Back to Journals » International Journal of Nanomedicine » Volume 20
Synthesis of Mesoporous Polydopamine-Coated Upconversion Nanoparticles for Dual-Enhanced Photodynamic and Photothermal Cancer Therapy
Authors Visetvichaporn V , Yu NY, Kang SW , Nguyen DT, Lim JP, Jang HS, Kim DD
Received 31 October 2024
Accepted for publication 6 February 2025
Published 26 February 2025 Volume 2025:20 Pages 2505—2519
DOI https://doi.org/10.2147/IJN.S503977
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Voradanu Visetvichaporn,1 Na-Young Yu,1 Seong-Wook Kang,1 Duy-Thuc Nguyen,2 Jun Pyo Lim,3,4 Ho Seong Jang,3,5 Dae-Duk Kim1
1College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul, 08826, Republic of Korea; 2Pritzker School of Molecular Engineering, University of Chicago, Chicago, IL, 60637, USA; 3Materials Architecturing Research Center, Korea Institute of Science and Technology, Seoul, 02792, Republic of Korea; 4Department of Materials Science and Engineering, Korea University, Seoul, 02841, Republic of Korea; 5Division of Nanoscience and Technology, KIST School, Korea University of Science and Technology (UST), Seoul, 02792, Republic of Korea
Correspondence: Dae-Duk Kim, College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul, 08826, Republic of Korea, Tel +82-2-880-7870, Fax +82-2-873-9177, Email [email protected]
Background: Photodynamic therapy (PDT) is a common cancer treatment strategy that combines the use of light, a photosensitizer, and oxygen to precisely generate reactive oxygen species (ROS). However, the efficacy of this method is limited by the shallow tissue penetration of the short-wavelength lasers involved, and combination therapy with other treatments such as photothermal therapy (PTT) or radiation therapy requires additional lasers or instruments. A new dual therapy approach using a single laser could minimize the treatment procedure.
Methods: Chlorin e6 (Ce6) loaded-NaYF4:Yb,Er@NaYF4:Yb,Nd upconversion nanoparticles@mesoporous silica@mesoporous polydopamine nanoparticles (U@MSC@MP NPs) were fabricated to achieve PDT and PTT combination cancer therapy using a single 808 nm laser. The NaYF4:Yb,Er@NaYF4:Yb,Nd upconversion nanoparticles (UCNPs) were coated with mesoporous silica (MS) for Ce6-loading and coated with mesoporous polydopamine (MP) as a PTT photosensitizer. The PDT and PTT effects were measured using ROS generation detection and a thermal camera, respectively; in vitro cytotoxicity studies and in vivo antitumor efficacy analysis using tumor xenograft mouse models were performed to confirm the dual effects.
Results and Discussion: The PDT-PTT UCNPs were successfully synthesized and emit photoluminescence spectra that can be absorbed by Ce6 to induce the PDT effect. Significant ROS generation was observed from U@MSC@MP NPs following 808 nm laser irradiation for 5 min, which corresponded to intracellular ROS detection in human colorectal adenocarcinoma HT-29 cells. The NPs significantly reduced HT-29 cell viability compared with PDT or PTT alone, demonstrating the potential of the designed UCNPs. Moreover, the in vivo antitumor efficacy analysis confirmed the dual effect with no signs of toxicity, supporting the safety and biocompatibility of the synthesized NPs.
Conclusion: These findings suggest that the combination of PDT and PTT using a single laser can be achieved with UCNPs. This approach is a promising strategy for simplifying the cancer treatment procedures in clinical applications.
Keywords: cancer treatment, chlorin e6, Ce6, dual therapy
Introduction
Cancer is a leading cause of mortality globally. Although conventional treatments such as chemotherapy are widely used, they are often associated with off-target effects and limited efficacies. Photodynamic therapy (PDT) offers a more targeted approach by using light, a photosensitizer (PS), and oxygen to generate reactive oxygen species (ROS), that selectively eliminate cancer cells. However, PDT is limited by the penetration depth of light, as most effective PSs are triggered at wavelengths below the near-infrared (NIR) range (eg ~665 nm (Chlorin e6) and 630 nm (5-aminolevulinic acid (5-ALA)).1,2 Therefore, PDT has been combined with other treatments such as photothermal therapy (PTT) or radiation therapy for better efficacy; however, these strategies require a minimum of two lasers or additional instruments.3–5 For example, a study of PDT-PTT combination therapy was reported utilizing two lasers of 670 nm and 808 nm.6 To simplify the administration procedure and enhance the efficacy of the treatment, the dual PDT-PTT delivery system with one laser was also developed utilizing upconversion nanoparticle (UCNP)-based strategy or PDT/PTT-induced novel photosensitizers.7–11 Recently, lanthanide-doped UCNPs were used with PDT owing to their ability to convert NIR rays into shorter wavelengths that can be absorbed by a PS. However, UCNPs studies mainly use a 980 nm laser, which overlaps with the absorption of water (950–1050 nm range) in biological tissue, causing retardation of the penetration depth, weakening of the excitation, and the generation of a severe local heating effect.12 Wavelengths lower than 700 nm are typically absorbed by tissue components such as hemoglobin; therefore, the safe window for laser application is between 700 and 950 nm.13 Studies have reported advantages of 800–808 nm lasers over the 980 nm units; for example, an 808 nm wavelength has a 20-fold lower water extinction coefficient and can penetrate bovine tissue twice as deeply.14,15 Consequently, several studies have focused on the use of neodymium (Nd3+)-sensitized UCNPs, which can be excited at approximately 800 nm, to improve safety and reduce energy loss.12,16–20 The UCNPs typically consist of dopant and host combinations. The dopant of UCNPs can be chosen and designed to emit wavelengths within the safe range based on the energy transfer of each lanthanide (Ln) element; therefore, the photoluminescence (PL) emission could feasibly match the absorbance of the PDT photosensitizer. The host matrices ideally have low phonon energies and high chemical stability.21,22 The ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) were widely used in many studies, including PDT, due to its efficient upconversion properties to produce the green (~540 nm) and red (~660 nm) emission.23–25 In particular, several studies reported the fabrication of NaYF4:Yb,Er UCNPs triggering by 980 nm laser for Ce6-mediated PDT.26,27 However, the previous UCNP-based application for PDT excited by 800–808 nm is limited by the long laser irradiation time (15 min), multiple irradiation (3–5 min every 7 days), or high laser intensity (6 W/cm2) required to show the efficacy, which made this approach challenging in clinical applications.7,16,19,28 In addition, the existing PDT-PTT UCNPs studies were fabricated for a 980 nm laser excitation which has drawbacks as mentioned earlier.29 Although 808 nm-induced PTT photosensitizer using iron phthalocyanine was proposed for dual PDT-PTT therapy with the same light exposure, long irradiation time (15 min) still remains to be overcome.7 Polydopamine (PDA) is a biodegradable and biocompatible material which absorbs NIR wavelengths and exhibits potent PTT effects. Additionally, mesoporous polydopamine (MP) NPs showed a stronger PTT effect than plain PDA NPs.30 Therefore, MP-coating was considered as a good candidate for PTT in combination with PDT-UCNPs to optimize their antitumor efficacy.
In this study, we hypothesized that a UCNP-based delivery system for PDT and PTT excited by a single 808 nm laser could be achieved using UCNPs loaded with a PS and MP-coating design (Figure 1). Core-shell UCNPs (NaYF4:Yb,Er@NaYF4:Yb,Nd) that were suitable for absorbing 808 nm radiation were synthesized using a thermal coprecipitation method aiming to emit light at approximately 660 nm for PDT activation by Ce6, a second-generation FDA-approved photosensitizer. Mesoporous silica (MS) was coated onto the UCNPs using the sol-gel method to allow for Ce6 loading; the MP was then designed to coat the surface of the NPs for the PTT effect. These dual PDT-PTT-UCNPs could provide simple and accurate light-controllable cancer treatment.
 |
Figure 1 Synthesis scheme and illustration of the designed mesoporous polydopamine coated-upconversion nanoparticles. |
Materials and Methods
Materials
The following compounds were purchased from Sigma Aldrich Co. (St. Louis, MO, USA): 1-octadecene, oleic acid (OA), yttrium (III) chloride hexahydrate (YCl3·6H2O), ytterbium (III) chloride hexahydrate (YbCl3·6H2O), erbium (III) chloride hexahydrate (ErCl3·6H2O), neodymium (III) chloride hexahydrate (NdCl3·6H2O), sodium hydroxide (NaOH), ammonium fluoride (NH4F), mineral oil, dopamine hydrochloride, pluronic F-127, 1,3,5-trimethylbenzene (TMB), hexadecyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), (3-aminopropyl)triethoxysilane (APTES), and 1,3-diphenylisobenzofuran (DPBF). The ammonia solution (28% in water) was purchased from Tokyo Chemical Industry (Tokyo, Japan), Ce6 was obtained from Cayman Chemical Co. (Ann Arbor, MI, USA), and the ethyl acetate and acetone were purchased from Duksan Pure Chemicals Co., Ltd. (Gyeonggi-do, Republic of Korea). The 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; DCFH-DA) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were obtained from Invitrogen (Carlsbad, CA, USA). CellTiter 96® AQueous One Solution was purchased from Promega (Madison, WI, USA). FITC Annexin V Apoptosis Detection Kit with 7-aminoactinomycin D (7-AAD) was obtained from BioLegend (San Diego, CA, USA). Fetal Bovine Serum was purchased from WELGENE Inc. (Gyeongsangbuk-do, Republic of Korea). All other chemicals and solvents were of analytical grade. Human colorectal adenocarcinoma HT-29 (KCLB no. 30038) was obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). A 808 nm laser (PSU-H-LED; MDL-H-808-4W) was purchased from Changchun New Industries Optoelectronics Tech. Co., Ltd. (Changchun, China).
Methods
Synthesis of NPs
Synthesis of NaYF4:Yb,Er UCNPs (Core Er UCNPs)
The core NaYF4:Yb,Er (Y:Yb:Er = 78:20:2 molar ratio) UCNPs were synthesized using the thermal coprecipitation method with slight modifications.31,32 An aqueous solution of lanthanides (Ln), LnCl3·6H2O [585 µL of YCl3·6H2O (2 M), 150 µL of YbCl3·6H2O (2 M), 150 µL of ErCl3·6H2O (0.2 M)], was added to a 100 mL three-neck round bottom flask and heated to 100 °C using a heating mantle while stirring (300 rpm) for 20 min to remove the water from the mixture. Following this, 1-octadecene (27 mL) and OA (9 mL) were added to the flask and the temperature was increased to 160 °C (400 rpm) for 30 min and then decreased to 50 °C. The solution became transparent due to the formation of rare earth (RE) oleates through the reaction of the RE chlorides with OA. While the flask was heating, NaOH pellets (150–160 mg) and NH4F (222.24 mg) were weighed and added to a 50 mL beaker filled with methanol (15 mL). The mixture was sealed with parafilm and stirred for 30 min. Once the flask had cooled, a glass dropping funnel filled with the methanol solution was connected to one neck of the flask, and dropwise additions were administered into the flask. The solution became cloudy as the NaYF4 crystals nucleated. The octadecene/methanol solution was then stirred for 1 h to ensure complete nucleation. The system was sealed by attaching a nitrogen (N2) tank through the glass adapter into a side port, inserting a reflux column to the center port (a T-valve glass adapter connected to a vacuum line and an oil bubbler attached to the top of the condenser), and slowly heated to 100 °C. The T-valve was then switched to place the system under a vacuum for approximately 10 min to remove methanol, after which the valve was switched back to the bubbler. The solution was rapidly heated to 300 °C and stirred for 1.5 h before cooling to 30 °C. The UCNPs were transferred to a 50 mL conical tube and collected via centrifugation (1200 ×g, 30 min). The crystals were then washed twice with cyclohexane and three times with ethanol via centrifugation (1200 ×g, 10 min each time). The UCNPs were dried in a 60°C oven overnight to evaporate the residual ethanol and stored in cyclohexane (50 mg/mL).
Synthesis of NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs (Core-Shell Er@Nd UCNPs; U)
Shell UCNPs NaYF4:Yb,Nd (Y:Yb:Nd = 65:5:30 molar ratio) were synthesized using the method described in the previous section. An aqueous solution of LnCl3·6H2O [487.5 µL of YCl3·6H2O (2 M), 75 µL of YbCl3·6H2O (1 M), 225 µL of NdCl3·6H2O (2 M)] was nucleated, the core UCNPs were added, and the mixture was stirred for 30 min before adding the methanol solution. The NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs (Er@Nd UCNPs; U) were dried in a 60 °C oven overnight to evaporate the residual ethanol and stored in cyclohexane (100 mg/mL).
Synthesis of Mesoporous Silica (MS)-Coated UCNPs (U@MS NPs) and Loading Ce6 (U@MSC NPs)
MS-coated UCNPs (U@MS NPs) were synthesized using the Stöber (sol-gel) method with slight modifications.33 NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs (U) in cyclohexane (3 mL, 10 mg/mL) were added to a 100 mL round bottom flask containing aqueous solution of CTAB (30 mL, 10 mg/mL). The mixture was sonicated in a water bath for 15 min to form an oil-in-water emulsion. The flask was subsequently heated to 80 °C, at which temperature it was maintained for 1 h to evaporate the cyclohexane. Double-distilled water (12 mL) and aqueous NaOH (2.8 mL, 2 M) were added to adjust the pH, and the mixture was stirred for 30 min. The temperature was then reduced to 70 °C and 0.77 mL of the TEOS:EA solution (1:6) was added dropwise under stirring at 0.11 mL/min. The reaction was maintained at 70 °C for 16 h. The nanoparticles were collected by centrifugation (1200 ×g, 30 min), washed thrice with ethanol (2592 ×g, 15 min), and redispersed in ethanol (30 mL). To extract CTAB from the U@MS NPs, 60 μL of HCl was added to the mixture to adjust the pH to ~1.3. The solution was stirred at 60 °C (300 rpm) for 3 h. The U@MS NPs were isolated by centrifugation (1200 ×g, 30 min) and washed thrice with ethanol (2592 ×g, 15 min). The surface of the U@MS NPs was modified with amine groups for better dispersion in water, according to a previous method.34 The product was re-dispersed in 5 mL of ethanol containing 100 μL of APTES. The amine-modified U@MS NPs were collected after 16 h of reaction, re-dispersed in water, and lyophilized.
Ce6 in a DMSO solution (0.2 mL, 20 mg/mL) was added with APTES for the preparation of Ce6-loaded-NPs (U@MSC NPs). After the reaction, the final NPs were collected by centrifugation (2592 ×g, 15 min). The supernatant was also collected to determine the untrapped Ce6 amount. The precipitated U@MSC NPs were washed thrice with ethanol, re-dispersed in water, and lyophilized. The Ce6 loading content was determined by UV-Vis microplate spectrophotometry. The absorbance at 662 nm was measured, and the Ce6 loading content and encapsulation efficiency were calculated using the following equations:
Ce6 loading content (%) = (Amount of Ce6 in NPs)/(Amount of NPs) ×100
Encapsulation efficiency (%) = (Amount of added Ce6- Amount of untrapped Ce6)/(Amount of added Ce6) ×100
Synthesis of Mesoporous Polydopamine (MP)-Coated NPs (U@MS@MP NPs) and U@MSC@MP NPs
Mesoporous polydopamine-coated NPs (U@MS@MP NPs) were synthesized using a previously reported method with modifications.35 TMB (0.32 mL) was added to a mixture of 10 mL Pluronic F127 aqueous solution (2 mg/mL) and 10 mL dopamine hydrochloride aqueous solution (2 mg/mL) in a glass bottle. The mixture was kept for bath sonication for 5 min to obtain a white emulsion. The U@MS NPs or U@MSC NPs were then added and the solution was stirred at room temperature for 60 min. The pH of the mixture was adjusted by adding 8 µL of ammonia solution under stirring. The color of the emulsion changed from white to brown. After 60 min, the nanoparticles were collected by centrifugation (2592 ×g, 5 min), washed thrice with an acetone:ethanol mixture (1:2) to remove the TMB template, and rinsed with water. The final product was dispersed in water and lyophilized. The Ce6 content in the U@MSC@MP NPs was determined by UV-Vis microplate spectrophotometry and calculated following the same procedure as in the previous section.
Characterization of the NPs
The synthesized NPs were analyzed using transmission electron microscopy (TEM; JEM-2100; Carl Zeiss, Jena, Germany), high-resolution transmission electron microscopy (HRTEM), energy dispersive spectroscopy (EDS) (JEM-F200 with FE-TEM, JEOL Co., Akishima, Japan), a particle size analyzer (Zetasizer Pro, ELSZ, Malvern Panalytical Ltd., Malvern, UK), powder X-ray diffractometry (XRD; D8 Advance; Bruker Corporation, Berlin, Germany), Fourier transform infrared spectroscopy (FTIR; FT/IR-4700, JASCO Inc., Tokyo, Japan), UV-Vis microplate spectrophotometry (Multiskan Go, Thermo Fisher Scientific Inc., CA, USA), UV-vis spectrophotometry (Orion Aquamate 8100, Thermo Fisher Scientific Inc., CA, USA), fluorescence spectrophotometry (F-7000, Hitachi High-Technologies Corp., Tokyo, Japan), and a Brunauer–Emmett–Teller (BET) surface area analyzer (ASAP2020, Micromeritics Instrument Corp., Norcross, GA, USA). The morphology and size of the NPs were observed by TEM at 200 kV. The size histograms were generated using Origin Pro software. The samples were dispersed in cyclohexane (UCNPs) or ethanol (U@MS and U@MS@MP NPs) and dropped onto a copper grid. The zeta potential was measured using a particle size analyzer. The uniform crystal lattice of the UCNPs was captured by HRTEM, and the composition of the UCNPs was determined using EDS. The photoluminescence (PL) of the UCNPs was analyzed using a fluorescence spectrophotometer with excitation at 808 nm. XRD patterns of UCNPs, U@MS, and U@MS@MP was determined with 2–theta range of 10–80° to identify the crystalline phase of UCNPs and coated NPs. The functionalized coating modification of the UCNPs was analyzed by FTIR spectroscopy. The porosities of the U@MS and U@MS@MP NPs were determined using a BET analysis. The UV-Vis absorption spectrum of Ce6 in NPs was obtained using UV-Vis spectrophotometry.
Determination of Total ROS Generation
The in vitro total ROS generation by NPs was determined using DPBF as the ROS detection reagent. DPBF in ethanol (20 µL, 1 mg/mL) was added to 1.96 mL of ethanol in a cuvette. After mixing with 20 µL of the NPs (20 mg/mL in DDW) followed by air bubbling, the UV-Vis spectra of the samples at the 300–500 nm range were measured at the designated time (every 1 min for 15 min) after 808 nm laser irradiation (1 W/cm2; laser - the cuvette distance = 0.5 cm). The absorbance at 410 nm was determined for DPBF absorption.
Intracellular ROS Generation Studies
The in vitro intracellular ROS generation by the NPs was determined using DCFH-DA as the ROS staining reagent. Human colorectal adenocarcinoma HT-29 cells (1 × 105 cells) were seeded onto 4-well slides. After 24 h, the media were replaced and incubated with Ce6, U@MSC NPs, and U@MSC@MP NPs (20 μg/mL as Ce6) in phenol-red-free cell culture medium for 4 h. After washing thrice with PBS, DCFH-DA solution (10 mm in PBS) was added, and the solution was incubated at 37 °C for 15 min and washed thrice with PBS. DAPI (300 nM in PBS) was added and the mixture was further incubated at 37 °C for 5 min and washed with PBS three times. The supernatant was then removed and replaced with phenol red-free cell culture medium. The cells were irradiated with an 808 nm laser (1 W/cm2; 5 min; laser - the bottom of the slide distance = 2 cm) and immediately mounted with a glass cover slip. Fluorescence signals were observed using confocal laser scanning microscopy (CLSM) (Leica TCS SP8, Leica Microsystems Ltd., Wetzlar, Germany) at an excitation wavelength of 488 nm using a fluorescein isothiocyanate (FITC) filter for DCFH-DA and 405 nm for DAPI.
Cellular Uptake Studies
The in vitro cellular uptake of NPs was determined using CLSM. HT-29 cells (1×105 cells) were seeded onto 4-well slides. After 24 h, the media were replaced and incubated with U@MSC@MP NPs (20 μg/mL as Ce6) in phenol-red-free cell culture medium for 0.5, 1, 2, and 4 h. After washing three times with PBS, DAPI (300 nM in PBS) was added and the mixture was incubated at 37 °C for 5 min and washed thrice with PBS. The slides were mounted on glass coverslips. The fluorescence signal of Ce6 was observed by CLSM at an excitation wavelength of 488 nm using a Cy5.5 filter for Ce6 and 405 nm for DAPI.
Photothermal Effect
The photothermal effect of the NPs was monitored by measuring their temperature at a designated time after 808 nm laser irradiation (1 W/cm2; laser - the bottom of the plate distance = 2.5 cm). Various concentrations of U@MSC@MP NPs were dispersed in water and 0.2 mL of the solution was added to a 96-well plate. Temperature changes were recorded using a thermal camera (FLIR ONE Pro; Teledyne FLIR LLC, Wilsonville, OR, USA).
In vitro Cytotoxicity Studies
HT-29 cells (5×103 cells/well) were seeded onto 96-well plates for the dark and 808 nm laser irradiation analyses. After 24 h, the media were replaced, and the solution was incubated with NPs in various concentrations or equivalent to 20 µg/mL as Ce6 for the comparison studies in a cell culture medium at 37 °C. For the no-laser condition, one plate was kept in an incubator (dark) for 24 h. For the laser groups, a second plate was put in the clean bench for 30 min post-incubation and irradiated with the 808 nm laser (1 W/cm2; 10 min; laser - the bottom of the plate distance = 2.5 cm) and then incubated for 24 h after finishing the laser irradiation. In addition, a third plate was irradiated with the same 808 nm laser on ice. In detail, the plastic zipper bag (13 × 15 cm) filled with water was put in the −20°C freezer a day before the experiment to make a flat ice pack (~1 cm thickness). The ice pack was put under the 96-well plate during the laser irradiation for the laser + ice group. The plates were then incubated at 37 °C for 24 h. The media was replaced, a cell viability reagent (CellTiter 96® AQueous One Solution) was added, and the solution was incubated for 1 h. The UV-Vis spectra were recorded at 490 nm.
In vivo Antitumor Efficacy Study in HT-29 Tumor Xenograft Mouse Model
A subcutaneous HT-29 tumor xenograft mouse model was prepared using female BALB/c nude mice (5-weeks old) acquired from Nara Biotech (Seoul, Republic of Korea). The study protocol (SNU-231023-3-3) followed the National Institutes of Health guidelines on the principles of laboratory animal care and was approved by the Institutional Animal Care and Use Committee of the College of Pharmacy, Seoul National University (Seoul, Republic of Korea). The mice were maintained at the Animal Center for Pharmaceutical Research (College of Pharmacy, Seoul National University) at 22 ± 2 °C with a 12 h light/dark cycle and a relative humidity of 55 ± 5%. The tumor-bearing mouse model was prepared by subcutaneous injection of 100 µL of an HT-29 cell suspension (5 × 106 cells) into the right hind limb region. The tumor volume (V) was determined using the following formula: V (mm3) = 0.5 × longest diameter × shortest diameter2. When the tumor volume reached approximately 280 mm3, the mice were randomized into seven groups: PBS, PBS + laser, Ce6 + laser, U@MS + laser, U@MSC + laser, U@MS@MP + laser, and U@MSC@MP + laser. Anesthesia was administered via isoflurane inhalation. The samples were intratumorally administered at a Ce6 dose of 5 mg/kg, and 24 h post-injection, an 808 nm laser (0.5 W/cm2; laser - tumor distance = 3 cm) was used to irradiate the samples for 5 min under tumor-temperature monitoring. To investigate the antitumor effect and toxicity of the NPs, tumor volume and body weight were measured every two days. The antitumor efficacy study was terminated before the tumor size reached the recommended value (less than 20 mm in any one dimension), as determined by the Institutional Animal Care and Use Committee, Seoul National University. On day 20, five major organs (the heart, lung, liver, kidney, and spleen) and tumor tissues were dissected and stained with hematoxylin and eosin (H&E). Tissue images were captured using a high-performance slide scanner (ZEISS Axioscan7, ZEISS, Oberkochen, Germany).
Statistical Analysis
All experiments were performed a minimum of three times, and the acquired data are presented as means ± standard deviations (S.D). The significance differences observed for the studies were determined using a two-way analysis of variance (ANOVA) and a subsequent assessment was performed using Sidak’s multiple comparison test with GraphPad PRISM® Software (GraphPad Prism, version 8.0.2, Boston, MA, USA) at a significance level of p < 0.05.
Results and Discussion
The Preparation and Characterization of NPs
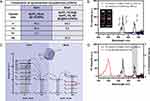
In this study, NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs was selected for 808 nm-induced PDT effect by Ce6 based on the previous report of Nd3+-sensitized UCNPs for 808 nm excitation, with modification.20 Figure 1 shows the synthesis scheme of the preparation of water-dispersible NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs@MS-Ce6@MP NPs (U@MSC@MP NPs) with an average size of 80 nm. Firstly, the NaYF4:Yb,Er (core Er) and NaYF4:Yb,Er@NaYF4:Yb,Nd UCNPs (core-shell Er@Nd) were synthesized using the thermal coprecipitation method. The hexagonal NaYF4 was selected as the host matrices due to its high chemical stability; the components of the UCNPs are displayed in Figure 2A. After synthesis of the core Er UCNPs that emit at around 665 nm after NIR light excitation at 980 nm (Figure S1), an active shell consisting of Nd3+ ions were epitaxially grown on the core UCNPs to allow for absorption of the 808 nm wavelength. The photoluminescence of the Er@Nd UCNPs after irradiation at 808 nm was confirmed by fluorescence spectrophotometry, which revealed green (~ 542 nm) and red (~ 658 nm) emissions (Figure 2B), the visible green color in the digital photograph. In contrast, the core Er UCNPs showed no emission because they were not excited by the 808 nm wavelength and showed only the red color of the 808 nm laser. Figure 2C is the schematic illustration of energy transfer process of UCNPs with 808 nm excitation, showing the absorbed energy transfer to the Yb3+ ion as an intermediary and the final transfer to Er3+ in the core. Figure 2D shows that the red emission from the Er@Nd UCNPs at approximately 660 nm overlapped with the activation range of the photosensitizer Ce6. Therefore, the Er@Nd UCNPs could activate the PDT effect of Ce6 using an 808 nm laser as opposed to the conventional 660 nm laser.
The Er and Er@Nd UCNPs showed good uniformity with particle sizes of 30.08 ± 1.81 nm and 44.55 ± 3.61 nm, respectively, as revealed by the TEM images (Figure 3A(i) and (ii)). Loading the PS onto the UCNPs creates some concerns regarding Förster resonance energy transfer (FRET), one of the energy transfer processes of UCNPs. FRET is only effective when the donor–acceptor distance is less than 10 nm; therefore, the distance between the UCNPs (donor) and Ce6 (acceptor) must be sufficiently close to activate the PDT effect.36 MS-coating on the surface of UCNPs was selected as the Ce6 carrier because Ce6 can be loaded into the porous structure of the MS, allowing it to be near the UCNPs. The U@MS NPs were synthesized by the sol–gel method using CTAB as a pore-enhancing template. The MP-coated NPs (U@MS@MP) for PTT were synthesized by the self-polymerization of a dopamine monomer using the π-electron-rich-TMB as a template for the porous structure under alkaline conditions. The π– π stacking interaction between the polydopamine and TMB created a porous structure that was deposited on the surface of the NPs. The mesoporous structure of the U@MS and U@MS@MP NPs was clearly observed by TEM and the particle sizes were 75.35 ± 5.35 and 79.68 ± 4.48, respectively (Figure 3A(iii) and (iv)). The size histogram shows the homogenous distribution of the prepared NPs. As shown in the average particles size of NPs (Figure 3B), the MS-coating was approximately 17 nm thick, and the MP was coated as a thin layer of 0.5–1 nm on the surface of the MS. Figure 3C presents the zeta potentials of the Er, Er@Nd, U@MS, U@MS@MP, U@MSC, and U@MSC@MP NPs as −12.21 ± 0.32, −22.99 ± 0.59, 11.30 ± 1.44, −4.65 ± 0.57, −4.62 ± 0.55, and −4.85 ± 0.73 mV, respectively. The positive charge of the U@MS NPs resulted from an amine functional group modification on their surface. However, the amine group was covered after Ce6 loading or coated with MP, causing the NPs to switch to a negative charge.
The HRTEM image (Figure 3D) of the Er and Er@Nd UCNPs revealed obvious lattice fringes with lattice spacings of 0.28 nm and 0.52 nm, respectively, corresponding to d-spacing between the (101) and (100) planes of the hexagonal NaYF4. EDS mapping was used to evaluate the distribution information of each element in the samples (Figure 3E). The elemental mapping images showed that the UCNPs were mainly composed of sodium (Na), yttrium (Y), and fluoride (F) doped with ytterbium (Yb) and erbium (Er) with the addition of neodymium (Nd) to the Er@Nd UCNPs. The Na, Y, and F elements were uniformly distributed in the host structure of the NPs, and all other lanthanide elements doped into the host (eg Yb, Er, and Nd) were also uniformly distributed in the NPs.
The XRD patterns (Figure 3F) further confirmed that the Er and Er@Nd UCNPs had a hexagonal structure, which corresponded to the reference standard for the hexagonal phase of NaYF4 (β-NaYF4) (Joint Committee on Powder Diffraction Standards; JCPDS No. 16–0334). After coating the UCNPs with a layer of mesoporous silica, the U@MS NPs showed the original diffraction peaks of the standard UCNPs with a broad peak at 2-theta approximately 22.0 ° (in red-dashed box) which corresponded to the amorphous silica. Moreover, MP-coating also did not change the original diffraction of the UCNPs, indicating that the UCNPs were well-coated with the MS and MP.
The FTIR spectra revealed that the Er and Er@Nd UCNPs presented bands at 2852 and 2924 cm−1 corresponding to the symmetric and asymmetric -CH2 stretches of oleic acid, respectively (Figure 3G). Additional bands at 1558 and 1452 cm−1 were found in the spectra of these two samples, representing the asymmetric and symmetric stretching vibrations of the carboxylic group of the oleate ligand of the UCNPs.37 These results confirm that the oleate ligands were bound to the surface of the NPs. After MS-coating, the strong bands at 1072 cm−1 (Si–O–Si asymmetric stretching) and 789 cm−1 (Si–O–Si symmetric stretching) represented the characteristic peaks of silica, thereby confirming that MS coated the surface of the UCNPs.28 After MP-coating, the peaks at 2923 and 2858 cm−1 were attributed to the stretching vibration of -CH- in MP.35 The broad peak at 3419 cm−1 (in red-dashed box) in the U@MS@MP and U@MSC@MP NPs spectra represented the characteristic adsorption of the amine N-H and phenolic O-H stretching vibrations, suggesting that MP was coated on the surface of the NPs.38
Considering that Ce6 was mainly loaded onto the pores of the MS, the surface area was important for the PS loading capacity. Therefore, the N2 adsorption–desorption isotherms and pore diameters were determined, and the N2 adsorption-desorption isotherms of both U@MS and U@MS@MP NPs fit Langmuir isotherm type IV, indicating that mesoporous structures of MS and MP were formed (Figure 3H). In addition, the BET analysis showed surface areas of U@MS and U@MS@MP of 311.6166 m²/g and 339.5563 m²/g, respectively. The high surface area of U@MS indicates that it could be a suitable PS carrier with a high loading capacity. Moreover, the Barrett-Joyner-Halenda (BJH) data showed that the average pore diameters of the U@MS and U@MS@MP NPs were 3.8534 and 5.7130 nm, respectively, suggesting that the porous-structured NPs were successfully fabricated (Figure 3I).
The Ce6 loading and encapsulation efficiencies of the U@MSC NPs were 10.04 ± 0.58% and 50.18 ± 2.92%, respectively. The Ce6 loading content of U@MSC@MP NPs were 6.26 ± 0.82%. Figure 3J shows that the green color of Ce6 was displayed in the U@MSC and U@MSC@MP NPs, corresponding to the distinct UV-Vis absorbance spectra of Ce6 at 400 and around 660 nm (Figure 3K). It is notable that absorbance at 660 nm also appeared in the Ce6-loaded U@MSC and U@MSC@MP NPs, indicating that these particles can efficiently activate PS Ce6 after absorbing the emission of around 660 nm from UCNPs upconverted by the irradiation of 808 nm laser.
Determination of Total ROS Generation
DPBF was used as a probe for detecting singlet oxygen (1O2), which corresponds to the total ROS generation. DPBF is fluorescent, strongly absorbs light at 410 nm, and reacts with 1O2 through 1, 4-cycloaddition to form endoperoxides, yielding colorless 1, 2-dibenzoylbenzene. This reaction is irreversible, and the UV-Vis absorbance measurement at 410 nm can be used to determine the percentage of DPBF bleaching, which is negatively correlated with ROS generation. In this study, DPBF was selected as the singlet oxygen sensor because it has no absorbance at 660 or 808 nm; therefore, it does not interfere with the functioning of UCNPs and Ce6 in relation to the PDT effect. The UV-Vis spectra in Figure 4A(i) and (ii) indicate that both the U@MSC and U@MSC@MPs showed gradual bleaching of the DPBF under 808 nm laser irradiation, indicating that ROS were generated. Figure 4B reveals that the absorbance at 410 nm significantly decreased after 5 and 6 min of 808 nm irradiation for the U@MSC NPs and U@MSC@MP NPs, respectively, compared to the DPBF + 808 nm laser group, indicating that MP-coating did not block the PDT effect of the NPs.
Intracellular ROS Generation Studies
DCFH-DA staining was used to detect intracellular ROS generation. The diacetate group of DCFH-DA can be cleaved by the esterase in the cells to produce a compound that can react with ROS and transform to 2’,7’-dichlorofluorescein, a fluorescent compound. The fluorescence intensity of DCF (green) in HT-29 cells was observed in the free Ce6, U@MSC NPs, and U@MSC@MP NPs with 808 nm laser irradiation (1 W/cm2) for 5 min compared to the groups without laser irradiation, as shown in Figure 4C. This result indicated that the PDT effect occurred in the cells and that ROS generation was triggered by the 808 nm laser irradiation.
Cellular Uptake Studies
The cellular uptake efficiency of the U@MSC@MP NPs was evaluated in HT-29 cells (Figure 4D). The Ce6 fluorescence intensity (red) gradually increased with incubation time. Initially, most Ce6 signals were located near the cell membrane, with little activity observed in the cytoplasm. After a longer incubation time, Ce6 showed granular distribution in the cytoplasm, with minimal distribution in the nucleus after 1 h and a higher intensity observed at 4 h of incubation.
Photothermal Effect
Figure 4E demonstrates the change in temperature during 10 min of 808 nm laser irradiation to the U@MSC@MP NPs. The increased temperature after longer laser exposure indicated that MP-coating had a PTT function. In addition, the elevated temperature was concentration-dependent, whereby higher concentrations showed a more rapid increase in the temperature compared to the lower concentrations. To ensure the stability of the PTT effect, the temperature was monitored by switching the laser on and off for three cycles, as shown in Figure 4F. The temperature change was consistent, suggesting that the effect of the PTT was stable under repeated laser irradiation.
In vitro Cytotoxicity Studies
Figures 5A–C illustrate the results of cytotoxicity studies involving HT-29 colon cancer cells after treatment with PDT (U@MSC), PTT (U@MS@MP), and PDT-PTT (U@MSC@MP NPs) (with and without 808 nm laser irradiation) in various concentration of NPs and incubation for 24 h. A significant reduction in cell viability was observed in the laser groups compared to the control groups, indicating that the cells died after exposure to 808 nm light, and this effect was dose-dependent. All of the NPs in concentrations up to 500 µg/mL showed no toxicity after treatment for 24 h in the dark, indicating high compatibility for an in vivo study. A comparison between various NPs treated at the same concentration of Ce6 (20 µg/mL) revealed that the U@MS NPs showed no signs of toxicity in either the dark environment or that with laser irradiation; thus, no PDT effect was evident without the PS (Figure 5D). The free Ce6 group showed high toxicity to the cells in both the dark and laser irradiation conditions at 20 µg/mL, whereas the same Ce6-loading U@MSC and U@MSC@MP NPs exhibited no toxicity under dark conditions, indicating that the cytotoxicity of Ce6 can be initiated only when 808 nm laser irradiation is applied. Moreover, the U@MSC@MP NPs (3.33% viable) significantly decreased the cell viability compared to the U@MSC (66.69% viable) or U@MS@MP NPs (60.52% viable), indicating that the dual-effect of PDT-PTT combination was achieved with synergistic effect. The possible mechanism for this synergistic PDT-PTT was reported through enhancing tumor cell death by the cell membrane damage in the previous study.39 To ensure that the PDT effect was not blocked by the MP-coating, an additional group was irradiated on ice to remove the PTT effect. The reduced cell viability of the U@MSC@MP NPs + laser on ice was similar to that of the U@MSC NPs, suggesting that the PDT effect was not influenced by MP modification. These results support the effectiveness of the PDT-PTT dual therapy with U@MSC@MP NPs in the cancer treatment. To understand the mechanism of cell death, the cellular apoptosis assay induced by the PDT and PDT-PTT treatment with U@MSC and U@MSC@MP NPs (with and without 808 nm laser irradiation) at the concentration of Ce6 (20 µg/mL) were performed using FITC Annexin V Apoptosis Detection Kit with 7-AAD. Annexin V, which detects phosphatidyl serine on the external membrane of apoptotic cells, and 7-ADD, which intercalates into the DNA of membrane-damaged cells, were used as markers of late apoptosis and dead cells. As shown in Figure S2, the dominant viable cell populations (Q4) were in the control groups (untreated and laser only), which were 99.7% and 99.2%, respectively, indicating that 808 nm laser alone did not affect cell viability. However, the proportion of the viable cells was decreased when treated with Ce6-mediated PDT (U@MSC NPs) and PDT-PTT (U@MSC@MP NPs) under laser irradiation compared to the same treatment without laser groups. In other words, the total apoptotic cells (early apoptosis (Q3)) plus late apoptosis/necrosis (Q2)) of the PDT and PDT-PTT treatment group with laser was considerably higher compared to theirs without laser group. In particular, the results showed that the PDT and PDT-PTT treatment with laser-induced HT-29 cell death via cell apoptosis and necrosis (indicated by (FITC Annexin V+/ 7-AAD+) in Figure S2. These findings were consistent with previous research reporting Ce6-mediated PDT-induced apoptotic cell death.40,41 Consequently, the synthesized 808 nm-response-UCNP-based NPs can substitute 660–670 nm laser for Ce6-sensitized PDT, inducing apoptotic cell death by singlet oxygen generated upon laser irradiation. In addition, the cell necrosis populations (Q1) of the PDT-PTT with laser were greater than PDT alone with laser, indicating that the cell membranes were damaged, which further confirmed the possible mechanism of enhancing cancer cell death by the synergistic PDT-PTT effect as mentioned previously.
In vivo Antitumor Efficacy Study Using HT-29 Tumor Xenograft Mouse Model
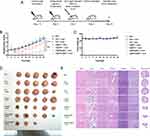
The efficacy of the U@MSC@MP NPs in dual PDT and PTT was further evaluated using an HT-29 tumor xenograft mouse model. Before evaluating the antitumor efficacy study, the preliminary testing was performed to find the optimum treatment-to-laser irradiation time. The U@MSC@MP NPs (Ce6 dose of 5 mg/kg) were intratumorally injected into the mouse, and the in vivo fluorescent images of Ce6 using Cy5.5 channel detection were captured, and the radiant efficiency was determined at the specific post-injection time by the VISQUE imaging (Gyeonggi-do, Republic of Korea). The tumor distribution of the nanoparticles based on the fluorescence of Ce6 was demonstrated in Figure S3A. The radiant efficiency at various time points after the treatment was summarized in Figure S3B. The result exhibited a homogenous distribution of the NPs in the tumor with stable fluorescence from 0.5 h up to 12 h after injection, showing the highest signal at 24 h. Therefore, 24 h post-injection was selected as the gap time for laser irradiation based on this preliminary result and following the previous study.19 When the tumor volume reached ~280 mm3, the mice were randomly divided into seven treatment groups: (1) PBS, (2) PBS + laser, (3) Ce6 + laser, (4) U@MS + laser, (5) U@MSC + laser, (6) U@MS@MP + laser, and (7) U@MSC@MP + laser. The samples were intratumorally administered at a Ce6 dose of 5 mg/kg. The control (PBS) was not exposed to laser irradiation, whereas the other groups were subjected to single 808 laser irradiation (0.5 W/cm2, 5 min) after 24 h post-injection to activate the PDT and/or PTT (Figure 6A). The laser intensity in the in vivo study was reduced from the in vitro study to prevent tissue damage due to overheating. In Figure 6B, the increase in the relative tumor volume of the PDT alone and PTT alone was evaluated on day 2, which was caused by a common side effect of the phototherapy-induced swelling of the tissue. This symptom recovered on day 4, as shown in Figure S4. In contrast, the other groups, including the PDT-PTT combination, showed no noticeable tissue swelling. Figure 6B also illustrates that the laser group (PBS + laser) showed no significant difference from the control (PBS) group, indicating that the 808 nm laser condition in this study had no antitumor effect without treatment. Notably, the Ce6 + laser also showed no difference in the relative tumor volume to the control group, unlike the high toxicity result in the in vitro cytotoxicity study. This phenomenon could relate to the Ce6 properties, including hydrophobicity and easy aggregation, which cause low stability in the physiological system.42 In addition, the treatment-to-laser interval of the in vitro and in vivo test were not the same (30 min post-treatment incubation and 24 h post-injection, respectively). Consequently, the Ce6 solution could be aggregated in the tumor tissue after 24 h, resulting in the loss of its phototoxicity. The U@MS + laser group (without Ce6) showed no significant tumor reduction compared to the control group, indicating that the cell death-inducing by the PDT effect could not be achieved without Ce6. The PDT-only (U@MSC + laser) group showed significantly greater antitumor efficacy than that of the control, Ce6 + laser, and U@MS + laser groups (*p < 0.05); however, it was not statistically different than the PBS + laser group. The PTT-only (U@MS@MP + laser) group demonstrated a significantly higher antitumor effect than the control, PBS + laser, and Ce6 + laser groups but showed no difference compared to the PDT group. Finally, the combination of PDT-PTT U@MSC@MP + laser exhibited significantly higher therapeutic efficacy than PDT alone (U@MSC + laser) or PTT alone (U@MS@MP + laser). Moreover, two mice from the U@MSC@MP + laser group demonstrated a complete response (CR). In detail, the CR of the PDT-PTT combination was observed on day 10 of the study, as shown in Figure S4. The other mice in the same group showed tumor regeneration after day 10, possibly due to the hypoxia (lack of oxygen) in the center of the 280 mm3-size tumors, resulting in less PDT effect. These results suggest that U@MSC@MP NPs with single 808 nm irradiation phototherapy are effective against human colorectal cancer. Therefore, the UCNP-based system could simplify the dual PDT-PTT to one laser (808 nm laser for PDT-PTT) compared to the previous reports using two lasers (670 nm laser for Ce6-mediated PDT and a 808 nm for polydopamine-mediated PTT).6 In addition, the in vivo laser irradiation condition in this study (808 nm laser; 0.5 W/cm2; 5 min; single irradiation) is advantageous with shorter time, lower intensity, or less frequency than the previous 808 nm-induced PDT or PDT-PTT studies, suggesting feasible dual PDT-PTT strategy for clinical applications.7,16,19,28 Furthermore, none of the BALB/c nude mice showed noticeable weight loss in any treatment group within the study period (20 days), indicating that the dose used in this study (5 mg/kg Ce6) was safe (Figure 6C). The tumors dissected on day 20 are shown in Figure 6D. The H&E staining of the tumor tissues with various treatments was performed further to illustrate the therapeutic efficacy (Figure 6E). The PDT (U@MSC + laser), PTT alone (U@MS@MP + laser), and PDT-PTT U@MSC@MP + laser slightly showed cell apoptosis and cell shrinking compared to the control group. In addition, the histological results of the major organs (heart, lung, liver, kidney, and spleen) of the various groups revealed no noticeable organ damage or lesions, indicating that the as-prepared U@MSC@MP NPs presented no signs of toxicity in mice.
Conclusions
U@MSC@MP NPs were successfully developed to create a highly biocompatible PDT and PTT combination to treat cancer using a single 808 nm laser. To the best of our knowledge, this is the first study to achieve the complete response results from PDT-PTT dual therapy activated by a single 808 nm laser in less than 15 min of light excitation for an in vivo test without noticeable toxicity. These results indicate that UCNP-based delivery systems can improve the efficacy of complicated treatment procedures. For clinical applications, further research on the biodistribution and long-term toxicity of UCNPs is required concerning intravenous administration or treatment regimen adjustments to optimize this promising approach.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (No. NRF-2020R1A2C2099983 and RS-2024-00432685).
Disclosure
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
1. Ormond AB, Freeman HS. Dye sensitizers for photodynamic therapy. Materials. 2013;6(3):817–840. doi:10.3390/ma6030817
2. Shabbirahmed AM, Kumaravel M, Somu P, Paul S, Khadria A. Recent advancements in nanomaterials for photodynamic therapy of cancers. In: Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Springer; 2022:1–24.
3. Ashkbar A, Rezaei F, Attari F, Ashkevarian S. Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci Rep. 2020;10(1):21206. doi:10.1038/s41598-020-78241-1
4. Sun H, Ai Y, Qi H, et al. Pt/Ag‐PEG‐Ce6 nanosystem with enhanced near‐infrared absorption and peroxidase‐like activity for synergistic photodynamic/photothermal therapy. Adv Therap. 2022;5(11):2200089. doi:10.1002/adtp.202200089
5. Yang J, Hou M, Sun W, et al. Sequential PDT and PTT using dual‐modal single‐walled carbon nanohorns synergistically promote systemic immune responses against tumor metastasis and relapse. Adv Sci. 2020;7(16):2001088. doi:10.1002/advs.202001088
6. Zhang D, Wu M, Zeng Y, et al. Chlorin e6 conjugated poly (dopamine) nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced cancer therapy. ACS Appl Mater Interfaces. 2015;7(15):8176–8187. doi:10.1021/acsami.5b01027
7. Zhang Y, Zhu X, Zhang J, Wu Y, Liu J, Zhang Y. Synergistic upconversion photodynamic and photothermal therapy under cold near-infrared excitation. J Colloid Interface Sci. 2021;600:513–529. doi:10.1016/j.jcis.2021.05.017
8. Song C, Tang C, Xu W, et al. Hypoxia-targeting multifunctional nanoparticles for sensitized chemotherapy and phototherapy in head and neck squamous cell carcinoma. Int J Nanomed. 2020;15:347–361. doi:10.2147/IJN.S233294
9. Song C, Ran J, Wei Z, et al. Organic near-infrared-II nanophotosensitizer for safe cancer phototheranostics and improving immune microenvironment against metastatic tumor. ACS Appl Mater Interfaces. 2021;13(3):3547–3558. doi:10.1021/acsami.0c18841
10. Song C, Xu W, Wei Z, et al. Anti-LDLR modified TPZ@ Ce6-PEG complexes for tumor hypoxia-targeting chemo-/radio-/photodynamic/photothermal therapy. J Mater Chem B. 2020;8(4):648–654. doi:10.1039/C9TB02248A
11. Wang K, Zhang Y, Wang J, et al. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci Rep. 2016;6(1):27421. doi:10.1038/srep27421
12. Wang YF, Liu GY, Sun LD, Xiao JW, Zhou JC, Yan CH. Nd3+-sensitized upconversion nanophosphors: efficient in vivo bioimaging probes with minimized heating effect. ACS Nano. 2013;7(8):7200–7206. doi:10.1021/nn402601d
13. Mettenbrink EM, Yang W, Wilhelm S. Bioimaging with Upconversion Nanoparticles. Adv Photonics Res. 2022;3(12). doi:10.1002/adpr.202200098
14. Shen J, Chen G, Vu AM, et al. Engineering the upconversion nanoparticle excitation wavelength: cascade sensitization of tri‐doped upconversion colloidal nanoparticles at 800 nm. Adv Opt Mater. 2013;1(9):644–650. doi:10.1002/adom.201300160
15. Hudson DE, Hudson DO, Wininger JM, Richardson BD. Penetration of laser light at 808 and 980 nm in bovine tissue samples. Photomed Laser Surg. 2013;31(4):163–168. doi:10.1089/pho.2012.3284
16. Ai F, Ju Q, Zhang X, Chen X, Wang F, Zhu G. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Sci Rep. 2015;5(1):10785. doi:10.1038/srep10785
17. Wiesholler LM, Frenzel F, Grauel B, Wurth C, Resch-Genger U, Hirsch T. Yb,Nd,Er-doped upconversion nanoparticles: 980 nm versus 808 nm excitation. Nanoscale. 2019;11(28):13440–13449. doi:10.1039/C9NR03127H
18. Zhan Q, Wang B, Wen X, He S. Controlling the excitation of upconverting luminescence for biomedical theranostics: neodymium sensitizing. Opt Mater Express. 2016;6(4):1011–1023. doi:10.1364/OME.6.001011
19. Zeng L, Pan Y, Zou R, et al. 808 nm-excited upconversion nanoprobes with low heating effect for targeted magnetic resonance imaging and high-efficacy photodynamic therapy in HER2-overexpressed breast cancer. Biomaterials. 2016;103:116–127. doi:10.1016/j.biomaterials.2016.06.037
20. Song N, Zhou B, Yan L, Huang J, Zhang Q. Understanding the Role of Yb3+ in the Nd/Yb Coupled 808-nm-responsive upconversion. Front Chem. 2018;6:673. doi:10.3389/fchem.2018.00673
21. Wang F, Liu X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev. 2009;38(4):976–989. doi:10.1039/b809132n
22. Wang M, Abbineni G, Clevenger A, Mao C, Xu S. Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomedicine. 2011;7(6):710–729. doi:10.1016/j.nano.2011.02.013
23. Wang F, Liu X. Upconversion multicolor fine-tuning: visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J Am Chem Soc. 2008;130(17):5642–5643. doi:10.1021/ja800868a
24. Qiu H, Tan M, Ohulchanskyy TY, Lovell JF, Chen G. Recent progress in upconversion photodynamic therapy. Nanomaterials. 2018;8(5):344. doi:10.3390/nano8050344
25. Bastos V, Oskoei P, Andresen E, et al. Stability, dissolution, and cytotoxicity of NaYF4-upconversion nanoparticles with different coatings. Sci Rep. 2022;12(1):3770. doi:10.1038/s41598-022-07630-5
26. Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32(26):6145–6154. doi:10.1016/j.biomaterials.2011.05.007
27. Park YI, Kim HM, Kim JH, et al. Theranostic probe based on lanthanide-doped nanoparticles for simultaneous in vivo dual-modal imaging and photodynamic therapy. Adv Mater. 2012;24(42):5755–5761. doi:10.1002/adma.201202433
28. Hu J, Shi J, Gao Y, et al. 808 nm Near-Infrared Light-Excited UCNPs@mSiO2-Ce6-GPC3 Nanocomposites for photodynamic therapy in liver cancer. Int J Nanomed. 2019;Volume 14:10009–10021. doi:10.2147/IJN.S221496
29. Akhtar N, Chen CL, Chattopadhyay S. PDT-active upconversion nanoheaters for targeted imaging guided combinatorial cancer phototherapies with low-power single NIR excitation. Biomater Adv. 2022;141:213117. doi:10.1016/j.bioadv.2022.213117
30. Li J, Zhang W, Luo X, et al. A comparison between mesoporous and nonporous polydopamine as nanoplatforms for synergistic chemo-photothermal therapy. Colloids Surf a Physicochem Eng Asp. 2022;653:130005. doi:10.1016/j.colsurfa.2022.130005
31. Han S, Beack S, Jeong S, et al. Hyaluronate modified upconversion nanoparticles for near infrared light-triggered on–off tattoo systems. RSC Adv. 2017;7(24):14805–14808. doi:10.1039/C6RA28600C
32. Das A, Corbella Bagot C, Rappeport E, Ba Tis T, Park W. Quantitative modeling and experimental verification of Förster resonant energy transfer in upconversion nanoparticle biosensors. J Appl Phys. 2021;130(2). doi:10.1063/5.0053464
33. He S, Krippes K, Ritz S, et al. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem Commun. 2015;51(2):431–434. doi:10.1039/C4CC07489K
34. Liu Z-Y, Tang X-Y, Huang C-Q, Zhang J, Huang W-Q, Ye Y. 808 nm NIR-triggered Camellia sapogein/curcumin-based antibacterial upconversion nanoparticles for synergistic photodynamic-chemical combined therapy. Inorg Chem Front. 2022;9(8):1836–1846. doi:10.1039/D1QI01569A
35. Gao M, Han Z, Zhang X, et al. Construction of double-shelled hollow Ag2S@polydopamine nanocomposites for fluorescence-guided, dual stimuli-responsive drug delivery and photothermal therapy. Nanomaterials. 2022;12(12):2068. doi:10.3390/nano12122068
36. Bednarkiewicz A, Chan EM, Prorok K. Enhancing FRET biosensing beyond 10 nm with photon avalanche nanoparticles. Nanoscale Adv. 2020;2(10):4863–4872. doi:10.1039/D0NA00404A
37. Cao T, Yang Y, Gao Y, Zhou J, Li Z, Li F. High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials. 2011;32(11):2959–2968. doi:10.1016/j.biomaterials.2010.12.050
38. Qin P, Meng Y, Yang Y, et al. Mesoporous polydopamine nanoparticles carrying peptide RL-QN15 show potential for skin wound therapy. J Nanobiotechnology. 2021;19(1):1–18. doi:10.1186/s12951-021-01051-8
39. Chen B, Xu Y, Agostinis P, De Witte PA. Synergistic effect of photodynamic therapy with hypericin in combination with hyperthermia on loss of clonogenicity of RIF-1 cells. Int J Oncol. 2001;18(6):1279–1285. doi:10.3892/ijo.18.6.1279
40. Xue Q, Wang X, Wang P, Zhang K, Liu Q. Role of p38MAPK in apoptosis and autophagy responses to photodynamic therapy with Chlorin e6. Photodiagnosis Photodyn Ther. 2015;12(1):84–91. doi:10.1016/j.pdpdt.2014.12.001
41. Yang W, Choi J, Choi SH, et al. A conjugate of chlorin e6 and cationic amphipathic peptoid: a dual antimicrobial and anticancer photodynamic therapy agent. Photochem Photobiol Sci. 2023;22(3):655–667. doi:10.1007/s43630-022-00343-8
42. Liu Y, Xu S, Lyu Q, Huang Y, Wang W. Ce6 nanoassemblies: molecular mechanism and strategies for combinational anticancer therapy: special collection: aggregation‐induced processes and functions. Aggregate. 2024;5(1):e443. doi:10.1002/agt2.443
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.