Back to Journals » Infection and Drug Resistance » Volume 18
Temporal Shifts in Etiological Agents and Trends in Antimicrobial Resistance of Bloodstream Infection in Southwest China from 2016 to 2023
Authors Long S, Zhong M, Huang X, Zhang J, Liu X, Yu H
Received 30 December 2024
Accepted for publication 6 March 2025
Published 11 March 2025 Volume 2025:18 Pages 1367—1379
DOI https://doi.org/10.2147/IDR.S514966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Shanshan Long,* Min Zhong,* Xiangning Huang, Jie Zhang, Xin Liu, Hua Yu
Department of Laboratory Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Yu, Department of Laboratory Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Department of Clinical Laboratory, No. 32, West Second Section, 1st Ring Road, Chengdu, Sichuan, 610072, People’s Republic of China, Email [email protected]
Objective: The purpose of this study was to assess the frequency distribution of bacterial pathogens causing bloodstream infections (BSIs) as well as the characteristics of antibiotic susceptibility and resistance to gain a deeper understanding of the drug resistance situation in the southwest China.
Methods: Non-duplicate pathogenic bacteria according to the American Clinical and Laboratory Standards Institute (CLSI) M100 and analyzed using WHONET 5.6 software.
Results: A total of 170,246 non-duplicated pathogenic bacteria were isolated from blood from 2016 to 2023. Gram-negative bacteria accounted for 59.5% and Gram-positive bacteria accounted for 40.5%. The top five detection rates were Escherichia coli (33.9%), coagulase-negative staphylococci (21.7%), Klebsiella pneumoniae (11.5%), Staphylococcus aureus (7.0%), and Enterococcus spp (5.4%). Streptococcus pneumoniae, Salmonella spp and β-Hemolytic Streptococcus were significantly more frequently isolated in pediatric patients than in adult patients. The rate of resistance to carbapenems was less than 1.2% in the Escherichia coli over the 8-year period, with a significant upward trend in the Klebsiella pneumoniae. The overall resistance rate of Pseudomonas aeruginosa to imipenem and meropenem had a slow decreasing trend. The resistance rate of Acinetobacter baumannii to imipenem and meropenem has been maintained at a high level, which is greater than 50%. The detection rate of MRSA was 27.70% and vancomycin- and linezolid-resistant strains were not found. The resistance rates of Enterococcus faecium and Enterococcus faecalis to vancomycin, linezolid and teicoplanin were less than 2.1%.
Conclusion: The pathogenic bacteria of bloodstream infection in southwest China were diversified, and the multi-drug resistant bacteria, especially Carbapenem-resistant Klebsiella pneumoniae (CRKP), had increased significantly, posing a serious challenge to clinical treatment. Additionally, the situation of Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococcus (VRE) also required close attention.
Keywords: bloodstream infections, bacterial resistance surveillance, multidrug-resistant bacteria, change of drug-resistant rate
Introduction
Bloodstream infections (BSIs) are associated with high morbidity and mortality.1 Globally, approximately 30 million people are infected each year.2 In the UK, approximately 100,000 people are hospitalized with sepsis and 37,000 die each year.3 Studies in the United States had shown that the incidence of bacteremia in hospitalized patients was 5.9% and the mortality rate was 15.6%.4 In childhood infections in Spain, the directly related 30-day mortality rate of BSIs was 10.5%.5 As the rate of resistance to bloodstream infections continues to rise, so do infection-related morbidity, mortality, and treatment costs, especially in the ICU setting.6 Another meta-analysis of 72 papers showed that the weighted combined all-cause mortality rate of BSI in China was 28.7%, and the mortality rate of hospital-acquired BSI was 26.8%, which was significantly higher than that of community-acquired BSI, which was 6.9%.7 Differences in the spectrum of pathogens and patterns of bacterial resistance causing healthcare-associated infections have been reported in different regions of the world. Data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) to be published in 20238 and the Korean Surveillance Network (KorGLASS) from 2016 to 20179 both showed that E. coli and S. aureus were the most common pathogens causing BSI. However, the most common pathogens in Malawi and Africa causing BSI were non-typhoid Salmonella, Salmonella typhi, and Streptococcus pneumoniae.10 However, E. coli, S. aureus, Streptococcus spp and Klebsiella spp were the most common pathogens according to Japanese data.11
Therefore, continuous and regional monitoring of the pathogenic spectrum of BSI and the trend of pathogenic bacterial drug resistance is of great significance for the effective control of the prevalence of multidrug-resistant bacteria, the development of new preventive methods, and optimizing treatment strategies. In our country, China Antimicrobial Surveillance Network (CHINET) and China Antimicrobial Resistance Surveillance System (CARSS) are responsible for the dynamic monitoring of pathogenic bacteria. CHINET focuses on the distribution of bacteria and drug resistance trends in major referral hospitals, whereas CARSS monitors bacterial drug resistance in different provinces and autonomous regions. The Antimicrobial Resistant Investigation Network of Sichuan Province (ARINSP) is a subordinate network unit of CARSS, including 109 healthcare institutions, and is responsible for monitoring the antibiotic resistance situation in the province, and the data of ARINSP represent the pattern of bacterial drug resistance in Southwest China. CARSS has reported the distribution of pathogenic bacteria and drug resistance rate of BSI in the whole country from 2014 to 2019,12 and some researchers had also analyzed the BSI in central China between 1998 and 2017,13 but currently the drug resistance of BSI in Southwest China has been rarely, except for Jie Zhang14 and Shanshan Long,15 who have reported. Therefore, we systematically analyzed the distribution of bacterial species and drug resistance in BSIs in Sichuan Province from 2016 to 2023 to further understand the situation in Southwest China.
Materials and Methods
Bacterial Isolates
Since isolates were collected from blood samples of outpatients and inpatients from 109 medical institutions in the Sichuan Provincial Antimicrobial Resistance Monitoring Network from 2016 to 2023. The blood culture systems used included Mérieux’s BacT/ALERT VIRTUO and BacT/ALERT 3D, BD’s BACTECTM 9000, Autobio’s BC60 and DL-Bt system. The range of blood culture bottles used included standard aerobic bottles, resinous aerobic bottles, standard anaerobic bottles and anaerobic bottles with special components.
The main reference for the diagnosis of bloodstream infections is the Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases (2024 edition), Inclusion criteria: 1) Clinical symptoms and signs: patients presenting with fever (body temperature >38°C) or hypothermia (body temperature <36°C) with chills, shock, organ dysfunction. 2) Blood culture: positive for pathogenic bacteria. 3) Other indicators: CRP >10mg/L, PCT >0.5ng/mL and other abnormal inflammatory indicators, imaging studies. Exclusion criteria: 1) Blood culture contamination: common skin colonising bacteria are cultured, only once in multiple blood cultures and the patient has no obvious signs and symptoms of infection. 2) Clear local foci of infection: there are clear local foci of infection and the symptoms and signs of infection can be fully explained by local infection. In order to exclude duplicate strains from the selected strains, only the first strain of the same pathogen is kept in the same patient, thus ensuring the validity and accuracy of the strain data.
Bacterial Identification and Susceptibility Testing
Bacterial identification was performed using VITEK2 automated system, BD Phoenix 100 system, or MALDI mass spectrometry system. Drug susceptibility testing was performed using the minimum inhibitory concentration (MIC) test, the Kirby-Bauer (K-B) method, and the E-test method according to Clinical Laboratory Standards Institute (CLSI) guidelines. All results were interpreted according to CLSI M100 guideline standards, except for tigecycline, which was interpreted according to FDA standards.
Interpretation of Results and Quality Control
QC testing was performed weekly according to CLSI. Quality control strains included but were not limited to Escherichia coli (ATCC25922), Klebsiella pneumoniae (ATCC700603), Staphylococcus aureus (ATCC25923), Pseudomonas aeruginosa (ATCC27853), Enterobacter cloacae (ATCC700323), Streptococcus pneumoniae (ATCC49619) and Haemophilus influenzae (ATCC49247).
Statistical Analysis
All results were analyzed using WHONET5.6 data analysis software. The general steps of WHONET5.6 for statistical analysis of drug susceptibility result data are as follows: 1) data entry: enter the strain information, antimicrobial drug name and corresponding drug susceptibility test results into the software; 2) analysis type selection: according to the statistical needs, select various types of analysis modes in the system, such as resistance analysis, sensitivity analysis, resistance spectrum analysis, etc. 3) Set analysis options: according to the data to be analysed, set the corresponding options, such as selecting the source of strains, types of strains, antimicrobial drugs of concern, etc.; 4) Generate reports; 5) Interpret the results; 6) Export the data.
Results
Bacterial Origin and Distribution
During 2016–2023, a total of 170,246 non-duplicated pathogenic bacteria were isolated from blood, of which 25,743 (15.1%) were from ICU sources and 11,445,503 (84.9%) were from non-ICU sources. Gram-negative bacteria detected were dominated by E. coli (33.9%), K. pneumoniae (11.5%), and P. aeruginosa (2.3%), while Gram-positive bacteria were mainly coagulase-negative staphylococci (CNS) predominantly (21.7%), followed by S. aureus (7.0%), and Enterococci spp (5.4%), and the variation of major clinical isolates is shown in Table 1. ICU isolates were dominated by CNS (32.4%) and non-ICU isolates were dominated by E. coli (36.8%), and the percentage of detected Enterococci spp was significantly higher in ICU (9.9%) than in non-ICU (4.6%), as shown in Table 2.
 |
Table 1 Changes of Main Clinically Isolated Bacteria in Blood Samples from 2016 to 2023 (%) |
 |
Table 2 Distribution of Bloodstream Infection Strains in ICU and Non-ICU Units |
Among the 170,246 pathogenic bacteria isolated from blood, 17,038 strains were recorded with age. Of these, 11.3% (19,287 strains) were from pediatric patients <18 years of age; 11.1% (18,896 strains) were from patients ≥18 to <45 years of age; 22.4% (38,040 strains) were from patients ≥45 to <60 years of age; and 55.2% (93,815 strains) were from patients ≥60 years of age. Among the isolates from pediatric patients <18 years old, CNS predominated. The remaining age group isolates were dominated by E. coli. S. pneumoniae, Salmonella spp and β-hemolytic streptococci were isolated more frequently in children than in adult patients. The differences in the major isolates from blood samples of patients of different age groups were significant, as shown in Table 3.
 |
Table 3 Distribution of Main Bacteria Isolated from Blood Samples in Different Age Groups from 2016 to 2023 (%) |
Sensitivity and Resistance Rates of Gram-Negative Bacteria to Antimicrobials
From 2016 to 2023, Escherichia coli had greater than 50.0% resistance to cefazolin, cefuroxime, ceftriaxone and trimethoprim-sulfamethoxazole and greater than 50.0% resistance to ertapenem, imipenem, meropenem, and amikacin was less than 1.8%, maintaining very high activity. The resistance rate to gentamicin had a decreasing trend year by year, while the resistance rate to quinolones fluctuated, with ciprofloxacin and levofloxacin resistance rates ranging from 42.0% to 48.8%, as detailed in Table 4. The resistance rates of K. pneumoniae to ertapenem, imipenem, and meropenem increased from 2.4%, 2.7%, and 4.1% to 9.8%, 10.0%, and 11.1%, showing a significant upward trend, and the resistance rate to amikacin, piperacillin and tazobactam maintained high sensitivity, as shown in Table 5. E. coli and K. pneumoniae showed low resistance to tigecycline (≤6.9%) and polymyxin B (≤4.2%) and were highly sensitive.
 |
Table 4 Changes of Resistance Rates of E. coli to Antimicrobial Agents in 2016–2023 |
 |
Table 5 Changes of Resistance Rates of K. pneumoniae to Antimicrobial Agents in 2016–2023 |
The resistance rate of P. aeruginosa to commonly used antimicrobial drugs showed an overall decreasing trend, in which the resistance rate to imipenem and meropenem decreased from 13.2% and 11.6% in 2016 to 9.1% and 8.1% in 2023, respectively, and the resistance rate to aztreonam decreased from 19.7% to 13.0%; the resistance rate to ceftazidime, cefepime around 10.0%; and remained highly sensitive to polymyxin B, with no resistant strains detected, as shown in Table 6. A. baumannii had resistance rates higher than 50.0% to most of the tested drugs, with resistance rates of ≥51.2% and 53.6% to imipenem and meropenem, respectively, and remained highly susceptible to tigecycline and polymyxin B only (resistance rates of 3.1% and 8.6%) as shown in Table 7.
 |
Table 6 Changes of Resistance Rates of P. aeruginosa to Antimicrobial Agents in 2016–2023 |
 |
Table 7 Changes of Resistance Rates of A. baumannii to Antimicrobial Agents in 2016–2023 |
Sensitivity and Resistance Rates of Gram-Positive Bacteria to Antimicrobials
There were 11966 strains of S. aureus isolated from the blood, and the detection rate of MRSA was 27.7%. The rates of resistance to penicillins, quinolones and aminoglycosides were higher in MRSA than in MSSA, and the rate of resistance to macrolides was the most significant; however, the rate of resistance to trimethoprim-sulfamethoxazole was slightly lower than that in MSSA. A small number of linezolid-resistant strains (0.4%) was isolated from MRSA, and a small number of teicoplanin-resistant strains (0.3%), in addition to a very small number of linezolid-resistant strains (0.1%), were also isolated from MSSA. No vancomycin-resistant Staphylococcus species were detected, as shown in Table 8.
 |
Table 8 Changes of Resistance Rate of Staphylococcus to Antibiotics in Blood Samples from 2016 to 2023 |
The 9130 strains of Enterococcus were mainly Enterococcus faecium (5141 strains, 56.3%) and Enterococcus faecalis (2888 strains, 31.6%). The resistance rate of E. faecium to most of the tested drugs was significantly higher than that of E. faecalis, especially ampicillin (87.9% vs 4.1%), and also maintained a high level of resistance to quinolones. E. faecalis had high drug resistance rate against rifampin (47.7%) but maintained high activity against vancomycin, linezolid and teicoplanin. Among the drug-resistant strains of vancomycin detected, E. faecium (1.5%) was more than E. faecalis (0.2%). For Linezolid, the drug resistance rate of E. faecium (0.5%) was lower than E. faecalis (2.1%), as shown in Table 9.
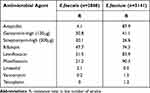 |
Table 9 Resistance Rates of Enterococcus to Antibiotics in Blood Samples from 2016 to 2023 |
Multidrug-Resistant Bacteria Changes
Among multidrug-resistant bacteria detected in 2016–2023, Carbapenem-resistant Escherichia coli(CRECO) and Carbapenem-resistant Klebsiella pneumoniae(CRKPN) showed a decreasing trend, Carbapenem-resistant Acinetobacter baumannii(CRAB) remained at a high level of detection and it showed a downward and then an upward trend. Methicillin-resistant Staphylococcus aureus(MRSA) showed a slight increasing trend and Vancomycin-resistant Enterococcus (VRE) remained at a low level (Figures 1 and 2).
Discussion
This surveillance showed that a total of 170,246 strains of non-repeatable pathogens of bloodstream infections were isolated from 109 healthcare institutions in Southwest China from 2016 to 2023, and the detected gram-negative organisms were dominated by E. coli (33.9%), K.pneumoniae(11.5%), and P. aeruginosa (2.3%), and the Gram-positive organisms were mainly coagulase-negative staphylococci (21.7%), followed by Staphylococcus aureus (7.0%) and Enterococcus spp. (5.4%). The percentage of gram-negative bacteria (59.5%) was higher than gram-positive bacteria (40.5%), which is consistent with other national reports.12,14–16 The 25743 pathogenic bacteria (15.1%) isolated from ICU patients were mainly coagulase-negative staphylococci (32.4%), and the 144503 pathogenic bacteria (84.9%) isolated from non-ICU patients were predominantly E. coli (36.8%), and the percentage of detected Enterococci was significantly higher in the ICU (9.9%) than in the non-ICU (4.6%). The distribution of isolates also differed significantly between children and adults, with CNS predominating in children and S. pneumoniae, Salmonella, and β-hemolytic streptococci being isolated at a much higher rate than in adult patients, and E. coli predominating in the rest of the age groups, with the highest number of strains being isolated in the ≥60-year age group.
In this study, it was found that the main pathogen causing BSI in this region was E. coli, which was consistent with the main cause of BSI in Europe.8 The resistance rate of E. coli to carbapenems was less than 1.4%, indicating high activity, but the resistance rates of E. coli to cefazolin, cefuroxime, ceftriaxone, and trimethoprim-sulfamethoxazole were all greater than 50.0%. The resistance rate to quinolones was more than 41.6%. The resistance rate of K. pneumoniae to ceftriaxone was higher than 24.8%, the resistance rate of cefuroxime was higher than 26.7% and the resistance rate of quinolones was higher than 14.8%. From 2016 to 2023, the CTX/CRO-R-ECO detection rate remained high (47.1–57.6%), and the CTX/CRO-R-KPN detection rate ranged from 25.0% to 36.0%. The emergence of third-generation cephalosporin (3GC) and fluoroquinolone resistant strains was highly affected. It is a major change in the epidemiology of E. coli and K. pneumoniae BSI due to the production of Extended-Spectrum β-Lactamase(ESBLs) or AmpC.17,18 In the World Health Organization’s (WHO) List of Pathogens of Concern for Bacteria 2024 (BPPL-2024),19 3GCRE has been separately classified as a key priority, a change that underscores the need for targeted policies and interventions to address this threat. The high failure rate of 3GCRE treatment and the increase in healthcare costs have increased the burden on low- and middle-income countries and vulnerable populations.
The data demonstrates that the detection rate of CRAB has remained at a high level (51.1–74.6%) and exhibits a high degree of drug resistance.16 In China, the clinical practice mainly relies on tigecycline, polymyxins and ceftazidime/avibactam. However, the suboptimal distribution and concentration of tigecycline in serum,20 heterogeneous resistance of polymyxins21 and ineffectiveness of ceftazidime/avibactam against most Acinetobacter baumanniihave caused significant challenges in the treatment of BSI.22 The prevalence of CRPA in our region is relatively low, ranging from 10.4% to 13.7%, which was much lower than other carbapenem-resistant strains. Despite the downgrading of CRPA priorities in BPPL-2024,19 there is still a need to continue to advance the development of new drugs.
In the past eight years, the resistance rate of K. pneumoniae to ertapenem, imipenem and meropenem increased from 2.4%, 2.7% and 4.1% in 2016 to 9.8%, 10.0% and 11.1% in 2023, showing a significant increase. The rate of CRKP detection in 2016–2019 had a significant decrease (15.7% to 9.7%) and then remained at 8.5%–10.5%, which was lower than the national level (25.5%) reported by CHINET.23 Among the carbapenem-resistant gram-negative bacteria (CRGNB) that cause BSI, CRKP is the most prominent.24–26 A prospective cohort study found that CRKP strains carried β-lactamase K. pneumoniae carbapenemase 2, and 73.1% of CRKP carried mucoid phenotype regulator genes A2 and iucABCD which could lead to the colonization and epidemic transmission of CRKP in surgical intensive care units.27 Hunan, Henan, and Changzhou have reported a high rate of CRKP resistance to most antibiotics in ICUs, with sensitivity only to polymyxin B and tigecycline, which poses a major challenge to clinical treatment.28–30 The study demonstrated that the primary resistance mechanism of CRKP in China is carbapenemase-producing enzyme, with blaKPC-2 being the predominant gene (89.4%), though the distribution of resistance genes exhibited variability across different regions. In the southwest and east, KL64 carrying blaKPC-2 was found to be particularly prevalent, while KL47 in the north and northeast, and some rare K-types, such as KL15 and KL51, carrying blaNDM resistant to ceftazidime-avibactam.31 Concurrently, a novel clone ST4495 (4-1-99-1-9-5-5) carrying blaNDM-1 was identified, disseminating in the southwestern region of China.32 Within the Indian region, NDM carbapenemases demonstrate a heightened prevalence, and in nations such as Japan and South Korea, beyond the enzyme-producing mechanism, the prevalence of altered outer membrane proteins, active exocytosis systems, and mucin resistance mechanisms is of heightened concern.33–35 In some European countries, the co-existence of OmpK35 and OmpK36 deletion and AcrAB-TolC efflux pump expression in certain CRKP strains has been observed to enhance drug resistance.36 In the context of China, there is currently a paucity of clinically available antibiotics for the treatment of CRKP, with only ceftazidime-avibactam and tigecycline being available on the market. In light of this, the KL64 and KL47 capsule types could be considered for future vaccine development.
The present data showed that Gram-positive bacteria causing BSI were dominated by CNS, followed by Staphylococcus aureus, which was slightly different from that in the central part of the country.13 The detection rate of CNS, as a normal colonizing flora of the human skin and mucous membranes, was much higher in ICUs (32.4%) than that in non-ICUs (19.8%), which was related to the fact that critically ill patients would undergo more invasive operations, such as tracheal intubation, indwelling catheters, and medical implantable devices. Thus, when determining whether CNS is a contaminating or infectious organism, it is necessary to combine the patient’s clinical symptoms and related indicators to make a comprehensive judgment. Considering that the data came from 109 hospitals in Sichuan province, there may be a lack of standardization in the collection of blood cultures, resulting in a high contamination rate. Therefore, while standardizing the collection method of blood cultures, it is recommended to puncture different parts of the body and send double bottles to multiple sets of tests to increase the positive rate and exclude contamination.
The detection rate of MRSA in this region was 27.7%, which was slightly lower than the national level, with a slight increasing trend.16 The resistance rate of MRSA to penicillins, quinolones and aminoglycosides was higher than that of the MSSA strains. No non-susceptible strains to vancomycin, ticlopidine, or linezolid were detected. A recent study reported that the MRSA subclone ST764-t002 detected in seven provinces and cities in China, including our region, has the ability to form biofilms and adhere to cells, and its deletion of the SCCmec II region may contribute to widespread transmission.37 Therefore, good hand hygiene and environmental cleanliness are essential, and rational drug administration based on drug susceptibility results may reduce the emergence and transmission of drug-resistant strains.
We also found that VRE strains detected decreased significantly from 2.9% in 2016 to 1.1% in 2020 but increased again in 2021–2023 (1.5% to 1.8%). This may be related to the following factors: (1) Some hospitals did not conduct VRE colonization screening for staff in key departments (such as ICU, surgery, anaesthesia) and long-term hospitalized patients; (2) The measures of gastrointestinal decolonization were not widely carried out; (3) there was insufficient space to fully isolate patients infected with or carrying the carriage organisms in a single room. As VRE bloodstream infections (VRE BSI) can lead to high mortality rates, clinical treatment with high-dose daptomycin is currently recommended, with better prognostic outcomes compared to linezolid.38,39 However, with increasing daptomycin resistance in VRE, combination therapy is required, and data suggest that combination with β-lactams, linezolid, aminoglycosides or tigecycline may have synergistic effects.40
Conclusion
Currently, the pathogenic bacteria of bloodstream infections in Southwest China are dominated by E. coli, K. pneumoniae, and CNS, and it is very important to continuously and dynamically monitor the development trend of multidrug-resistant bacteria, especially CRGNB, MRSA, and VRE. Hospitals also need to strengthen the awareness of infection prevention and control, and continuously improve the preventive and control measures, in order to slow down the production of drug-resistant bacteria. The hospitals also need to strengthen the awareness of infection prevention and control and continuously improve the preventive and control measures to slow down the emergence of drug-resistant bacteria.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China (No. 2024733). The ethics committee waived the need for written informed consent provided by participants due to the retrospective nature of this study. Patients’ anonymous information was provided from the microbiology hospital laboratory, which isolated the strains. The study completely followed the principles outlined in the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang S, Zhang X, Yu W, et al. Infection biomarkers in assisting the judgement of blood stream infection and patient prognosis: a retrospective study incorporating principal components analysis. Ann Transl Med. 2020;8(23):1581. doi:10.21037/atm-20-3425
2. Lamy B, Sundqvist M, Idelevich EA; ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis (ESGBIES). Bloodstream infections - Standard and progress in pathogen diagnostics. Clin Microbiol Infect. 2020;26(2):142–150. doi:10.1016/j.cmi.2019.11.017
3. Stevenson M, Pandor A, Martyn-St James M, et al. Sepsis: the LightCycler SeptiFast Test MGRADE®, SepsiTest™ and IRIDICA BAC BSI assay for rapidly identifying bloodstream bacteria and fungi - a systematic review and economic evaluation. Health Technol Assess. 2016;20(46):1–246. doi:10.3310/hta20460
4. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi:10.1001/jama.2017.13836
5. Ara-Montojo MF, Escosa-García L, Alguacil-Guillén M, et al. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J Antimicrob Chemother. 2021;76(1):220–225. doi:10.1093/jac/dkaa397
6. Munari M, Franzoi F, Sergi M, et al. Extensively drug-resistant and multidrug-resistant gram-negative pathogens in the neurocritical intensive care unit. Acta Neurochir. 2022;164(3):859–865. doi:10.1007/s00701-020-04611-3
7. Yang ZY, Zhan SY, Wang B, et al. Fatality and secular trend of bloodstream infections during hospitalization in China: a systematic review and meta-analysis. Beijing da Xue Xue Bao Yi Xue Ban. 2010;42(3):304–307.
8. European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2022. Stockholm: ECDC; 2023.
9. Lee H, Yoon EJ, Kim D, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018;23(42):1800047. doi:10.2807/1560-7917.ES.2018.23.42.1800047
10. Musicha P, Cornick JE, Bar-Zeev N, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998-2016): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–1052. doi:10.1016/S1473-3099(17)30394-8
11. Hattori H, Maeda M, Nagatomo Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: a single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–e79. doi:10.1016/j.ajic.2018.06.019
12. China Antimicrobial Resistance Surveillance System. Change in antimicrobial resistance of pathogens from blood specimens: surveillance report from China antimicrobial resistance surveillance system in 2014–2019. Chin J Infect Control. 2021;20(02):124–133. doi:10.12138/j.issn.1671-9638.20216173
13. Tian L, Zhang Z, Sun Z. Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: a 20-year surveillance study (1998-2017). Antimicrob Resist Infect Control. 2019;8:86. doi:10.1186/s13756-019-0545-z
14. Jie Z, Xiangning H, Shanshan L, et al. Analysis of distribution and drug resistance of pathogens in bloodstream infection in Sichuan Antibiotic Resistance Monitoring Network in 2016. Chin J Evid-Based Med. 2017;17(09):1011–1014. doi:10.7507/1672-2531.201708119
15. Shanshan L, Xiangning H, Jie Z, et al. Analysis of distribution and drug resistance of pathogens in bloodstream infections in Sichuan Antibiotics Resistance Monitoring Network from 2016 to 2020. Herald of Medicine. 2021;(08):1053–1059. doi:10.3870/j.issn.1004-0781.2021.08.012
16. CHINET. China’s clinical strain detection distribution and drug resistance rate change data. Available from: https://www.chinets.com/Data/AntibioticDrugFast.
17. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7):e00355–19. doi:10.1128/AAC.00355-19
18. Peirano G, Pitout JDD. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79(14):1529–1541. doi:10.1007/s40265-019-01180-3
19. Jesudason T. WHO publishes updated list of bacterial priority pathogens. Lancet Microbe. 2024;5(9):100940. doi:10.1016/j.lanmic.2024.07.003
20. Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and safety of high dose tigecycline for the treatment of severe infections: a systematic review and meta-analysis. Adv Ther. 2020;37(3):1049–1064. doi:10.1007/s12325-020-01235-y
21. Infectious Diseases Society of China; Chinese Thoracic Society; Chinese Society of Critical Care Medicine; Chinese Society of Hematology, et al. [Multi-disciplinary expert consensus on the optimal clinical use of the polymyxins in China]. Zhonghua Jie He He Hu Xi Za Zhi. 2021;44(4):292–310. doi:10.3760/cma.j.cn112147-20201109-01091 Danish
22. Tang B, Cui N. [Several key points that need to be concerned in the clinical application of ceftazidime/avibactam]. Zhonghua Yi Xue Za Zhi. 2021;101(41):3365–3370. Chinese. doi:10.3760/cma.j.cn112137-20210531-01240 Danish
23. Zhuo CY, Guo YY, Zhuo C, et al. Changing distribution and resistance profiles of Klebsiella strains in hospitals across China: results from the CHINET Antimicrobial Resistance Surveillance Program, 2015-2021. Chin J Infect Chemother. 2024;24(04):418–426. doi:10.16718/j.1009-7708.2024.04.007
24. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
25. Satlin MJ, Chen L, Patel G, et al. Multicenter clinical and molecular epidemiological analysis of bacteremia due to Carbapenem-Resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother. 2017;61(4):e02349–16. doi:10.1128/AAC.02349-16
26. Sabino S, Soares S, Ramos F, et al. A cohort study of the impact of carbapenem-resistant Enterobacteriaceae infections on mortality of patients presenting with sepsis. mSphere. 2019;4(2):e00052–19. doi:10.1128/mSphere.00052-19
27. Chu W, Hang X, Li X, et al. Bloodstream infections in patients with rectal colonization by carbapenem-resistant Enterobacteriaceae: a prospective cohort study. Infect Drug Resist. 2022;15:6051–6063. doi:10.2147/IDR.S383688
28. Wang F, Zou X, Zhou B, et al. Clinical characteristics of carbapenem-resistant Klebsiella pneumoniae infection/colonisation in the intensive care unit: a 9-year retrospective study. BMJ Open. 2023;13(6):e065786. doi:10.1136/bmjopen-2022-065786
29. Wang S, Wang L, Jin J, et al. Genomic epidemiology and characterization of carbapenem-resistant Klebsiella pneumoniae in ICU inpatients in Henan Province, China: a multicenter cross-sectional study. Microbiol Spectr. 2023;11(3):e0419722. doi:10.1128/spectrum.04197-22
30. Feng C, Zhang L, Wang Y, et al. Variation of Klebsiella pneumoniae drug resistance rate in intensive care medicine and comparison of Chinese and foreign databases. J Infect Control. 2024;23(10):1241–1248. Chinese.
31. Hu F, Pan Y, Li H, et al. Carbapenem-resistant Klebsiella pneumoniae capsular types, antibiotic resistance and virulence factors in China: a longitudinal, multi-centre study. Nat Microbiol. 2024;9(3):814–829. doi:10.1038/s41564-024-01612-1
32. Li Z, Ding Z, Yang J, et al. Carbapenem-resistant Klebsiella pneumoniae in Southwest China: molecular characteristics and risk factors caused by KPC and NDM producers. Infect Drug Resist. 2021;14:3145–3158. doi:10.2147/IDR.S324244
33. Gupta V, Garg R, Kumaraswamy K, Datta P, Mohi GK, Chander J. Phenotypic and genotypic characterization of carbapenem resistance mechanisms in Klebsiella pneumoniae from blood culture specimens: a study from North India. J Lab Physicians. 2018;10(2):125–129. doi:10.4103/JLP.JLP_155_16
34. Hara Y, Iguchi M, Tetsuka N, et al. Multicenter survey for carbapenemase-producing Enterobacterales in central Japan. Nagoya J Med Sci. 2022;84(3):630–639. doi:10.18999/nagjms.84.3.630
35. Cheong HS, Kim SY, Seo J, Wi YM, Peck KR, Ko KS. Colistin resistance and extensive genetic variations in PmrAB and PhoPQ in Klebsiella Pneumoniae Isolates from South Korea. Curr Microbiol. 2020;77(9):2307–2311. doi:10.1007/s00284-020-02074-4
36. Budia-Silva M, Kostyanev T, Ayala-Montaño S, et al. International and regional spread of carbapenem-resistant Klebsiella pneumoniae in Europe. Nat Commun. 2024;15(1):5092. doi:10.1038/s41467-024-49349-z
37. Xiao Y, Han W, Wang B, et al. Phylogenetic analysis and virulence characteristics of methicillin-resistant Staphylococcus aureus ST764-SCCmec II: an emerging hypervirulent clone ST764-t1084 in China. Emerg Microbes Infect. 2023;12(1):2165969. doi:10.1080/22221751.2023.2165969
38. Hayakawa K, Martin ET, Gudur UM, et al. Impact of different antimicrobial therapies on clinical and fiscal outcomes of patients with bacteremia due to vancomycin-resistant enterococci. Antimicrob Agents Chemother. 2014;58(7):3968–3975. doi:10.1128/AAC.02943-14
39. White BP, Barber KE, Chastain DB. Treatment decisions in VRE bacteraemia: a survey of infectious diseases pharmacists. JAC Antimicrob Resist. 2023;5(3):dlad063. doi:10.1093/jacamr/dlad063
40. Avery LM, Kuti JL, Weisser M, et al. Pharmacodynamic analysis of daptomycin-treated enterococcal bacteremia: it is time to change the breakpoint. Clin Infect Dis. 2019;68(10):1650–1657. doi:10.1093/cid/ciy749
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



