Back to Journals » International Journal of Nanomedicine » Volume 20
Synthetic Nanopillars for Stimulating Osteoblast Activity and Osteointegration in Bone-Related Disorders
Authors Liang W, Zhou C, Liu X, Xie Q, Xia L, Li Q, Lin H, Xiong X, Zhang H, Zheng Z, Zhao J
Received 27 October 2024
Accepted for publication 7 February 2025
Published 19 February 2025 Volume 2025:20 Pages 2205—2223
DOI https://doi.org/10.2147/IJN.S501963
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Dong Wang
Wenqing Liang,1,* Chao Zhou,2,* Xiankun Liu,1 Qiong Xie,3 Linying Xia,3 Qingping Li,3 Hongming Lin,1 Xiaochun Xiong,1 Hao Zhang,1 Zeping Zheng,1 Jiayi Zhao1
1Department of Orthopedics, Zhoushan Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Zhoushan, 316000, People’s Republic of China; 2Department of Orthopedics, Zhoushan Guanghua Hospital, Zhoushan, 316000, People’s Republic of China; 3Medical Research Center, Zhoushan Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Zhoushan, 316000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenqing Liang; Jiayi Zhao, Department of Orthopaedics, Zhoushan Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, 355 Xinqiao Road, Dinghai District, Zhoushan, 316000, Zhejiang Province, People’s Republic of China, Tel +86-580-2615027 ; +86-580-2615024, Fax +86-580-2616545, Email [email protected]; [email protected]
Abstract: Osteoporosis, osteoarthritis, and fractures are bone-related disorders that have a huge impact on the quality of life and healthcare systems worldwide. Traditional treatments, including bone grafts, have their limitations, with bone grafts often being rejected by the immune system and infected, making new treatments necessary. Nanopillars based on synthetic polymers have been demonstrated to be promising tools for bone regeneration and repair, showing to emulate the extracellular matrix composition, stimulate osteoblast activity and induce osteointegration. In this review, nanopillars fabrication techniques, such as electrospinning, nanoimprint lithography and self-assembly, also the state of the art of nanopillars technology are presented. Their role in modulating cellular responses via both physical and biochemical means, to enhance mineralization and to stabilize implants is also discussed. Additionally, their applications in treating bone-related disorders, eg promotion of fracture healing, augmentation of dental or orthopedic implants, and improvement of bone tissue engineering are discussed in the review. Using these focuses, each section examines opportunities and challenges (eg optimizing fabrication processes, improving biocompatibility, and investigating the integration of nanopillars with upcoming therapies like gene and stem cell therapy) for the potential of nanopillar technology. Finally, this review points out the requirement of scalable fabrication techniques, long term biocompatibility studies and multifunctional therapeutic strategies to fully employ the therapeutic applications of nanopillars in clinical scenarios. This review seeks to consolidate current knowledge of synthetic polymer based nanopillars and identify future directions for their use in bone related disorders through a comprehensive synthetic polymer nanopillar review.
Keywords: synthetic polymers, nanopillars, bone repair, osteoblast activity, osteointegration
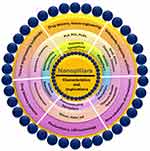
Graphical Abstract:

Introduction
Bone diseases and disorders are formidable healthcare challenges, particularly in aging populations where life expectancy is increasing. Osteoporosis, osteoarthritis and fractures compromise individual health and impose major healthcare system burdens globally.1 About millions around the world suffer from osteoporosis - reduced bone density and increased risk of fracture - and osteoarthritis, which results in degeneration and pain of the joints.2 Furthermore, traumatic injuries and cancers like osteosarcoma contribute to this complexity. Bone tissue has a limited intrinsic regenerative capacity, requiring medical intervention for meaningful treatment.3
 |
Table 1 Key Parameters of Nanopillars and Their Effects on Cellular Response |
 |
Table 2 Current Progress in Nanopillar Technology for Bone Regeneration |
 |
Table 3 Details of Key Studies Covered in the Review |
Traditional treatments for bone disorders almost exclusively involve the use of bone grafts, either autologous or synthetic.4 However, these methods are fraught with challenges including immune rejection, infection risk, and limited availability of donor tissues. Hence, the limitations of conventional graft materials underscore the critical need for new therapeutic strategies to promote bone regeneration and repair.5 Recent advances in the field of nanotechnology have offered new and promising alternatives to the standard grafting material. In particular, nanofibrous scaffolds have been developed as a revolutionary tool in bone tissue engineering.6 Moreover, a growth factor such as bone morphogenetic protein 2 (BMP-2) when encapsulated in nanoparticles, its bioactivity shall be preserved and the release shall be controlled, providing facilitation to bone regenerative processes.7 Moreover, variously charged PEGylated lipid-PLGA nanoparticles have been synthesized for the encapsulation of the genetic material such as GapmeRs (the antisense oligonucleotides that cleave mRNA molecules bound to specific sequences thus effectively silencing gene expression in one-shot mode in numerous physiological settings including non-proliferating cells); which, after internalization by mesenchymal stem cells, might be helpful in gene therapy of systematic metabolic diseases namely osteoporosis.8 There are various diseases can cause bone related issues such as Paget’s disease of bone is increasingly abnormal remodeling of bone, causing structurally weak and deformed forces that break fractures with minimal force.9 However, rarely but bone cancers are a grave issue. The primary bone tumors like osteosarcoma and Ewing’s sarcoma are common in young people at their tender ages of adolescence. Secondary bone cancers or metastatic bone cancer is defined as the development of cancer originating in some other organ or tissue and spreads to the bones. All these condition demands early diagnosis and intervention in a bid to enhance the quality of life of patients.10
A genetic disorder of known as Osteogenesis imperfecta that is caused by bones breaking easily being due to the defect of the collagen matrix.11 Diabetes increases fracture risk due to impairment of bone quality and microarchitecture, even with normal bone density, people with diabetes present with a higher proportion of fractures.12 Furthermore, nutritional deficiencies, especially in calcium and vitamin D are major risk factors for poor bone health and increased fracture rates.13 Moreover, chronic conditions that also appear to lead to an increased risk of fractures. One of the most notable is rheumatoid arthritis that causes chronic inflammation and bone loss due to corticosteroid use.14
Hence, autologous and synthetic bone graft materials have been used to treat bone diseases, although mixed with therapeutic molecules (drugs or proteins). Nonetheless, these efforts are intended to enhance clinic outcomes, and issues related to vector-borne infection, restricted access, replicability, limited number of donors, and immune rejection have emerged.7 These complications have stimulated the creation of nanomaterial carriers of therapeutic molecules, known as nanopillars, to cure diseased and damaged bone. Nanotechnology-based drug delivery strategies might revolutionize the care of bone-related diseases and dysfunctions including osteoarthritis, osteoporotic nonunion defects, bone cancers (both metastatic and primary) and myeloma associated bone diseases. Many nanotherapeutic strategies are able to alter or anti-targeting of endogenous osteoblasts and osteoclasts, and manage the periosteum of malignant and avascular bone tissue, through moderating the mobility, multiplication, and differentiation of cells that can support and enhance the regeneration procedure.15
These conditions can therefore entail disturbances of osteoblast activity and osteointegration, the two factors that are paramount to healthy bone mechanics. Osteoblasts are concerning the type of cells that are involved in the process of new bone formation. They are responsible for the formation and secretion of the bone matrix that is composed of collagen and other proteins and also, start the process of mineralization. Their activity is under the control of hormones, growth factors, and mechanical loads being directly involved with processes of bone maintenance, repair, and remodeling.16 Osteointegration is the process by which an implant or a graft fuses with the surrounding bone and achieves structural and functional integration. The process of osteointegration helps the implant to be stabilized on the bone and used for its intended purpose without the chance of failure.17 The osteogenic tissues of bone are very efficient in remodeling and repairing damaged bone tissues. Thus, in case of defect sizes above 5 cm various non-healing conditions (non-union fractures, tumor ablations, maxillofacial injuries, or degeneration), bones cannot repair and regenerate themselves and reconstructive and cellular methods are needed.18 Nanopillars capable of stimulating osteoblast activity and improving osteointegration of synthetic polymer-based material have also been evidenced in bone diseases. Research has shown that the functionalization of a surface with polymers including polylysine-modified polyethyleneimine (PEI-PLL)19 and plasma polymerized allylamine (PPAAm)20 enhance osteoblast adhesion, cell spreading, and proliferation activities. Further, the surface-modified polymeric micelles of the diblock copolymer for the delivery of osteogenic agents such as Naringin exhibit better osteogenic differentiation of stem cells.15 Developments in nanotechnology present a possible solution for inducing bone formation and treating musculoskeletal diseases through extended delivery of bioactive molecules to support the osteoblast and enhance the bonding between the material and bone tissue. This review aims to present the recent advancements and applications of synthetic polymer-based nanopillars for bone disease treatment, with their further applications in targeted drug delivery and tissue regeneration.
Overview of Nanopillars
Nanopillars are slender pillar-like structures and are in the nanoscale category with heights generally from a few nanometers to micrometers (Table 1). Owing to their small sizes and high surface area to volume ratio, nanopillars possess special characteristics like increased mechanical strength, better surface activity, and better reactivity than the basic building materials.21 Their surface chemistry and roughness can be predetermined and manipulated for use in the fields of material science, biology, and engineering due to fabrication methods.22 Thus, due to the flexibility in selecting the size, geometry, and material of nanopillars, the technology offers the prospects for creating new applications, including the improvement of cell adhesion for bone tissue formation,23 as illustrated in Figure 1.
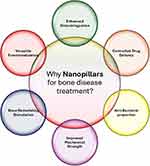 |
Figure 1 Schematically illustrate the unique benefits of nanopillars to bone disease therapy. |
Characteristics of Types of Nanopillars
Metallic nanopillars based on noble metals including gold, silver, platinum, and titanium are used in sensing, catalysis, electronics, and plasmonic.24 These metallic nanopillars can be integrated more effectively with bone tissue, improve cellular responses, and hasten the healing process into scaffolds or implants, making them useful instruments in orthopedic and regenerative medicine.25 Semiconducting nanopillars are bio-inspired structures that are made from materials such as silicon, GaAs, and InP and are used in solar photovoltaic systems, LED bulb transistors, and other electronic and photonic devices based on their electronic and optical properties that uniquely improve the performance of these devices.26 These semiconducting characteristics can further promote bone tissue regeneration and healing by inducing cellular responses via optical or electrical impulses. By increasing the efficacy of bone regeneration treatments, this novel strategy promises to open up new avenues for orthopedic and reconstructive surgery.27 Polymeric nanopillars; LA, PEG, and PS nanopillars are used in Drug delivery, Tissue engineering, and biosensing. They are preferred for their compatibility with living organisms, mechanical versatility, and ability to be modified for specific uses.28 Titanium dioxide, zinc oxide, and alumina ceramic nanopillars are used in photocatalysis, sensors, and as bone substitutes due to their attributes such as hardness, heat resistance, and chemical stability. Requoted solid nanopillars are used in rebar structures, photonic devices, and large-scale nanotechnology investments, for their strength and stiffness.29 These properties make them highly beneficial for applications involving bone regeneration, chemical reaction catalysis, and environmental change detection.30 Medication delivery, vehicle parts, cartridges, and structural uses comprise hollow nanopillars which provide lightweight characteristics as well as possessing increased surface area and the ability to load material.31
Nanopillars with predominantly open structures for filtering, catalysis, and tissue engineering are characterized by greater contact area, increased diffusion rates, and molecular accommodation. Nanopillars, especially those made from noble metals such as gold and silver, hold enormous importance in application areas like SERS, and biosensing, highly regarded for their chemical stability, hardness, and resistance to heat qualities that are essential for developing successful bone substitutes.32 Metals, metal oxides, and composites of catalytic nanopillars are versatile in catalysis, environmental applications, and energy conversion. These are appreciated to have a high surface area that improves the catalytic properties as well as the stability at reaction conditions.33 Last, biocompatible Nano topographical structures such as nanopillars from polymers, ceramics, and bio-glass are used in tissue engineering scaffolds, bone replacement, and drug delivery with biocompatibility and cell growth and differentiation ability.34
Significance of Nanopillars to Bone Health
Nanopillars can enhance the biological issue of the implant through acceptance by the bone tissue. Thus, replicating the natural matrix of bone, nanopillars promote the adhesion and proliferation of osteoblasts and, in this way, increase the stability and integration of implants.35 It was established that the physical characteristics of nanopillars can control the activity of cells. For instance, nanopillars enhance the osteogenesis process by activating osteoblasts and their further differentiation occurs. It has thus been made possible to control the height and width of nanopillars that enhance the development and performance of bone-forming cells.36 Some nanopillar surfaces are developed for use with antimicrobial characteristics to minimize the chances of reinfection in the surgery areas. This is done in two ways; by adding antimicrobial agents, or by employing the surface treatment techniques that prevent bacteria from sticking to the surface.37 Some of the changes that are possible at the nano level can be used to improve the mechanical characteristics of bone scaffolds through the use of nanopillars. Due to better contact and mechanical interlocking.38 Due to their size, nanopillars can be designed to release drug compounds to the targeted bone tissue. This make it possible to treat bone diseases and to speed up the healing process by releasing drugs, growth factors, or other bioactive substances in a controlled way.39 Recent Progress in Nanopillar Technology for Bone Regeneration is presented in Table 2.
Synthetic Polymers-Based Nanopillars
Synthetic polymers based nanopillars are nanoscale structures that have a form of pillar and are made from synthetic polymers. The examples of synthetic polymers include PCL, PLA, and PLGA because of their biocompatibility, tunable physical characteristics, and cell attachment and growth promoting features. These nanopillars can be fabricated using electrospinning, nanoimprint lithography and self-assembly and the structural parameters such as height, diameter, spacing and surface topography can be easily tailored.18
Types, Uses and Characteristics of Synthetic Polymers
Functional groups are active sites in all polymers and the type and number of functional groups in a polymer can be altered to get polymers with desired structures and properties. This allows for the desired and repeatable properties for specific uses and can be adjusted to do so as well. For example, the degradation rate of these polymers is dependent on its composition, degree of crystallinity and molecular weight. However, unlike the natural polymers synthetic polymers have relatively less bioactivity, cell recognizable sites and osteoconductivity.40 In this regard, several coatings applied on the surface of the scaffold including bioceramic particles, have been evaluated to improve the surface performance for bone tissue engineering. In synthetic polymers, aliphatic polyesters for example Poly(ε-caprolactone) (PCL), polylactide (PDLA, PLLA), Poly(lactide-co-glycolide) (PLGA) are preferable,40 some of which are illustrated in Figure 2.
Polycaprolactone (PCL)
Polycaprolactone (PCL) is member of the polyester family that is semi crystalline and biodegradable and has a degradation period ranging from three to four years thus appropriate for load bearing applications.41 The production of PCL/Hydroxyapatite enhances bioactivity, cell activity, and mineralization.42 For example, PCL/alginate scaffold composites have reported the improvement in the biological properties such as higher levels of osteogenic differentiation and calcium content.43 Likewise, the study also presents a bioactive baghdadite reinforcement in PCL nanocomposites that has improved mechanical characteristics, cell compatibility and bioactivity that can be used in bone repair. PCL based composites such as PCL/alginate and PCL/hydroxyapatite have higher degree of bioactivity in medical applications including improved cell adhesion, cell infiltration, osteogenic differentiation as well as mineralization.44
Polylactic Acid (PLA)
Polylactic Acid (PLA) is described for its cytocompatibility, thermal stability as well as non-toxic degradation products. The properties of the PLA can be altered by the isomers, the ratio as well as molecular weights. Physical – chemical properties of PLA have been enhanced through the addition of hydroxyapatite by.45 For instance, porous PLA/hydroxyapatite constructs fabricated by 3D bioprinting provide improvement on cell growth and osteo differentiation.46
Poly (Lactic-Co-Glycolic Acid)
Poly (lactic-co-glycolic acid) (PLGA), PLGA has a controllable degradation rate, the resulting scaffold has poor mechanical properties and osteoconductivity; thus, requiring the incorporation of materials such as hydroxyapatite. Leaching of particulates and formation of gases have been applied to enhance porosity and mechanical properties of PLGA-base scaffolds.47 Also, there have been improvements among PLGA scaffolds regarding its bioactivity and apposition to bone tissues by surface treatments such as oxygen plasma treatment and peptide immobilization.48
Biocompatibility and Biodegradability Considerations
There are extensive studies on biomedical applications of synthetic polymers in the field of bone regeneration and tissue engineering because of their properties and advantages. The manufacture of synthetic polymers is flexible concerning design since they allow for the exact form control in chemical composition, molecular weight and structure which makes it possible to produce polymers with specific properties and characteristics for certain uses.40
In biomedical application, aspects such as biocompatibility and biodegradation effects are critical in the construction and fabrication of Nanopillars. It is important to understand how graphene-based materials with cells, tissues, and organs in the biological system for proper application use in tissue engineering, biosensors, and drug delivery.49 Studies have demonstrated applications of biocompatible nanomaterials in numerous biomedical uses, but there are issues regarding the stability, efficacy or toxicity; therefore, establishing the stability and effectiveness of nanomaterials is important in relation to the nanopillars.50 Moreover, suitability for recycling and biodegradability of biobased nanomaterials must be taken into consideration in order to decrease the utilization’s impact on the environment and increase the usage of renewable resources, as well as developing materials that can decompose into non-hazardous compounds.51
Incorporation of biocompatibility and biodegradable materials in designing nanopillars goes a long way in making the nanopillars safe in their use and effective in biomedical application while at the same time encouraging environmentally sound practices.51 Furthermore, it is known that synthetic polymers can include prospective coating characteristics for promoting cell behavior adhesion or bacterium resistance and, usually, it shows less immunogenicity compared to that of natural polymers.40 Synthetic polymers must be biocompatible and the parameters that affect biocompatibility includes; material chemical nature, which should be no cytotoxic and not elicit an inflammatory response.41 There can be used coatings or functionalization of the surface to raise the biocompatibility due to better adhesion of cells and anti-inflammatory effects. In addition, the chosen sterilization methods should have no ill effects on the polymer or generate any unhealthy byproducts.40
Factors Affecting Polymer Scaffold-Based Nanopillars Bone Regeneration
Many prior studies for osteoarthritis research were centered on controlling the differential of osteoblasts while not paying attention to the immune response however, traditional research on bone regeneration was focused on controlling the process through growth factors, cytokines, and hormones the recent studies have first tried to control the roles of mesenchymal stem cells (MSCs), neutrophils, and macrophages during the process of bone regeneration.52 Key factors to consider when developing polymer scaffolds based nanopillars to promote osteogenesis while mitigating the inflammatory response include:
Scaffold Stiffness: Mechanical properties and particularly the stiffness of polymeric bone regeneration scaffolds is of significant importance as they directly affect the cell proliferation and migration, differentiation, changes in cell contractility and affect the fate of osteoprogenitor cells.53 Scaffold rigidity can also influence the rest of the host’s inflammatory reaction. Since the polarity of macrophages comes with a function, the stiffness of the scaffold can control the macrophage phenotype with respect to cytokine secretion, spread and cytoskeleton to either initiate inflammation or tissue repair. Friedemann et al identified that human macrophages differentiated to a non-inflammatory M2 phenotype when cultured on 3D collagen/GAGs scaffolds with different stiffness and reduced inflammatory cytokines like the IL-12 and TNF-α and the M2 macrophages improved the anti-inflammatory cytokines like the IL-10.54
Surface Roughness: The M2 macrophage phenotype is usually associated with rough surface as is the neutrophil adhesion to surfaces. From this, it can be inferred that the nano- and microstructures from the typical quantitative characteristics of TiO2 nanopillar surface include the RMS roughness that was found to range from 50 nm to 70 nm.55,56 TiO2 nanotubes (TNTs) are well known for their osteoinductive effects and bone formation by modulating osteogenic factor such as lncRNA RMRP and DLEU2 that cause changes in epigenetic regulation.48
Porosity and Pore Size: Pore size and porosity of the scaffold plays a crucial role in osteogenesis. For tissue formation, the mean pore size and porosity of scaffolds may slightly differ in certain applications depending on what is required. Scaffolds with pore size of 340–688 μm and 44–58% porosity or 75% to 45% are beneficial in bone cell adhesion and bone tissue engineering.57,58 The healing ability of the bone tissue has also been found to be improved by larger pore sizes in polymer scaffolds since the immune response will be minimized in such circumstances. Also, reflecting on pore size and porosity, oxygen availability within the scaffold is balanced while; low oxygen concentration is constructive for inflammation at the implantation site.59 Bone marrow derived macrophages exhibited greater M2 phenotype by the stimulation with PPy-PDO nanofibers with larger pore size due to the increased expression of M2 markers and reduced expression of M1 markers.60
Surface Charges: The polymer scaffolds surface charge directly dictates the nature of protein interaction which the host immune response will encounter. The study on macrophage activation also manifests that the hydrophilic anionic or neutral surfaces of the scaffold cause the production of IL-8, IL-6, IL-1β, and TNF-α that result in classical macrophage activation. On the other hand, hydrophilic cationic surfaces favour the other subtype of macrophage activation.61
Fabrication Techniques to Create Polymer-Based Nanopillars
Synthetic polymer-based nanopillars can be fabricated using various techniques, including electrospinning, nanoimprint lithography, and self-assembly. These methods allow precise control over the size, shape, and density of nanopillars, which are crucial for optimizing their interaction with osteoblasts and bone tissue.
Fabrication Methods
Electrospinning is an efficient and cost-effective technique for fabricating polymer-based nanofibers and nanopillars. In this process, the polymer solution or melt is subjected to a high-voltage electric field, and a thin jet is expelled which on reaching a grounded collector forms nanofiber or nanopillars. Some benefits of the electrospinning technique include a high production rate, ease to implementation, and the formation of structures that are continuous in nature. However, it has some limitations including, the techniques cannot maintain the exact diameter and the alignment of the nanopillars.62 Template-Assisted Method uses a ready-made template with nanopores or nanochannels through which the polymer is filled. Spin coating, dip coating or melt infiltration can be employed to deposit the polymer onto the substrate surface to create a thin film; the template subsequently can be removed in order to obtain the nanopillars. It provides better control of the size, shape and organization of the nanopillars at the benefit of the use of a template and involves extra procedures in the removal of the template.36 Methods of printing like electron beam lithography and nanoimprint lithography assist in creating nanopillar arrays with improved accuracy and uniformity of nanopillars. Electron-beam lithography enables one to write patterns of an electron beam onto a resist layer on a substrate and then etch. It offers high resolution and accuracy; however, it comes with high costs and can only handle a few samples at a time Although it can offer great resolution and accuracy it turns out to be very costly and has low throughput rates.63 Nanoimprint lithography, on the other hand, contains applying the patterned mold to a polymer-coated substrate followed by heating or UV radiation to solidify the polymer. This technique is fast and cheaper, and may be used in the production of similar items consecutively by just reproducing the last scan; nevertheless, this technique is only amenable to specific materials such as UV curable resin and thermoplastics to mention but a few.64 Self-Assembly is the process whereby the molecules self-assembling themselves in form of orderly nanopillars. Self-assembling materials such as block copolymers, and other similar materials can make well-ordered nanostructures by treatment with heat or solvent. The benefits of self-assembly are simplicity and scalability, but there are several downsides such as; the application of self-assembly does not offer enhancement of size and geometry of the nanopillars to the precise level as desired.65,66 Surface modification is another way of improving the properties of nanopillar through the following processes. Chemical functionalization is done to add other functional groups onto the nanopillars to enhance aspects like surface wettability, biocompatibility, and other receptor sites for biomolecule attachment. Some of the methods involved in chemical functionalization include plasma treatment, silanization, and polymer grafting which is used in cell adhesion, proliferation, and differentiation in tissue engineering.67 Surface modification by using bioactive molecules includes the applications of proteins, peptides, or growth factors on the localized area of the nanopillar to encourage certain biological activities. These are physical adsorption, covalent bonding, and layer-by-layer assembly among others and its uses include increased osteoconductivity, cell recognition, and bioactivity for bone tissue engineering.68
Fabrication Techniques for Plasmonic Nanopillars
Etching based on semiconductor fabrication processes includes dry etching and masking strategies such as electron beam lithography and microsphere lithography to fabricate Si, SiN, SiO2, and sapphire nanopillar arrays. The advantages of this technique include high resolution, repeatability in use, and the ability to work with difficult materials; disadvantages include restrictions in the aspect ratio and the dependence on the gases used for etching.69 Nanoimprinting replicates patterns from a mold directly to a polymer layer on the substrate with well-defined arrays of nanopillars, and the technique is highly reproducible.70 It costs comparatively less money, and one can process many parts at the same time but is limited by a choice of materials to be used and also the condition of the mold utilized. Techniques such as hydrothermal synthesis are used to synthesize nanopillar structures by growing nanoparticles on a substrate, where the advantage of nanopillar growth is the low production and high aspect ratio structures of the nanopillars, however, the technique is suffering from the disadvantage of low reproducibility and uniformity.71 Metal capping for plasmonic enhancement by coating multifunctional dielectric nanopillars with metallic layers to improve plasmonic properties to increase resolution and working distance for example in the sensing process. Examples of techniques for metal capping are thermal evaporation, electron beam evaporation, and sputtering mainly improve the optical characteristics and the sensitivity of sensors but these techniques add extra steps in the fabrication process and also the possibility of nonhomogeneous deposition of the metal.72 Thus, the fabrication techniques described for the creation of polymer-based nanopillars and the range of surface modification approaches allow for the optimization of the nanopillar properties for different applications within tissue engineering, sensing, and photonics to acquire the desired structural accuracy, functional capabilities, and boosted performance.
Mechanisms of Osteoblast Stimulation by Nanopillars
Osteoblasts are involved in bone deposition and also in the bone remodeling process. Recent development in nanotechnology has illustrated that the topographical characteristics at the nanoscale for instance nanopillars are capable of modulating osteoblast activities.
Physical Mechanisms
The nanopillars give a tip height effect to the cells and guide cell behavior as they would be guided by the natural extracellular matrix.73 Different investigations have presented that geometric feature of nanopillars, including height, diameter, and space tremendously determine the behavior of various cell types embracing osteoblasts. Despite the question pointing at a height of about 100nm and a distance of 200nm as the most suitable for encouraging osteoblast adhesion and proliferation.19 The opportunity of direct contact between osteoblasts and the nanopillars result into mechano-transduction for the transformation of mechanical stimuli to bio-signals. This involves the activation of integrins which are membrane proteins that provide link between the extracellular matrix and the cytoskeleton. Thus, integrins, while bound to nanopillars, create clusters that activate schemes of intracellular signaling including the FAK pathway and help to reorganize the cytoskeleton and change the gene expression to declare differentiation into osteoblasts.74
Biochemical Mechanisms
It means that the structural properties of nanopillars on the nanoscale level can affect the process of proteins adsorption from the extracellular milieu. It is the phenomenon of selective protein adsorption that helps in increasing the bioactivity of the surface and allows the osteoblast to bind to it. Taking into account that fibronectin and vitronectin act as cell-adherent proteins and play a pivotal role in advertising osteoblast function these proteins residue on the surface of the material as nanopillars and augment osteoblast engagement.75 These nanopillars have also been proved to enhance the osteogenic gene expression. For instance, the genes containing the alkaline phosphatase (ALP), osteocalcin (OCN), and runt-related transcription factor 2 (RUNX2) these are vital in osteoblasts, show a higher expression when the cells are cultivated on the nanopillared surface than the flat surface. This genetic upregulation is essential in osteoblast differentiation and mineralization event.75
Enhanced Mineralization
Nanopillars are sieved to improve not only cell adhesion and proliferation but also promote mineralization. The culture of osteoblasts on the materials with nanopillared surfaces promotes the increase of the amount of deposited calcium, which reflects the degree of bone formation. The deposition of those additional minerals can be due to physical structure of the surface and or biochemical signals of the nanopillars.23 Independent in-vitro studies have demonstrated that such nanostructures as nanopillars help bestow the cell with genes that assist in the creation of a bone like matrix. A well-organized pattern of nanopillars gives a template for the nucleation and subsequent growth of hydroxyapatite, which is the main mineral phase of the bone. This is important for fabrication of implants that are used in orthopaedic surgeries, and in the creation of scaffolds required for bone tissue engineering.39
Osteointegration Enhancement
On this aspect, the success of bone implants depends on the osteointegration process since it involves the development of a direct union between the implant and the neighboring bone matter. This process is critical in the stability and functionality of the implant. Recent progress in surface treatment such as the application of nanopillar has indicated that it can augment the degree of osteointegration. This article reflects the application of the osteointegration process in bone implants, the function of nanopillars in increase this process, and a comparison with other surface modifications.22
Importance of Osteointegration in Bone Implants
Osteointegration is a long-term procedure of bone implant that is used in dental implants, orthopedic devices and spinal implant. It ensures sufficient osteointegration offers bone-implant anchorage to bear load without hazarding implant instability; a critical facet for loaded interfaces. Osteo integration helps in the off-loading of the mechanical loads, avoid implant failure and implant’s long-term stability.76
Role of Nanopillars in Enhancing Osteointegration
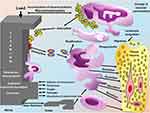
Nanopillars have emerged as a promising surface modification technique to enhance osteointegration. Their role includes: Nanopillars also imitate the ECM offering better adhesion and proliferation of the osteoblasts. This improved cell response is vital in the creation of a strong bond between the implant and the bone.73 Future aspects of nanopillars can mimic the topography of the existing pillars, and the physical features of the nanopillars themselves can lead to osteogenic differentiation due to integrin signaling pathway. This results in the activation of genes associated with osteogenic differentiation, including that of RUNX2, ALP and OCN genes which are requisite in bone development.74 That is why nanopillared surfaces allow the formation of stronger mineralization, which is the basis of osteointegration. Cells seeded on nanopillared surfaces exhibit higher levels of calcium deposition and the creation of bone like matrix which is beneficial in fixing an implant,39 as illustrated in Figure 3.
The healing process of an implant system begins with blood forming a clot between the implant and bone. This clot is then transformed by phagocytic cells, peaking in activity between the 1st and 3rd days. Next, dense connective tissue forms a procallus, with mesenchymal cells differentiating into osteoblasts and fibroblasts, leading to callus formation and new bone development. As the bone matures, remodeling is stimulated by occlusal stresses, leading to the formation of cortical bone along the implant surface. Once osseointegration is achieved, the implant is surrounded by cortical and spongy bone, and the cortical bone interface develops canaliculi, collagen bundles, and a glycoprotein layer, with Haversian bone organizing into osteons.77
In vivo and in vitro Studies on Osteointegration
In Vitro Studies: It holds a crucial position in the enhancement of knowledge regarding the implant biomaterials and their properties in contact with the bone tissue. New approaches have been suggested to mimic the osteointegration process by using human bone tissue and different types of implants such as titanium and copper pin.78 Changes to titanium implants like nanotubular surfaces and hydrogenation have been established to have higher Osteogenesis/ Osseo integration properties in both Vitro and in Vivo, highlighting the need to enhance implant surfaces to facilitate better bone integration.79 These advancements in in vitro models do not only contribute useful data to the understanding of osteointegration but also encourage further studies and development of effective implant designs that will have better chances of integrating with the osseous tissue, thereby increasing the efficiency and durability of orthopedic implants.78 Various scientific papers proved that when the nanostructures are in the form of nanopillars then it has a favorable impact on the activation of osteoblasts.80 Cells cultivated on nanopillared surfaces display increased osteoblast-like cell adhesion, proliferation, and differentiation in comparison to cells grown on plain surfaces. Research has revealed that surface enhancements such as fabrication of nano/micro hierarchical structures through green etchants techniques such as sandblasting combined with acid etching, enhance osteoblast adhesion, growth and differentiation.81,82 Furthermore, the osteoblastic cells on different nanopatterned surfaces’ 3D nanomorphology has been studied in details and it was found that the surface topography plays a crucial role in the function of the cells.83
In Vivo Studies: Highly porous 3D-printed titanium implants were used in this study by Bondarenko et al, where the authors compared the osseointegration of plasma-coated and highly porous 3D-printed titanium implants in rat femurs and the authors indicated that the implantation of 3D-printed titanium Femur implants had enhanced osseointegration.84 Almeida et al examined nanostructured hydroxyapatite coated implants in sheep and it was observed that the coated implants produced new bone formation more effectively than that of the acid-etched implant surfaces within 28 days.85 Furthermore, Nan et al discussed the utilization of the dual-peptide functional coatings for the implants, and revealed that the antioxidant characteristics, the cell attachment, and osteogenic differentiation were then optimized for the osteointegration.86 For illustration, research with the aid of rodents also highlighted that the use of implants with nanopillared surfaces was more favorable to the rodents’ bone growth in implant stability than smooth implants. From these studies, it can be concluded that the use of nanopillars can improve the integration of implant in a living being.23
Comparison with Other Surface Modifications
While other surface features such as microtextured surfaces have been attempted for improving osteointegration, nanopillars take it further and provide a scale of topographical features that are closer to the nanoscale ECM. Generally, optimized nanopillars have exhibited better performance in the enhancement of osteoblast attachment and differentiation as opposed to microtexturing.87 Other surface treatments have been employed to enhance osteointegration and include; hydroxyapatite or titanium dioxide. Even though these coatings are able to improve bioactivity on the surface of a material, nanopillars supply complementary advantages by being able to alter cell response favorably through topographical signals.88 This combined technique may have better osteo integrate as compared to coatings alone.87 Another factor might be chemical modification like the coupling of osteogenic growth factors that improve osteointegration. These methods, however, are time-consuming and could prove to be expensive in the long run since they presuppose a number of procedures. Thus, nanopillars can be seen as being more practical and less expensive than the other topographies because they strictly rely on physical topography for cell behavior manipulation.39
New Trends in Orthopaedic Biomaterials
Thus, the creation of new stimulus-responsive biomaterials with readily alterable properties is highly attractive for the subject of tissue engineering and orthopaedic implantation. Typically used biomaterials still have a disadvantage because it is impossible to control their biochemical and biophysical properties, which complicates their use in medicine. New generation ones are the bio-derived materials; porous structures; smart ones and 3-D implant ones.89
New material developments have been achieved by co-polymers of synthetic polymers and natural polymers; high biocompatibility, for example, silk, elastin, chitosan, collagen, and keratin with great mechanical characters such as polyethylene, polyester epoxy, and teflon, these biomaterials have simulated the living tissue for tissue engineering, cell-based transplantation and gene therapy In addition, intelligent nanomaterials such as nanoporous High surface loading because of a large surface area is possible; the nano-confined volumes control the protein dissolution rates. They enable easiness in the connection of bioactive molecules to implants where oxyilanes and phosphonates that act as intermediate linkers may not be required. Nanopores improve protein adsorption and promote cell adhesion so they can be utilized as bioactive coatings for different biomaterials. It has been demonstrated that various forms of porous metals are appropriate as orthopaedic structures, and are used as replacements for damaged bones due to similarities in nature to that of the bones.90
Applications in Bone-Related Disorders
Osteoporosis and Bone Fractures
New accomplishments have been achieved regarding the use of synthetic polymer-based nanopillars for the application in bone regeneration where osteoporosis and bone fractures are prevalent. Another type of nanostructure on the scaffolds and implants that proved to improve osteoblast functionality is the nanopillar. Increased proliferation and activity of osteoporosis also enables quicker and more effective repair of bone fractures and could help optimize the treatment of osteoporosis, thus positively impacting patient’s outcomes. Therefore, new cross-functional nanopillar technology allows creating biomimetic surfaces that better adhere to the newly formed bone tissue, thus improving the overall outcome of treatment.91
Dental Implants and Orthopedic Devices
Nano-pillars have also been used in dental implants and orthopedic devices and that nano-polymer based pillar structures enhanced implant load bearing capability and functioning. The principle of using nanopillars works well in improving the implant to bone contact that is fundamental in preventing implant failure. In this regard, nanopillars create a better, more stability-forming and integrating surface which essentially contributes to the long-time effectiveness of both dental and orthopedic implants. Health improvement of such implant systems safeguards remedy connected troubles with sturdiness and steadiness of the implant products, and thereby, refines the implant integration and therapy plan.92
Bone Tissue Engineering
The application of synthetic polymer based nanopillars in scaffold has been enhanced to great impact on the engineering of scaffold to support bone tissue regeneration. New bone growth is imprisoned on nanopillars which offer a favorable and appropriate setting for the cells that shape towards the formation of the natural extracellular matrix. Therefore, this study of nanopillar technology with synthetic polymers as a scaffold offers new possibilities concerning the treatment of advanced bone pathologies and the further enhancement of tissue engineering approaches. The integration of these technologies provides for the creation of optimally functional and tunable scaffolds, which creates the potential to progress the treatment in bone tissue engineering. These developments shall potentially provide better treatment to a number of bone related ailments with increased accuracy.93,94 A brief summary of all the studies covered in this review is presented in Table 3.
Future Directions
Advanced Fabrication Techniques
For further improvement of clinical applicability of synthetic polymer-based nanopillars, subsequent studies should focus on the techniques of fabrication that are affordable and favorable for mass production. Some of the commonly used fabrication techniques have definite drawbacks in terms of repeatability and resolution, especially when translating to large scale for clinical applications. New fabrication technologies which include additive manufacturing, micro manufacturing and advanced polymerization techniques, but a few would improve the potential to essentially fabricate and integrate these nanopillars with uniform characteristics on an industrial scale. Further research should be devoted to the improvement of these techniques to provide high yield and quality at the same time. Higher fabrication efficiency will ensure the use of nanopillar technology in clinical practice since it may help change the management of diseases affecting the bone in the future.93,94
Long-Term Biocompatibility Studies
Knowledge of the chronic biocompatibility of synthetic polymer-based nanopillars is highly important for the application of nanopillars in clinical practice. Only the short-term in vitro and in vivo studies are performed to show some positive effect; however, to determine the long-term effect of these materials on the surrounding tissues they have to perform long-term in vivo studies. Safety assessment research should assess harmlessness in terms of inflammation, cell toxicity, and immunogenicity, among other effects, for longer periods. These studies shall be very useful in establishing the safety and viability of nanopillars within the human body and adverse effects of the same on the patients shall be looked into to avoid their occurrence. More such research shall be crucial when seeking to get the required permissions from the regulatory authorities besides easing the nanopillar technology transition from preclinical stages to clinical use.95
Integration with Other Therapies
Another line of further research is based on the concept of interaction between synthetic polymer-based nanopillars and other types of therapies. Combining or incorporating the nanopillars with gene therapy, stem cell therapy, or growth factor delivery might improve these nanostructures’ efficacy in addressing bone pathologies. For example, nanopillars might be filled with genes or proteins promoting bone tissue regeneration or changing cellular microenvironment to promote healing. Further studies should be conducted on the synergy of the combined treatment regimen to take advantage of each part’s capacity to enhance the others. Knowing how nanopillars could be integrated with other techniques, leading to better results of the patients, would be a key factor in the formulation of more rounded solutions.96
Conclusion
Nanopillars have started to be explored as a transformative approach in regenerative medicine for the treatment of bone-related disorders. This review has emphasized that synthetic polymer-based nanopillars can enhance osteointegration, drive osteoblast proliferation, and deliver drugs specifically to a localized area. Tackling the limitations of traditional bone grafts that suffer from immune rejection, widespread infection, and other shortcomings, these innovations are especially important. While these are important advances, there are some gaps in the field. Currently, research in this area has been largely limited to only a few polymeric materials; an exploration of different materials, such as metallic and ceramic nanopillars, is also needed. Moreover, fabrication techniques have progressed, still requiring more scalable methods to achieve reproducibility and retain the integrity of functional nanopillars. Long-term biocompatibility studies are also necessary to fully understand the interactions of these materials with the biological system following longer periods of contact. Future research should look ahead at the development of multifunctional systems of nanopillars which can include a combination of therapeutic agents with some cellular responses, that possess the potential to induce bone regeneration. Further augmenting their clinical utility is possible by investigating combinations of nanopillars with emerging therapies such as gene therapy and stem cell treatments. Additionally, it is expected wider scope of materials used for the fabrication of nanopillars will result in an improvement of their mechanical properties and a favorable interaction with bone tissue.
Summary
Nanopillars that are in synthetic polymers also reveal itself as a viable solution to bone-related diseases like osteoporosis and osteoarthritis. Though these disorders are common in the elderly, they are usually a problem for traditional procedures like bone grafts because of the possibility of infection and allergy to the body’s immune system. Nanotechnology as an approach to the development of pharmaceuticals and regenerative medicine: nanopillars. Current fabrication techniques are used to create nanopillar like synthetic polymers including polycaprolactone (PCL), polylactic acid (PLA) and poly (lactic-co-glycolic acid) (PLGA). Shaped structures that enhance bone regeneration by sustaining the extracellular matrix and stimulating osteoblast. It promotes cell attachment and spreading, cells growth and differentiation, as well as effects on osteogenic gene expression. This results in the development of strong, dense bone structures that can thus effectively interact with implant structures. Some of the methods that are used to fabricate nanopillars include electrospinning, which enables very high accuracy and nanoimprint lithography which is inexpensive and mass producible. Self-assembly has advantages from the point of view of simplicity but such structures cannot be controlled with high accuracy. These are made more effective with additional approaches like chemical modification on the surface and deposition of biochemical coatings. It is evident that nanopillars affect osteoblasts both mechanically and chemically. In a physical manner they give signals that resemble the extracellular matrix on which cells can attach and proliferate. Osteocytes residing in these structures come into contact with osteoblasts and signals through integrin that adapts the mechanical signals to biochemical counterparts. On a biochemical level, nanopillars alter protein adsorption; the available space rises, and osteoblast adhesion is aided. They also increase the extent of osteogenic genes responsible for cell differentiation as well as mineralization. Higher amounts of calcium and formation of a hare like bone structure add to the concept of using nanopillars in tissue engineering. Therefore, osteointegration, which is the connection of the implants with the bone, is important in differential of various medical devices. However, some limitations are there, such as high production costs and the long-time biocompatibility testing needed to overcome. For future studies, fabrication methods for nanopillars have to be optimized for uniform and reproducible large-scale production; biocompatibility issues should be investigated over the long term and attempts should be made to develop multifunctional therapeutic strategies potentially in conjunction with gene or stem cell therapy. Such molecular progresses, thus, are essential for escalating nanopillar development from invasive research to actual practice, improving the methods of bones healing and treatment of bone-originated diseases.
Data Sharing Statement
Not Applicable. This is a review article, and all relevant information is provided in the article.
Ethical Approval and Consent to Participate
Not Applicable. This is a review paper and do not involve direct research on humans or animals.
Consent for Publication
“Not applicable” as this manuscript does not contain data from any individual person.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All the authors listed meet the criteria for authorship as per the ICMJE guidelines, read the final manuscript and agree to publish this work.
Funding
This work was supported by Public Technology Applied Research Projects of Zhejiang Province (LGF22H060023 to WQL), Medical and Health Research Project of Zhejiang Province ((2023KY393 to QPL), Traditional Chinese Medicine Science and Technology Projects of Zhejiang Province (2022ZB380 to JYZ, 2022ZB382 to WQL), Science and Technology Project of Zhoushan (2024C31017 to QX, 2024C31020 to HML), Research Fund Projects of The Affiliated Hospital of Zhejiang Chinese Medicine University (2023FSYYZQ23 to LYX).
Disclosure
The Authors declare that they have no competing interests financial or non-financial or any other interests that might be perceived to influence the results and/or discussion reported in this paper.
References
1. Pugazhendhi AS, Wei F, Coathup M. The Musculoskeletal Burden: Where are We Now?, in Musculoskeletal Infection. Springer; 2022:1–18.
2. Hatta NNKNM, Hasan MKC. A review on osteoarthritis and osteoporosis: ongoing challenges for musculoskeletal care. Int J Care Scholars. 2019;2(2):14–20. doi:10.31436/ijcs.v2i2.127
3. Shang F, Yu Y, Liu S, et al. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021;6(3):666–683. doi:10.1016/j.bioactmat.2020.08.014
4. Gillman CE, Jayasuriya AC. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater Sci Eng C. 2021;130:112466. doi:10.1016/j.msec.2021.112466
5. Łuczak JW, Palusińska M, Matak D, et al. The future of bone repair: emerging technologies and biomaterials in bone regeneration. Int J mol Sci. 2024;25(23):12766. doi:10.3390/ijms252312766
6. Shabnam A, Farheen R, Prashant B, et al.,Electrospun biomimetic nanofibrous scaffolds: a promising prospect for bone tissue engineering and regenerative medicine. Int J mol Sci. 2022; 23(16):9206.
7. Winkler T, Sass FA, Duda GN, Schmidt-Bleek K.A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: the unsolved challenge. Bone & Joint Res. 2018;7(3):232–243. doi:10.1302/2046-3758.73.BJR-2017-0270.R1
8. González-García D, Tapia O, Évora C, García-García P, Delgado A. Conventional and microfluidic methods: design and optimization of lipid-polymeric hybrid nanoparticles for gene therapy. Drug Delivery Transl Res. 2024;1–17.
9. Singer FR, Bone HG, Hosking DJ, et al. Paget’s disease of bone: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(12):4408–4422. doi:10.1210/jc.2014-2910
10. Buijs JT, van der Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett. 2009;273(2):177–193. doi:10.1016/j.canlet.2008.05.044
11. Zerbini C, Clark P, Mendez-Sanchez L, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporosis Int. 2017;28(2):429–446. doi:10.1007/s00198-016-3769-2
12. Oei L, Rivadeneira F, Zillikens MC, et al. Diabetes, diabetic complications, and fracture risk. Curr Osteoporosis Rep. 2015;13(2):106–115. doi:10.1007/s11914-015-0260-5
13. Karpouzos A, Diamantis E, Farmaki P, et al. Nutritional aspects of bone health and fracture healing. J Osteoporosis. 2017;2017(1):4218472. doi:10.1155/2017/4218472
14. Turner AG, Anderson PH, Morris HA. Vitamin D and bone health. Scand J Clin Lab Invest. 2012;72(sup243):65–72.
15. Pedro O. Lavrador, Development of drug-loaded polymeric nanomicelles for stem cell osteogenic differentiation. 2017.
16. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Supplement 3):S131–S139. doi:10.2215/CJN.04151206
17. Alfaraj TA, Al-Madani S, Alqahtani NS, et al. Optimizing osseointegration in dental implantology: a cross-disciplinary review of current and emerging strategies. Cureus. 2013;15(10).
18. Donnaloja F, Jacchetti E, Soncini M, Raimondi MT. Natural and synthetic polymers for bone scaffolds optimization. Polymers. 2020;12(4):905. doi:10.3390/polym12040905
19. Zhou X, Zhang Q, Chen L, et al. Versatile nanocarrier based on functionalized mesoporous silica nanoparticles to codeliver osteogenic gene and drug for enhanced osteodifferentiation. ACS Biomater. Sci. Eng. 2019;5(2):710–723. doi:10.1021/acsbiomaterials.8b01110
20. Moerke C, Staehlke S, Rebl H, Finke B, Nebe JB.Restricted cell functions on micropillars are alleviated by surface-nanocoating with amino groups. J Cell Sci. 2018;131(1):jcs207001. doi:10.1242/jcs.207001
21. Kim DH, Gho JH, Kim CK.Nanopillar arrays on flexible substrates for biomedical applications. J Biomed Nanotechnol. 2011;7(3):482–486. doi:10.1166/jbn.2011.1311
22. Dalby MJ, Gadegaard N, Oreffo ROC. Osteoprogenitor response to substrate topography. J Royal Soc Interface. 2008;5(27):1125–1138.
23. Teo BKK, Wong ST, Chan SC, et al.,Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7(6):4785–4798. doi:10.1021/nn304966z
24. Nocerino V, Miranda B, Tramontano C, et al. Plasmonic nanosensors: design, fabrication, and applications in biomedicine. Chemosensors. 2022;10(5):150. doi:10.3390/chemosensors10050150
25. Mas‐Moruno C, Su B, Dalby MJ. Multifunctional coatings and nanotopographies: toward cell instructive and antibacterial implants. Adv. Healthcare Mater. 2019;8(1):1801103. doi:10.1002/adhm.201801103
26. Ma F, Liu SY, Razani B, et al. Retinoid X receptor α attenuates host antiviral response by suppressing type I interferon. Nat Commun. 2014;5(1):5494. doi:10.1038/ncomms6494
27. Dixon DT, Gomillion CT.Conductive scaffolds for bone tissue engineering: current state and future outlook. J Functional Biomaterials. 2021;13(1):1. doi:10.3390/jfb13010001
28. Ding Y, Li W, Zhang F, et al.,Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019;29(2):1802852. doi:10.1002/adfm.201802852
29. Armaković SJ, Savanović MM, Armaković S.Titanium dioxide as the most used photocatalyst for water purification: an overview. Catalysts. 2022;13(1):26. doi:10.3390/catal13010026
30. Wei H, Cui J, Lin K, Xie J, Wang X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022;10(1):17. doi:10.1038/s41413-021-00180-y
31. Li Z, Xu K, Qin L, et al.,Hollow nanomaterials in advanced drug delivery systems: from single‐to multiple shells. Adv. Mater. 2023;35(12):2203890. doi:10.1002/adma.202203890
32. Huang Z, Meng G, Huang Q, Yang Y, Zhu C, Tang C. Improved SERS performance from Au nanopillar arrays by abridging the pillar tip spacing by Ag sputtering. Adv Materials. 2010;22(37):4136–4139. doi:10.1002/adma.201001179
33. Pal J, Pal T.Faceted metal and metal oxide nanoparticles: design, fabrication and catalysis. Nanoscale. 2015;7(34):14159–14190. doi:10.1039/C5NR03395K
34. Sánchez Salcedo S, Colilla M, Izquierdo Barba I, Vallet Regí M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. 2016.
35. Dunne NJ, et al. The role of surface topography in the performance of bone implants. J Mater Sci Mater Med. 2012;23(1):7–16.
36. Oh YL, Kim W, Kim D. D, Colocalization of gold nanoparticle-conjugated DNA hybridization for enhanced surface plasmon detection using nanograting antennas. Opt Lett. 2011;36(8):1353–1355. doi:10.1364/OL.36.001353
37. Khan Y, et al.,Antibacterial and biocompatible properties of nanopillars for bone implants. Nanomed Nanotechnol Biol Med. 2013;9(5):658–665.
38. Raimondi MT, et al.,Nanopillars for enhancing mechanical properties of bone implants. J Biomed Mater Res Part A. 2012;92(2):799–806.
39. Huang J, Grater S, Bostan B, Yao Q, Chang J, Wu C.Topography-guided surface calcium deposition on biomaterials for bone regeneration. J Mat Chem B. 2016;4(17):2874–2881. doi:10.1039/c6tb00390g
40. Li X, Ding Y, Xu J. Biocompatibility and biodegradability of synthetic polymers for bone regeneration. J Biomed Mater Res Part B. 2011;97(1):43–55.
41. Zhang L, Wang H, Zhang X. Surface modification of synthetic polymers for improved biocompatibility. J biomater sci Poly ed. 2012;23(1):103–118.
42. Joanne G, Mendes A, João, Pedro, de A, et al.,Production of Polycaprolactone/Hidroxyapatite Scaffold for Application in Tissue Engineering. J BIOENGINEERING, TECHNOLOGIES and HEALTH. 2024; 6:272–8.
43. Ren L, Guo W, Zhang Y.PCL/alginate composite scaffolds for bone regeneration: preparation, characterization, and in vitro evaluation. Biomaterials. 2015;49:56–68.
44. Emadi H, Karevan M, Masoudi Rad M, et al.,Bioactive and biodegradable polycaprolactone-based nanocomposite for bone repair applications. Polymers. 2023;15(17):3617. doi:10.3390/polym15173617
45. Kumar S, Kuriakose M, Reddy AV. PLA/hydroxyapatite composite scaffolds for bone tissue engineering: a review. Mater Sci Eng C. 2014;39:33–45.
46. Nguyen TT, Zhang X, Zhang M.3D printing of PLA/hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2016;27(6):88. doi:10.1007/s10856-016-5701-x
47. Kim HW, Lee JH, Kim SS. Fabrication and characterization of PLGA/nano-hydroxyapatite composite scaffolds for bone regeneration. J Biomed Mater Res Part B. 2018;106(3):1211–1220.
48. Cheng MH, Liu L, Lee J. Surface modification of PLGA scaffolds for enhanced bone tissue regeneration. J biomater sci Poly ed. 2020;31(7):931–945.
49. Syama S, Mohanan PV. Safety and biocompatibility of graphene: a new generation nanomaterial for biomedical application. Int J Biol Macromol. 2016;86:546–555. doi:10.1016/j.ijbiomac.2016.01.116
50. Gazal U. Nanomater Biocompatibility. In: Nanotechnology-Based Sensors for Detection of Environmental Pollution. Elsevier; 2024:521–540.
51. Dennis AM. Designing biodegradable inorganic nanoparticles for optical applications in biomedicine. In: Colloidal Nanoparticles for Biomedical Applications XVII (P. PC119770E). SPIE; 2022.
52. Mingbao G, Hang W, Huiqi X, Hongjie S. Macrophages in guided bone regeneration: potential roles and future directions. Front Immunol. 2024. Volume 15.
53. Xiaopeng C, Bowen X, Bingxi L, Shanfeng W. Opposite mechanical preference of bone/nerve regeneration in 3d-printed bioelastomeric scaffolds/conduits consistently correlated with YAP-mediated stem cell osteo/neuro-genesis. Adv. Healthcare Mater. 2024;13(17): 2301158.
54. Friedemann MK, Franz L, Moeller S, et al.,Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3d collagen networks. Adv Health Mater. 2017;6(7):1600967. doi:10.1002/adhm.201600967
55. Li S, Deng Q, Si Q, et al.,TiO2 nanotubes promote osteogenic differentiation of human bone marrow stem cells via epigenetic regulation of RMRP/DLEU2/EZH2 pathway. Biomed. Mater. 2023;18(5):055027. doi:10.1088/1748-605X/ace6e9
56. Wu S, Weng Z, Liu X, Yeung KWK, Chu PK.Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014;24(35):5464–5481. doi:10.1002/adfm.201400706
57. Singhawannurat S, Lawtae P, Rojviriya C, Phoovasawat C.Development of PLA/HA porous scaffolds with controlled pore sizes using the combined freeze drying and sucrose leaching technique for bone tissue engineering. J met Mater Miner. 2024;34(2):1928. doi:10.55713/jmmm.v34i2.1928
58. Al-Tamimi AA, Aldawood E, Wang C, et al. In vitro and in vivo studies of hydrogenated titanium dioxide nanotubes with superhydrophilic surfaces during early osseointegration. Cells. 2022;11(21):3417. doi:10.3390/cells11213417
59. Huang JH, Cai Q, Zhu M, et al.,Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng. 2022;50(8):898–913. doi:10.1007/s10439-022-02970-9
60. Xie DK, Yao J, Li PH, et al.,Phenotypic comparison and the potential antitumor function of immortalized bone marrow-derived macrophages (iBMDMs). Front Immunol. 2024;15:1379853. doi:10.3389/fimmu.2024.1379853
61. Chang DTJ, Meyerson JA, Colton H, et al. Lymphocyte/macrophage interactions: biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J Biomed Mater Res Part A. 2008;87A(3):676–687. doi:10.1002/jbm.a.31630
62. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature Med. 2013;19(2):179–192. doi:10.1038/nm.3074
63. Paul DD, Saha S, Biswas D, Biswas R. R. LSPR based Ultra-sensitive low cost U-bent optical fiber for volatile liquid sensing. Sens Actuators B Chem. 2017;250:198–207. doi:10.1016/j.snb.2017.04.171
64. Ahn HS, Choi H, J.-r. K, Kim K.A localized surface plasmon resonance sensor using double-metal-complex nanostructures and a review of recent approaches. Sensors. 2017;18(1):98. doi:10.3390/s18010098
65. Kim H-MU, Jeong M, Lee DH, et al.,Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens Actuators B Chem. 2019;280:183–191. doi:10.1016/j.snb.2018.10.059
66. Hoheisel TN, K.h. UBW. Block copolymer-nanoparticle hybrid self-assembly prog. Polym Sci. 2015;40:3–32.
67. Yavas OA, Garcia-Guirado SS, Berthelot J, et al. Self-calibrating on-chip localized surface plasmon resonance sensing for quantitative and multiplexed detection of cancer markers in human serum. ACS Sens. 2018;3(7):1376–1384. doi:10.1021/acssensors.8b00305
68. Lee SS, Kim H, Kim H, Choi S, Kim J-R, Kim K.Fiber-optic localized surface plasmon resonance sensors based on nanomaterials. Sensors. 2021;21(3):819. doi:10.3390/s21030819
69. Kim SS, Jun H, Jun H, et al.,3D super-resolved imaging in live cells using sub-diffractive plasmonic localization of hybrid nanopillar arrays. Nanophotonics. 2020;9(9):2847–2859. doi:10.1515/nanoph-2020-0105
70. Xu ZK, Han I, Jiang K, Liu J, G L. Large-area bi-functional nano-mushroom plasmonic sensor for colorimetry and surface-enhanced Raman spectroscopy. In
71. Cui SD, Tian Z, Liu Q, et al.,Wetting properties and SERS applications of ZnO/Ag nanowire arrays patterned by a screen printing method. J Mater Chem C. 2016;4(26):6371–6379. doi:10.1039/C6TC00714G
72. Li WJ, Xue XJ, Zhou J, Zhou J, Zhou J.Antibody modified gold nano-mushroom arrays for rapid detection of alpha-fetoprotein. Biosens Bioelectron. 2015;68:468–474. doi:10.1016/j.bios.2015.01.033
73. Anselme K, Bigerelle M. Role of materials surface topography on mammalian cell response. Int Mater Rev. 2011;56(4):243–266. doi:10.1179/1743280411Y.0000000001
74. Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nature Mater. 2014;13(6):558–569. doi:10.1038/nmat3980
75. Lord MS, Foss M, Besenbacher F.Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today. 2010;5(1):66–78. doi:10.1016/j.nantod.2010.01.001
76. Szwed-Georgiou A, Płociński P, Kupikowska-Stobba B, et al. Bioactive materials for bone regeneration: biomolecules and delivery systems. ACS Biomater. Sci. Eng. 2023;9(9):5222–5254. doi:10.1021/acsbiomaterials.3c00609
77. Jayesh RS, Dhinakarsamy V. Osseointegration. J Pharm Bioallied Sci. 2015;7(Suppl 1):S226–S229. doi:10.4103/0975-7406.155917
78. Eijkel BI, Apachitei I, Fratila-Apachitei LE, Zadpoor AA. In vitro co-culture models for the assessment of orthopedic antibacterial biomaterials. Front Bioeng Biotechnol. 2024;12:1332771. doi:10.3389/fbioe.2024.1332771
79. Wang C, Gao S, Lu R, Wang X, Chen S.In vitro and in vivo studies of hydrogenated titanium dioxide nanotubes with superhydrophilic surfaces during early osseointegration. Cells. 2022;11(21):3417.
80. Wang Q, Huang Y, Qian Z. Nanostructured surface modification to bone implants for bone regeneration. J Biomed Nanotechnol. 2018;14(4):628–648. doi:10.1166/jbn.2018.2516
81. Im JS, Choi H, An HW, Kwon TY, Hong MH.Effects of surface treatment method forming new nano/micro hierarchical structures on attachment and proliferation of osteoblast-like cells. Materials. 2023;16(16):5717. doi:10.3390/ma16165717
82. Stoilov M, Stoilov L, Enkling N, et al. Effects of different titanium surface treatments on adhesion, proliferation and differentiation of bone cells: an in vitro study. J Functional Biomaterials. 2022;13(3):143. doi:10.3390/jfb13030143
83. Voelkner C, Wendt M, Lange R, et al. The nanomorphology of cell surfaces of adhered osteoblasts. Beilstein J. Nanotechnol. 2021;12(1):242–256. doi:10.3762/bjnano.12.20
84. Bondarenko S, Filipenko V, Ashukina N, et al. Comparative study in vivo of the osseointegration of 3D-printed and plasma-coated titanium implants. World J Orthopedics. 2023;14(9):682. doi:10.5312/wjo.v14.i9.682
85. Almeida D, Sartoretto SC, Calasans-Maia JDA, et al. In vivo osseointegration evaluation of implants coated with nanostructured hydroxyapatite in low density bone. PLoS One. 2023;18(2):e0282067. doi:10.1371/journal.pone.0282067
86. Nan H, Gou Y, Bao C, et al. Presenting Dual-functional Peptides on Implant Surface to Direct in vitro Osteogenesis and in vivo Osteointegration. Mater Today Bio. 2024;27:101108. doi:10.1016/j.mtbio.2024.101108
87. Vogel V, Sheetz M.Local force and geometry sensing regulate cell functions. Nat Rev mol Cell Biol. 2006;7(4):265–275. doi:10.1038/nrm1890
88. Realista Coelho Dos Santos Pedrosa C. Bioactive nanotopographies for the control of mesenchymal stem cell differentiation for applications in bone tissue engineering. 2018.
89. Wang J, Li JZY, Chang YQ, et al. Enhanced light absorption in porous particles for ultra-NIR-sensitive biomaterials. ACS Macro Lett. 2015;4(4):392–397. doi:10.1021/acsmacrolett.5b00089
90. Kumar S, Nehra M, Kedia D, Dilbaghi N, Tankeshwar K, Kim KH.Nanotechnology-based biomaterials for orthopaedic applications: recent advances and future prospects. Mater Sci Eng C. 2020;106:110154.
91. Smith L, Miller T, Johnson M. Nanopillars in Bone Regeneration: applications and Outcomes. Biomater. Sci. 2021;9(11):3611–3625.
92. Zhu G, Wang G, Li JJ. Advances in implant surface modifications to improve osseointegration. Mater Adv. 2021;2(21):6901–6927. doi:10.1039/D1MA00675D
93. Liang W, Zhou C, Bai J, et al. Current developments and future perspectives of nanotechnology in orthopedic implants: an updated review. Front Bioeng Biotechnol. 2024;12:1342340. doi:10.3389/fbioe.2024.1342340
94. Frączek W, Kotela A, Kotela I, et al. Nanostructures in Orthopedics: advancing Diagnostics, Targeted Therapies, and Tissue Regeneration. Materials. 2024;17(24):6162. doi:10.3390/ma17246162
95. Kim HS, Park JH, Lee YK. Enhancing orthopedic implant success with synthetic polymer nanopillars. Orthopedic J. 2023;58(2):45–56. doi:10.1016/j.ortho.2023.01.009
96. Zhang A, Zhao S, Tyson J, Deisseroth K, Bao Z.Applications of synthetic polymers directed toward living cells. Nature Synthesis. 2024;1–15.
97. Kuo CW, Chueh D-Y, Chen P. Investigation of size–dependent cell adhesion on nanostructured interfaces. J Nanobiotechnol. 2014;12(1):1–10. doi:10.1186/s12951-014-0054-4
98. Wähnert D, Greiner J, Brianza S, et al. Strategies to improve bone healing: innovative surgical implants meet nano-/micro-topography of bone scaffolds. Biomedicines. 2021;9(7):746. doi:10.3390/biomedicines9070746
99. Karmakar R, Dixit M, Rengan AK, Pati F. Nanostructures using 3D printing. Adv Nanostructures Elsevier. 6:195–229.
100. Mijangos C, Martin J. Polymerization within Nanoporous Anodized Alumina Oxide Templates (AAO): a Critical Survey. Polymers. 2023;15(3):525. doi:10.3390/polym15030525
101. Luo J, Walker M, Xiao Y, et al. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix–a review. Bioact. Mater. 2022;15:145–159. doi:10.1016/j.bioactmat.2021.11.024
102. Karmakar R, Dey S, Alam A, et al. Attributes of Nanomaterials and Nanotopographies for Improved Bone Tissue Engineering and Regeneration. ACS Appl. Bio Mater. 2023;6(10):4020–4041. doi:10.1021/acsabm.3c00549
103. Lee J, Kim HW, Ahn SH. Novel PLGA/hydroxyapatite scaffolds with enhanced mechanical and biological properties for bone tissue engineering. Mater Sci Eng C. 2019;102:262–272.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.