Back to Journals » Infection and Drug Resistance » Volume 18
Synergy and Mechanism of the Action of the Combination of Essential Oils and Antibiotics Against Antibiotic-Resistant Food Borne Disease Bacteria in Burkina Faso
Authors Sodéré P , Somda MK, Zongo L , Mihin HB, Mogmenga I, Akakpo AY, Dicko MH
Received 22 January 2025
Accepted for publication 19 May 2025
Published 30 May 2025 Volume 2025:18 Pages 2743—2763
DOI https://doi.org/10.2147/IDR.S518717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Paul Sodéré,1,2 Marius K Somda,1,2 Luc Zongo,3,4 Henriette B Mihin,1 Iliassou Mogmenga,2,5 Agbémébia Y Akakpo,1 Mamoudou H Dicko2
1Laboratory of Microbiology and Microbial Biotechnology, Department of Biochemistry and Microbiology, University Joseph KI-ZERBO, Ouagadougou, 03 BP: 7021, Burkina Faso; 2Laboratory of Biochemistry Biotechnology Food Technology and Nutrition, Department of Biochemistry Microbiology, University Joseph KI-ZERBO, Ouagadougou, 03 BP: 7021, Burkina Faso; 3Hospital Saint Camille of Ouagadougou (HOSCO), Ouagadougou, 09 BP 444, Burkina Faso; 4Faculty of Health Sciences, University Saint Thomas d’Aquin (USTA), Ouagadougou, 06 BP 10212, Burkina Faso; 5Banfora University Centre, University Nazi Boni, Bobo-Dsso, 01 BP: 1091, Burkina Faso
Correspondence: Paul Sodéré, Laboratory of Microbiology and Microbial Biotechnology, Department of Biochemistry and Microbiology, University Joseph KI-ZERBO, Ouagadougou, 03 BP: 7021, Burkina Faso, Email [email protected]
Background: Bacterial resistance to antibiotics is an important developing threat for human health. Therefore, searching for alternatives through the synergistic combination of essential oils with conventional antibiotics is one of the relevant approaches. This study aims to investigate the mechanism of action of essential oils isolated from local common medicinal plants of Burkina Faso (Hyptis suaveolens and Laggera aurita) combined with three antibiotics (ciprofloxacin, amoxicillin + clavulanic acid and colistin) against resistant-bacterial strains (08) involved in toxi–intoxic effects (Staphylococcus aureus ATCC 2523, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 9027, Enterococcus faecalis 0366 V, Salmonella typhi SKN 1152, Bacillus cereus 0998 V, Bacillus subtilis 0486 V, Yersinia enterocolitica 0938 V).
Methods: The methodological approach consisted of evaluating the antibacterial activity followed by investigating antibacterial mechanism.
Results: The MICs (minimum inhibitory concentration) of Laggera aurita and Hyptis suaveolens ranged from 4.05 mg/mL to 64.92 mg/mL and from 9.57 mg/mL to 38.28 mg/mL, respectively. Checkerboard assays revealed synergistic effects resulting in reductions of 93.69% and 87.73%, respectively, in amoxicillin+ clavulanic acid and colistin MICs. Protein and nucleic acid leakage assays demonstrated that peptidoglycan and cytoplasmic membrane damage induced by the synergistic combination were significantly greater than those in the control. The viable count of bacteria for this combination showed a complete killing profile after 12 hours and further.
Conclusion: It appears from this study that the combinations (essential oil-antibiotic) reduced the MICs of the antibiotics and eradicated antibiotic-resistant bacteria completely. This combination could constitute an excellent means against bacterial resistance to antibiotics.
Keywords: antibacterial activity, antibiotics, essential oils, Hyptis suaveolens, Laggera aurita, mechanisms
Introduction
According to the World Health Organization (WHO), antimicrobial resistance (AMR) is one of the ten global threats to public health.1 Indeed, the WHO estimates that nearly 50,000 people die per day worldwide from infectious diseases caused by bacteria and fungi and the hospital treatment burden is around 380 million euros.2 Antibiotics have exhibited inhibitory effects, but very soon after their introduction as therapeutic agents, resistance were observed.3 Indeed, the frequency of resistance to first-line antimicrobial agents has been increasing worldwide in recent decades in both human and veterinary medicine.4 In Africa and in Burkina Faso in particular, recent studies revealed that the prevalence of resistance to colistin was 1.2% and 1.5% for Klebsiella pneumoniae and Enterococcus cloacae, respectively.5 Indeed, Ba et al and Nadembega et al6,7 reported that 32% of S. aureus were resistant to methicillin, 98.3% of Escherichia coli and 94.7% of K. pneumoniae were resistant to amoxicillin + clavulanic acid, 36.44% of E. coli and 26.3% of K. pneumoniae were resistant to cephalosporins in the third-generation. The resistance rate of beta-lactamase-producing gram-negative bacteria was 35%. Giving the alarming number of reported cases of bacterial resistance, in 2015, the WHO, in collaboration with the Food and Agriculture Organization and the World Organization for Animal Health declared a “global plan”, encouraging each state member to build a national strategy against bacterial resistance to antibiotics from “One Health” perspective.1 In Burkina Faso for instance, many actions have been undertaken through the organization of national symposia to combat antimicrobial resistance.
Almost 30% of antibiotics prescriptions are considered to be of little or no effectiveness.8 To increase the effectiveness of antibiotics, many researchers focused on the use of plants to combat bacterial resistance. The use of essential oils as antimicrobial agents is of increasing interest in research.9 In fact, essential oils have a wide spectrum of inhibitory effects on gram-negative and gram-positive bacteria.10 Antibiotics combinations against antibiotic resistance have been ineffective because of multidrug-resistant bacteria and the use of essential oils in combination with antibiotics seems to improve the efficacy and may “save antibiotics”.11,12 The synergism between antimicrobial agents and plant extracts is a novel concept and has been recently reported.13,14 Many studies focused on the antibacterial activities of the combination of essential oils and antibiotics on antibiotic-resistant bacteria.15 But the number of studies focused on the antibacterial mechanism remains low.16,17 The present study aimed to elucidate the synergistic mechanism effect of essential oils from H. suaveolens and L. aurita combined with antibiotics against antibiotic-resistant bacteria. In Burkina Faso, previous studies showed that these plants were very widespread and have traditional medicine use. Indeed, Laggera aurita and Hyptis suaveolens are proliferating herbaceous plants and possess significant antibacterial activity against Gram-positive and Gram-negative bacteria.18–22 The antibacterial activity of the combination of essential oils and antibiotics was then investigated to determine synergistic combinations. To elucidate the antimicrobial mechanism, antibiotic-resistant bacteria were treated with synergistic combinations and the release of bacterial material that absorbs at 260 and then 595 nm and the viable cell count were monitored.
Materials and Methods
Material
Essential Oils
Leaves of H. suaveolens and L. aurita were identified by Mr OUEDRAOGO Karim a botanist of the National Center for Scientific and Technology Research (CNRST) in OUAGADOUGOU. The references of the voucher specimens of L. aurita and H. suaveolens are n° 152A preserved in the National Herbarium of Burkina (HNBU) and n° 110 preserved in the herbarium of University Joseph KI-ZERBO at the Laboratory of Ecology and plant Biology (LaBEV) respectively. Leaves were harvested from September to November 2018 in the outlying north of city of Ouagadougou, more precisely in Bassinko.
Figure 1 shows H. suaveolens and L. aurita plants.
 |
Figure 1 Leaves of Hyptis suaveolens (a) and Laggera aurita (b). |
Antibiotics
Ciprofloxacin, amoxicillin + clavulanic acid (Amoxiclav) and colistin procured from a pharmacy in Burkina Faso were used for this study.
The different bacterial targets of inhibition (wall bacteria, cytoplasmic membrane and cytoplasm) and availability in local pharmacy led to the choice of these antibiotics in our study.
Bacterial Strains
The following microorganisms were used as test strains: Staphylococcus aureus ATCC 2523, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 9027 provided by the American Type Culture Collection (ATCC), Enterococcus faecalis 0366 V, Salmonella typhi SKN 1152, Bacillus cereus 0998 V, Bacillus subtilis 0486 V, and Yersinia enterocolitica 0938 V provided by the Food and Nutritional Biological Sciences Research Center at University Joseph-KI ZERBO in Ouagadougou.
The food-borne diseases caused by these bacteria led to the choice of these pathogens for our study.
Methods
Hydrodistillation
Leaves were washed after collection several times with clean water to remove adhering debris. In the shaded area, the leaves were air dried and then dried in an oven at approximately 37°C. The dried leaves were ground into fine particles and then stored for further extraction in plastic bags at room temperature.
Essential oils were extracted from H. suaveolens and L. aurita by hydrodistillation.23
Ten kilograms of each grounded fine particles leave type were placed in an alembic containing 100 liters of water. The alembic was hermetically sealed and the mixture boiled for 3 hours. The essential oils rich vapors passing through the alembic column were collected in separatory funnel. The essential oils were recovered by simple decantation. Density of each essential oils was calculated as follows:
Density = Mass of essential oil/Volume of essential oils.
L. aurita and H. suaveolens concentrations of 0.7657 g/mL and 0.6493 g/mL, respectively, were obtained and placed in bottles wrapped with aluminum foil and stored at 4°C in a refrigerator away from ultraviolet rays.
Bacterial Strains Culture Conditions
All bacterial cultures were stored in Mueller–Hinton (MH) liquid media supplemented with 25% (v/v) glycerol at 20°C. Cultures were revived on Mueller–Hinton (MH) agar plates as necessary. After incubation, a few well-isolated bacterial colonies were picked to prepare the standard 0.5 Mac Farland suspension. The optical density was read at 625 nm via a spectrophotometer. The inoculum was adjusted to 1.5.106 CFU/mL for the bacterial suspension.
Preparation of Antibiotic and Essential Oil Concentrations
Antibiotic solutions were prepared by dissolving 500 mg of each antibiotic in 1 mL of sterile tween 80. The mixture (tween 80 and antibiotic) was transferred to a sterile flask containing 199 mL of sterile distilled water to obtain a concentration of 2.5 mg/mL according to CLSI.24
Antibacterial Activity Determination via Well Diffusion Method
Antibacterial activity was measured via the well diffusion method according to Braga et al.25 Briefly, plates containing approximately 25–30 mL of Mueller–Hinton agar medium were inoculated with an 18–24h-old culture of the bacterial strains via a cotton swab. Wells (6 mm diameter) were punched in the agar and filled with 10 µL of essential oils or antibiotics. The plates were incubated at 37°C for 18–24 h. The antibacterial activity was assessed by measuring the inhibition zone diameter (mm) around the well.26 The bacterial susceptibility to antibiotics or essential oils was determined via the critical diameters and critical concentrations reported in Table 1.
 |
Table 1 Susceptibility of Bacteria to Antibiotics and Essential Oils |
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
The MIC and MBC values of the essential oils and antibiotics were determined via the broth microdilution method.27 Mueller–Hinton broth (MHB) supplemented with tween 80 was used to promote bacterial growth. A volume of 190 µL of this medium was introduced into all the wells of the first column, and a volume of 100 µL was added to each of the remaining wells.
Ten serials twofold dilutions of each antibacterial agent (L. aurita, H. suaveolens ciprofloxacin, amoxicillin + clavulanic acid or colistin) were made from the essential oils and antibiotic concentrations previously prepared to obtain final concentrations ranging from 32.46 to 0.03 mg/mL for H. suaveolens, 38.28 to 0.01 mg/mL for L. aurita and 125 to 0.06 µg/mL for each antibiotic. The wells of the broth without antimicrobials served as controls. The 96-well microplates were incubated at 37°C for 24 hours and turbidity or growth was observed. The lowest concentration with no turbidity among the test-wells was recorded as the MIC.28
The MBC was considered as the lowest antimicrobial concentration that killed 99.9% of the bacterial inocula after 24 h of incubation at 37°C.29 MBC values were determined by subculturing the contents of test-wells without turbidity or no visible growth from MIC determinations to neutral sterile Mueller–Hinton agar plates.
Synergistic Effects of the Checkerboard Method
The interaction types between the essential oils compounds and the antibiotics were evaluated via the checkerboard method.30 The checkerboard method was only used on bacteria resistant to the antibiotics. Serial twofold dilutions of each essential oil were made horizontally, and those of each antibiotic were made vertically in the 96-well microplates to obtain serial combinations with different concentrations. Each well contained a unique combination of a binary essential oil mixture (50 µL) and an antibiotic (50 µL). Then, 100 µL of bacterial suspension at 1.5.106 CFU/mL, equivalent to 0.5 McFarland, was added to each well and incubated at 37°C for 24h.
The plates were incubated at 37°C. The lowest concentration of combination (essential oils and antibiotics), which did not contain any visually detectable bacterial growth was considered as MIC. Besides, the control (containing test bacteria but no essential oils/antibiotics) was used to assess the growth of bacteria, while the negative control was taken to assess the clarity/turbidity in the combination. The checkerboard generates many effective combinations.
The interaction between the essential oils and antibiotics was calculated by the fractional inhibitory concentration (FIC) index of the combinations. The FIC of each antibacterial agent was calculated on the basis of the complete growth inhibition of the microorganisms in the combination well. The FICI was calculated as follows:
FIC of essential oil = MIC of essential oil in the combination/MIC of essential oil alone; FIC of antibiotic = MIC of antibiotic in the combination/MIC of antibiotic alone.
FIC index = FIC of essential oil + FIC of antibiotic as described by.31
Synergy (FICI ˂ 0.5); addition (0.5 ≤ FICI ≤ 1); indifference (1 ˂ FICI ≤ 4) and antagonism (FICI > 4).
Bacterial Fixation Test
The purpose of this test is to measure in the spectrophotometer, the absorbance of effective combinations of antibiotics and essential oils before and after brief contact with bacteria.32 Indeed, E. coli bacterial suspensions of 1.5 × 106 and 1.5 × 108 bacteria/mL were treated for 15 min with the effective combinations of antibiotics and essential oils and then were centrifuged at 12000 × g for 2 min.
The absorbance of the supernatants from the suspensions of essential oils and antibiotics in contact with bacteria was measured at 280 nm and compared to that of the samples without bacterial contact.
Proteins Leakage Estimation
Proteins released by bacterial cells were assessed during treatment with MIC synergistic values of antimicrobials (antibiotics alone and in combination with essential oils). The release of proteins was examined through colorimetric methods as described by Bradford.33 The 96-well plate was incubated at 37°C for 30 min, and the absorbance was measured at 595 nm via a microtiter plate reader (Epoch; BioTeK spectrophotometer) following the manufacturer’s instructions. The standard calibration curve (y = 0,0184x + 0,0017 R² = 0,9992) was prepared using bovine serum albumin (BSA) at 0–250 µg/mL, protein solutions and used for calculating the protein released by bacteria treated with synergistic combinations in the supernatant. PBS containing washed bacteria was used as a control. Each experiment was performed independently three times.
Cytoplasmic Membrane (CM) Permeabilization Assays
Molecules released by treated bacteria and absorbed at 260 nm are probably nucleic acids.34 The CM permeabilization experiment was performed with some modifications to confirm the results as previously described by Rhayour.32 Shortly after the FIC index was determined by the checkerboard assay, the MIC values for essential oil and antibiotic combinations that indicated synergistic FIC indices were selected against antibiotic-resistant bacteria to measure CM permeability. This method was performed by measuring the release of 260 nm-absorbing material (Epoch; BioTeK spectrophotometer) following the manufacturer’s instructions.
The effects of essential oils combined with antibiotics on the cell membrane integrity of bacterial strains were measured following the method described by35 with some modifications. Overnight bacterial cultures (1.5.106 CFU/mL) at 37°C were washed and resuspended in PBS. The strains were treated with synergistic combinations of essential oils and antibiotics while the untreated strains served as negative controls. After incubation at 37°C for 2 h, the cell suspensions were centrifuged at 13.000 rpm for 10 min, and the supernatants were collected and diluted appropriately. The optical density (OD) values at 260 nm were recorded via spectrophotometer.
The bacterial solutions were incubated at 37°C with shaking at 200 rpm and then centrifuged at 6.000 × g for 5 min. Next, 100 µL of the supernatant was removed at 1/4, 1/2, 2, 4, 12 and 24 h. The absorption at 260 nm was measured via a 96-well plate reader. Each experiment was performed independently three times.
Kill Curve Determination (Viable Count)
Bacteria were inoculated into sterile MH liquid media. Synergistic combinations of essential oils and antibiotics were added to the bacterial mixture to obtain the synergistic concentration combinations previously determined via the checkerboard method. Serial twofold dilutions of bacterial solutions were made to obtain a range of 30–300 counted colonies.36
The bacterial solutions were incubated at 37°C with shaking at 200 rpm. Next, 100 µL from each treated bacterial solution was removed after 15 min, 30 min, 2h, 4h, 12h and 24h of treatment and inoculated onto MH agar and then incubated. After 24 h, colonies were counted.37 A control group without the addition of the essential oils or antibiotics was then established for comparison. The number of viable cells was monitored by counting the CFUs after incubation for 24 h at 37°C.
Statistical Analysis of Data
Analysis of variance (ANOVA) was used to compare variable means. Fisher’s Least Significant Difference (LSD) test was used for pairwise multiple comparisons of means where there was a significant difference between all means. The difference between means was considered significant when the p-value was less than 0.05. XLSTAT version 7.5.2 2016 software was used for statistical analysis.
Results
Antibacterial Activity of Antibiotics
The antibacterial activity of antibiotics against bacteria was assessed in vitro via the well diffusion method. All the antibiotics used in this study showed antibacterial activity against the investigated test organisms (Table 2). All the strains were sensitive to ciprofloxacin, 50% of the bacteria tested were resistant to amoxicillin + clavulanic acid and 87.50% were resistant to colistin.
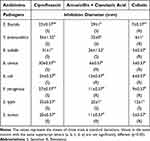 |
Table 2 Antibacterial Activity of Antibiotics |
Ciprofloxacin had the maximum zone of inhibition (36 mm) against Y. enterocolitica. Colistin showed the minimum zone of inhibition (05 mm) against B. cereus, B. subtilis and S. aureus.
MIC/MBC Values of Antibiotics
The results from the broth microdilution tests for the in vitro antibacterial activity of the antibiotics are presented in Table 3. The MICs of amoxicillin + clavulanic acid ranged from 0.02 µg/mL to 29.66 µg/mL and the MICs of colistin ranged from 0.03 µg/mL to 5 µg/mL.
 |
Table 3 Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Antibiotics |
The amoxicillin + clavulanic acid MBC ranged from 6 µg/mL to 96 µg/mL and the colistin MBC ranged from 7.5 µg/mL to 60 µg/mL.
The lowest MIC (0.02 µg/mL) was obtained with amoxicillin + clavulanic acid on S. typhi and the highest MIC (29.66 µg/mL) was obtained with the same antibiotic on P. aeruginosa.
The lowest MBC (6 µg/mL) was obtained with amoxicillin+ clavulanic acid on S. typhi and the highest MBC (96 µg/mL) was obtained with amoxicillin+ clavulanic acid on P. aeruginosa.
MIC and MBC Values for Essential Oil Determination
The MICs of H. suaveolens essential oil ranged from 9.57 mg/mL against S. typhi, B. cereus and P. aeruginosa to 38.28 mg/mL against E. faecalis, Y. enterocolitica, E. coli, and S. aureus. The MBC ranged from 19.14 mg/mL with S. typhi to 76.56 mg/mL with E. coli and P. aeruginosa (Table 4).
 |
Table 4 Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Essential Oils |
The MIC of L. aurita essential oil, ranged from 1.69 mg/mL against S. aureus to 64.92 mg/mL against E. coli. The MBCs ranged from 16.23 mg/mL against S. typhi to 64.92 mg/mL against E. faecalis and Y. enterocolitica (Table 4).
Synergistic Effects of Essential Oils and Antibiotics
The assessed FIC indices for the essential oils of H. suaveolens and L. aurita plus antibiotics against pathogen strains tested revealed synergistic effects. These findings suggest that H. suaveolens and L. aurita not only have some antibacterial activity against these strains but also have the ability to reverse the resistance of such bacterial strains via synergy with antibiotics. However, it’s not only synergistic effects that have been observed. Indeed, in addition to synergistic effects, addition, indifference and antagonism effects have been also observed when combining essential oils to antibiotics and tested against antibiotic-resistant bacterial strains (Table 5). Twelve synergistic effects were observed when essential oils were combined with two antibiotics.
 |
Table 5 Synergistic Effects of Essential Oils and Different Antibiotics Against Pathogens |
On the other hand, the synergistic combination of essential oils with antibiotics considerably reduced the MICs of antibiotics.
Bacteria Fixation
For bacterial test fixation, 280 nm was chosen. The effects of combinations of essential oils and antibiotics bacterial fixation were assessed by comparing their absorbances in the supernatant after contact with bacteria (1.5 × 106 bacteria/ mL) and that without contact and by comparing the absorbances of the supernatants from different density bacterial suspensions. The results are reported in Tables 6 and 7.
 |
Table 6 Bacteria Fixation of Essential Oils and Antibiotics Before and After Bacterial Contact |
 |
Table 7 Bacteria Test Fixation of Essential Oils and Antibiotics at Different Bacterial Densities |
Spontaneous Lysis and Viability of Bacteria in the Absence of Bactericidal Agents
A suspension of washed and untreated bacteria was used as a control. The results are shown in Table 8. Very little release of bacterial material absorbing at 260 nm and 595 nm was observed. The bacterial mortality rate was also very low.
 |
Table 8 260 nm and 595 nm Absorbing Materials in the Cells and Spontaneous Bacterial Mortality |
Analysis of the Release of Proteins by Treated Bacteria
Washed bacterial suspensions were treated with combinations of essential oils and antibiotics at various concentrations. The results of the release of proteins during the treatment are shown in Figure 2. Compared with the combinations of antibiotics alone or the control, the combination of synergistic agents caused greater release of proteins (Table 8). Indeed, the average maximum quantities of released proteins induced by combinations of amoxicillin + clavulanic acid and H. suaveolens, amoxicillin + clavulanic and L. aurita, colistin and H. suaveolens, colistin and L. aurita and that induced by colistin, amoxicillin + clavulanic acid and the control were 16.06 µg/mL, 17.95 µg/mL, 14.44 µg/mL, 17.24 µg/mL, 5.86 µg/mL, 7.52 µg/mL and 0.04 µg/mL respectively.
Release of 260 nm Absorbing Material Analysis
To explore the mechanism of action of the combination of essential oils and antibiotics, we tested the leakage of bacterial nucleotides to verify that essential oils and antibiotics affect the integrity and the permeability of bacterial cell membranes. Soon nucleotides are released after the plasma membrane rupture, these exudates were quantified by monitoring the absorbance at 260 nm.
The results of the loss of 260 nm absorbing material in the cells of the bacterial strain are shown in Figure 3.
Compared with antibiotics alone or the control, synergistic combinations caused greater release of nucleotides. Indeed, the average maximum quantity of nucleotides released by measuring the absorbance induced by combinations of amoxicillin+ clavulanic acid and H. suaveolens, amoxicillin+ clavulanic acid and L. aurita, colistin and H. suaveolens, colistin and L. aurita and that induced by colistin, amoxicillin+ clavulanic acid and the control were 0.33, 0.37, 0.35, 0.31, 0.11, 0.03 and 0.02 respectively.
Kill Curve Assays
The results of the combined effects of essential oils and antibiotics on the viable counts of bacteria are presented in Figures 4. All the synergistic combinations and all the antibiotics resulted in a reduction in the number of CFU/mL inoculum, unlike the control, which resulted in no reduction but an increase in the number of CFU/mL inoculum from the control inoculum (between 0 and 24 h). Clearly, the synergistic combination greatly decreased the cell count. Indeed, synergistic combinations increased the number of cells to approximately 0 after 12 hours of treatment. Amoxicillin + clavulanic acid and colistin tested against antibiotic-resistant bacteria did not induce total inhibition.
Discussion
Antibacterial Effects of Antibiotics
The high sensitivity of bacteria to ciprofloxacin could be explained by the fact that ciprofloxacin is an antibiotic of the fluoroquinolone class that crosses the wall and the plasma membrane by passive diffusion to interact with the DNA and cause the death of the bacteria.38 On the other hand, the tested strains were not able to efflux ciprofloxacin which is a one of the mechanisms of resistance to ciprofloxacin.
The resistance rate of amoxicillin + clavulanic acid was similar to that reported by Compaoré,39 who reported a 33% resistance rate to amoxiclav by bacterial strains isolated from gray water from Ouagadougou. The resistance to amoxicillin + clavulanic, a beta-lactam, could be explained by the production of metallo-β-lactamases (MβLIs) by certain bacterial strains.40
The high resistance (87.50%) of bacteria to colistin could be explained by the intensive use of antibiotics in animal breeding, which selects multiresistant strains in the commensal flora of these animals, strains that then spread to humans. This is also illustrated by the extreme antibiotic resistance of bacteria.40,41 The resistance observed with colistin for most bacteria could be explained by chromosomal mutations in various genes leading to modifications in the charge of LPS, which prevents the binding of colistin.
In our study, the prevalence of the resistance to ciprofloxacin was nonexistent. However, this does not exclude the existence of resistance to this antibiotic. Indeed, the National Observatory of Epidemiology and Bacterial Resistance to Antibiotics (ONERBA) reported a prevalence of resistance to ciprofloxacin of 5.5%.38
MIC Determination of Antibiotics
The MIC of colistin was significantly (p < 0.05) lower than that of amoxicillin + clavulanic acid. This could be explained by differences in their antimicrobial sites of action. Indeed, the inner membrane is the target of colistin and the outer membrane is the target of beta lactams.4,15 The inner membrane being less accessible than the outer membrane could explain the higher resistance of bacteria to colistin. The high resistance of bacteria to colistin compared with beta lactams was also reported by Benameur et al42 in their study of the synergistic effects of medicinal plants and antibiotics on pathogenic bacteria. The percentages of amoxicillin + acid clavulanic and colistin resistance in the solid media were slightly different from those in the liquid media. These differences occur because an antibiotic inhibits a microorganism via direct contact does not react in the same way in a liquid medium.43
The P. aeruginosa MBC was significantly greater than the other bacterial MICs were (ANOVA, P < 0.05). A high MBC of P. aeruginosa was also reported by Gang-Joon et al.44 Our results are similar to those of Lambert,45 who reported high MICs of P. aeruginosa on 40 strains tested.
MIC Determination of Essential Oils
H. suaveolens and L. aurita oils showed important antibacterial activity against bacteria-resistant bacteria. This antibacterial activity was observed by Mihin et al.15 Similarly, Dian-Hong et al46 reported an important antibacterial effects of H. suaveolens on E. coli, P. aeruginosa, B. subtilis and S. aureus strains. The antibacterial activity of L. aurita was also reported by Dibala et al18 in the evaluation of the antimicrobial properties of extracts from L. aurita.
Essential oils compounds have two ways of action (action on bacterial envelops or action on bacterial cytoplasmic). Indeed, to inhibit bacteria, essential oils compounds can disrupt the cytoplasmic membrane or bacterial cell wall or modify the cytoplasmic membrane increasing cell leakage and permeability. They can also inhibit bacterial ATP synthetases or interfere with quorum-sensing regulatory mechanisms.
ANOVA revealed no significant difference between the MICs of both essential oils tested against bacterial strains at the 5% significance level. However, The MIC of H. suaveolens oil was relatively greater than that of L. aurita. The high antibacterial activity of L. aurita can be explained by its high content of phenolic and alcoholic compounds.47 Phenolic and alcoholic compounds act on bacteria by inhibiting glycoprotein biosynthesis.48 The relatively low activity of H. suaveolens can be explained by its high content of hydrocarbon compounds.15 Indeed, the main compounds of H. suaveolens, namely β-caryophyllene, sabinene, terpinolene and limonene, which are hydrocarbon compounds, have less antibacterial activity than phenols and ketones do.49 Hydrocarbon compounds act on the bacterial membrane by disrupting lipophilic compounds.50
Compared with Dian-Hong et al,46 essential oils of L. aurita and H. suaveolens from Burkina Faso have important activities with MICs that are slightly superior. These differences can be explained by differences in plant location, plant age harvest period, the mode of essential oil extraction and environmental factors.51
Synergistic Effects of Essential Oils and Antibiotics
Table 5 shows the considerable reduction in the MICs of antibiotics caused by synergistic combinations.
The greatest reduction in the MICs of amoxicillin+ clavulanic acid was from 29.66 µg/mL to 1.87 µg/mL (93.69%) in combination with L. aurita against P. aeruginosa and the greatest reduction in the MICs of colistin was from 3.75 µg/mL to 0.46 µg/mL (87.73%) in combination with L. aurita against E. faecalis.
Most combinations have synergistic effects on P. aeruginosa, which is considered as one of the strains most resistant to antimicrobial agents.45
The various types of combinations observed (Synergistic, additive, indifferent, antagonistic) could be explained by the fact that essential oil compounds have different ways of reacting when combined with antibiotics against bacteria.52
Many researchers have also reported synergistic effects on other plants.53,54 Indeed, the synergistic effect between antimicrobial agents and plant extracts may be due to the possible activities of substances found in plant extract(s) on ribosome structure and bacterial enzyme inhibition.54 However, obtained results of this study revealed the necessity of further investigation and justification of the synergism between antibiotics and plants to overcome antimicrobial drug-resistance.
The different synergies observed could be explained by the synergy between antibiotics and essential oils. Indeed, Oussalah et al55 reported that monoterpenes, particularly the phenols in essential oils, cause damage to the outer membrane of bacteria, resulting in increased membrane permeability to protons and potassium ions, reduced intracellular ATP reserves, disrupted proton-motive forces and denatured intracellular proteins.45,56 The same applies to ampicillin, amoxicillin and amoxicillin+ clavulanic acid which inhibit bacterial wall synthesis (peptidoglycan synthesis) by inactivating the main enzymes involved in this process.57
All the combinations do not produce synergistic effects; therefore, combinations must be tested in vitro before being used to treat ailment.
Mechanism of Essential Oil and Antibiotic Combination
The exact antibacterial mechanism of essential oils is still poorly understood. Indeed, it is even more difficult to propose an exact antibacterial mechanism of essential oil-antibiotic combinations. Each essential oil has a particular chemical composition and specific biological properties, and each type of microorganism has a distinct sensitivity.58 Several methods can be employed to assess the cellular damage induced in bacterial cells and the release of proteins or bacterial genetic material after exposure to essential oil and antibiotic combinations.32,35
To study the antibacterial mechanism of the essential–antibiotic combination, the release of bacterial material absorbing at 260 nm and 595 nm and bacterial mortality were monitored at the same time.
Spontaneous Lysis and Viability of Bacteria in the Absence of Bactericidal Agent
The results of protein and nucleic acid release and viable counts of untreated bacteria are presented in Table 8. The control showed neither the release of proteins nor the release of cytoplasmic contents at 260 nm nor bacterial mortality. This can be considered as an index of the integrity of the envelope of bacteria that are washed once and suspended in PBS as reported by Newton.34 The results revealed that multiple washes and suspensions in 1% NaCl resulted in the spontaneous release of 10% of their contents after 3 hours and 30% after 24 hours.59,60
Bacterial Fixation Results
Table 6 reveals that after centrifugation, highly significant differences in essential oil and antibiotic combination absorbances were detected before and after brief contact (2 min) with bacteria.
Table 6 reveals a significant decrease (from 08% to 24%) in the absorbance of essential oils and antibiotics after contact with bacteria. This can be explained by a decrease in the concentration of these molecules in the supernatant after centrifugation. This rapid decrease in absorbance was due to sedimentation of the 280 nm-absorbing essential oils and antibiotics fixed on bacteria.
Table 7 also reveals that after centrifugation, highly significant differences in essential oil and antibiotic combination absorbances in contact with bacteria at different concentrations (1.5 × 106 and 1.5 × 108 bacteria/mL) were detected.
The decrease in absorbance is due to the absorption of essential oils and antibiotics or their penetration into bacteria. To confirm this hypothesis, the subinhibitory concentrations were first brought into contact with a low bacterial density and then with a high bacterial density.
Table 7 reveals that the absorbance of these molecules is significantly greater after contact with a low number of bacteria (1.5 × 106 bacteria/mL) than after contact with a 100-fold greater number of bacteria (1.5 × 108 bacteria/mL). These findings suggest that essential oils and antibiotics adsorb to the bacterial surface or penetrate the bacterial cytoplasm. Since essential oils are hydrophobic molecules, they are likely to interact predictably interact with bacterial membranes and walls.
These results corroborate those of Rhayour,32 who also demonstrated the binding of essential oils and antibiotics to bacteria.
These findings that the antibacterial mechanism of the essential oil and antibiotic combination begins with adsorption to the bacterial surface or penetration into the bacterial cytoplasm.
We then concluded from previous results that the binding of essential oils and antibiotics to bacteria was a function of time as well as bacterial density.61
Protein Leakage Analysis
The results (Figure 2) revealed a very rapid initial release during the first 30 minutes of treatment with the synergistic combination, which represented 34.16% of the release at 24 hours, followed by a slow release during the rest of the treatment. This mechanism of action was similar to the mechanism of action observed by Rhayour32 when bacterial strains (E. coli, B. subtilis, Mycobacterium phlei and Mycobacterium fortuitum) were treated with oregano and clove essential oils. Untreated bacteria failed to release detectable quantities of protein (Table 8). This confirmed the occurrence of membrane destabilization triggered by antibiotics and their combination with essential oils.
The expose of bacteria to colistin and amoxicillin+ clavulanic acid caused the release of approximately 5.86 µg and 7.52 µg protein per mL of supernatant, respectively. The release of proteins from bacteria treated with colistin and amoxicillin+ clavulanic acid was also reported in the works of Chen et al, Ronald and Ian,61,62 who reported the release of proteins from bacteria treated with polymyxin B and oxacillin which are glycopeptide and beta-lactam drugs, respectively. However, the quantity of protein released was lower than that.
The leakage of gram-negative bacteria proteins was greater (ANOVA, P < 0.05) than that of gram-positive bacteria proteins. These results corroborate those of Burt.63 This difference in the leakage of proteins between the two types of bacteria may be explained by the complexity of the cell envelopes of gram-negative bacteria, which possess a double membrane that is richer in proteins than the simple membrane structure of gram-positive bacteria.64
The kinetics of protein release induced by antibiotics alone and synergistic combinations were similar. However, protein release induced by synergistic combinations was significantly greater than that induced by antibiotics alone (ANOVA, P < 0.05). These results are consistent with the results of Phitaktim65 who suggested that the synergistic activity of α-mangostin isolated from Garcinia mangostana essential oils plus oxacillin (antibiotic) resulted in increased cytoplasmic membrane permeability of cellular metabolites.66,67
The protein leakage results provide evidence that one of the important mechanisms of action of L. aurita and H. suaveolens is disruption of the cytoplasmic membrane or bacterial cell wall. This disruption in turn leads to deactivation of the β-lactamase activity, amoxicillin+ clavulanic acid binding to the penicillin-binding proteins (PBPs) or the modifications in the charge of LPS.65,68
These results led us to attribute the mechanism of action of L. aurita and H. suaveolens oils to the damage to the bacterial envelopes. The majority of essential oils are responsible for the antibacterial activity.49,69
The maximum protein concentration released when bacterial strains were treated with synergistic combinations was 16.49 µg/mL. This quantity was lower than that reported in.35,61 This led us to think that synergistic combinations resulted in increased cytoplasmic membrane permeability by making small holes in the cell envelope that led to disruption of the integrity of the cytoplasmic membrane of bacteria, leading to the loss of intracellular components.70
The mechanism of action of the L. aurita and H. suaveolens essential oils was similar to that of Inula graveolens and Santolina Corsica. Indeed, as Inula graveolens, L. aurita essential oil is rich in oxygen compounds and H. suaveolens is rich in hydrocarbon compounds such as Santolina Corsica essential oils. L. aurita and H. suaveolens essential oils such as Inula graveolens and Santolina Corsica essential oils act simultaneously on the cell wall and plasma membrane leading to the loss of cytoplasmic material.9,71,72 Bacteria resisting to colistin and amoxicillin+ clavulanic acid by producing beta-lactam or by multiple bacterial membrane modifications became sensitive when treated with synergistic combination of essential oil and antibiotic. The increase in cytoplasm permeability induced by bacteria envelops damage when bacteria are treated with synergistic antibiotic-essential oil combinations has also been reported in several works.65,73
Nucleic Acid Leakage Analysis
Nucleic acid release was assessed by monitoring the release of bacteria at 260 nm. The release of bacterial material absorbed at 260 nm can suggest bacterial cell lysis.74,75 The results (Figure 3) revealed a very rapid initial release during the first 30 min, which represented 59.23% of the release at 24 h, followed by a slow release during the rest of the treatment with a synergistic combination of essential oils and antibiotics. The drastic leakage of 260 nm absorbing material during the first 30 min of treatment was also reported by Phitaktim et al, Li et al,65,73 when essential oils (which chemical compounds are similar to L. aurita and H. suaveolens) were combined with antibiotics.
Compared with that of the control, the absorbance of the supernatant from bacteria were treated with synergistic combinations was significantly (p < 0.01) greater. This result led us to attribute the release of nucleic acids to synergistic combination and not to weakening of the cell wall and the subsequent rupture of the cytoplasmic membrane due to osmotic pressure.74 The loss of 260 nm absorbing material in the gram-positive cells was significantly greater (ANOVA p < 0.05) than that in the gram-negative cells. This result corroborates the results of Mihin et al, Sharma et al,15,76 who reported that gram-positive bacteria have a less complicated cell wall structure than do gram-negative bacteria.77,78 The loss of cytoplasmic bacteria material absorbed at 260 nm by different combinations of essential oils and antibiotics was not significantly different (ANOVA p < 0.05). The release of nucleic acid was performed according to the Gram of bacteria. However, this was not due to the type of synergistic combination of essential oils and antibiotics.
Owing to the release of nucleic acids (260 nm) from the resistant bacterial culture initiated by the combination of essential oils (L. aurita, H. suaveolens) and antibiotics (amoxicillin+ clavulanic acid and colistin), the results indicated that synergistic combinations might cause the loss of the integrity of the bacterial membrane, thereby increasing the permeability of cells making bacteria previously resistant to antibiotics sensitive. These findings suggest that L. aurita and H. suaveolens oils could inhibit β-lactamase activity and LPS charge changes.
Compared with those treated with antibiotics alone, resistant bacterial strains treated with synergistic combinations lost significant amounts of 260 nm-absorbing material (ANOVA p < 0.05). The increase in cytoplasmic permeability when bacterial strains were treated with essential oils and antibiotic combinations compared with antibiotics alone was previously reported by Eumkeb and Chukrathok,79 where the combination of ceftazidime plus galangin led to damage to the cell ultrastructure and integrity of the cell wall and an increase in the cell size of ceftazidime-resistant S. aureus.
The mechanism of action of colistin and amoxicillin+ clavulanic acid is known.80,81 Our results suggest that the targets of L. aureus and H. suaveolens oils remain the envelope cells of bacteria as do many other similar essential oils.65,74,82–84
The combination of L. aurita and H. suaveolens and antibiotics may attach to the cell membrane, resulting in lysis, resulting in cell entry, which causes the DNA to unwind, ultimately leading to cell death. On the other hand, this may also be due to the bonding reaction between the components of these essential oils and the antibiotics.
L. aurita and H. suaveolens combined with colistin and amoxicillin + clavulanic acid led to the release of intracellular contents outside of the cell. Similar essential oils combined with polymyxin B and piperacillin, glycopeptide and beta lactam respectively, present the same mechanism.85,86 We can conclude that the combination of L. aurita and H. suaveolens with colistin and amoxicillin+ clavulanic acid has the potential for causing membrane disruption and cytoplasmic leakage. L. aurita and H. suaveolens combined with colistin and amoxicillin+ clavulanic acid may be able to enter bacterial cells and damage DNA. The same mechanism was suggested by El-Deeb et al; Abdul- Rahim et al.87,88 However, the possibility remains that sites of action other than the cytoplasmic membrane exist. Given the heterogeneous compositions of L. aurita and H. suaveolens and the antimicrobial activities of many of their components, it seems unlikely that there is only one mechanism of action or that only one component is responsible for the antimicrobial action. Further work is needed to fully understand the mechanisms involved.
Kill Curve Assays
Bacterial mortality was monitored in parallel with the release of proteins and the absorption of cytoplasmic material at 260 nm. Bacteria killing curve assays confirmed that leakage of cytoplasmic contents induced by synergistic combinations led to bacterial death.
The control resulted in no reduction in the number of CFUs from the control inoculum. The bacterial growth curve in the control group rose slowly from 0 h and increased rapidly during the exponential phase from 2 to 4 h. Subsequently, the bacterial growth curve reached the stationary phase with a relatively stable CFU/ mL. At 4 h, the CFU/ mL peaked, reaching an average value of 2.74 × 106 CFU/mL. These results are consistent with those of Hassan et al,89 who reported exponential phase of the growth of lactic Streptococci in milk occurred between 1 h and 6h.
The viable counts of the cells treated with the essential oil-antibiotic combinations were significantly lower than those of the cells treated with the antibiotic alone (between 0 and 24 h).
Clearly, all the antibiotic and essential oil combinations greatly decreased the cell count to 0 CFU/mL after a maximum of 4 h of treatment. These results confirmed the checkerboard assay results, which indicated synergistic activity in which the combination produced a decrease of ≥2 log10 CFU/mL compared with colistin and amoxicillin+ clavulanic acid treatment alone.90,91
The rapid action of the combination pair observed in the time-kill analysis indicated treatment-induced gross cell damage. This confirmed that the synergistic combinations were bactericidal at 100% against the tested resistant-bacteria, namely, gram-negative and gram-positive bacteria, after 0–12 h of exposure. The leakage of proteins and nucleic acid with bactericidal effects against bacteria has also been reported in many studies.92
In the literature,82,93–95 the major components of essential oils, including carvacrol, citronellol, geraniol, and thymol, reportedly cause gross membrane damage and provoke whole-cell lysis. These components are not found in L. aurita or H. suaveolens oils.46,96 However, studies on the effects of chemical constituents such as terpinen-4-ol, linalool and 1,8-cineole which are also found in L. aurita and H. suaveolens indicate that the mechanism of action of these components damages the lipid layer of the cell membrane which results in bacterial cell leakage and permeability.97,98
Conclusion
In conclusion, our findings provide evidence that L. aurita and H. suaveolens oils not only have important activities against multidrug-resistant bacteria but also inhibit synergistic activity with antibiotics against resistant bacteria. This synergistic activity of essential oils plus beta lactams and glycopeptides may involve two modes of action. First, there are potential effects of cytoplasmic membrane disruption and increased permeability. Secondly, the inhibition of β-lactamase activity or the inhibition of LPS charge changes ultimately results in peptidoglycan damage. This antibacterial mechanism led to a bactericidal effect against bacteria that previously resisted to the two antibiotics amoxicillin + clavulanic acid and colistin.
Most of these efficacious essential oil and antibiotic mixtures enhanced the antimicrobial activity of the two tested antibiotics but to varying degrees and differed with different bacterial strains. Overall, these results represent the basis for further in vivo investigations, which could ultimately lead to the development of new antimicrobial agents based on L. aurita and H. suaveolens oils and therefore contribute to enhance the efficacy of antibiotics in controlling multidrug-resistant pathogenic bacteria. However, additional investigations are needed to determine the compounds responsible for the antibacterial activity in complex mixtures and to elucidate the likely modes of action responsible for their synergistic effect when combined with antibiotics.
The variability in essential oils composition due to plant growth conditions, extraction methods and storage added to the diversity of molecules in essential oils leading to a difficult identification of the exact molecular mechanism constitute potential limitations to our studies.
Disclosure
The authors report no conflicts of interest in this work.
References
1. MboleJ, Aba’aM, NdzieP, MinyemA, NgolsuF, NgaE. Antimicrobial resistance. Health Sci Dis. 2023;24(12). doi:10.5281/hsd.v24i12
2. El-sakhawyMA, Al-zabanMI, AlharbiMA, AbdelazimNS, ZainME. Synergistic effect of medicinal plants and antibiotics on pathogenic bacteria. Int J Biol Pharm Allied Sci. 2015;4(9):5792–5800.
3. SalamMA, Al-AminMY, SalamMT, et al.Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 1946:11. doi:10.3390/healthcare11131946
4. MezghaniMS, RekikMM, MahjoubiFHA. Epidemiological study of Enterobacteriaceae resistance to colistin in Sfax (Tunisia). Med Mal Infect. 2012;42:256–263. doi:10.1016/j.medmal.2012.04.008
5. PatersonD. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Ctrl. 2006;34:20–28. doi:10.1016/j.ajic.2006.05.238
6. BaKA, DiendereA, SanouM, et al.Antibiotics resistance of Staphylococcus aureus strains and enterobacteria isolated at the LNSP in Ouagadougou (Burkina Faso). Afr J OnLine. 2017;42(1):83–94.
7. NadembegaWMC, DjigmaF, OuermiD, BelemgnegreM, KarouDS, SimporeJ. Bacterial resistance profile at Saint Camille hospital in Ouagadougou. Afr J Online. 2017;19(4):91–101.
8. Centers of Disease Control Prevention. Be antibiotics aware: smart use, best care features; 2021. Available from: www.cdc.gov/patientsafety/features/be-antibiotics-aware.html.
9. GuinoiseauE. Antibacterial molecules from essential oils: séparation, identification and mode of action. University of Corse; 2011. Available from: https://theses.hal.science/tel-00595051v.
10. RemmalA, TantaouiEA, BouchikhiT, RhayourK, EttayebiM. Improved method for the determination of antimicrobial activity of essential oils in Agar medium. J Essent Oil Res. 1993;5:179–184. doi:10.1080/10412905.1993.9698197
11. HemaiswaryaS, KruthiventiAK, DobleM. Synergism between natural products and antibiotics against infectious diseases. J Phytomédécine. 2010;16:997–1005.
12. RosatoA, PiarulliM, CorboF, et al.In-vitro synergistic action of certain combinations of gentamicin and essential oils. Curr Med Chem. 2010;17(28):3289–3295. doi:10.2174/092986710792231996
13. ChangPC, LiHY, TangHJ, LiuJW, WangJJ, ChuangYC. In vitro synergy of baicalein and gentamicin against vancomycin resistant Enterococcus. J Microbiol Immunol Infect. 2007;40:56–61.
14. EsimoneCO, IrohaIR, IbezimEC, OkehCO, OkpnaEM. In vitro evaluation of the interaction between tea extracts and penicillin G against Staphylococcus aureus. Afr J Biotechnol. 2006;5:1082–1086.
15. MihinHB, SomdaK, KaboreK, et al.Contribution to the study of essential oils of two aromatic plants from Burkina Faso (Laggera aurita (L.f.) Benth. Ex C.B. Clarke and Hyptis suaveolens Poit) for food industry. Afr J Biotechnol. 2019;18(29):808–818.
16. ChangS, ChenP, WangS, WuH. Antimite activity of essential oils and their constituents from Taiwania cryptomerioides. J Med Entomol. 2001;38(3):445–447. doi:10.1603/0022-2585-38.3.455
17. UltéeA, GorrisLM, SmidE. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J Appl Microbiol. 1998;85:211–218. doi:10.1046/j.1365-2672.1998.00467.x
18. DibalaCI, DickoMH, DiaoM, KonatéK, OuedraogoM. Phytoconstituents Analysis, antioxidant capacity and antimicrobial properties of extracts from Laggera aurita L. (ASTERACEAE). Int J Pharm Pharm Sci. 2014;6(7):172–178.
19. NjanNAM, SaotoingP, TchouankeuJC, MessiJ. Effect of essential oils of six local plants used insecticide on adults of Anopheles gambiae. J Entomol. 2007;4(6):444–450. doi:10.3923/je.2007.444.450
20. OkhaleSE, OdiniyaEO, KunleOF. Preliminary phytochemical and pharmacognostical investigation of pediatrics antimalarial Laggera pterodonta (DC) Sch. Bip.: Asteraceae of Nigerian origin. Ethnobot Leafl. 2010;14:457–466.
21. NacoulmaOG. Medicinal Plants and Traditional Medical Practices in Burkina Faso. Case of Central Plateau. University of Ouagadougou. Vol. II. Université de Ouagadougou; 1996.
22. BurkillIH. A Dictionary of the Economic Products of the Malay Peninsula.
23. BayalaB. Study of the Antioxidant, Anti-Inflammatory, Anti-Proliferative and Anti-Migratory Properties of Essential Oils From Medicinal Plants of Burkina Faso Against Prostate Cancer and Glioblastoma Cell Lines. University Blaise Pascal- Clermont-Ferrand II, Université Joseph Ki-Zerbo; 2014. https://theses.hal.science/tel-01166321.
24. CLSI. Methods antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. CLSI NCCLS. 2006;26:M7–A7.
25. BragaFG, BouzadaMLM, FabriRLO, et al.Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J Ethnopharmacol. 2007;111(2):396–402. doi:10.1016/j.jep.2006.12.006
26. CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clin Lab Stand Inst. 2015;35(3):M100–S25.
27. CLSI. Performance standards for antimicrobial susceptibility testing. CLSI. 1999;19(18):M100–S25.
28. MayachiewP, SakamonD. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. J Food Sci Technol. 2008;41:1153–1159.
29. MandalS, ManishaD, NishithK, KrishnenduS. Synergistic anti–Staphylococcus aureus activity of amoxicillin in combination with Emblica officinalis and Nymphae odorata extracts. Asian Pac J Trop Med. 2010;3(9):711–714. doi:10.1016/S1995-7645(10)60171-X
30. CanillacN, MoureyA. Antibacterial activity of the essential oil of Picea excelsa on Listeria monocytogenes, Staphylococcus aureus and coliform bacteria. J Food Microbiol. 2001;18(3):261–268. doi:10.1006/fmic.2000.0397
31. SchelzZ, JosephM, JudithH. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia. 2006;77(4):279–285. doi:10.1016/j.fitote.2006.03.013
32. RhayourK. Study of the Bactericidal Action Mechanism of Essential Oil on Escherichia coli, Bacillus Subtilis, Mycobacterium Phlei and Mycobacterium Fortuitum. University Sidi Mohamed Ben Abdellah. Sciences Faculty Dhar Mehraz -Fès; 2002.
33. BradfordMM. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi:10.1016/0003-2697(76)90527-3
34. NewtonBA. The release of soluble constituents from washed cells of Pseudomonas aeruginosa by the action of polymyxin. J Gen Microbiol. 1953;9:54–65. doi:10.1099/00221287-9-1-54
35. AhmadSJ, LianHH, BasriDF, ZinNM. Mode of action of Endophytic Streptomyces sp. SUK 25 extracts Against MRSA.; Microscopic, Biochemical and Time-Kill Analysis. Int J Pharm Sci Rev Res. 2015;30:11–17.
36. MilesA, MisraS, IrwinJ. The estimation on the bactericidal power of blood. J Hyg Camb. 1938;38(6):732–749. doi:10.1017/s002217240001158x
37. CaillonD. Living bacteria counting. Bactericidal action. Theoretical and therapeutic aspects. Bactéricidies. 1991:127–137.
38. LastourV, FantinB. Resistance to Fluoroquinolone in 2010: what impact for intensive care prescription?Elsevier Masson. 2010;19:347–353.
39. CompaoréCOT, DabreD, MaigaY, et al.Assessing greywater characteristics in the sahel region and perception of the local population on its reuse in agriculture. Heliyon. 2024;33473. doi:10.1016/j.heliyon.2024.e33473
40. WalshTR, TolemanMA, PoirelL, NordmannP. Metallo-β-lactamase: the quiet before the storm. Clin Microbiol Rev. 2005;18:306–325. doi:10.1128/CMR.18.2.306-325.2005
41. GalesAC, JonesRN, SaderHS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother. 2011;66:2070–2074. doi:10.1093/jac/dkr239
42. BenameurQ, Ben-MahdiMH, Boutaiba BenklaouzM, et al.Analysis of high levels of multidrug resistant Escherichia coli from healthy broiler chickens in Western Algeria. Afr J Microbiol Res. 2016;10:1792–1797. doi:10.5897/AJMR2016.8284
43. BendjilaliB, Tantaoui-ElarakiA-G, AyadiA, LhlalM. Method to study antimicrobial effects of essential oils: application to the antifungal activity of six Moroccan essences. J Food Prot. 1984;47(10):748–752. doi:10.4315/0362-028X-47.10.748
44. Gang-JoonH, De SilvaBCJ, JungWG, HossainS, WimalasenaSHMP, PathiranaHNKS. Antimicrobial property of Lemongrass (Cymbopogon citratus) oil against pathogenic bacteria isolated from pet turtles. Lab Anim Res. 2017;33(2):84–91. doi:10.5625/lar.2017.33.2.84
45. LambertPA, MehamdiaN, MouassaS. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;95:22–26.
46. XuD-H, HuangY-S, JiangD-Q, YuanK. The essential oils chemical compositions and antimicrobial, antioxidant activities and toxicity of three Hyptis species. Afr J Biotechnol. 2013;5(9):1125–1130. doi:10.3109/13880209.2013.781195
47. SamatéAD. Chemical Compositions of Essential Oils Extracted From Aromatic Plants in Soudanneen Zone of Burkina Faso: Valorization. University of Ouagadougou;; 2002.
48. OuridaC. Chemical Composition and Antibacterial Activity of Essential Oils From Glycyrrhiza Glabra. University of Oran; 2012.
49. KalembaD, KunickaA. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi:10.2174/0929867033457719
50. CowanM. Plant products as antimicrobial agents. J Med Plant. 1999;16:122–125.
51. MusyimiDM, OgurJA, MuemaPM. Phytochemical compounds and antimicrobial activity of extracts of aspilia plant (Aspilia mossambicensis) (Oliv) wild. Int J Bot. 2018;4(1):56–61. doi:10.3923/ijb.2008.56.61
52. GallucciN, CaseroC, OlivaM, ZygadloJ, DemoM. Interaction between terpenes and penicillin on bacterial strains resistant to beta-lactam antibiotics. Mol Med Chem. 2006;10:30–32.
53. KamatouGPP, ViljoenAM, VanVSF, VanZRL. In vitro evidence of antimicrobial synergy between Salvia chamelaeagnea and Leonotis leonurus. South Afr J Bot. 2006;72:634–636. doi:10.1016/j.sajb.2006.03.011
54. ElaineJ, BetoniC, MantovaniR, BarbosaL, Di StasiL, JuniorA. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem Inst Oswaldo Cruz Rio Jan. 2006;101(4):387–390. doi:10.1590/S0074-02762006000400007
55. OussalahM, CailletS, LacroixM. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J Food Prot. 2006;69(5):1046–1055. doi:10.4315/0362-028X-69.5.1046
56. BakkaliF, AverbeckS, AverbeckD, IdaomarM. Biological effects of essential oils a review. Food Chem Toxicol. 2008;95:22–26.
57. NourM, MastouriM, NejmaMB. Methicillin-resistant Staphylococcus aureus: emergence and molecular bases. Pathol Biol. 2005;53(6):334–340. doi:10.1016/j.patbio.2004.08.001
58. BouhdidS, AbrinniJ, BaudouxD, ManresaA, ZhiriA. Essential oils of compact oregano and ceylon cinnamon: antibacterial activity and mechanism of action. J Pharm Clin. 2012;31(3):141–148.
59. TeuberM. Lysozyme-dependant production of spheroplast-like bodies from polymixin-B treated Salmonella thyphimurium. Arch Mikrobiol. 1970;70:139–146. doi:10.1007/BF00412204
60. WarrenGH, GrayJ, YurchencoJA. Effect of polymixin on lysis of Neisseria catarrhalis by lysozyme. J Bacteriol. 1957;74:788–793. doi:10.1128/jb.74.6.788-793.1957
61. ChenY, ZhaoJ, LuiC, WuD, WangX. In-vitro antibacterial activity and mechanism of Monarda didyma essential oils against Carbapenem-resistant Klebsiella pneumoniae. BMC Microbiol. 2023;23(263). doi:10.1186/s12866-023-03015-4
62. RonaldAD, IanC. Leakage of periplasmic proteins from Escherichia coli Mediated by Polymyxin B nonapeptid. Am Soc Microbiol. 1986;29(5):781–788.
63. BurtS. Essential oils: their antibacterial properties and potential applications in foods. IntJ Food Microbiol. 2004;94:223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
64. NgomS, DiopM, MbengueM, FayeF, KornprobstJM, SambA. Chemical composition and antibacterial properties of essential oils from Ocimum basilicum and Hyptis suaveolens (L.) Poit collected in the Dakar region of Senegal. Afr Sci. 2014;10(4):109–117.
65. PhitaktimS, EumkebG, SirichaiwetchakoonK, DunkhunthodB, ChomnawangM, HobbsG. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016;16(195):109–117. doi:10.1186/s12866-016-0814-4
66. ShenL, LiuD, LiM, JinF, DinM, ParnellLD. Mechanism of action of recombinant acc-royalisin from royal jelly of Asian honeybee against gram-positive bacteria. PLoS One. 2012;7(10):7194. doi:10.1371/journal.pone.0047194
67. ZhouK, ZhouW, LiP, LiuG, ZhangJ, DaiY. Mode of action of pentocin 31-1: an antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control. 2008;19:817–822. doi:10.1016/j.foodcont.2007.08.008
68. RhoumaM, BeaudryF, ThériaultW, LetellierA. Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and One Health perspectives. Front Microbiol. 2016:7. doi:10.3389/fmicb.2016.01789
69. DormanHJD, DeansSG. Antimicrobial agents from plants: antibacterial oils, activity of plant volatile. J Appl Microbiol. 2000;88(2):308–316. doi:10.1046/j.1365-2672.2000.00969.x
70. KohJJ, QiuS, ZouH, LakshminarayananR, LiJ, ZhouX. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta. 2013;1828:834.
71. EL-SayedAIM, El-SheekhMM, MakhlofMEM. Synergistic antibacterial effects of Ulva lactuca methanolic extract alone and in combination with different antibiotics on multidrug-resistant Klebsiella pneumoniae isolate. BMC Microbiol. 2023;23(106). doi:10.1186/s12866-023-02854-5
72. HammerMU, BrauserAM, OlakC, et al.Lipopolysaccharide interaction is decisive for the activity of the antimicrobial peptide NK-2 against Escherichia coli and Proteus mirabilis. Biochem J. 2010;427:477–488. doi:10.1042/BJ20091607
73. LiQQ, ChaeHS, KangOH, KwonDY. Synergistic antibacterial activity with conventional antibiotics and mechanism of action of Shikonin against methicillin-resistant Staphylococcus aureus. Int J Mol Sci. 2022;23(7551). doi:10.3390/ijms23147551
74. CarsonCF, BrianJM, ThomasVR. Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by Time-Kill, lysis, leakage, and salt tolerance assays and electron microscopy. Am Soc Microbiol. 2011;1914–1920.
75. HugoWB, LongworthR. Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol. 1964;16:655–662. doi:10.1111/j.2042-7158.1964.tb07384.x
76. SharmaPP, RoyRK, AnuragGD, SharmaVK. Hyptis suaveolens (L.) poit: a phyto-pharmacological review. International Journal of Chemical and Pharmaceutical Sciences. 2013;4(1).
77. BouankB, KerouazM. Assessment of Antibacterial Activity of Essential Oils of Some Species of Lamiaceae and Effect of Their Association with Antibiotics. University of Med -Seddik Benyahia -Jijel;; 2016.
78. DortetL, BonninR, JoussetA, GauthierL, NaasT. The emergence and spread of plasmid-mediated resistance to colistin in Enterobacteriaceae. J Anti-Infect. 2016;18:139–149. doi:10.1016/j.antinf.2016.09.003
79. EumkebG, ChukrathokS. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine. 2013;20:262–269. doi:10.1016/j.phymed.2012.10.008
80. AndreB. Les peptides. Médecine Thérapeutique Hors Sér. 1997:3.
81. YalaD, MeradAS, MohamediMN, Ouar-KorichMN. Classification and mode of action of antibiotics. Médecine Maghreb. 2001;91:5–12.
82. HorneDS, HolmM, ObergC, ChaoS, YoungDG. Antimicrobial effects of essential oils on Streptococcus pneumoniae. J Essent Oil Res. 2001;13:387–392. doi:10.1080/10412905.2001.9712241
83. MouwakehA, KincsesA, NovéM. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother Res. 2019;33(4):1010–1018. doi:10.1002/ptr.6294
84. OnawunmiGO, OgunlanaEO. Effects of lemon grass oil on the cells and spheroplasts of Escherichia coli NCTC 9001. Microbios Lett. 1985;28:63–68.
85. AleksicV, Mimica-DukicN, SiminN, NedeljkovicNS, KnezevicP. Synergistic effect of Myrtus communis L. essential oils and conventional antibiotics against multi-drug-resistant Acinetobacter baumannii wound isolates. Phytomedicine. 2014;21(12):1666–1674. doi:10.1016/j.phymed.2014.08.013
86. PollySXY, ThibaK, Kok-GanC, SweeHEL. Antibacterial Mode of Action of Cinnamomum verum Bark Essential Oil, Alone and in Combination with Piperacillin, Against a Multi-Drug-Resistant Escherichia coli Strain. J Microbiol Biotechnol. 2015;25(8):1299–1306. doi:10.4014/jmb.1407.07054
87. El-DeebB, Al-TalhiA, MostafaN, Abou-assyR. Biological Synthesis and Structural Characterization of Selenium Nanoparticles and Assessment of Their Antimicrobial Properties. Am Sci Res J Eng Technol Sci. 2018;45(1):135–170.
88. Abdul- RahimN, ZhuY, CheahS, et al.Synergy of the polymyxin-chloramphenicol combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae is predominately driven by chloramphenicol. CS Infect Dis. 2015;7(6):1584–1595.
89. HassanAI, DeschampsN, RichardJ. Original article precision of growth rate measurements of lactic Streptococci in milk based on the microbial count method by colony formation. Reference study with Lactococcus lactis. ElsevierINRA Lait. 1989;69:433–447. doi:10.1051/lait:1989529
90. EliopoulosGM, MoelleringRC. Antimicrobial combinations. In: Lorian. Antibiot Lab Med; 1996:330–396.
91. LorianV. Antibiotics in Laboratory Medicine. Lippincott Williams Wilkins; 2005:365–405.
92. SinghG, KatochM. Antimicrobial activities and mechanism of action of Cymbopogon khasianus (Munro ex Hackel) Bor essential oil. BMC Complement Med Ther. 2020;20:331. doi:10.1186/s12906-020-03112-1
93. DenyerSP, HugoW. Biocide-induced damage to the bacterial cytoplasmic membrane. Blackwell Sci Publ Oxf U K. 1973:171–187.
94. WindholzM, BudavariSRFB, OtterbeinES. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals.
95. RussellAD, MorrisA, AllwoodMC. Methods for assessing damage to bacteria induced by chemical and physical agents. Methods Microbiol. 1973;13:387–392.
96. DjiboKA. Analysis of the Essential Oils of Some Plants From the Flora of Burkina Faso Belonging to the Families Lamiaceae (Hyptis Spicigera Lam, Hyptis Suaveolens Poit. Ocimum Americanum L.) and Poaceae (Cymbopogon Schoenanthus L.) Spreng, Cymbopogon Giganteus, Chiov, Cymbopogon Citratus (DC) Staph). University of Ouagadougou; 2001.
97. De RapperS, ViljoenA, van VuurenS. The In Vitro Antimicrobial Effects of Lavandula angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid Based Complement Altern Med. 2016;2016:2752739. doi:10.1155/2016/2752739
98. SaviucC, GheorgheI, CobanS, et al.Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR strains. Rom Biotechnol Lett. 2016;21:11782–11790.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




