inflammation in vivo.22 Moreover, this magnetic marker is capable of tracking the temporal and spatial migration of stem cells and other cellular tissues.23
Combidex®
Combidex® is a solution of dextran-coated iron oxide nanoparticles, with particles ranging in size from 20–50 nm, and is approved for clinical lymph node metastasis imaging. Early research results showed that the overall sensitivity (90.5%) of MRI based on Ferumoxtran-10 lymphocyte-affinity superparamagnetic nanoparticles was significantly higher than that of conventional MRI (35.4%) while the diagnostic specificity was improved from 90.4% to 97.8%.24 In the past two decades, Ferumoxtran-10 has gone from approval to deregistration, was reintroduced to the market in 2013, and has achieved encouraging clinical results in clinical use in patients.25 For example, in a direct comparison of ultrasmall superparamagnetic iron oxide particle-enhanced MRI (USPIO-MRI) and prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT), USPIO-MRI has shown greater sensitivity in detecting suspicious lymph nodes (LN) <5 mm.26
Gastromark®
Gastromark®27 is a silica-coated superparamagnetic iron oxide suspension, featuring particles sized between 200–400 nm. Unlike other injectable contrast agents, Ferumoxsil functions as a gastrointestinal contrast agent administered orally, remaining unabsorbed by the gastrointestinal tract. Its effectiveness manifests within half an hour post-administration, with excretion occurring via feces within 7–8 hours subsequent to gastrointestinal retention. The findings from two clinical trials involving a total of 186 patients demonstrate that over half of the participants exhibited improved upper gastrointestinal and gastric imaging following the administration of Ferumoxsil for 30 minutes, while also observing enhanced colonic visualization and improved delineation of anatomical markers in MRI scans.27 Nevertheless, the unpleasant taste of oral iron remains a significant barrier to its widespread clinical use. Furthermore, there is currently insufficient evidence regarding potential drug interactions and distribution in specific patient populations for Ferumoxsil.
Feraheme®
Unlike the majority of iron-based nanoparticles utilized in MRI, Feraheme® has received approval for treating iron deficiency anemia (IDA) commonly observed in patients with renal insufficiency, while concurrently undergoing clinical trials as MRI CAs. Previous preclinical studies have indicated that Ferumoxytol is expected to serve as a promising MR cell tracer in cell therapy, such as tracking Ferumoxytol-labeled stem cells28 and macrophages29 by MRI to monitor cell migration and rejection reactions. Results from a small-scale clinical trial’s histopathological analysis have validated the clinical feasibility and applicability of Ferumoxytol-enhanced MRI for the quantitative detection, and monitoring of macrophages in tumors.30 In addition to providing new non-invasive quantitative biomarkers for tumor treatment stratification and assessment of treatment response, studies have demonstrated that Ferumoxytol can induce phenotypic transformation of M2 macrophages into M1 macrophage subtypes characterized by high CD86 and TNFα positivity,31 and M1 polarization triggers the Fenton reaction, thereby inhibiting BC growth.32 It can be speculated that Feraheme®-mediated tumor immunomodulation is expected to synergize with chemotherapy and potentially achieve new breakthroughs in clinical practice.
Currently, commercial CAs based on MIONPs are limited by low crystallinity or poor surface modification, thus limiting their widespread adoption in BC diagnosis. MagTrace® (MT), currently in clinical trials, is primarily utilized for sentinel lymph node biopsy (SLN) in early-stage BC axillary staging, aiming to enhance identification rates and diminish complication occurrences.33 As early as 2013, The SentiMAG Multicentre Trial compared MT with standard techniques (radioactive isotopes with blue dye (BD) or radioactive isotopes alone) for sentinel lymph node biopsy. MT demonstrated a recognition rate of 94.4%, marginally lower (0.6%) than standard methods (95%), with LN retrieval values closer to actual counts.34 A recent network meta-analysis quantitatively assessed various sentinel lymph node identification techniques and their respective false-negative rates, comprehensively comparing detection and false-negative rates across all methods. Findings indicate that MT outperforms BD alone in lymphatic tracing, exhibiting comparable sentinel lymph node detection and false-negative rates to dual tracer methods.35
At present, there are still some SPIO (Small particles of iron oxide) and USPIO (Ultrasmall particles of iron oxide) in the clinical trial stage, as summarized in Table 1.
 |
Table 1 MIONPs-Based CAs in Clinical Trials in the Last Decade |
Application of Iron Oxide Nanoparticles in BC Diagnosis and Treatment
MIONPs possess integrated design capabilities for optimized imaging, cell tracing, and targeted therapy. This combination renders MIONPs an ideal platform for both BC diagnosis and treatment. The MRI enhancement effect of MIONPs significantly depends on the size of the nanoparticle,17 aggregation state,47 and surface conjugates, impacting their biocompatibility and MRI effect. Studies have demonstrated that cubic nanoparticles exhibit superior performance in terms of crystallinity, single crystal size, saturation magnetization, and T2 relaxation rate compared to spherical nanoparticles of the same size.48 These high-quality nanocrystals hold great potential as candidates for diagnostic imaging and treatment in BC.49 For instance, 225Ac radionuclide-labeled MIONPs (225Ac@Fe3O4-CEPA-trastuzumab) can serve as a valuable tool for fluoroscopy in HER2-positive BC, combining trastuzumab with α-radioimmunotherapy and magnetic hyperthermia.50 NO-responsive MIONPs nanoprobes,51 modified with o-phenylenediamine (OPA) on the surface, are capable of monitoring dynamic changes in the phenotype of M2/M1 macrophages in tumors, offering insights into the prognostic evaluation of cancer treatments based on macrophage-mediated immune responses. Additionally, superparamagnetic iron oxide nanoparticles (SPIONs) are capable of replacing traditional radioactive substances such as Technetium-99m (Tc99) and BD for SLN detection and localization in primary BC metastasis assessment,33,52,53 enabling completion with an ultra-low dose intradermal injection method54 while improving sensitivity, specificity, and accuracy compared to conventional approaches.55 Another critical marker for tumor metastasis is circulating tumor clusters (CTC). The tracking, capture, and elimination of CTC can be facilitated by MIONPs leveraging cancer cell-related biomarkers or the electrostatic binding affinity between cancer cells and positively charged surfaces56 (Figure 1). Furthermore, the comprehensive diagnostic strategy of combining MRI with magnetic particle imaging (MPI), fluorescence molecular imaging (FMI), or near-infrared fluorescence imaging (NIRF) of MIONPs, based on their unique size and adjustable functional composition, is paving the way for advancing BC prevention and treatment.57
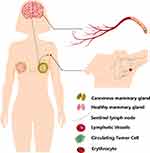 |
Figure 1 Schematic diagram of brain metastasis and sentinel lymph node metastasis of BC. |
Representative applications of MIONPs applied to BC diagnosis are outlined in Table 2.
 |
Table 2 Summary of Representative Applications of MIONPs |
More importantly, the inherent Brownian and Neel relaxation mechanisms of MIONPs efficiently convert the energy of an alternating magnetic field (AMF) into thermal energy, resulting in significant thermal effects.67–70 Within the treatment range, temperatures can be elevated to a range of 42–46 °C.71 Local temperature extremes are poorly tolerated by tumor cells, leading to protein denaturation,72 increased permeability of cell membrane,73 swelling of cell organelles,74 and DNA damage75 irreversible damage, with little or no effect on normal tissue.76,77 These findings strongly support the effectiveness of MIONPs-based magnetic hyperthermia (MHT) as a promising physical anti-tumor modality78 (Figure 2). Furthermore, the incorporation of one or more therapeutic drugs onto the surface of MIONPs represents a promising strategy for targeted drug delivery in cancer treatment. Nevertheless, suboptimal MHT and combined drug therapy approaches may yield unintended consequences: concurrent drug administration can lead to multidrug resistance,79 while excessive heat shock protein exposure may promote cancer metastasis.80,81 Designing precise and controllable strategies for combining magnetic hyperthermia with drug therapies is crucial to addressing issues such as low efficacy, drug resistance, aggressiveness, and recurrence commonly encountered in BC treatment.
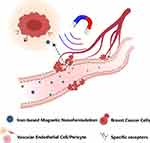 |
Figure 2 Schematic diagram of targeted drug delivery for magnetothermal therapy. |
Targeted delivery system based on surface modification of MIONPs exhibits significant potential as an advanced platform for early clinical diagnosis, real-time imaging, and precision adjuvant therapy of BC, owing to its inherent MRI, biocatalytic activity (nanoenzymes), MHT, and homologous targeted delivery of chemotherapeutic and gene therapy drug.47
Surface Engineering of MIONPs
Most iron oxide nanoparticles are inherently hydrophobic, necessitating surface modification for biomedical applications. The targeting strategies for nanoparticles encompass passive targeting via the enhanced permeability and retention (EPR) effect, and active targeting relying on specific targeting ligands. Challenges at the tumor site, such as uncontrolled angiogenesis, high interstitial pressure, dense tumor cells, and the extratumoral matrix, impede drug penetration to the tumor core and hinder optimal drug efficacy. Achieving targeted tumor delivery involves four critical steps: effective retention, phagocytosis evasion, precise targeting, and controlled release. MIONPs leverage an external magnetic field for guided navigation. The modified material coating the iron core acts as a carrier, enhancing biocompatibility and providing docking sites for drugs, fluorescent markers, proteins, enzymes, antibodies, or nucleic acids82 that bind to tumor cell-specific receptors. By navigating the intricate tumor microenvironment and evading macrophage phagocytosis, MIONPs accumulate significantly in target tissues and facilitate rapid drug release directly into deep-seated lesions. This targeted accumulation minimizes off-target site deposition, while also preventing nanoparticle aggregation under physiological conditions, thus avoiding potential blockages in blood vessels.83
Meanwhile, leveraging their inherent enzymatic catalytic activity, MIONPs can facilitate the conversion of endogenous H2O2 into reactive oxygen species (ROS), thereby triggering a novel iron-dependent form of programmed cell death known as ferroptosis. The hydrodynamic diameter, surface charge, morphology, surface coating, and modifications play pivotal roles in modulating the intrinsic enzymatic catalytic activity of MIONPs. For example, a magnetic near-infrared nano-photosensitive micelle (CSO-SS-Cy7-Hex/SPION/Srfn) was developed as a strategy for tumor imaging-guided ferroptosis therapy (FT). Excess lipid peroxides (LPO) accumulate through three pathways: mitochondrial membrane oxidation/reduction reactions, depletion of GSH by disulfide bonds, SPION-induced release of Fe2+ initiating the Fenton reaction, and near-infrared light irradiation. Ultimately, these processes trigger mitochondrial collapse and irreversible ferroptosis.84,85 The unique dual therapeutic approach combines an aldehyde dehydrogenase inhibitor targeting cancer stem cells (CSCs) with ferroptosis, effectively eliminating malignant breast tumors by enhancing ferroptosis through glutathione (GSH) depletion.86 Additionally, surface-engineered MIONPs induce tumor cell pyroptosis. For example, the virus spike tumor-activated hyperthermia agent (VTPA) accumulates at tumor sites via systemic circulation, triggering intracellular lysosomal rupture and release of iron cores that induce ROS production. Lysosomal rupture and ROS synergistically activate the caspase-1 pathway, leading to the cleavage of Gasdermin family members (such as GSDMD, GSDME), ultimately disrupting the plasma membrane of cancer cells87,88 (Figure 3).
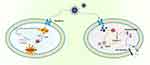 |
Figure 3 MIONPs mediate iron death/cellular scorch death. |
The surface modification of the nucleus of MIONPs can lead to nanoparticles that can be used for specific applications (targeting specific tissues, loading drugs, and inducing exogenous biological processes). For example, organic polymers can provide MIONPs with excellent biocompatibility and loading capacity, and inorganic small molecule coatings can improve MIONP stability and control size distribution. The modification of nanoparticle surfaces with natural products, polymeric, organic, or inorganic coating was made by one-pot or layer-by-layer methods. Different types of reactions were used for modified IONPs surface, such as click chemistry,89 UGI multicomponent reaction,90 bioconjugation,91 and others.92 Therefore, This review summarized the recent progress of various organic and inorganic molecules as surface engineering materials for MIONPs.
Organic Nanomaterials
Polymers
Polymer-encapsulated MIONPs have been extensively investigated as an exceptional drug carrier with their remarkable performance. Their advantages can be summarized as follows: (1) Drug delivery assisted by an external magnetic field using a magnetic core surpasses the limitations of traditional diffusion and transmembrane transport methods. (2) The polymer coating exhibits excellent biocompatibility, ensuring prolonged circulation time of drugs and enhancing specific anticancer drug concentrations at tumor sites compared to other locations by facilitating interaction with target molecules on nanocarrier surfaces. (3) Prevention of spin disorder, uncontrolled oxidation, and nanoparticle aggregation improves the stability of nanoformulations. (4) Bio-based polymers are cost-effective and less toxic, promising for future industrial production.
Polyurethane
Polyurethane (PU)93 is a well-established biocompatible and blood-compatible biomaterial. Nevertheless, the hydrophobic nature of PU surfaces renders them prone to nonspecific protein adsorption and platelet adhesion, potentially resulting in the formation of blood clots.94 Techniques for surface modification, such as isocyanate chemical coupling, surface plasma modification, and hydrogen bond interaction, are employed to confer upon PU the ability to resist nonspecific antigen adhesion.
Waterborne polyurethane is an innovative green polyurethane system that utilizes water as a dispersion medium instead of organic solvents. This approach offers several advantages, including cost-effectiveness, safety, and non-toxicity. The main chain of waterborne polyurethane effectively stabilizes hydrophobic drugs through electrostatic forces, playing a unique role in drug delivery. When iron oxide nanoparticles are incorporated into the PU matrix, they can provide structural support for the matrix. The polyurethane structure exhibits high elasticity, good conductivity, magnetizability, and other properties, making it suitable for utilization as a novel composite nanomaterial to enhance MRI quality.95 The most crucial aspect is that their integration confers magnetic and antibacterial properties96 to the polymers, enabling them to catalyze Fenton-like and Haber-Weiss reactions, leading to oxidative stress and cellular damage. For example, aqueous polyurethane-based nanomicelles co-encapsulated with hydrophobic SPIONs and the anticancer agent doxorubicin (DOX) exhibited good superparamagnetic behavior and magnetic responsiveness,97 and experimental results showed that the MRI contrast effect was concentration-dependent.
Despite the benefits of various surface modification technologies in augmenting the biological functionalities of PU-coated MIONPs, persistent challenges exist: (1) Modified polymers may degrade due to exposure to the multifactorial body environment, necessitating further investigation into long-term stability and durability; (2) The chemical coupling method could potentially induce cytotoxicity due to incomplete reactions, necessitating safety evaluation; (3) Ensuring the reproducibility in clinical settings and stable large-scale industrial production technology remains crucial.
Polyethylene Glycol
Modified MIONPs with polyethylene glycol (PEG) is a widely employed strategy. PEG, being a flexible hydrophilic polymer capable of forming segments and densely bound chains,98 serves as an effective tool for extending drug circulation in vivo and safeguarding therapeutics against adverse immunogenicity.99 Moreover, the spatial repulsion effect of PEG chains confers an advantage in enhancing drug stability.100 Siloxane crosslinked PEG-coated MIONPs with small particle sizes release 50% of the drug within the first 4 hours and completely release all drugs within 10 hours in a weak acidic environment, effectively achieving controlled drug release101 (Figure 4). The PEG coating not only shields nucleic acid drugs from degradation in the body but also enhances their accumulation at tumor sites through increased aggregation. For example, CaP/PEG-polyanion/siRNA magnetic composite nanoparticles (MHNPs) were prepared with CaP serving as an interlayer to enhance biocompatibility, and polyethylene glycol-polyaspartic acid (PEG-PAsp) as an outer stabilizer layer for delivering siRNA targeted to the cytoplasm of BC cells. MHNPs effectively reduced the expression of tumor cell vascular endothelial growth factor (VEGF) compared to free siRNA.102 Magnetic nanoparticles, based on calcium phosphate-polyethylene glycol block copolymer (CaP-PEG) and coated with caffeic acid (Caf-MCaP), were synthesized by using a co-precipitation method, resulting in a nearly neutral surface zeta potential. Magnetization curves demonstrated that Caf-MCaP exhibited superparamagnetic behavior and low coercivity. By encapsulating siRNA within CaP-PEG, efficient delivery across biological barriers to the cytoplasm of tumor cells was achieved under magnetic guidance, leading to enhanced silencing of the HER2 gene.103
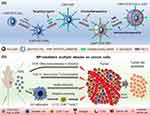 |
Figure 4 (a) Schematic illustration of preparation of the EBP-modified and DOX/Poly IC loaded nanocarrier. (b) Mechanisms of targeted and combined chemo-immuno therapy for TNBC. Reprinted from Materials Today. Volume 50. Mu Q, Lin G, Jeon M, et al. Iron Oxide Nanoparticle Targeted Chemo-Immunotherapy for Triple Negative Breast Cancer. Page numbers: 149–169. Copyright (2021), with permission from Elsevier.101 |
It is well known that drug-targeted controlled release largely hinges on the interaction between the bound ligand and the receptor specifically expressed at the molecular level on the target site. However, the receptor-guided strategy faces several disadvantages, including cancer cell heterogeneity,104 and the presence of binding site barriers.105 Combining PEG surface modification with physiologically triggered drug release mechanisms (such as pH or enzyme concentration) represents an optimization strategy to overcome these challenges. A SiO2-modified MRI probe covered with a PEG functionalized coating, and established a pH-responsive intelligent system through pH-low insertion peptide (pHLIP) modification. Histological analysis revealed that the additional pHLIP-modified MNPs were localized in the amphisomes, autolysosomes, and multilamellar bodies within the tumor acidic microenvironment.106
Although PEGylation is considered the “gold standard” for surface modification of iron oxide nanoparticles, within the complex pathophysiological environment of BC, the interaction between PEG and plasma proteins critically regulates circulation time, biodistribution, drug release, and immunotoxicity of MIONPs. Challenges arising from these interactions should not be underestimated. The first is the ubiquitous IgM adsorption.107 PEG-functionalized MIONPs may be naturally recognized as potential pathogens by IgM while entering the bloodstream. This recognition prompts splenic B lymphocytes to produce anti-PEG IgM antibodies, which in turn activate complement and trigger the mononuclear phagocyte system (MPS), resulting in accelerated blood clearance (ABC). Secondly, there is a propensity to induce adverse reactions, notably hypersensitivity reactions. Third, the protein corona formed by the adsorption of PEG and plasma proteins may significantly change the colloidal stability and biological fate of nanoparticles, such as shielding the targeting affinity of specific antibodies and ligands, known as the “off-target effect”.108 To mitigate adverse clinical reactions, attention must be directed towards understanding the specific immune responses elicited by PEG coating, encompassing its physiological induction mechanisms and the impact of structural characteristics of PEGylated nanoparticles.109
Carbohydrate Polymers
Carbohydrate polymers110 are distinguished by their extensive surface area, biodegradability, and environmentally friendly properties, rendering them promising support materials for iron oxide nanoparticles.
Carbohydrate polymers can be categorized into three main groups: (1) natural polysaccharides, (2) synthetic polysaccharides derived from natural sources, and (3) sugar polymers. The combination of carbohydrate polymers with iron oxide nanocores results in the manifestation of distinctive biological activities, including the silencing of carcinogenic epidermal growth factor receptors,111 antibacterial properties, enhanced immunogenicity,112 and targeted delivery to tumor cells.113 β-cyclodextrin (β-CD) with poly (D, L-lactic acid-co-glycolide) PLGA were conjugated by using chemical functional groups to develop a biodegradable polymer with a high drug loading capacity. This highly efficient magnetic carrier has an average size of 30 nm. Both the β-CD cavity and the polymer chain are capable of binding camptothecin. Experimental data demonstrate that the drug encapsulation rate can reach 89%. In addition, PLGA has the characteristics of pH responsiveness and photothermal responsiveness. The sustained release of camptothecin in vivo is facilitated by an external magnetic field in conjunction with the weakly acidic microenvironment of BC, ensuring the maintenance of an effective drug concentration.114 While the efficacy of the preparation has been confirmed in in vitro experiments involving folate receptor-overexpressing MCF7 cells, further studies are required to assess its in vivo effectiveness. Agar, a linear polymer composed of 1,3-linked β-D-galactose and 1,4-linked 3,6-endoether-L-galactose units, can form chelates with metal ions in a strongly alkaline environment, utilizing its free hydroxyl groups. The cytotoxicity of composite nanoparticles (Fe3O4/agar/Au NPs), created by coating the surface of MIONPs with a biopolymer consisting of gold ions and agar, demonstrates a concentration-dependent pattern. Comparison of IC50 values of Fe3O4/agar/Au NPs across various cell lines, including breast adenocarcinoma (MCF-7), breast cancer (Hs 578Bst), invasive ductal carcinoma (Hs 319.T), and metastatic carcinoma (MDA-MB-453), reveals that the Hs 319.T cell line exhibits the highest cytotoxicity, with an IC50 value of 165 μg/mL.115
Molecularly Imprinted Polymers
Molecularly imprinted polymers (MIPs) mimic the natural antibody-antigen mechanism to create binding sites for specific “template molecules” at the interface of cancer cell membranes or the extracellular matrix (ECM), demonstrating predetermined selectivity toward the target site.116 The dosage of crosslinker significantly affects both the drug loading capacity and stability of MIPs.117 In comparison to traditional bioligand modification strategies, MIPs offer enhanced stability and cost efficiency, making them promising candidates for large-scale production.116 MIPs outperform non-imprinted polymers due to their superior selectivity and affinity. Magnetic MIPs (MMIPs), which integrate a magnetic iron core with a MIP shell, can be guided effectively using an external magnetic field to achieve targeted drug release and local magnetic hyperthermia.118 In addition, the high specific surface area of MIONPs facilitates rapid and targeted delivery of MIPs within the organism,119 reducing permeability issues and enhancing resistance to interference. Some scholars have designed and synthesized an MMIP using MIP technology, employing methacrylic acid (MAA) and itaconic acid (ITA) as pH-responsive monomers for efficient delivery of Zidovudine (AZT). The pH sensitivity of MMIPs was demonstrated in the treatment of BC. AZT-loaded MMIPs exhibited significantly higher cytotoxicity compared to free AZT, approximately 49 times greater, and induced apoptosis in BC cell lines by participating in the caspase-dependent apoptosis signaling pathway120 (Figure 5).
 |
Figure 5 Schematic illustration of the synthesis of MMIOs-based MIONPs and mechanism diagram of Anti-BC. Reprinted from Mater Sci Eng C Mater Biol Appl. Volume 103. Hassanpour A, Irandoust M, Soleimani E, Zhaleh H. Increasing the Anticancer Activity of Azidothymidine toward the Breast Cancer via Rational Design of Magnetic Drug Carrier Based on Molecular Imprinting Technology. Page number: 109771, Copyright (2019), with permission from Elsevier.120 |
The magnetic core preparation is identified as pivotal in MMIP synthesis. Future development of MMIP programs should prioritize optimization of the magnetic core preparation process and identification of appropriate functional monomers, templates, and cross-linking agents.
In addition to the polymers mentioned above, materials such as chitosan, Poly (methyl acrylic acid carboxyl betaine) (pCBMA), polyaniline, and others have been utilized as modifiers for MIONPs (Table 3). Polymer-encapsulated MIONPs hold significant promise in the fields of drug-targeted delivery and BC visualization imaging.
 |
Table 3 Review of MIONPs Based on Different Polymer Coatings as a Platform for BC Diagnosis and Treatment |
Lipids
Lipids constitute vital components of cell membranes. Biomimetic vesicle structures can be formed by phospholipid molecules, enabling the encapsulation of MIONPs and creating a natural protective barrier.
As an innovative drug delivery system, magnetic liposomes combine the dual advantages of metal nanoparticle magnetohydrodynamics and liposome technology, playing a key role in the field of targeted drug delivery: (1) Thermosensitive drug release can be achieved. Closed double-layer magnetic thermosensitive liposomes, with nano-sized iron oxide at their core, enable the modulation of drug release by altering the liposomal membrane structure at varying temperatures.128 For instance, magnetic liposomes loaded with betulinic acid (BA), prepared via the thin layer hydration method, demonstrate increased cytotoxicity against MDA-MB-231 cells under elevated temperature conditions compared to BA alone. With a saturation magnetization intensity of 48.7 emu/g, these liposomes exhibit both thermosensitive drug release and superparamagnetism, making them ideal for hyperthermia-induced and remotely controlled drug delivery.129 (2) The magnetomotive force of iron oxide is an effective strategy to achieve the fixed-point thermosensitive drug release under the action of an external magnetic field. Nanostructured lipid carriers (NLCs) incorporating MIONPs have been demonstrated to enhance the release and efficacy of chemotherapeutic agents through magnetic hyperthermia.130 It was observed that exposure to SPIONs-NLC and MTX-SPIONs-NLC under external magnetic field stimulation increased the apoptosis rates of cancer cells from 35% (with MTX-SPIONs-NLC alone) to 50% and 65%, respectively. Moreover, cell viability demonstrated time-dependent behavior, suggesting that the enhanced cytotoxicity of MTX-loaded NLC co-formulations may be attributed to MTX release within the NLC or potentially to lipid matrix degradation. (3) External liposomes of iron oxide core were employed to enhance drug loading capacity. Development of calcium ferrite nanoparticles (CaFe2O4) for creating solid or aqueous magnetic liposomes through lipid bilayer coating or encapsulation. Solid magnetic liposomes showed superior superparamagnetic behavior, whereas aqueous magnetic liposomes exhibited approximately 10% higher drug encapsulation efficiency (EE%) compared to solid counterparts. These findings underscore the promising magnetic properties and high EE% of drug-loaded nanocarriers containing biocompatible calcium ferrite nanoparticles, suggesting their potential for dual therapy combining chemotherapy and magnetic hyperthermia in BC treatment.131 (4) The flexibility of the lipid bilayer allows the incorporation of a PEG “stealth layer” to increase blood circulation time, making it an excellent candidate material for contrast agents.132 Magnetic liposomes are engineered to form nanoclusters in a controlled manner, typically resulting in heightened local magnetic field inhomogeneity, prolonged water residence time, and increased spin-spin relaxation rate (r2 or 1/T2 description) of protons in the environment. This approach facilitates achieving an optimal r2/r1 ratio,133 enhancing the intensity of dark contrast signals in T2-weighted MRI.
In addition to the synthetic phospholipids used above, another type that can be included in the scope of magnetic liposomes is bionic MIONPs. This approach offers an opportunity to merge the characteristics of natural cell membranes with those of artificial iron oxide core materials. This bionic phospholipid membrane replicates the physiological composition and motility of normal cells, enabling successful evasion of non-specific RES uptake and offering significant advantages in the realm of targeted delivery using MIONPs. For example, recent research utilized a magnetic core modified with extracellular vesicles (EVs)134,135 extracted from BC cells and employed MPI to monitor and characterize brain metastasis in BC. This study demonstrated that EV-modified MIONPs can traverse the blood-brain barrier (BBB), serving as both an imaging tool and a drug delivery carrier for targeted brain metastasis. With the cancer cell membrane (CM) as a shelter of polyethylene imine bionic magnetic nano gel (NG) system can effectively achieve (DTX) and antibody (CD47 siRNA) homologous targeted delivery136 (Figure 6). By downregulating the “don’t eat me” signal of cancer cells, it induced immunogenic cell death (ICD) and promoted macrophage M1 polarization, while reversing the immunosuppressive microenvironment137 caused by immune checkpoint inhibition, promoting dendritic cell (DCs) maturation and cytotoxic T lymphocytes (CTLs) infiltration into tumor sites, and effectively inhibiting the growth and lung metastasis of primary BC.
 |
Figure 6 Schematic illustration of the synthesis of DTX/USIO@PEI NGs/siCD47@CM for synergistic chemo-immunotherapy and MRI of tumors in vivo. Reprinted from Li L, Gao Y, Zhang Y, et al. A Biomimetic Nanogel System Restores Macrophage Phagocytosis for Magnetic Resonance Imaging-Guided Synergistic Chemoimmunotherapy of Breast Cancer. Adv Healthc Mater. 2023;12 (26): e2300967. © 2023 Wiley-VCH GmbH.136 |
Due to the intricate nature of the tumor microenvironment, hybrid bio-mimetic membranes coated with a combination of two or more cell membranes exhibit superior tumor-targeting efficiency compared to pure bio-mimetic membranes.138 A hybrid biomimetic membrane, modified with osteoclasts and tumor cells, is effectively targeted towards osteoclasts and tumor cells at bone metastasis sites via a homologous targeting mechanism. This approach is complemented by sonodynamic therapy and chemodynamic therapy to mitigate malignant bone metastasis in BC. Nevertheless, mere biofilm modification proves insufficient. Excessive use of immune cell membrane-coated MIONPs may induce or exacerbate inflammation through interactions with the immune system, resulting in the release of pathological mediators. Hence, the development of more intelligent and effective biofilm-coated MIONPs is imperative to facilitate potential industrial production and clinical translation.
Proteins and Amino Acids
In comparison to polymers, lipids, and other materials, protein/amino acids-based nanocarriers exhibit superior biofunctionality, biomolecular recognition ability, and renewable sourcing potential.
Proteins play a crucial role in gene delivery due to their distinct advantages. Traditional gene therapy methods often rely on retroviruses or adenoviruses, which inherently possess toxicity and induce immunogenic responses upon frequent or prolonged administration. Protein-based nanocarriers, utilizing proteins closely resembling natural proteins as shells, address the drawbacks of viral systems and serve as alternatives to viral vectors in gene therapy. Furthermore, protein-coated MIONPs are straightforward to prepare and scale up in production. A polyethyleneimine (PEI)-sericin-modified MIONPs have been reported for delivering siRNA to the tumor site, demonstrating the positive effect of tyrosine kinase-like orphan receptor (ROR1) gene downregulation on TNBC treatment in both in vivo and in vitro experiments.139 The surface cationic properties of PEI enhance particle stability and promote effective transfection of ROR1 siRNA. Sericin, on the other hand, mitigates the binding of NPs to plasma proteins, thereby reducing their inflammatory potential compared to other commonly used biodegradable polymers.140 Another study involves SPIONPs (SP-AH) coupled with Argonaute 2 protein and anti-HER2 antibody as carriers of autophagy-inhibiting microRNA (MIR376B), which effectively downregulates autophagy-related targets of miRNA and inhibits autophagy activity in BC cells, representing a novel adjuvant therapeutic agent for BC.141 In addition to serving as drug delivery carriers, protein coatings are also considered for the fabrication of high-performance MIONPs T2 CAs, such as casein,142 known for their high permeability, proton affinity, and plentiful hydrated groups. The T2 relaxation time of MIONPs coated with casein is 2–3 times longer than that of MIONPs coated with amphiphilic polymers.
Amino acid-coated MIONPs are mostly used as carriers for chemotherapeutic drugs. For example, lysine-coated MIONPs loaded with tamoxifen (TMX), the data showed that the apoptosis induced by F-Lys-TMX NPs treatment for 48 and 72 h (53.1% and 58.6%, respectively) was significantly higher than that induced by free TMX treatment for 48 and 72 h (36.1% and 45.7%, respectively), and the apoptosis induced by HER2 in F-Lys-TMX NPs treatment significantly reduced the expression levels in cells.143 Another interesting feature of amino acid carriers is their diversity of functional groups, which allows for the coupling of targeted ligands or drug molecules.12 The NPs are also characterized by a variety of functional groups. In addition, the tumor acidic microenvironment facilitates the cleavage release of the affixed drug, suggesting that amino acid-based MIONPs have a better chance of targeting the drug to the tumor site. When glutamic acid is in-situ encapsulated on the surface of iron oxide magnetic nanoparticles, high density of amine and carboxyl functional groups of glutamic acid provide abundant coupling sites for drug molecules. The DOX and MTX dual drug coupling exhibits the potential to track and kill cancer cells under pH-dependent release and thermal activation of external AC magnetic field.144
MIONPs based on proteins or amino acids also present certain limitations:145 Firstly, natural proteins vary widely in molecular weight. This variability can impact the particle size distribution of MIONPs during industrial production across different protein batches. The advent of recombinant protein technology146 is expected to effectively mitigate this challenge. Compared to natural proteins, recombinant proteins offer consistent batch-to-batch performance, abundant availability, and the capability to be engineered for specific properties such as solubility, stability, and efficacy. Secondly, certain proteins or amino acids can be prone to degradation because of their natural hydrophilicity, which hinders controlled drug release. The use of chemical cross-linkers to enhance controlled release poses the risk that incompletely reacted cross-linkers may persist within MIONPs. This persistence could hinder biodegradation or lead to the formation of toxic byproducts during circulation in organisms. Therefore, exploring novel non-toxic and degradable cross-linkers is crucial for mitigating these issues.
Inorganic Nanomaterials
Silicon Dioxide (SiO2)
Silica is widely regarded as one of the most ideal coatings for MIONPs due to several advantages. Firstly, it offers excellent stability in tumor acidic microenvironment. When administered intravenously under physiological conditions, bare iron oxide nanoparticles release iron ions that accumulate in the liver. This iron ions overload increases ROS, causing oxidative stress that damages mitochondria, induces protein denaturation, and accelerate damage to cell membranes and DNA.147 Silica coating serves as an ionic surface modifier, reducing cytotoxicity by enhancing spatial repulsion between particles and preventing aggregation. Secondly, mesoporous silica structure significantly enhances drug loading. For instance, the Co-loading of two different drugs, letrozole and curcumin, was achieved by coating the magnetic NiCoFe2O4 core with a thin layer of silica and niosomal structure. The niosomal structure confers pH responsiveness, while silica provides a high loading capacity.148 Thirdly, the physical properties of silica coating can be precisely controlled by adjusting reaction conditions, allowing for modulation of the surface roughness of MIONPs. This enhancement improves their capability to bind to biological molecules and increases sensitivity to electron radiation, potentially augmenting the effectiveness of radiotherapy (RT).149 For example, the near-infrared light and magnetic field dual-effect anticancer drug PSiNPs@(Fe3O4/Au) showed certain advantages in the treatment of multidrug resistance in BC,150 achieving a synergistic “magnetothermal + photothermal” anti-tumor enhancement effect. Another notable study involves integrating plasma AuNPs and silica-coated iron oxide NPs (Fe3O4/SiO2NPs) as magnetically aligned components within a temperature and pH dual-responsive polyvinyl alcohol (PVA) gel network. This preparation exploits the swelling and collapse of the PVA gel and the plasma-induced heating of AuNPs to facilitate the capture and release of the highly selective cytotoxic anticancer agent DOX in targeted tissues. Experiments indicate that compared to the control group, Au/Fe3O4/PVA-10%DOX achieves a tumor inhibition rate of (70±6.3%) under near-infrared light irradiation and magnetic field exposure.151 Even though external magnetic fields can accelerate the aggregation of mesoporous silica-MIONPs at tumor sites, they still lack specific localization functions. The abundant silanol groups on the silica surface provide ideal anchor points for the covalent bonding of specific ligands. For example, the modification of mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles (HA-MSNs) with hyaluronic acid (HA) effectively addresses these localization function shortcomings.152 HA is chemically or physically cross-linked with functionalized nanoparticles through covalent bonds or non-covalent means. This modification enables targeted inhibition of tumor infiltration and metastasis through cell surface receptors such as CD44 and RHAMM.153 Furthermore, HA imparts intrinsic anti-inflammatory properties154 and reshapes the ECM, which is the key to regulating the immune system and cancer monitoring.
Gold
Gold coating will induce surface plasmon resonance to generate local heat flow under the stimulation of light. Unlike chemotherapy,155 gold-coated Fe3O4 composite nanoparticles can reshape the tumor microenvironment by mediating a photothermal effect that reduces cancer-associated fibroblasts (CAF).156 Additionally, they enable photothermal-controlled rapid drug release and targeted recognition in vitro.
A comprehensive diagnostic and therapeutic technique based on gold-coated MIONPs for photoacoustic/MRI dual model imaging diagnosis and photothermal therapy157 offers the advantages of short-term repeatable treatment, safety, and low drug resistance. A magnetic-optical iron-gold core-shell nanoparticle with a distinct Raman reporting factor can serve as a four-color surface-enhanced Raman scattering (SERS) nanolabel to quantitatively identify and detect four surface protein markers on individual tumor cells in whole blood simultaneously, providing a means for detecting and monitoring cancer metastasis.158 Other researchers have designed a magnetically targeted 198Au radionuclide therapeutic drug designed to target HER2-positive BC by coupling with the biomacromolecule trastuzumab (Tmab).159 Combining magnetic hyperthermia with radionuclide therapy can enhance blood flow in tumors and induce the generation of ROS that damage DNA under ionizing radiation. This Fe3O4@198Au-Tmab radioactive bioconjugate enables a multimodal platform for combined ionizing radiation and magnetic hyperthermia treatment. It has been observed in studies that gold coating also induces the expression of HSP70. Elevated levels of this immunogenic protein can enhance immune cell recognition of tumor cells and promote ICD.160 The gold coating can effectively improve the efficiency of photothermal conversion and cooperate with MRI for accurate tumor detection and monitoring treatment. Compared with other metals (copper and silver), the drawback of gold coating is its high cost, which limits its suitability for industrial production.
Graphene Oxide
Graphite interacts with strong oxidizing agents to produce graphene oxide (GO), which contains oxygen-containing functional groups such as -OH, -COOH, and epoxy groups on its surface. These groups are responsible for the hydrophilicity and efficient drug-loading capacity of GO.161 The GO-Fe3O4 hybrid nanoparticles can be synthesized by interacting GO with the surface amino groups of MIONPs coated with short-chain amino acids. The synthesis of non-covalent (nc-GO-Fe3O4-HCPT) based on the physical adsorption of Hydroxycamptothecin (HCPT) on the surface of GO-Fe3O4 nanocomposites based on π-π interactions possessed more potent antitumor activity than the covalent (c-GO-Fe3O4-HCPT) synthesized based on the chemical binding of modified HCPT to GO-Fe3O4. The superiority of nc-GO-Fe3O4-HCPT over c-GO-Fe3O4-HCPT in NRU analysis was evidenced by a more pronounced reduction in mitochondrial activity.162 Another gefitinib-loaded GO exhibited 64% drug-carrying capacity through nanocomplexes formed by covalently linking with amine-functionalized iron oxide nanoparticles, and at a concentration of 40 ppm, GOIGF had an incremental inhibitory potential of ~10% over pure GF.163 The good electrical and thermal conductivity of GO synergistically enhanced the specific absorption rate (SAR).164 For instance, it was developed that tumor-targeted magnetic nanoparticles (FVIOs-GO-CREKAMTD) with a high SAR and enhanced ROS generation. The combination of MIONPs-mediated magnetothermal dynamics (MTD) and ROS-mediated ICD demonstrated that reducing the exposure time to low doses of alternating magnetic fields could achieve comparable anti-tumor efficacy as conventional magnetothermal therapy. Furthermore, this approach promoted the infiltration of CD4 helper proteins and CD8 cytotoxic T lymphocytes into tumors.165 Additionally, the size of MIONPs significantly influenced GO performance, where smaller MIONPs enhanced GO surface reactivity and exhibited superparamagnetic behavior. Moreover, their pharmacokinetics, stability, and toxicity characteristics were also affected.166 These size-dependent changes in application performance highlight the importance of selecting appropriate GO-MIONPs conjugates in biomedical fields such as MRI.
Metal Oxides/Metal Sulphide
Metal oxides/metal sulfides have emerged as highly promising candidates in the field of MIONPs-related bionanomedicine owing to their distinctive physicochemical properties.
Nanoscale metal oxides, such as MgO, NiO, CdO, and ZnO, have found extensive applications in antibacterial and anticancer research due to their substantial pore volume, non-toxic nature, cost-effectiveness, exceptional thermal stability, and chemical durability. The underlying mechanisms encompass but are not limited to DNA chain disruption in cancer cells,167 cell wall damage168 and regulation of cellular oxidative homeostasis.169 Four novel high-purity α-Fe2O3-ZnO nanostructures were synthesized (including disk-shaped (FZ-ND), spindle-shaped (FZ-NSP), rod-shaped (FZ-NR) and spherical (FZ-NS) structures) by simple solvothermal method combined with heat treatment. Among these nanostructures, the FZ-NSP sample exhibited the highest cytotoxicity to MCF- cells at all concentrations, showing a concentration-dependent effect, reaching 80% at a concentration of 250 μg/mL.170 Transition metal chalcogenides (TMDs), particularly those operating within the near-infrared window (NIR-II), are promising candidates in the field of photoacoustic (PA) imaging. These materials can enhance PA/MR imaging when combined with MIONPs. Moreover, they have the potential to trigger ICD, which can reverse immunosuppression and enhance the therapeutic efficacy against primary, distant, and metastatic tumors.171 This dual capability makes TMDs valuable for advancing diagnostic and therapeutic strategies in BC.
It is noteworthy that, in comparison to the protein corona formed on the surface of PEG-MIONPs, the sulfide coating demonstrates a reduction in protein corona formation on the surface of MIONPs. Moreover, the protein corona specifically adsorbed on the sulfide-coated MIONPs exhibits a decreased likelihood of participating in nanoparticle-targeted transport and immune response mediation, while also displaying enhanced resistance to phagocytosis by macrophages, resulting in an extended in vivo circulation time.69
Summary and Outlook
In recent decades of clinical research on BC, significant efforts in optimizing the structure of MIONPs have primarily aimed to enhance their effectiveness as excellent T2 contrast agents (T2CAs). However, the defects of MIONPs as T2CAs have been gradually exposed in the process of clinical practice: (1) As a negative contrast agent, the dark signal generated by MIONPs can be easily confused with signals from hematoma, calcification, air, and other phenomena within the body. (2) Their high magnetic moment can induce magnetization artifacts known as the “halo effect”, distorting the background around lesion tissues. (3) Larger MIONPs exhibit slow clearance from the body, potentially leading to cumulative toxicity with prolonged use. In recent years, increasing attention has been directed toward engineering the crystal structure of MIONPs to enhance their T1 relaxivity and develop T1 contrast agents (T1CAs) based on MIONPs. In recent studies, iron oxide nanoparticles ranging from 1.9 to 4.9 nm in size have been synthesized, with 3.6 nm identified as optimal for serving as T1-weighted MRI CAs.70 In addition, some experiments compared different coatings, synthesis methods, and dopants to improve the T1 contrast enhancement of MIONPs. Despite numerous demonstrations of their potential as T1-weighted CAs, the complexity of parameter control has hindered the clinical validation of suitable MIONPs. Moreover, the ultra-small particle size may lead to heightened surface energy within the body, potentially resulting in aggregation, and these potential risks and challenges will increase in proportion with the scale of production.
To address these challenges, there is growing interest in T1/T2 dual-mode CAs.172 For instance, DNA was utilized as a precisely controllable and stable ligand to assemble dimeric ultra-small magnetic nanoparticles. Under ultra-high field MRI, DMA exhibits moderate local magnetic field homogeneity while maintaining superparamagnetic behavior, with an r2/r1 value of approximately 5.93, falling within the ideal range for T1/T2 dual-mode contrast agents (5 < r2/r1 < 10). DMA exhibits excellent dual-mode imaging output, filtering out magnetization artifacts with high spatial resolution under ultra-high magnetic fields. Furthermore, a model representing various stages of hematoma development adjacent to the tumor assesses DMA’s in vivo MR precision imaging for BC, highlighting its accurate tumor recognition. Subsequent toxicological analysis confirms DMA’s excellent biosafety profile in vivo. The established principles of surface modification effects on T1 and T2 remain pertinent for T1/T2 dual-functional contrast agents, emphasizing attributes like high hydrophilicity, optimal bioavailability, and stable surface coatings. However, further extensive research is necessary to fully assess the advantages of dual-mode imaging methodologies in models that closely mimic clinical conditions relevant to breast cancer. Despite the fact that 2D/3D models have demonstrated good BC diagnosis and treatment results by using MIONPs-based drug delivery systems, achieving clinical translation remains the biggest existing challenge for MIONPs. In fact, we still lack in vitro/in vivo models that summarize the detailed characteristics of BC itself and the complex microenvironment, and most studies rely on animal models, where inter-species differences inevitably affect researchers’ judgment of whether drugs with good pharmacokinetic performance in animal models will produce the expected results if applied in humans. In recent years, new progress has been made in the in vitro humanization model of BC, and the emerging cutting-edge technology of “organoid/organ chip/microfluidic organ chip technology” can simulate and reproduce the microenvironment of the human body under actual physiological and pathological conditions. This new frontier technology can simulate and reproduce the microenvironment of the human body under actual physiological and pathological conditions, which can be helpful for the preclinical research of surface-engineered MIONPs as well as for the promotion of clinical research.
Surface modifications of MIONPs have all been developed to reduce the challenges of traditional drug delivery systems. The ideal surface-engineered MIONPs function as a multifunctional vehicle, ensuring precise release and retention of therapeutic agents at the appropriate dosage, location, and timing to maximize efficacy while minimizing side effects. Furthermore, surface-engineered MIONPs offer enhanced contrast, sensitivity, and spatial resolution for clinical imaging modalities such as MRI and PA imaging, as mentioned earlier. With the advancement of environmentally friendly and intelligent surface-engineered MIONPs for targeted drug delivery systems, there will be groundbreaking developments in the diagnosis and treatment of BC. The emergence of organoid models as a novel platform signals an increasing role for MIONPs in cutting-edge clinical solutions.
Abbreviations
MIONPs, Magnetic iron oxide nanoparticles; IARC, International Agency for Research on Cancer; BC, Breast Cancer; MRI, Magnetic Resonance Imaging; TNBC, Triple Negative Breast Cancer; RES, Reticuloendothelial System; CAs, Contrast Agents; SPIO, Small particles of iron oxide; USPIO, Ultrasmall particles of iron oxide; SPIONs, Superparamagnetic Iron Oxide Nanoparticles; Tc99, Technetium-99m; BD, Blue Dye; SLN, Sentinel Lymph Node; MT, MagTrace®; FM, Ferumoxytol®; SUH, Sahlgrenska University Hospital; FAHNMU, The First Affiliated Hospital with Nanjing Medical University; CTC, Circulating Tumor Clusters; MPI, Magnetic Particle Imaging; FMI, Fluorescence Molecular Imaging; NIRF, Near-infrared Fluorescence; AMF, Alternating Magnetic Field (AMF); MHT, Magnetic Hyperthermia; EPR, Enhanced Permeability and Retention; ROS, Reactive Oxygen Species; FT, Ferroptosis Therapy; LPO, Lipid Peroxides; VTPA, Virus Spike Tumor-activated Hyperthermia Agent; PU, Polyurethane; DOX, Doxorubicin; PEG, Polyethylene Glycol; VEGF, Vascular Endothelial Growth Factor; RES, Reticuloendothelial System; pHLIP, pH low insertion peptide; MPS, Mononuclear Phagocyte System; ABC, accelerated blood clearance; MIPs, Molecularly Imprinted Polymers; ECM, Extracellular Matrix; MAA, methacrylic acid; ITA, Itaconic Acid; AZT, Zidovudine; pCBMA, poly (Carboxybetaine Methacrylate); MVA, mevalonate; GPX4, Glutathione Peroxidase 4; TAMs, Tumor-associated Macrophages; EMT, Epithelial-mesenchymal Transition; BA, Betulinic Acid; GT, Gene Therapy; PTT, Photothermal Therapy; BA, Betulinic Acid; EVs, Extracellular Vesicles; BBB, Blood-brain Barrier; NG, nanogel; NLC, Nanostructured Lipid Carriers; TMX, Tamoxifen; CAF, Cancer-associated Fibroblasts; ICD, Immunogenic Cell Death; DCs, Dendritic Cells; CTLs, Cytotoxic T Lymphocytes; PEI, Polyethyleneimine; TMX, tamoxifen; HA, Hyaluronic Acid; SERS, Surface Enhanced Raman Scattering; GO, Graphene Oxide; HCPT, Hydroxycamptothecin; TMDs, Transition Metal Chalcogenides; MTD, Magnetothermal Dynamics;; ECM, Extracellular Matrix; ITA, Itaconic Acid.
Acknowledgments
Mengjie Xie, Fansu Meng and Panpan Wang are co-first authors for this work.
Funding
This work was supported by the National Key R&D Program of China (International Programs & Strategic Innovative Programs, Grant No.2023YFE0112200); Key Program of International (Regional) Cooperative Research Projects of the National Natural Science Foundation of China (Grant No.82020108033); General Program of Natural Science Foundation of China (Grant No.82274255); International (Regional) Cooperation and Exchange Program of National Natural Science Foundation of China (Grant No.82211540013,82411530061); Natural Science Foundation of Guangdong Province (Grant No.2023A1515220221); International Joint Research Center for Anti-tumor Nanomedicine Innovation and Application (Grant No.2023A0505090002); Foundation of Zhongshan Hospital of Traditional Chinese Medicine (Grant No.YN2024A001, YN2024A004); The Fifth National Traditional Chinese Medicine Excellent Talents Training Program (2022).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Siegel R L, Miller K D, Wagle N S, Jemal A. Cancer Statistics. CA A Can J Clin. 2023;731:17–48. doi:10.3322/caac.21763
2. Heil J, Kuerer HM, Pfob A, et al. eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol. 2020;31(1):61–71. doi:10.1016/j.annonc.2019.10.012
3. Kerr AJ, Dodwell D, McGale P, et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat Rev. 2022;105:102375. doi:10.1016/j.ctrv.2022.102375
4. Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. Ca a Cancer J Clinicians. 2022;72(6):524–541. doi:10.3322/caac.21754
5. Burstein HJ, Curigliano G, Thürlimann B, et al. Panelists of the St Gallen Consensus Conference. customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi:10.1016/j.annonc.2021.06.023
6. Choong GM, Cullen GD, O’Sullivan CC. Evolving Standards of care and new challenges in the management of HER2-positive breast cancer. Ca a Can J Clin. 2020;70(5):355–374. doi:10.3322/caac.21634
7. Laurent S, Forge D, Port M, et al.. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem Rev. 2008;108(6):2064–2110. doi:10.1021/cr068445e
8. Besenhard MO, Storozhuk L, LaGrow A, et al. High temperature flow synthesis of iron oxide nanoparticles: Size tuning via reactor engineering. Chem Eng J. 2023;473:144542. doi:10.1016/j.cej.2023.144542
9. Kubániová D, Brázda P, Závěta K, Kmječ T, Klementová M, Kohout J. Identification of ferric oxide polymorphs in nanoparticles prepared by sol-gel method and maximization of ε-Fe2O3 Content. J Magn Magn Mater. 2019;472:96–103. doi:10.1016/j.jmmm.2018.09.107
10. Tong S, Quinto CA, Zhang L, Mohindra P, Bao G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano. 2017;11(7):6808–6816. doi:10.1021/acsnano.7b01762
11. Gao H, Zhang T, Zhang Y, et al. ellipsoidal magnetite nanoparticles: A new member of the magnetic-vortex nanoparticles family for efficient magnetic hyperthermia. J Mater Chem B. 2020;8(3):515–522. doi:10.1039/c9tb00998a
12. Purushothaman K, B. P, M. U, M K, B MS. Magnetic Casein-CaFe2O4 Nanohybrid Carrier Conjugated with Progesterone for Enhanced Cytotoxicity of Citrus Peel Derived Hesperidin Drug towards Breast and Ovarian Cancer. Int J Biol Macromol. 2020;151:293–304. doi:10.1016/j.ijbiomac.2020.02.172
13. Naumenko V, Nikitin A, Garanina A, et al. Neutrophil-mediated transport is crucial for delivery of short-circulating magnetic nanoparticles to tumors. Acta Biomater. 2020;104:176–187. doi:10.1016/j.actbio.2020.01.011
14. Liang Y-J, Xie J, Yu J, Zheng Z, Liu F, Yang A. Recent advances of high performance magnetic iron oxide nanoparticles: controlled synthesis, properties tuning and cancer theranostics. Nano Select. 2021;2(2):216–250. doi:10.1002/nano.202000169
15. Zou T, Lu W, Mezhuev Y, et al. A review of nanoparticle drug delivery systems responsive to endogenous breast cancer microenvironment. Eur J Pharm Biopharm. 2021;166:30–43. doi:10.1016/j.ejpb.2021.05.029
16. Jeon M, Halbert MV, Stephen ZR, Zhang M. Iron Oxide Nanoparticles as T1 Contrast Agents for magnetic resonance imaging: fundamentals, challenges, applications, and prospectives. Adv Mater. 2021;33(23):e1906539. doi:10.1002/adma.201906539
17. Lee N, Hyeon T. Designed Synthesis of Uniformly Sized Iron Oxide Nanoparticles for Efficient Magnetic Resonance Imaging Contrast Agents. Chem Soc Rev. 2012;41(7):2575–2589. doi:10.1039/c1cs15248c
18. Lu X, Zhou H, Liang Z, et al. Biodegradable and biocompatible exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging of tumors. J Nanobiotechnology. 2022;20(1):350. doi:10.1186/s12951-022-01562-y
19. Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. chem soc rev. 2015;44(23):8576–8607. doi:10.1039/c5cs00541h
20. Feridex I.V. (ferumoxides injectable solution). Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded&type=display.
21. Arbab AS, Ichikawa T, Sou H, et al. Ferumoxides-Enhanced Double-Echo T2-Weighted MR Imaging in Differentiating Metastases from Nonsolid Benign Lesions of the Liver. Radiology. 2002;225(1):151–158. doi:10.1148/radiol.2251011090
22. Wu YJ, Muldoon LL, Varallyay C, Markwardt S, Jones RE, Neuwelt EA. In Vivo Leukocyte Labeling with Intravenous Ferumoxides/Protamine Sulfate Complex and in Vitro Characterization for Cellular Magnetic Resonance Imaging. Am J Physiol Cell Physiol. 2007;293(5):C1698–C1708. doi:10.1152/ajpcell.00215.2007
23. Arbab AS, Yocum GT, Kalish H, et al. Efficient Magnetic Cell Labeling with Protamine Sulfate Complexed to Ferumoxides for Cellular MRI. Blood. 2004;104(4):1217–1223. doi:10.1182/blood-2004-02-0655
24. Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–2499. doi:10.1056/NEJMoa022749
25. Fortuin AS, Brüggemann R, van der Linden J, et al. Ultra-Small Superparamagnetic Iron Oxides for Metastatic Lymph Node Detection: back on the Block. Wiley Interdiscip Rev Nano Nanobio. 2018;10(1):e1471. doi:10.1002/wnan.1471
26. Fortuin A, van Asten J, Veltien A. Small Suspicious Lymph Nodes Detected on Ultrahigh-Field Magnetic Resonance Imaging (MRI) in Patients with Prostate Cancer with High Risk of Nodal Metastases: the First In-Patient Study on Ultrasmall Superparamagnetic Iron Oxide–Enhanced 7T MRI. Europ urol. 2023;83(4):375–377. doi:10.1016/j.eururo.2023.01.002
27. Gastromark: Package Insert - Drugs.com. Available from: https://www.drugs.com/pro/gastromark.html.
28. Daldrup-Link HE, Chan C, Lenkov O, et al. Detection of stem cell transplant rejection with ferumoxytol mr imaging: correlation of mr imaging findings with those at intravital Microscopy. Radiology. 2017;284(2):495–507. doi:10.1148/radiol.2017161139
29. Nejadnik H, Tseng J, Daldrup-Link H. Magnetic resonance imaging of stem cell-macrophage interactions with ferumoxytol and ferumoxytol-derived nanoparticles. Wiley Interdiscip Rev Nano Nanobio. 2019;11(4):e1552. doi:10.1002/wnan.1552
30. Iv M, Samghabadi P, Holdsworth S, et al. Quantification of Macrophages in High-Grade Gliomas by Using Ferumoxytol-Enhanced MRI: A pilot study. Radiology. 290(1):198–206. doi:10.1148/radiol.2018181204
31. Zanganeh S, Hutter G, Spitler R, et al. Iron Oxide Nanoparticles Inhibit Tumour Growth by Inducing Pro-Inflammatory Macrophage Polarization in Tumour Tissues. Nat Nanotechnol. 2016;11(11):986–994. doi:10.1038/nnano.2016.168
32. Sindrilaru A, Peters T, Wieschalka S, et al. An Unrestrained Proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. doi:10.1172/JCI44490
33. Alvarado MD, Mittendorf EA, Teshome M, et al. SentimagIC: A non-inferiority trial comparing superparamagnetic iron oxide versus technetium-99m and blue dye in the detection of axillary sentinel nodes in patients with early-stage breast cancer. Ann Surg Oncol. 2019;26(11):3510–3516. doi:10.1245/s10434-019-07577-4
34. Douek M, Klaase J, Monypenny I, et al. SentiMAG trialists group. sentinel node biopsy using a magnetic tracer versus standard technique: the sentimag multicentre trial. Ann Surg Oncol. 2014;21(4):1237–1245. doi:10.1245/s10434-013-3379-6
35. Mok CW, Tan S-M, Zheng Q, Shi L. Network Meta‐analysis of Novel and Conventional Sentinel Lymph Node Biopsy Techniques in Breast Cancer. BJS Open. 2019;3(4):445–452. doi:10.1002/bjs5.50157
36. Bagge RO Sentinel Lymph Node Localisation With an Ultra-Low Dose of Superparamagnetic Iron Oxide Nanoparticles in Patients With Breast Cancer. Clinical trial registration NCT06169072; clinicaltrials.gov; 2024. Available from: https://clinicaltrials.gov/study/NCT06169072.
37. Karakatsanis A, Daskalakis K, Stålberg P, et al. Superparamagnetic Iron Oxide Nanoparticles as the Sole Method for Sentinel Node Biopsy Detection in Patients with Breast Cancer. Br J Surg. 2017;104(12):1675–1685. doi:10.1002/bjs.10606
38. Bagge RO Sentinel node localization and staging with low dose superparamagnetic iron oxide-enhanced magnetic resonance imaging and magnetic probe in patients with breast cancer. Clinical trial registration NCT05359783; clinicaltrials.gov; 2024. Available from: https://clinicaltrials.gov/study/NCT05359783.
39. Karakatsanis A Sentinel lymph node biopsy in ductal cancer in situ or unclear lesions of the breast and how to not do it. an open-label, phase 3, randomised controlled trial. (SentiNot 2.0).; Clinical trial registration NCT04722692; clinicaltrials.gov; 2023. Available from: https://clinicaltrials.gov/study/NCT04722692.
40. Michalak S application of nanoparticles for cyclic hyperthermia in adjuvant therapy of glioblastoma multiforme. (ANCHIALE); Clinical trial registration NCT06271421; clinicaltrials.gov; 2024. Available from: https://clinicaltrials.gov/study/NCT06271421.
41. Li C A novel ferumoxytol-enhanced cardiac magnetic resonance imaging for the detection of intracardiac thrombus in patients with ventricular aneurysm and after percutaneous ventricular reconstruction. Clinical trial registration NCT06146751; clinicaltrials.gov; 2023. Available from: https://clinicaltrials.gov/study/NCT06146751.
42. Peter MacCallum Cancer Centre, Australia. CAR-T Cell therapy in relapsed/refractory myeloma with extramedullary disease an in vivo imaging and molecular monitoring study. Clinical trial registration NCT05666700; clinicaltrials.gov; 2024. Available from: https://clinicaltrials.gov/study/NCT05666700.
43. Li C Clinical study of domestic polysaccharide superparamagnetic iron oxide nanoparticle injection for renal contrast-enhanced magnetic resonance imaging. Clinical trial registration NCT05045872; clinicaltrials.gov; 2023. Available from: https://clinicaltrials.gov/study/NCT05045872.
44. Chia Tai Tianqing Pharmaceutical Group Co. Ltd. A Single-Center, Randomized, Open-Label, Single-Dose Study of Polysaccharide Superparamagnetic Iron Oxide Injection in Healthy Subjects to Evaluate Magnetic Resonance Imaging in Multiple Dose Groups. Clinical trial registration NCT06010537; clinicaltrials.gov; 2023. Available from: https://clinicaltrials.gov/study/NCT06010537.
45. Maranchie J Virtual histology of the bladder wall for bladder cancer staging; A novel intravesical contrast-enhanced mri for bladder cancer staging. Clinical trial registration NCT04369560; clinicaltrials.gov; 2023. Available from: https://clinicaltrials.gov/study/NCT04369560.
46. Kirichenko A Adaptive Stereotactic radiotherapy with superparamagnetic iron oxide nanoparticles (SPION) cellular magnetic Resonance Imaging on MR-Linac (MR-L-SPION) for primary and metastatic hepatic malignancies with assessment of treatment response. Clinical trial registration NCT04682847; clinicaltrials.gov; 2024. Available from: https://clinicaltrials.gov/study/NCT04682847.
47. Zhu X, Li J, Peng P, Hosseini Nassab N, Smith BR. Quantitative drug release monitoring in tumors of living subjects by magnetic particle imaging nanocomposite. Nano Lett. 2019;19(10):6725–6733. doi:10.1021/acs.nanolett.9b01202
48. Zhen G, Muir B, Moffat B, et al. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J Phyl Chem C. 2011;115(2):327–334. doi:10.1021/jp104953z
49. Zhong D, Zhao J, Li Y, et al. Laser-Triggered Aggregated Cubic α-Fe2O3@Au Nanocomposites for Magnetic Resonance Imaging and Photothermal/Enhanced Radiation Synergistic Therapy. Biomaterials. 2019;219:119369. doi:10.1016/j.biomaterials.2019.119369
50. Cędrowska E, Pruszyński M, Gawęda W, et al. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules. 2020;25(5):1025. doi:10.3390/molecules25051025
51. Liu X, Wang M, Jiang Y, et al. Magnetic resonance imaging nanoprobe quantifies nitric oxide for evaluating M1/M2 Macrophage Polarization and prognosis of cancer treatments. ACS Nano. 2023;17(24):24854–24866. doi:10.1021/acsnano.3c05627
52. Jedryka MA, Klimczak P, Kryszpin M, Matkowski R. Superparamagnetic Iron Oxide: a Novel Tracer for Sentinel Lymph Node Detection in Vulvar Cancer. Int J Gynecol Cancer. 2020;30(9):1280–1284. doi:10.1136/ijgc-2020-001458
53. Wärnberg F, Stigberg E, Obondo C, et al. Long-term outcome after retro-areolar versus peri-tumoral injection of superparamagnetic iron oxide Nanoparticles (SPIO) for sentinel lymph node detection in breast cancer surgery. Ann Surg Oncol. 2019;26(5):1247–1253. doi:10.1245/s10434-019-07239-5
54. Mirzaei N, Wärnberg F, Zaar P, Leonhardt H, Olofsson Bagge R. Ultra-low dose of superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in patients with breast cancer. ann Surg Oncol. 2023;30(9):5685–5689. doi:10.1245/s10434-023-13722-x
55. Motomura K, Tabuchi Y, Enomoto Y, et al. Accurate Axillary Staging by Superparamagnetic Iron Oxide-Enhanced MRI at 1.5 T with Fat-Suppression Sequence as an Alternative to Sentinel Node Biopsy in Breast Cancer. Br J Surg. 2021;108(11):e359–e360. doi:10.1093/bjs/znab277
56. Li Z, Ruan J, Zhuang X. Effective Capture of Circulating Tumor Cells from an S180-bearing mouse model using electrically charged magnetic nanoparticles. J Nanobiotechnology. 2019;17(1):59. doi:10.1186/s12951-019-0491-1
57. Du Y, Liu X, Liang Q, Liang X-J, Tian J. Optimization and design of magnetic ferrite nanoparticles with uniform tumor distribution for highly Sensitive MRI/MPI Performance and improved magnetic hyperthermia therapy. Nano Lett. 2019;19(6):3618–3626. doi:10.1021/acs.nanolett.9b00630
58. Du J, Zhang Y, Jin Z, et al. Targeted NIRF/MR Dual-Mode Imaging of Breast Cancer Brain Metastasis Using BRBP1-Functionalized Ultra-Small Iron Oxide Nanoparticles. Mater Sci Eng C Mater Biol Appl. 2020;116:111188. doi:10.1016/j.msec.2020.111188
59. Wang G, Li W, Shi G, et al. Sensitive and Specific Detection of Breast Cancer Lymph Node Metastasis through Dual-Modality Magnetic Particle Imaging and Fluorescence Molecular Imaging: a Preclinical Evaluation. Eur J Nucl Med Mol Imaging. 2022;49(8):2723–2734. doi:10.1007/s00259-022-05834-5
60. Tran MV, Susumu K, Medintz IL, Algar WR. supraparticle assemblies of magnetic nanoparticles and quantum dots for selective cell isolation and counting on a smartphone-based imaging platform. Anal Chem. 2019;91(18):11963–11971. doi:10.1021/acs.analchem.9b02853
61. Cui H, Li R, Du J, et al. Rapid and Efficient Isolation and Detection of Circulating Tumor Cells Based on ZnS: Mn2+ Quantum Dots and Magnetic Nanocomposites. Talanta. 2019;202:230–236. doi:10.1016/j.talanta.2019.05.001
62. Wang Z, Sun N, Liu H, et al. High-Efficiency Isolation and Rapid Identification of Heterogeneous Circulating Tumor Cells (CTCs) Using Dual-Antibody-Modified Fluorescent-Magnetic Nanoparticles. ACS Appl Mater Interfaces. 2019;11(43):39586–39593. doi:10.1021/acsami.9b14051
63. Wang Y, Jiang L, Zhang Y, et al.. Fibronectin-targeting and cathepsin b-activatable theranostic nanoprobe for mr/fluorescence imaging and enhanced photodynamic therapy for triple negative breast cancer. ACS Appl Mater Interfaces. 2020;12(30):33564–33574. doi:10.1021/acsami.0c10397
64. Wang Y, Liu H, Yao D, et al. 18F-Labeled Magnetic Nanoparticles for Monitoring Anti-Angiogenic Therapeutic Effects in Breast Cancer Xenografts. J Nanobiotechnology. 2019;17(1):105. doi:10.1186/s12951-019-0534-7
65. Chen S, Zhang Q, Sun H, et al. A cation exchange strategy to construct a targeting nanoprobe for enhanced T1-weighted mr imaging of tumors. J Mater Chem B. 2020;8(37):8519–8526. doi:10.1039/d0tb01632b
66. Li L, Wu C, Pan L, et al. Bombesin-Functionalized Superparamagnetic Iron Oxide Nanoparticles for Dual-Modality MR/NIRFI in Mouse Models of Breast Cancer. Int J Nanomed. 2019;14:6721–6732. doi:10.2147/IJN.S211476
67. Sotiriou GA, Visbal-Onufrak MA, Teleki A, et al. Thermal energy dissipation by sio2-coated plasmonic-superparamagnetic nanoparticles in alternating magnetic fields. Chem Mater. 2013;25(22):4603–4612. doi:10.1021/cm402896x
68. Ilg P, Kröger M. Dynamics of interacting magnetic nanoparticles: effective behavior from competition between brownian and Néel relaxation. Phys Chem Chem Phys. 2020;22(39):22244–22259. doi:10.1039/D0CP04377J
69. Xu X, Huang X, Chang Y, et al. Antifouling surfaces enabled by surface grafting of highly hydrophilic sulfoxide polymer brushes. Biomacromolecules. 2021;22(2):330–339. doi:10.1021/acs.biomac.0c01193
70. Shen Z, Chen T, Ma X, et al. Multifunctional theranostic nanoparticles based on exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging and chemotherapy. ACS Nano. 2017;11(11):10992–11004. doi:10.1021/acsnano.7b04924
71. Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi:10.1016/j.jconrel.2011.06.001
72. Peiravi M, Eslami H, Ansari M, Zare-Zardini H. Magnetic Hyperthermia: Potentials and Limitations. J Indian Chem Soc. 2022;99(1):100269. doi:10.1016/j.jics.2021.100269
73. Alvarez-Berríos MP, Castillo A, Mendéz J, Soto O, Rinaldi C, Torres-Lugo M. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int J Nanomed. 2013;8:1003–1013. doi:10.2147/IJN.S38842
74. Hannon G, Bogdanska A, Volkov Y, Prina-Mello A. Comparing the effects of intracellular and extracellular magnetic hyperthermia on the viability of BxPC-3 Cells. Nanomaterials (Basel). 2020;10(3):593. doi:10.3390/nano10030593
75. Zastko L, Petrovičová P, Račková A, et al. DNA damage response and apoptosis induced by hyperthermia in human umbilical cord blood lymphocytes. Toxicol In Vitro. 2021;73:105127. doi:10.1016/j.tiv.2021.105127
76. Vorotnikova E, Ivkov R, Foreman A, Tries M, Braunhut SJ. The Magnitude and Time-Dependence of the Apoptotic Response of Normal and Malignant Cells Subjected to Ionizing Radiation versus Hyperthermia. Int J Radiat Biol. 2006;82(8):549–559. doi:10.1080/09553000600876678
77. Chu KF, Dupuy DE. Thermal Ablation of Tumours: biological Mechanisms and Advances in Therapy. Nat Rev Cancer. 2014;14(3):199–208. doi:10.1038/nrc3672
78. Chandrasekharan P, Tay ZW, Hensley D, et al. Using Magnetic Particle Imaging Systems to Localize and Guide Magnetic Hyperthermia Treatment: tracers, Hardware, and Future Medical Applications. Theranostics. 2020;10(7):2965–2981. doi:10.7150/thno.40858
79. Bukowski K, Kciuk M, Kontek R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi:10.3390/ijms21093233
80. Liu Y, Suo X, Peng H, et al.. Multifunctional Magnetic Nanoplatform Eliminates Cancer Stem Cells via Inhibiting the Secretion of Extracellular Heat Shock Protein 90. Adv Healthc Mater. 2019;8(13):e1900160. doi:10.1002/adhm.201900160
81. Visvader JE, Lindeman GJ. Cancer Stem Cells: current Status and Evolving Complexities. Cell Stem Cell. 2012;10(6):717–728. doi:10.1016/j.stem.2012.05.007
82. Li Z, Guo T, Zhao S, Lin M. The Therapeutic Effects of MUC1-C shRNA@Fe3O4 magnetic nanoparticles in alternating magnetic fields on triple-negative breast cancer. Int J Nanomed. 2023;18:5651–5670. doi:10.2147/IJN.S426849
83. Vakili-Ghartavol R, Momtazi-Borojeni AA, Vakili-Ghartavol Z, et al. Toxicity Assessment of Superparamagnetic Iron Oxide Nanoparticles in Different Tissues. Artif Cells Nanomed Biotechnol. 2020;48(1):443–451. doi:10.1080/21691401.2019.1709855
84. Sang M, Luo R, Bai Y, et al. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics. 2019;9(21):6209–6223. doi:10.7150/thno.36283
85. Chen Y, Li X, Luo K, et al. Hyperthermia/glutathione-triggered ferritin nanoparticles amplify the ferroptosis for synergistic tumor therapy. Mater Today Bio. 2024;26:101085. doi:10.1016/j.mtbio.2024.101085
86. Wu M, Zhang X, Zhang W, et al. Cancer Stem Cell Regulated Phenotypic Plasticity Protects Metastasized Cancer Cells from Ferroptosis. Nat Commun. 2022;13(1):1371. doi:10.1038/s41467-022-29018-9
87. Wang X, Hua P, He C, Chen M. Non-Apoptotic Cell Death-Based Cancer Therapy: molecular Mechanism, Pharmacological Modulators, and Nanomedicine. Acta Pharmaceutica Sinica B. 2022;12(9):3567–3593. doi:10.1016/j.apsb.2022.03.020
88. S N, C Y, Y D, et al. A Virus-Spike Tumor-Activatable Pyroptotic Agent. Small. 2021;17:8. doi:10.1002/smll.202006599
89. Ultrasmall superparamagnetic iron oxide nanoparticles as nanocarriers for magnetic resonance imaging: Development and in vivo characterization | acs applied nano materials. Available from: https://pubs.acs.org/doi/10.1021/acsanm.2c01835.
90. Augusto-Jimenez YE, González-Montoya M, Naranjo-Feliciano D, et al. Antioxidant activity of bioactive peptide fractions from germinated soybeans conjugated to Fe3O4 Nanoparticles by the Ugi Multicomponent Reaction. Molecules. 2021;26(19):5726. doi:10.3390/molecules26195726
91. Freis B, Ramirez MDLA, Kiefer C, et al. Effect of the size and shape of dendronized iron oxide nanoparticles bearing a targeting ligand on mri, magnetic hyperthermia, and photothermia properties-from suspension to in vitro studies. Pharmaceutics. 2023;15(4):1104. doi:10.3390/pharmaceutics15041104
92. Comanescu C. Recent advances in surface functionalization of magnetic nanoparticles. Coatings. 2023;13(10):1772. doi:10.3390/coatings13101772
93. Zhang J, Lv S, Zhao X, Ma S, Zhou F. Surface Functionalization of Polyurethanes: a Critical Review. Adv. Colloid Interface Sci. 2024;325:103100. doi:10.1016/j.cis.2024.103100
94. Horbett TA. Chapter 13 Principles Underlying the Role of Adsorbed Plasma Proteins in Blood Interactions with Foreign Materials. Cardiovasc Pathol. 1993;2(3):137–148. doi:10.1016/1054-8807(93)90054-6
95. Gradinaru LM, Barbalata Mandru M, Drobota M, et al. Composite materials based on iron oxide nanoparticles and polyurethane for improving the quality of MRI. Polymers. 2021;13(24):4316. doi:10.3390/polym13244316
96. Diez-Pascual AM. Antibacterial nanocomposites based on thermosetting polymers derived from vegetable oils and metal oxide nanoparticles. Polymers. 2019;11(11):1790. doi:10.3390/polym11111790
97. Mahdieh A, Yeganeh H, Motasadizadeh H, et al.. Waterborne polyurethane magnetic nanomicelles with magnetically governed functions for breast cancer therapy. Int J Pharm. 2023;645:123356. doi:10.1016/j.ijpharm.2023.123356
98. Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55(3):403–419. doi:10.1016/s0169-409x(02)00226-0
99. Shi D, Beasock D, Fessler A, et al. To PEGylate or Not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079. doi:10.1016/j.addr.2021.114079
100. Chen J, Rizvi A, Patterson JP, Hawker CJ. Discrete libraries of amphiphilic poly(ethylene glycol) graft Copolymers: synthesis, assembly, and bioactivity. J Am Chem Soc. 2022;144(42):19466–19474. doi:10.1021/jacs.2c07859
101. Mu Q, Lin G, Jeon M, et al. Iron Oxide Nanoparticle Targeted Chemo-Immunotherapy for Triple Negative Breast Cancer. Mater Today. 2021;50:149–169. doi:10.1016/j.mattod.2021.08.002
102. Dalmina M, Pittella F, Sierra JA, et al. Magnetically responsive hybrid nanoparticles for in vitro siRNA delivery to breast cancer cells. Mater Sci Eng C. 2019;99:1182–1190. doi:10.1016/j.msec.2019.02.026
103. Cristofolini T, Dalmina M, Sierra JA, et al. multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA Targeting the HER2 Gene in breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2020;109:110555. doi:10.1016/j.msec.2019.110555
104. Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–345. doi:10.1038/nature12625
105. Saga T, Neumann RD, Heya T, et al. Targeting cancer micrometastases with monoclonal antibodies: A binding-site barrier. proc natl acad sci u s a. 1995;92(19):8999–9003. doi:10.1073/pnas.92.19.8999
106. Demin AM, Pershina AG, Minin AS, et al. Smart Design of a pH-Responsive System Based on pHLIP-modified magnetite nanoparticles for tumor MRI. ACS Appl Mater Interfaces. 2021;13(31):36800–36815. doi:10.1021/acsami.1c07748
107. Wang H, Lin S, Wu X, Jiang K, Lu H, Zhan C. Interplay between Liposomes and IgM: Principles, challenges, and opportunities. Adv Sci. 2023;10(20):2301777. doi:10.1002/advs.202301777
108. Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi:10.1158/0008-5472.CAN-05-4199
109. Guo C, Yuan H, Wang Y, et al. The Interplay between PEGylated Nanoparticles and Blood Immune System. Adv Drug Delivery Rev. 2023;200:115044. doi:10.1016/j.addr.2023.115044
110. Chen G. The Past Ten Years of Carbohydrate Polymers in ACS Macro Letters. ACS Macro Lett. 2021;10(9):1145–1150. doi:10.1021/acsmacrolett.1c00526
111. Diaz-Dussan D, Peng -Y-Y, Kumar P, Narain R. Oncogenic epidermal growth factor receptor silencing in cervical carcinoma mediated by dynamic sugar-benzoxaborole polyplexes. ACS Macro Lett. 2020;9(10):1464–1470. doi:10.1021/acsmacrolett.0c00599
112. Gracia R, Marradi M, Salerno G, et al.. Biocompatible Single-Chain Polymer Nanoparticles Loaded with an Antigen Mimetic as Potential Anticancer Vaccine. ACS Macro Lett. 2018;7(2):196–200. doi:10.1021/acsmacrolett.8b00052
113. Zhao C-M, Wang K-R, Wang C, He X, Li X-L. Cooling-induced nir emission enhancement and targeting fluorescence imaging of biperylene monoimide and glycodendrimer conjugates. ACS Macro Lett. 2019;8(4):381–386. doi:10.1021/acsmacrolett.9b00095
114. Kaliyamoorthy K, Pillai AS, Alexander A, Arivarasu A, Enoch IVMV, Ramasamy S. β-Cyclodextrin-Folate Functionalized Poly(Lactic-Co-Glycolide)-Superparamagnetic Ytterbium Ferrite Hybrid Nanocarrier for Targeted Delivery of Camptothecin. Mater Sci Eng C Mater Biol Appl. 2021;122:111796. doi:10.1016/j.msec.2020.111796
115. Sun T, Gao J, Shi H, et al. Decorated Au NPs on Agar Modified Fe3O4 NPs: Investigation of its catalytic performance in the degradation of methylene orange, and anti-human breast carcinoma properties. Int J Biol Macromol. 2020;165(Pt A):787–795. doi:10.1016/j.ijbiomac.2020.09.157
116. Dinc M, Esen C, Mizaikoff B. recent advances on core–shell magnetic molecularly imprinted polymers for biomacromolecules. TrAC Trends in Analytical Chemistry. 2019;114:202–217. doi:10.1016/j.trac.2019.03.008
117. Muhammad T, Nur Z, Piletska EV, Yimit O, Piletsky SA. rational design of molecularly imprinted polymer: The choice of cross-linker. analyst. 2012;137(11):2623–2628. doi:10.1039/c2an35228a
118. Cazares-Cortes E, Nerantzaki M, Fresnais J, Wilhelm C, Griffete N, Ménager C. magnetic nanoparticles create hot spots in polymer matrix for controlled drug release. Nanomaterials (Basel). 2018;8(10):850. doi:10.3390/nano8100850
119. Wackerlig J, Lieberzeit PA. Molecularly imprinted polymer nanoparticles in chemical sensing – synthesis, characterisation and application. Sensors and Actuat B Chem. 2015;207:144–157. doi:10.1016/j.snb.2014.09.094
120. Hassanpour A, Irandoust M, Soleimani E, Zhaleh H. Increasing the anticancer activity of azidothymidine toward the breast cancer via rational design of magnetic drug carrier based on molecular imprinting technology. Mater Sci Eng C Mater Biol Appl. 2019;103:109771. doi:10.1016/j.msec.2019.109771
121. Yao X, Xie R, Cao Y, et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnology. 2021;19(1):311. doi:10.1186/s12951-021-01058-1
122. Nascimento C, Castro F, Domingues M, et al. Reprogramming of tumor-associated macrophages by polyaniline-coated iron oxide nanoparticles applied to treatment of breast cancer. Int J Pharm. 2023;636:122866. doi:10.1016/j.ijpharm.2023.122866
123. Amani S, Mohamadnia Z, Mahdavi A. pH-Responsive hybrid magnetic polyelectrolyte complex based on alginate/bsa as efficient nanocarrier for curcumin encapsulation and delivery. Int J Biol Macromol. 2019;141:1258–1270. doi:10.1016/j.ijbiomac.2019.09.048
124. Bulatao BP, Nalinratana N, Jantaratana P, Vajragupta O, Rojsitthisak P, Rojsitthisak P. Lutein-Loaded Chitosan/Alginate-Coated Fe3O4 nanoparticles as effective targeted carriers for breast cancer treatment. Int J Biol Macromol. 2023;242(Pt 1):124673. doi:10.1016/j.ijbiomac.2023.124673
125. Shahriari M, Sedigh MA, Mahdavian Y, et al. In Situ Supported Pd NPs on biodegradable chitosan/agarose modified magnetic nanoparticles as an effective catalyst for the ultrasound assisted oxidation of alcohols and activities against human breast cancer. Int J Biol Macromol. 2021;172:55–65. doi:10.1016/j.ijbiomac.2021.01.037
126. Pathania K, Sah SP, Salunke DB, et al. Green synthesis of lignin-based nanoparticles as a bio-carrier for targeted delivery in cancer therapy. Int J Biol Macromol. 2023;229:684–695. doi:10.1016/j.ijbiomac.2022.12.323
127. Zhang T, Li Y, Hong W, et al. Glucose oxidase and polydopamine functionalized iron oxide nanoparticles: combination of the photothermal effect and reactive oxygen species generation for dual-modality selective cancer therapy. J Mater Chem B. 2019;7(13):2190–2200. doi:10.1039/c8tb03320j
128. Alawak M, Mahmoud G, Dayyih AA, et al. Magnetic Resonance Activatable Thermosensitive Liposomes for Controlled Doxorubicin Delivery. Mater Sci Eng C. 2020;115:111116. doi:10.1016/j.msec.2020.111116
129. Farcas CG, Dehelean C, Pinzaru IA, et al. thermosensitive betulinic acid-loaded magnetoliposomes: a promising antitumor potential for highly aggressive human breast adenocarcinoma cells under hyperthermic Conditions. Int J Nanomed. 2020;15:8175–8200. doi:10.2147/IJN.S269630
130. Ong YS, Bañobre-López M, Costa Lima SA, Reis S. A multifunctional nanomedicine platform for co-delivery of methotrexate and mild hyperthermia towards breast cancer therapy. Mater Sci Eng C Mater Biol Appl. 2020;116:111255. doi:10.1016/j.msec.2020.111255
131. Pereira DSM, Cardoso BD, Rodrigues ARO, et al. Magnetoliposomes containing calcium ferrite nanoparticles for applications in breast cancer therapy. Pharmaceutics. 2019;11(9):477. doi:10.3390/pharmaceutics11090477
132. Guo Y, Zhang Y, Ma J, et al. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J Control Release. 2018;272:145–158. doi:10.1016/j.jconrel.2017.04.028
133. Tang W, Zhen Z, Yang C, et al.. Fe5C2 nanoparticles with high mri contrast enhancement for tumor imaging. Small. 2014;10(7):1245–1249. doi:10.1002/smll.201303263
134. Toomajian VA, Tundo A, Ural EE, Greeson EM, Contag CH, Makela AV. Magnetic particle imaging reveals that iron-labeled extracellular vesicles accumulate in brains of mice with metastases. ACS Appl Mater Interfaces. 2024;16(24):30860–30873. doi:10.1021/acsami.4c04920
135. Ren Y, Nie L, Zhu S, Zhang X. Nanovesicles-mediated drug delivery for oral bioavailability enhancement. Int J Nanomed. 2022;17:4861–4877. doi:10.2147/IJN.S382192
136. Li L, Gao Y, Zhang Y, et al. A Biomimetic nanogel system restores macrophage phagocytosis for magnetic resonance imaging-guided synergistic chemoimmunotherapy of breast cancer. Adv Healthc Mater. 2023;12(26):e2300967. doi:10.1002/adhm.202300967
137. Gong C, Yu X, Zhang W, et al. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J Nanobiotechnology. 2021;19(1):58. doi:10.1186/s12951-021-00805-8
138. Zhang Y, Wang Y, Zhu A, Yu N, Xia J, Li J. Dual-Targeting Biomimetic Semiconducting Polymer Nanocomposites for Amplified Theranostics of Bone Metastasis. Angew Chem Int Ed 2024;63(2):e202310252. doi:10.1002/anie.202310252
139. Shirangi A, Mottaghitalab F, Dinarvand S, Atyabi F. Theranostic Silk Sericin/SPION Nanoparticles for Targeted Delivery of ROR1 siRNA: Synthesis, characterization, diagnosis and anticancer effect on triple-negative breast cancer. Int J Biol Macromol. 2022;221:604–612. doi:10.1016/j.ijbiomac.2022.09.020
140. Altman GH, Diaz F, Jakuba C, et al. Silk-Based Biomaterials. Biomaterials. 2003;24(3):401–416. doi:10.1016/S0142-9612(02)00353-8
141. Unal O, Akkoc Y, Kocak M, et al. Treatment of Breast Cancer with Autophagy Inhibitory microRNAs Carried by AGO2-Conjugated Nanoparticles. J Nanobiotechnology. 2020;18(1):65. doi:10.1186/s12951-020-00615-4
142. Huang J, Wang L, Lin R, et al. Casein-coated iron oxide nanoparticles for high mri contrast enhancement and efficient cell targeting. ACS Appl Mater Interfaces. 2013;5(11):4632–4639. doi:10.1021/am400713j
143. Rostami S, Tafvizi F, Kheiri Manjili HR. High Efficacy of Tamoxifen-Loaded L-Lysine Coated Magnetic Iron Oxide Nanoparticles in Cell Cycle Arrest and Anti-Cancer Activity for Breast Cancer Therapy. Bioimpacts. 2022;12(4):301–313. doi:10.34172/bi.2021.23337
144. Dutta B, Nema A, Shetake NG, et al. Glutamic Acid-Coated Fe3O4 nanoparticles for tumor-targeted imaging and therapeutics. Mater Sci Eng C Mater Biol Appl. 2020;112:110915. doi:10.1016/j.msec.2020.110915
145. Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161(1):38–49. doi:10.1016/j.jconrel.2012.04.036
146. Carreno A, Guerrero-Yague R, Casal E, Mendoza R, Corchero JL. Tuning Plasmid DNA amounts for cost-effective transfections of mammalian cells: When Less Is More. Appl Microbiol Biotechnol. 2024;108(1):98. doi:10.1007/s00253-024-13003-x
147. Nikzamir M, Akbarzadeh A, Panahi Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J Drug Delivery Sci Technol. 2021;61:102316. doi:10.1016/j.jddst.2020.102316
148. Jamshidifar E, Eshrati Yeganeh F, Shayan M, et al. super magnetic niosomal nanocarrier as a new approach for treatment of breast cancer: A case study on SK-BR-3 and MDA-MB-231 Cell Lines. Int J Mol Sci. 2021;22(15):7948. doi:10.3390/ijms22157948
149. Fathy MM, Fahmy HM, Saad OA, Elshemey WM. Silica-coated iron oxide nanoparticles as a novel nano-radiosensitizer for electron therapy. Life Sci. 2019;234:116756. doi:10.1016/j.lfs.2019.116756
150. Li J, Zhang W, Gao Y, et al. Near-Infrared Light and Magnetic Field Dual-Responsive Porous Silicon-Based Nanocarriers to Overcome Multidrug Resistance in Breast Cancer Cells with Enhanced Efficiency. J Mater Chem B. 2020;8(3):546–557. doi:10.1039/c9tb02340b
151. Taheri-Ledari R, Zhang W, Radmanesh M, et al. Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small. 2020;16(41):e2002733. doi:10.1002/smll.202002733
152. Fang Z, Li X, Xu Z, et al. Hyaluronic Acid-Modified Mesoporous Silica-Coated Superparamagnetic Fe3O4 Nanoparticles for Targeted Drug Delivery. Int J Nanomed. 2019;14:5785–5797. doi:10.2147/IJN.S213974
153. Soleymani M, Velashjerdi M, Shaterabadi Z, Barati A. One-Pot Preparation of Hyaluronic Acid-Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia Therapy and Targeting CD44-Overexpressing Cancer Cells. Carbohydr Polym. 2020;237:116130. doi:10.1016/j.carbpol.2020.116130
154. Zamboni F, Vieira S, Reis RL, Miguel Oliveira J, Collins MN. The Potential of Hyaluronic Acid in Immunoprotection and Immunomodulation: chemistry, Processing and Function. Pro Mater Sci. 2018;97:97–122. doi:10.1016/j.pmatsci.2018.04.003
155. Sanad MF, Meneses-Brassea BP, Blazer DS, Pourmiri S, Hadjipanayis GC, El-Gendy AA. Superparamagnetic Fe/Au Nanoparticles and their feasibility for magnetic hyperthermia. Appl Sci. 2021;11(14):6637. doi:10.3390/app11146637
156. Zheng D, Wan C, Yang H, et al. Her2-Targeted multifunctional nano-theranostic platform mediates tumor microenvironment remodeling and immune activation for breast cancer treatment. Int j Nanomed. 2020;15:10007–10028. doi:10.2147/IJN.S271213
157. Dong Q, Yang H, Wan C, et al. Her2-functionalized gold-nanoshelled magnetic hybrid nanoparticles: A theranostic agent for dual-modal imaging and photothermal therapy of breast cancer. Nanoscale Res Lett. 2019;14(1):235. doi:10.1186/s11671-019-3053-4
158. Wilson RE, O’Connor R, Gallops CE, et al. immunomagnetic capture and multiplexed surface marker detection of circulating tumor cells with magnetic multicolor surface-enhanced raman scattering nanotags. ACS Appl Mater Interfaces. 2020;12(42):47220–47232. doi:10.1021/acsami.0c12395
159. Żuk M, Podgórski R, Ruszczyńska A, et al. Multifunctional nanoparticles based on iron oxide and gold-198 designed for magnetic hyperthermia and radionuclide therapy as a potential tool for combined her2-positive cancer treatment. Pharmaceutics. 2022;14(8):1680. doi:10.3390/pharmaceutics14081680
160. Li T-F, Li K, Zhang Q, et al. dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials. 2018;181:35–52. doi:10.1016/j.biomaterials.2018.07.035
161. Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C. 2008;112(45):17554–17558. doi:10.1021/jp806751k
162. Jedrzejczak-Silicka M, Szymańska K, Mijowska E, Rakoczy R. The Influence of Graphene Oxide-Fe3O4 Differently Conjugated with 10-Hydroxycampthotecin and a Rotating Magnetic Field on Adenocarcinoma Cells. Int J Mol Sci. 2024;25(2):930. doi:10.3390/ijms25020930
163. More MP, Deshmukh PK. development of amine-functionalized superparamagnetic iron oxide nanoparticles anchored graphene nanosheets as a possible theranostic agent in cancer metastasis. Drug Deliv Transl Res. 2020;10(4):862–877. doi:10.1007/s13346-020-00729-0
164. Li X, Feng J, Du Y, et al. One-Pot Synthesis of CoFe2O4/Graphene Oxide Hybrids and Their Conversion into FeCo/Graphene Hybrids for Lightweight and Highly Efficient Microwave Absorber. J Mater Chem A. 2015;3(10):5535–5546. doi:10.1039/C4TA05718J
165. Liu X, Yan B, Li Y, et al. Graphene oxide-grafted magnetic nanorings mediated magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano. 2020;14(2):1936–1950. doi:10.1021/acsnano.9b08320
166. Mai S, Inkielewicz-Stepniak I. Graphene oxide nanoparticles and organoids: A prospective advanced model for pancreatic cancer research. Int J Mol Sci. 2024;25(2):1066. doi:10.3390/ijms25021066
167. Kannan K, Radhika D, Sadasivuni KK, Reddy KR, Raghu AV. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv Colloid Interface Sci. 2020;281:102178. doi:10.1016/j.cis.2020.102178
168. Magdalane CM, Kaviyarasu K, Vijaya JJ, Siddhardha B, Jeyaraj B. Photocatalytic activity of binary metal oxide nanocomposites of CeO2/CdO Nanospheres: investigation of optical and antimicrobial activity. J Photochem Photobiol B. 2016;163:77–86. doi:10.1016/j.jphotobiol.2016.08.013
169. Chakraborty N, Gandhi S, Verma R, Roy I. Emerging prospects of nanozymes for antibacterial and anticancer applications. Biomedicines. 2022;10(6):1378. doi:10.3390/biomedicines10061378
170. Jana TK, Jana SK, Kumar A, et al.. The antibacterial and anticancer properties of zinc oxide coated iron oxide nanotextured composites. Colloids Surf B Biointerfaces. 2019;177:512–519. doi:10.1016/j.colsurfb.2019.02.041
171. Fu Q, Li Z, Ye J, et al. Magnetic Targeted Near-Infrared II PA/MR Imaging Guided Photothermal Therapy to Trigger Cancer Immunotherapy. Theranostics. 2020;10(11):4997–5010. doi:10.7150/thno.43604
172. Liu X, Liang Z, Du H, et al. DNA-mediated magnetic-dimer assembly for fault-free ultra-high-field magnetic resonance imaging of tumors. Nano Lett. 2024;24(22):6696–6705. doi:10.1021/acs.nanolett.4c01389
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

