Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 15
Surge in Mycoplasma Pneumoniae infection and Respiratory Viruses Co-infection in Children With Community-Acquired Pneumonia in the Post-Pandemic
Authors Chi J , Tang H, Wang F, Wang Y, Chen Z
Received 13 April 2024
Accepted for publication 3 September 2024
Published 7 September 2024 Volume 2024:15 Pages 279—288
DOI https://doi.org/10.2147/PHMT.S473669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Laurens Holmes, Jr
Jie Chi, Heng Tang, Fang Wang, Yuxuan Wang, Zhifeng Chen
Department of Pharmacy, Tongling Municipal Hospital, Tongling, Anhui, People’s Republic of China
Correspondence: Zhifeng Chen, Department of Pharmacy, Tongling Municipal Hospital, Tongling, Anhui, People’s Republic of China, Tel +8613856231378, Email [email protected]
Purpose: During the COVID-19 pandemic, multifaceted non-pharmaceutical interventions have not only reduced the transmission of SARS-CoV2 but also affected the prevalence of other respiratory pathogens. With the lifting of many restrictions, a surge in cases of pneumonia in children has been reported in many hospitals in China. The study assessed the changes in pathogen and symptoms of children with community-acquired pneumonia (CAP) before and after the adjustments of prevention and control measures of epidemic and provided recommendations for CAP in children.
Patients and methods: Children diagnosed with CAP were enrolled in the study from 2022 to 2023. A cross-sectional retrospective study was conducted in a general hospital. We analyzed the data about demographic data, clinical symptoms, pathogens, and medical treatments. The Chi-square and Mann–Whitney U-test were used to assess the statistical significance of groups.
Results: We studied 1103 children, 339 in 2022 and 764 in 2023. Compared with children in 2022, more children were diagnosed with CAP in 2023 and these children had a higher body temperature and levels of CRP and PCT, which indicated these children got severe inflammation. The positive rate of the pathogen was also higher in 2023, especially the detective rate of Mycoplasma pneumoniae. The number of children infected with more than two pathogens was higher in 2023, especially those co-infected with the virus and M. Pneumoniae. Concerning the medicine therapy, the usage of β-lactam antibiotics, Macrolide antibiotics, and antiviral drugs kept rapid growth.
Conclusion: After the adjustment of epidemic prevention and control policies in 2023, more children got CAP with severe clinical symptoms, and more antibiotics and antiviral drugs were used. Further study is needed to explore the reasons for the increase in children with CAP and to explore the rationality of treatment.
Keywords: community-acquired pneumonia, children, post-pandemic, pathogen
Introduction
Community-acquired pneumonia (CAP) is one of the most common respiratory illnesses in children and is an important cause of morbidity in children in developing countries.1 According to the World Health Organization (WHO), CAP is an important cause of death in children under 5 years ago. More than 19% of children under 5 years ago died from CAP.2 In late 2019, the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) broke out in China,3 China has taken various non-pharmaceutical interventions and measures to curb the spread of this virus, such as wearing masks, washing hands frequently, paying attention to indoor ventilation, maintaining social distance and supporting employees to work and study at home. There was a significant decrease in the number of children with CAP hospitalized in the pandemic, especially among school-age children.4 In non-pandemic period, children, especially the school-age and preschool children, would spend more time to study or play with other children in their same age groups, thereby facilitating common respiratory pathogens transmission through spreading of droplets via close contacts in the same age group. But in the pandemic, strict interventions and measures had not only reduced the spread of COVID-19, but also common respiratory pathogens.5 The conductors deduced that the occurrence of CAP was effectively prevented in school-age children due to strong mitigation measures.
Since mid-October 2023, the WHO has been monitoring data from Chinese surveillance systems that have been showing an increase in respiratory illness in children in northern China.6 Cases of pneumonia in children surged and it was reported that some hospitals in the major cities of China were overwhelmed by pediatric emergency admissions.7 Chinese health authorities held the opinion that this was the seasonal peak in a trend of rising respiratory infections in children. Epidemiological evidence supported that, one or more re-emerging infections, such as respiratory syncytial virus (RSV), influenza virus, or Mycoplasma pneumoniae are the cause, rather than a novel respiratory pathogen in children.6 Bacterial pneumonia is a major complication of influenza infection, but bacterial co-infection and secondary infection are not limited to influenza viruses, as many other viruses, including RSV, parainfluenza virus, rhinovirus, adenovirus, and human coronavirus, have also been associated with secondary bacterial pneumonia.8 Respiratory viruses replicate in the respiratory tract and induce a strong cytokine response, causing severe airway inflammation and viral shedding duration.9 Viral infections can induce epithelial damage in the respiratory tract, enhance bacterial colonization of the upper and lower respiratory tracts, and cause dysfunction of the immune response, leading to increased susceptibility to secondary bacterial infections.10
Even the respiratory tract infections of children were caused by common pathogens, the clinical features of the newly occurring infections and their potential threat to children have never been thoroughly investigated after the COVID-19 pandemic. This study collected the medical records of pneumonia patients under 18 years old, who were admitted to a hospital in Anhui Province, China, from 2022 to 2023, aimed to analyze the etiology and clinical characteristics of these children and provide more information for clinical treatment.
Methods
Study Design
The study was a cross-sectional retrospective study conducted in a general hospital in Anhui province, China. Children between≥28 days and 18 years of age who completed pathogen testing within 48 hours after admission and met the diagnostic criteria for CAP from January 2022 to December 2023 were included in this study. We collected demographic data and clinical information of enrolled CAP cases, including laboratory results, treatments, and outcomes. The study protocol complied with the Declaration of Helsinki and was approved by the Ethics Committee of Tongling Municipal Hospital. Individual patient-informed consent was not required for this retrospective study using anonymous data, so written informed consent was waived.
Study Population and Definitions
Patients were enrolled in the study following the procedure. First, children diagnosed with pneumonia during the study period and admitted to our hospital were searched. Then, we excluded duplicates and cases of bronchiolitis, hospital-acquired infections, tuberculosis, and aspiration pneumonia. Thirdly, we reconfirmed the cases that satisfied the clinical definition of CAP, referring to the “Guideline for diagnosis and treatment of community-acquired pneumonia in Children” (2019 version). The diagnostic criteria11 for CAP in children were as follows: (1) cough, expectoration, wheezing, and dyspnea recently with or without chest pain or aggravation of the original respiratory symptoms; (2) fever; (3) signs of pulmonary consolidation and/or moist rales; and (4) patchy, infiltrative shadows or interstitial changes in the lung with or without pleural effusion in the chest X-ray. CAP was clinically diagnosed when the criteria for (1) or (2) were combined with the criteria for (3) or (4). Finally, children with incomplete information were excluded.
A total of 1128 children were diagnosed with pneumonia during the study period and admitted to our hospital. According to the above exclusion criteria, and 1103 cases were included in this study (Figure 1).
According to their age, children were divided into four groups: an infant group (≥ 28 days to < 1 year of age), a young age group (1 to 3 years of age), a preschool-age group (4 to 7 years of age), and a school-age group (7 to 18 years of age). For the season variable, the children were divided into a spring group (March 1–May 31), summer group (June 1–August 31), autumn group (September 1–November 30) and winter group (December 1–February 28).
Etiological Detection and Data Collection
For specimen collection, a throat swab or sputum specimen was routinely collected from all children with CAP. Bronchoalveolar lavage fluid (BALF) was collected from children with extensive pulmonary consolidation. But bronchoalveolar lavage (BAL) was indispensable and consent from the child’s parents was required before the implementation of BAL. A pleural effusion sample was taken from children with a large amount of pleural effusion. The clinician decided which specimen should be collected.
Etiological detection of CAP was conducted using laboratory tests and standard techniques. Sputum smear and culture are commonly used in clinical, and sputum specimens must be qualified. If there is a dominant growth through a semi-quantitative culture, it can be considered a pathogenic bacteria. BALF is important evidence for bacterial pneumonia, but it is not recommended for all pneumonia patients because it is invasive.
Multiplex reverse transcriptase quantitative polymerase chain reaction (mRT-qPCR) is the most commonly used and reliable method for the detection of respiratory viruses from respiratory samples, which can be used for early and rapid pathogen diagnosis. Targeted viruses were adenovirus (AdV), influenza virus (Flu), human metapneumovirus (HMpV), parainfluenza virus (PIV), respiratory syncytial virus (RSV), and human rhinovirus/enterovirus (HRV). In this study, HRV was excluded from the analysis because it can often be detected even in asymptomatic children, and thus, the significance of their detection from respiratory specimens is still not evident, particularly in children.12
Mycoplasma pneumoniae, Chlamydophila pneumoniae, Chlamydophila trachomatis, and Legionella pneumophila were detected from respiratory specimens (eg: throat swab and BALF). For M. pneumoniae, the results of serologic tests were also used. Serologic diagnosis for M. pneumoniae infection was primarily made by a 4-fold increase in antibody titers between the acute and convalescent stages. When only one serologic test result was available, we regarded M. pneumoniae as detected if the titer was ≥ 1:160 in an indirect agglutination test (IAT).11
Common respiratory tract pathogens were detected by PCR kits (Bitron, China), and titers were detected by diagnostic kit to measure antibodies to M. pneumoniae (FUJIREBIO INC., Japan).
Statistical Analyses
The power analysis was performed using G.power (Germany). The results of the priori analysis showed that the number of enrolled children in the study was sufficient for statistical analyses. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). All categorical variables were presented as numbers (percentages) except the total number of patients enrolled, and continuous variables were presented as medians and interquartile ranges (IQRs). The chi-square and the Mann–Whitney U-test were used to assess the statistical significance of groups. All tests were two-sided, and a P value of < 0.05 was considered statistically significant.
Results
General Description of Children with CAP
According to the enrollment criteria above, 1103 children diagnosed with community-acquired pneumonia were registered in the present study, 339 in 2022 and 764 in 2023 (Table 1). No significant difference in sex distribution was observed (P>0.05). The ratio of males to females in 2022 was 1.26:1 and in 2023 was 1.28:1, respectively. In age distribution, patients in 2023 were older than in 2022 (P<0.05), the median age was 4 years old in 2022 and 5 years old in 2023. There was a significant difference in the number of patients in the infant group in 2023 compared with 2022 (P<0.05). The number of children diagnosed with CAP began to increase in the winter of 2022 and continued to increase in the spring, summer, and autumn of 2023, the growth curve showed a downward trend in winter of 2023. The median duration of hospitalization in 2022 and 2023 was both 7 days.
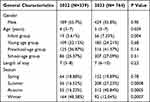 |
Table 1 General Characteristics of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
Clinical Characteristics of Children with CAP
Comparing clinical symptoms of children diagnosed with CAP in 2022 with 2023, a high fever (defined as ≥ 39°C), maximum body temperature (T max), and dyspnea present significant differences (P<0.001) (Table 2). In 2023, more children got a high fever and dyspnea compared with children in 2022. The maximum body temperature was also higher than that in 2022, presented at 38.5 °C and 37.6 °C respectively. Compared with 2022, the white blood cell (WBC) count of children in 2023 was lower, but the NEUT% was higher. However, there was no significant difference in the WBC and NEUT% of children with CAP in 2022 and 2023. C-reactive protein (CRP) and procalcitonin (PCT) in 2023 were higher than that in 2022 and exhibited significant differences (P<0.001).
 |
Table 2 Clinical Characteristics of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
Positives Rates of Pathogens and Co-Infection
Among the 1103 children with CAP, a total of 794 pathogenic tests were positive, with 214 in 2022 and 580 in 2023 (Table 3). It was obvious the detective rate was higher in 2023 than that in 2022. The proportion of both viral and M. pneumoniae CAP increased, especially mycoplasmal pneumonia in 2023. The rate of single pathogens in 2023 was higher than that in 2022. So was the co-infection rate. In 2023, the co-infection rate of the virus and M. pneumoniae increased, and the difference was significant (P< 0.05). In Table 4, the titers of M. pneumoniae were analyzed. The number of children with titers of 1:640 and 1:1280 increased. As the titers of antibodies were positively correlated with the severity of M. pneumoniae infection,13 those may be the reason why the clinical symptoms of children with CAP in 2023 were more severe than those in 2022.
 |
Table 3 Positives Rates of Pathogens and Co-Infection of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
 |
Table 4 The Antibody Titres of M. Pneumoniae of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
Use of Drugs
Among the total of 1103 children, 1101 (99.81%) children received antibiotics therapy and the main used antibiotics were β-lactam antibiotics and macrolide antibiotics (Table 5). Among the 338 children who received antibiotics therapy in 2022, 36.58% of children got antibiotics combination therapy and there was no difference between that in 2023 (P=0.662). In 2022, 91.45% of children received β-lactam antibiotics therapy and the usage decreased to 75.92% in 2023 (P<0.001). In contrast, the utilizatio of macrolide antibiotics significantly ascended from 42.77% in 2022 to 59.29% in 2023 (P<0.001). In 2023, 28.29% of children received antiviral therapy which was significantly higher than that in 2022 (P<0.001) (Table 5), this may be because more children have been tested for respiratory virus infections in 2023. Although the clinical symptoms of children with CAP in 2023 appeared to be more severe than those in 2022, such as higher body temperature, CRP, and PCT, there was no significant difference in the use of glucocorticoids compared to 2022 (P=0.276). Among the children who received antibiotics, 3rd-generation cephalosporin antibiotics were the most frequently used, both in 2022 and 2023 (Table 6). Meanwhile, penicillin and first- and second-generation cephalosporin antibiotics were also commonly used in 2022.
 |
Table 5 Use of Drugs of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
 |
Table 6 Use of β-Lactam Antibiotics of Children Hospitalized with Community-Acquired Pneumonia, 2022–2023 |
Discussion
In December 2022, the Chinese government downgraded the classification of COVID-19 from Class A infectious disease to Class B and canceled the earlier mandatory quarantine measures. The number of people infected with CAP continued to increase in 2023, especially among children.14 In this retrospective study, we compared the basic characteristics, clinical symptoms, pathogens, and clinical medication of children with CAP before and after the adjustment of the COVID-19 epidemic prevention policy. Our study showed that there was a significant increase in the number of children with CAP after the strict epidemic prevention policies were canceled. The clinical symptoms of affected children became severe, and there was a notable increase in the positive detection rate of pathogenic bacteria, especially M. mycoplasma pneumoniae. This phenomenon of a rising number of people infected with M. pneumoniae was not only happening in China but also has been reported abroad.15 Additionally, the rate of mixed infections also increased significantly, leading to a higher utilization of antibiotics and antiviral drugs. Viral co-infections are important contributors to CAP susceptibility and progression.16 Viral co-infection could affect inflammatory states, cardiac function, liver function, and kidney function, possibly due to the virus’s potential to damage tissues, and was associated with a high level of WBC, PCT, lactate dehydrogenase (LDH), creatine kinase (CK), platelet (PLT), indicating that co-infection may affect the immune response, leading to the inflammation.17
Our study found that the number of children infected with CAP began to increase in the winter of 2022, a time when city lockdown and other non-pharmaceutical measures were gradually relaxed, and this increasing wave continued until the winter of 2023. Among the age groups of infected people, there was a significant increase in the proportion of infections in the Infant group (Table 1). The immune status may affect the susceptibility to various viruses infections, and children in the infant group may be more susceptible to respiratory viruses because their immune systems are not fully developed.
The clinical symptoms of infected children in 2023 were significantly more severe than in 2022 (Table 2). We compared the clinical symptoms of the infected children, such as fever and dyspnea, and did not count the other common symptoms of CAP, like cough, expectoration, and runny nose, mainly because the number of medical records was not enough for statistical analysis. The number of infected children in 2023 with a high fever was larger than children in 2022, so the number of children in 2023 with dyspnea was. The maximum body temperature of children in 2023 was higher than children in 2022, 37.6°C and 38.5°C respectively. As mentioned above, the immature immune system and co-infection may contribute to severe inflammation.
Typically, blood tests are useful in the observation of alterations in the number, morphology, and distribution of blood cells, aiding in the identification of bacterial infection, viral infection, or M. mycoplasma pneumonia infection. Both the elevation and the depression of white blood cell (WBC) count and NEUT% are used to aid in the diagnosis of infection.18 A combination of blood tests, C-reactive protein (CRP), and procalcitonin (PCT) is commonly employed to ascertain the type and severity of pneumonia infection.
C-reactive protein (CRP) is a pentameric protein, with 5 identical noncovalently bound subunits, synthesized by hepatocytes (molecular mass 118,000 Da). Infection or inflammation can generate cytokine release (IL-6, IL-1, and TNF-a), which then stimulates CRP synthesis.19 It is positively correlated with the degree of inflammation. Importantly, CRP exhibits relative insensitivity to viral infections and is a clinically significant differentiator between bacterial and viral infections.20
Another commonly used biomarker for the diagnosis and treatment of pneumonia is procalcitonin (PCT), a 116-amino acid peptide reactant produced by the liver during the acute phase and a reactant of thyroid C cells and lung K cells.21 PCT is highly sensitive to bacterial infection, with significantly higher levels in infected compared with non-infected patients associated with infection severity.22 Conversely, viral infection stimulates the release of interferon-G, which, along with other cytokines, down-regulates this PCT release pathway. Thus, although PCT may have an increased response to aseptic inflammation or viral infection, it is not as profound as other biomarkers.23 Likewise, procalcitonin is considered a useful additional tool for diagnosing bacterial diseases in children.24 In this study, there was no significant difference in WBC count and NEUT% between 2022 and 2023 (Table 2). However, the CRP and PCT were significantly higher in 2023 (P<0.001), which meant more infected children got bacterial infections in 2023.
Although inflammatory markers, like CRP and PCT, suggested that bacterial infections were more common in 2023, the detective rate of the bacterium was low in both 2022 and 2023 (Table 3). The difficulty of obtaining sputum specimens in children may be one of the reasons. In the common pathogen of CAP, the positive rate of M. Pneumoniae was significantly higher in 2023 (P<0.001). The same situation is happening not only in other regions of China but also in other countries of the world.15 The resurgence of M. Pneumoniae is thought to be driven by increased population susceptibility as well as relaxation of enhanced public health measures, the so-called “immune gap”. Under enhanced public health measures, the vulnerability of an individual or population was cumulative due to the lack of acquired immunity to M. Pneumoniae and other pathogens.25 Thus, the relaxation of measures during the pandemic may inadvertently create favorable conditions for the resurgence of infectious diseases, including the M. Pneumoniae.26
In M. Pneumoniae pneumonia, the levels of CRP and PCT rise and are related to the severity of M. Pneumoniae pneumonia in children.27 We compared the antibody titer of M. pneumoniae (Table 4), which was a reference standard with a serum antibody titer≥ 1:160 for M. Pneumoniae recent infection or acute infection by the particle agglutination method,28 and the titers of antibody were positively correlated with the severity of M. Pneumoniae infection. The number of children with antibody titer was 1:640 was significantly higher in 2023 (P<0.001), which may be related to the rising levels of CRP and PCT in 2023.
Similarly, as inflammatory biomarkers and detective rate of the pathogen indicate severe infections in children, more broad-spectrum antibiotics will be used in 2023. In this study, we found that only 2 children did not take antibiotics. Moreover, up to 35.12% of children received more than one kind of antibiotic in 2023, the rate of drug-combination was 36.58% in 2022 (Table 5).
It was worth noting that the use of antiviral drugs in 2023 was significantly higher than that in 2022 (P<0.001) (Table 5). Although the number of children infected virus has increased, there was no significant difference in the detection rate of the virus. This paradox may be due to the low positive rate of common respiratory viruses.
In the Guideline for Diagnosis and Treatment of community-acquired pneumonia in Children” (2019 version),11 in children with mild symptoms, children do not have non-pathogenic complications, amoxicillin/clavulanate, 1st and 2nd generation cephalosporins are the top choices, the 3rd generation cephalosporins only be selected if children were highly suspect to infect gram-negative bacilli. In our study, no children were diagnosed with complications, and there was no reason to conclude that up to 70% of children were infected with gram-negative bacilli due to the lack of the results of sputum culture (Table 6). So, the reasonableness of the use of the 3rd generation cephalosporins needs to be further assessed.
There were some limitations in our study. First, the positive rate of bacteria was very low, which may lead to bias in our results, and this may be related to difficulties in obtaining sputum samples from children. Second, the data for this study were all from one hospital, and the sample size may be small. Third, past medical history, especially respiratory infections, was not included in this study because lacking of sufficient data. More studies were needed in the mechanisms of pathogen-to-pathogen interactions in co-infection, the changes of drug resistance in pathogens, and the subsequent effects of “immune debt”,29 which means a larger post-pandemic epidemic than the pre-pandemic epidemic, during the pandemic in the future.
Conclusion
The number of children infected with CAP has significantly increased during the post-pandemic, especially M. Pneumoniae pneumonia and the symptoms of infected children in 2023 were more severe. According to our study, during the COVID-19 pandemic, the average cases of these diseases caused by common respiratory pathogens decreased significantly when enhanced public health measures were used, and after the pandemic, there was a rebound in the average cases of these diseases when there measure were not available. The “immune debt” observing in young children may be the reason of increased number of people infected with CAP. Therefore, there is a need for continuous and appropriate public health management strategies to prevent the resurgence of serious infectious diseases in the post-pandemic period. Since the implementation of public health management has a double-edged sword effect on the spread of infectious diseases and economic development, appropriate alternative strategies such as hand hygiene, social distancing, and wearing masks should be considered. Multi-center studies and data about drug-resistance and genotype of pathogen are needed in the future.
Acknowledgments
This study was supported by Health Commission of Anhui Province (AHWJ2022c042).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qin Q, Shen KL. Community-acquired Pneumonia and its Complications. Indian J Pediatr. 2015;82(8):745–751. doi:10.1007/s12098-015-1785-4
2. McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7(1):e47–e57. doi:10.1016/S2214-109X(18)30408-X
3. Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
4. Zhang LN, Cao L, Meng LH. Pathogenic changes of community-acquired pneumonia in a children’s hospital in Beijing, China before and after COVID-19 onset: a retrospective study. World J Pediatr. 2022;18(11):746–752. doi:10.1007/s12519-022-00592-8
5. Cheng Y, Cheng Y, Dai S, et al. The Prevalence of Mycoplasma Pneumoniae Among Children in Beijing Before and During the COVID-19 Pandemic. Front Cell Infect Microbiol. 2022;12:854505. doi:10.3389/fcimb.2022.854505
6. World Health Organization (WHO) Disease Outbreak News. Upsurge of respiratory illnesses among children in northern China, 2023. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON494.
7. Parums DV. Editorial: outbreaks of Post-Pandemic Childhood Pneumonia and the Re-Emergence of Endemic Respiratory Infections. Med Sci Monit. 2023; 29:e943312.
8. Falsey AR, Becker KL, Swinburne AJ, et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208(3):432–441. doi:10.1093/infdis/jit190
9. Chen Q, Lin L, Zhang N, Yang Y. Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front Pediatr. 2024;12:1337786. doi:10.3389/fped.2024.1337786
10. Sumitomo T, Kawabata S. Respiratory tract barrier dysfunction in viral-bacterial co-infection cases. Jpn Dent Sci Rev. 2024;60:44–52. doi:10.1016/j.jdsr.2023.12.006
11. National Health Commission of the People’s Republic of China, State Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of community-acquired pneumonia in children (2019 version). Chin J Clin Infect Dis. 2019;12:6–13.
12. Self WH, Williams DJ, Zhu Y, et al. Respiratory Viral Detection in Children and Adults: comparing Asymptomatic Controls and Patients With Community-Acquired Pneumonia. J Infect Dis. 2016;213(4):584–591. doi:10.1093/infdis/jiv323
13. Lee WJ, Huang EY, Tsai CM, et al. Role of Serum Mycoplasma pneumoniae IgA, IgM, and IgG in the Diagnosis of Mycoplasma pneumoniae-Related Pneumonia in School-Age Children and Adolescents. Clin Vaccine Immunol. 2017;24(1):e00471–16. doi:10.1128/CVI.00471-16
14. Xing FF, Chiu KH, Deng CW, et al. Post-COVID-19 Pandemic Rebound of Macrolide-Resistant Mycoplasma pneumoniae Infection: a Descriptive Study. Antibiotics. 2024;13(3):262. doi:10.3390/antibiotics13030262
15. Urbieta AD, Barbeito Castiñeiras G, Rivero Calle I, et al. Martinón Torres F. Mycoplasma pneumoniae at the rise not only in China: rapid increase of Mycoplasma pneumoniae cases also in Spain. Emerg Microbes Infect. 2024;13(1):2332680. doi:10.1080/22221751.2024.2332680
16. Smith DK, Kuckel DP, Recidoro AM. Community-acquired pneumonia in children: rapid evidence review. Am Fam Physician. 2021;104(6):618–625.
17. Mao S, Wu L. Coinfection of viruses in children with community-acquired pneumonia. BMC Pediatr. 2024;24(1):457. doi:10.1186/s12887-024-04939-0
18. Dudognon D, Levy C, Chalumeau M, et al. Diagnostic Accuracy of Routinely Available Biomarkers to Predict Bacteremia in Children With Community-Acquired Pneumonia: a Secondary Analysis of the GPIP/ACTIV Pneumonia Study in France, 2009-2018. Front Pediatr. 2021;9:684628. doi:10.3389/fped.2021.684628
19. Zhang GM, Gu YY. Diagnostic value of Procalcitonin, C-reactive protein-to-lymphocyte ratio (CLR), C-reactive protein and neutrophil-to-lymphocyte ratio (NLR) for predicting patients with Bacteraemia in the intensive care unit. J Crit Care. 2024;81:154538. doi:10.1016/j.jcrc.2024.154538
20. Zhou B, Wen X, Zhou J, Lv X. Assessing Diagnostic Significance of White Blood Cell Count, Serum C-Reactive Protein, and Procalcitonin in Neonatal Pneumonia: a Comparative Analysis. Altern Ther Health Med. 2024; 2024: 9954.
21. Bréchot N, Hékimian G, Chastre J, Luyt CE. Procalcitonin to guide antibiotic therapy in the ICU. Int J Antimicrob Agents. 2015;46(Suppl 1):S19–24. doi:10.1016/j.ijantimicag.2015.10.012
22. Saura O, Luyt CE. Procalcitonin as a biomarker to guide treatments for patients with lower respiratory tract infections. Expert Rev Respir Med. 2023;17(8):651–661. doi:10.1080/17476348.2023.2251394
23. Sungurlu S, Balk RA. The Role of Biomarkers in the Diagnosis and Management of Pneumonia. Infect Dis Clin North Am. 2024;38(1):35–49. doi:10.1016/j.idc.2023.12.005
24. Van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4(10):620–630. doi:10.1016/S1473-3099(04)01146-6
25. Silva SJRD, Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13:100287. doi:10.1016/j.onehlt.2021.100287
26. Xu Y, Yang C, Sun P, et al. Epidemic features and megagenomic analysis of childhood Mycoplasma pneumoniae post COVID-19 pandemic: a 6-year study in southern China. Emerg Microbes Infect. 2024;13(1):2353298. doi:10.1080/22221751.2024.2353298
27. Chen Q, Hu T, Wu L, Chen L. Clinical Features and Biomarkers for Early Prediction of Refractory Mycoplasma Pneumoniae Pneumonia in Children. Emerg Med Int. 2024;2024:9328177. doi:10.1155/2024/9328177
28. Tuo W, Guo X, Wu M, et al. Application value of antibody titres and RNA detection in the early prediction of Mycoplasma pneumoniae pneumonia in children: a retrospective study. BMC Infect Dis. 2023;23(1):220. doi:10.1186/s12879-023-08161-8
29. Lee PI, Hsueh PR, Chuang JH, Liu MT. Changing epidemic patterns of infectious diseases during and after COVID-19 pandemic in Taiwan. J Microbiol Immunol Infect. 2024;2024:
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Impact of mNGS Technology in the Etiological Diagnosis of Severe Pneumonia in Children During the Epidemic of COVID-19
Yu C, Guo W, Zhang Z, Ma Y, Cao X, Sun N, Cui Y, Wang Y, Cui W, Xu Y, Zhan J
Infection and Drug Resistance 2023, 16:2395-2402
Published Date: 21 April 2023
Clinical Characteristics of Necrotizing Pneumonia Caused by Different Pathogens
Luo Y, Wang Y
Infection and Drug Resistance 2023, 16:3777-3786
Published Date: 14 June 2023


