Back to Journals » Risk Management and Healthcare Policy » Volume 18
Temporal Trends in the Burden of Disease for Male Infertility from 1990 to 2021 in the BRICS
Authors Xu R, Wang XJ, Lin QC, Zhuang YT, Zhou QY, Xu NF, Zheng DQ
Received 13 November 2024
Accepted for publication 23 April 2025
Published 24 May 2025 Volume 2025:18 Pages 1721—1733
DOI https://doi.org/10.2147/RMHP.S506211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kyriakos Souliotis
Ran Xu,1 Xin-Jun Wang,2 Qing-Cheng Lin,3 Yan-Ting Zhuang,4 Qing-Ying Zhou,1 Nai-Fen Xu,1 Ding-Qin Zheng1
1Department of Urology, Pingyang Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Department of Urology, Zhongshan Hospital Xiamen University, School of Medicine, Xiamen University, Xiamen, People’s Republic of China; 3Department of Xiaojiang, Pingyang Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 4Department of Hepatobiliary and Pancreatic Surgery, Zhongshan Hospital Xiamen University, School of Medicine, Xiamen University, Xiamen, People’s Republic of China
Correspondence: Ran Xu, Department of Urology, Pingyang Hospital of Wenzhou Medical University, No.555 Kunao Avenue, Wenzhou, Zhejiang, 325400, People’s Republic of China, Email [email protected]
Background: Over the past three decades, male infertility has become a significant burden on global public health. As an international organization with nearly half of the world’s population, BRICS plays a crucial role in global health. This study investigates the trend of male infertility burden in BRICS countries from 1990 to 2021, providing valuable information for prevention and treatment strategies.
Methods: Data on male infertility in BRICS countries were obtained from the Global Burden of Disease database. Joinpoint regression, decomposition analysis, and prediction models were applied to analyze the data and assess the disease burden trends.
Results: The global prevalence of male infertility has worsened significantly between 1990 and 2021, with projections indicating this trend will continue for the next 15 years. While this global trend is based on data from a range of countries, the results of this study specifically focus on the BRICS countries. In these countries, while China and the Russian Federation have had high prevalence rates, improvements were observed over the past 30 years. India and Brazil, though unable to control male infertility in this period, have managed to halt its worsening in recent years. South Africa experienced substantial fluctuations from 2001 to 2015, with further significant changes projected in the next 15 years.
Conclusion: This study provides valuable insights into the evolving burden of male infertility in BRICS countries. It underscores the importance of targeted prevention and treatment strategies for these countries based on national and global trends.
Keywords: male infertility, global burden of disease, joinpoint regression, brics, prevalence
Introduction
Infertility is defined by the World Health Organization as the inability of a couple to conceive naturally after one year of regular, unprotected intercourse.1 Male factors alone account for 20% of infertility cases, while a combination of male and female factors contributes to 30%.2,3
On a global scale, male infertility presents a significant public health burden, affecting nearly 56 million people worldwide.4 Beyond health, male infertility has far-reaching economic and social consequences. It contributes to a decline in fertility, which exacerbates population aging and threatens the socio-economic stability and vitality of societies.5 The economic implications are particularly evident in the rising healthcare costs and the strain on social security systems. Additionally, male infertility is linked to mental health issues such as depression and anxiety, further impacting individuals’ well-being.6,7
A number of sociocultural factors, such as societal pressure and stigma around infertility, particularly in developing countries, further exacerbate the effects of male infertility. In many societies, men with infertility may face discrimination and social exclusion, affecting their mental health and relationships.8,9 Lifestyle factors, including smoking, alcohol consumption, and obesity, are also known to contribute significantly to male infertility.10–12 Environmental pollutants, such as endocrine-disrupting chemicals, and genetic factors (such as congenital anatomical abnormalities) can affect testicular function, sperm production, and overall fertility.13,14 Recent studies have highlighted the rising incidence of hormonal imbalances and sperm disorders, which are increasingly recognized as critical factors contributing to infertility in men.15,16
Moreover, international organizations and governments have implemented various strategies to address male infertility. Programs focused on public education, lifestyle changes, and improved access to fertility treatments have been introduced in several countries to reduce infertility rates and mitigate its social consequences.17 However, further efforts are needed to tackle the underlying causes of male infertility and to improve healthcare policies that support affected families.
In addition to these efforts, advances in assisted reproductive technologies (ART) and genetic research have provided promising new treatments for male infertility, such as sperm retrieval techniques and genetic screening, offering hope to affected individuals and couples.18,19
Given the global significance of male infertility, it is important to focus on specific regions to better understand its burden and develop targeted interventions. The BRICS countries, which include Brazil, the Russian Federation, India, China, and South Africa, are of particular importance due to their large populations and unique socio-economic contexts.20 These countries face significant challenges in public health, economic development, and social welfare, making them crucial to understanding the broader impact of male infertility.21
In light of these challenges, studying the burden of male infertility in the BRICS countries provides valuable insights into the global public health landscape. Therefore, building upon our previous research on the burden of male infertility in China,22 our research team used data from the recently updated Global Burden of Disease (GBD) database to conduct an epidemiological assessment of the disease burden in the BRICS countries and project trends for the coming decades.
Methods
Data Resources and Definitions
Data on the burden of male infertility in the BRICS countries included in this study were obtained from the public database GBD 2021, a database initiated and compiled by organisations such as the World Bank and the World Health Organization to assess the burden of various diseases and injuries on a global scale. GBD 2021, the latest release in 2024, contains data on 371 diseases and injuries and their 88 major risk factors from 204 countries and territories. The data are available online at http://ghdx.healthdata.org/gbd-results-tool.
In the 11th edition of the International Classification of Diseases (ICD-11), male infertility, coded GB04, is defined as any disorder of the male reproductive system characterised by ejaculatory dysfunction or abnormal loss of measurable sperm in semen. This category is further divided into three subcategories: azoospermia (GB04.0), other specifically related to male infertility (GB04.Y) and not specifically related to male infertility (GB04.Z). In addition, the code for drug-induced male infertility due to hypotesticular dysfunction was 5A81.1; the codes for male infertility due to cystic fibrosis include CA25.0, CA25.1 and CA25.Z.
Descriptive Analysis
In order to better describe the burden of male infertility in the BRICS countries between 1990 and 2021, this study examined and obtained two statistical parameters: the prevalence number and the age-standardised prevalence rate (ASPR). The age-standardized prevalence rate was calculated using the direct standardization method, which involves applying the age-specific prevalence rates from each country to a standard population to account for differences in age distribution.
For the standard population, we used the World Health Organization’s World Standard Population. This approach ensures that comparisons between countries are not biased by differences in the age structures of their populations. The age groups used for standardization were: 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49 years.
The ASPR was calculated by multiplying the age-specific prevalence rate (Pi) in each age group by the weight (Wi) of that age group in the standard population, summing these products across all age groups, and then normalizing the calculation by dividing by the total weight (Wtotal) of all age groups in the standard population. The formula for calculating the age-standardized prevalence rate (ASPR) is as follows:
This method provides a more accurate comparison of male infertility burden across countries with different population age distributions.
Joinpoint Regression Models
In this study, temporal trends in the disease burden of male infertility in the BRICS countries were analysed using the Joinpoint regression model, which analyses trend turning points and identifies inflection points in time series data, thereby decomposing the overall trend into sub-segmental trends.23–25 As a statistical method for analysing local disease trends, the Joinpoint regression model is more suitable for public health and epidemiological studies than other methods for analysing overall disease trends. The results of the Joinpoint regression model are expressed as annual percentage change (APC) and average annual percentage change (AAPC). An APC or AAPC greater than 0 indicates that the burden of disease has tended to increase over the period; conversely, that the burden of disease has tended to decrease. p-values less than 0.05 were considered statistically significant.
We used the Joinpoint software to perform the analysis, which automatically detects the change points and fits the data to piecewise linear trends. The software’s default settings were applied, and we selected the simplest model that best fit the data, as indicated by the outputs. While the software internally handles assumptions such as the distribution of the data and the linearity of trends, we did not explicitly test or verify these assumptions before applying the model.
To ensure transparency, we have included the charts generated by the Joinpoint software, which display the data with the identified change points and the corresponding fitted regression lines.
Decomposition Analysis
Decomposition analysis is a method used to understand patterns and changes in data. Unlike traditional statistical methods such as linear regression, decomposition analysis can assess in detail the contribution of each factor to the overall change in the burden of disease, helping us better understand the impact of different factors on the overall change.26 In this study, we used the Das Gupta method,27 a classic decomposition technique, to analyze the change in the burden of male infertility in the BRICS countries between 1990 and 2021, along three dimensions: population age structure, population growth, and epidemiological trends.
Population age structure refers to the shift in the population’s age distribution, which can influence the burden of male infertility as the proportion of older individuals increases. Additionally, population growth captures the increase in overall population size, contributing to the burden of male infertility as the number of individuals at risk rises. Furthermore, epidemiological trends reflect changes in male infertility rates due to factors such as healthcare improvements, environmental influences, and other social determinants.
The Das Gupta method was chosen because it effectively analyzes how these factors—population growth, age structure, and epidemiological trends—contribute to changes in the overall disease burden. By analyzing these trends, we gain a clearer understanding of the underlying drivers of the changes in the burden of male infertility.
Prediction Models
The Autoregressive Integrated Moving Average (ARIMA) model is a commonly used time series analysis method that combines three components - autoregression (AR), integration (I) and moving average (MA) - to effectively capture trends and seasonal changes in time series data.28–30 The parameters of an ARIMA model are usually denoted as ARIMA (p, d, q),31 where
- p (autoregressive coefficient) indicates that the values from the previous P time points are used to predict the current value. The autoregressive part means that the current value depends on a linear combination of the previous P values.
- d (integration coefficient) indicates how many integration operations are needed to make the data a smooth series. Integration means calculating the difference between neighbouring observations to remove the trend or seasonal component from the time series.
- q (moving average order) indicates that the first q error terms are used to predict the current value. The moving average component implies that the current value depends on a linear combination of the first q error terms.
Therefore, this study proposes to use the ARIMA model to predict and analyse the burden of male infertility in the BRICS countries over the next 15 years.
Results
Description of the Disease Burden
As shown in Figure 1A and Supplementary Table 1, the global burden of male infertility increases significantly between 1990 and 2021, with the prevalence of the disease reaching 55.00 million (95% UI: 32.61 to 88.73) in 2021, an increase of 74.66% compared with 31.49 million (95% UI: 18.73 to 50.17) in 1990. The burden of male infertility in South Africa shows a decreasing-increasing-decreasing-increasing pattern over time, with the highest prevalence of 0.34 million (95% UI: 0.19 to 0.58) in 2010 and the lowest of 0.17 million (95% UI: 0.09 to 0.30) in 1994. Brazil also shows a significant increase between 1990 and 2021, from 0.60 million (95% UI: 0.33 to 1.03) in 1990 to 1.34 million (95% UI: 0.75 to 2.22) in 2021. The burden of male infertility in China increased steadily between 1990 and 2007 and then declined over time between 2008 and 2021, peaking at 13.42 million in 2007 (95% UI: 7.37 to 22.61). The burden of disease in India increases over time and will surpass that of China in 2020, peaking at 12.35 million (95% UI: 7.08 to 20.28) in 2021, from a low of 4.11 million (95% UI: 2.32 to 6.80) in 1990. Russia is the only BRICS country where the burden of disease is decreasing and declining over time, with a level of 1.50 million (95% UI: 0.82 to 2.53) in 2021, an 18% decrease from the 1990 level of 1.77 million (95% UI: 0.96 to 3.01).
 |
Figure 1 Trends in the burden of male infertility globally and in BRICS countries between 1990 and 2021. (A) Prevalence number; (B) ASPR. |
According to Figure 1B and Supplementary Table 2, the global ASPR for male infertility did not change significantly between 1990 and 2010 and gradually increased after 2010 to reach 1354.76/100,000 in 2021 (95% UI: 802.12 to 2174.77). The burden of disease in South Africa shows a decreasing trend over the periods 1990–1995 and 2010–2015, and an increasing trend over the period 2001–2010. The highest value was 1242.56/100,000 (95% UI: 675.09 to 2159.69) in 2010 and the lowest value was 722.59/100,000 (95% UI: 377.22 to 1360.38) in 2021. The ASPR for Brazil first decreased over time, reaching a low of 4.09/100,000 in 2000 (95% UI: 1.48 to 9.98), then the burden increased and finally reached an equilibrium level, with a maximum value of 1,129.52/100,000 (95% UI: 640.25 to 1,898.58) in 2015. The ASPR for male infertility in China increased significantly only between 1990 and 1994 and has remained relatively stable since then, with a maximum of 1624.91/100,000 (95% UI: 908.34 to 2723.29) in 1997 and a minimum of 1517.39/100,000 (95% UI: 834.93 to 2599.79) in 1990. In India, the burden of disease increased rapidly between 2010 and 2015, peaking at 1519.57/100,000 (95% UI: 878.85 to 2497.76) in 2016. Although Russia’s ASPR has not changed significantly over time, it remains the highest among the BRICS countries and well above the global level. Its lowest value was 2142.99/100,000 (95% UI: 1172.22 to 3604.65) in 2010 and its highest value was 2171.13/100,000 (95% UI: 1195.99 to 3632.18) in 1990.
Analysis of the Joinpoint Regression Models
The results of the Joinpoint regression analyses are shown in Figure 2 and Table 1, and the AAPCs are statistically significant (p < 0.05) both globally and for the BRICS countries.
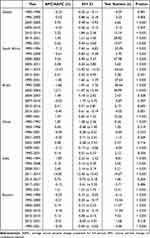 |
Table 1 Joinpoint Regression Analysis: APC and AAPC of ASPR for Male Infertility in Global and BRICS From 1990 to 2021 |
The global analysis of male infertility showed an overall increasing trend in ASPR with an AAPC of 0.52 (95% CI: 0.44 to 0.60). In terms of sub-trends, there was a decreasing trend in the periods 1990–1996, 1996–2000 and 2005–2010, and an increasing trend in the periods 2000–2005, 2010–2014 and 2014–2021. Brazil shows an overall increasing trend with an AAPC of 1.03 (95% CI: 0.83 to 1.22). Its sub-trends show a decreasing trend in 1990–2000, 2007–2010 and 2015–2021, and an increasing trend in 2000–2004, 2004–2007 and 2010–2015. The ASPR for male infertility in China showed an overall increasing trend, with an AAPC of 0.14 (95% CI: 0.01 to 0.27). The 1990–1993, 1993–1996 and 2005–2009 periods showed an increasing trend, whereas the 1996–2001, 2001–2005 and 2009–2021 periods showed a decreasing trend. In addition, the burden of disease in India increased rapidly between 2011 and 2014, which had a significant impact on the overall trend. The AAPC for India was 1.51 (95% CI: 1.21 to 1.75).
The regression analysis for South Africa shows an overall downward trend in its ASPR, with an AAPC of −1.50 (95% CI: −1.60 to −1.39). The periods 1990–1994, 1994–2000 and 2011–2015 show a downward trend, while the periods 2000–2005, 2005–2011 and 2015–2021 show an upward trend. Another country with a reduced burden of disease, Russia, had an AAPC of −0.03 (95% CI: −0.04 to −0.02), although it increased in some periods (eg 2000–2005 and 2010–2015).
Decomposition Analysis
As shown in Figure 3 and Table 2, the prevalence of male infertility increased significantly worldwide over the last two decades (1990–2021), with ageing, population growth and epidemiological changes accounting for 34.71%, 45.74% and 19.55% of the increase respectively. Among the BRICS countries, India had the largest increase in prevalence over the two decades, with the smallest contribution from ageing (8.54%) and the largest from population growth (47.12%). The second largest increase in the number of people with the disease was in China, where, in contrast to India, ageing accounted for most of the increase (71.02%), while population growth accounted for only 22.92%. In Brazil, ageing accounted for 13.98%, population growth for 47.61% and epidemiological changes for 38.40% of the increase in cases.
 |
Table 2 Results of Decomposition Analyses of the Changing Burden of Male Infertility Globally and in the BRICS Countries |
The results of the data analysed for South Africa and Russia are interesting. Although South Africa had the smallest increase in prevalence, ageing, population growth and epidemiological changes accounted for 123.98%, 136.22% and −160.20% of the increase in prevalence, respectively. In addition, Russia is the only BRICS country with a negative increase in prevalence, with ageing, population growth and epidemiological changes accounting for 79.37%, 18.17% and 2.46% of the negative increase, respectively.
Predictive Analyses of the ARIMA Models
According to the ARIMA prediction models (Figure 4), the global and BRICS ASPR for male infertility will show different trends over the next 15 years. First, the global male infertility ASPR shows a steady trend of gradual increase over the next 15 years. Both Brazil and India have relatively stable trends in ASPR, with no significant increases or decreases. In terms of future disease burden, South Africa, China and Russia are more similar in that they all show an initial elevated trend, with the difference that South Africa has an estimated 10 years of elevation before a slow decline, while China has an elevated trend for the first three years and then an orderly decline. In the case of Russia, the ASPR shows an upward trend, then a downward trend, then an upward trend.
Discussion
The global prevalence of male infertility has worsened dramatically between 1990 and 2021, and this trend is predicted to continue for the next 15 years. On the other hand, although China and Russia still have high ASPR, their prevalence number has gradually improved over these 30 years. In addition, although India and Brazil have failed to control male infertility over the past 30 years, it is reassuring to see that both countries have gradually controlled the deterioration in prevalence in recent years, and this will continue over the next 15 years. Finally, the burden of disease is most specific to South Africa, which has experienced large fluctuations over the period 2001–2015 and is projected to experience significant changes again over the next 15 years.
Statistically, India has the highest increase in male infertility among the BRICS countries at 8.51 million. A combination of unchecked population growth and poorly controlled epidemiology has played a crucial role. Studies show that India is expected to overtake China as the world’s most populous country in the next decade, reaching 1.5 billion people by around 2025.32 With this population explosion, the demand for reproductive health services in India has increased dramatically. However, due to socio-cultural constraints, assisted reproductive technology (ART) and other infertility treatments have been slow to take off in India and are highly regulated.33–35 In addition, India faces enormous challenges in building its healthcare system. According to studies, India has only about 860 hospital beds per million people, far below the World Health Organization’s global average of 3960 beds per million people.36 This lack of healthcare resources limits the diagnosis and treatment of male infertility. In addition, due to uneven economic development and rising healthcare costs, around a quarter of India’s population does not have access to healthcare services and 60 million Indians have fallen below the poverty line,37,38 further exacerbating the poor state of male infertility in India.
In the last 30 years, the prevalence number of male infertility in Brazil has increased by 121.62% and the age-standardised prevalence by 36.32%. In Brazil, the federal constitution guarantees health care as a right for all citizens, and the state is required to cover all health-related expenditures within the national health system.39 Despite the significant economic progress that Brazil has made in these three decades, the country’s economic situation remains bleak, and poverty rates and the gap between rich and poor in Brazil remain high. In addition, despite Brazil’s significant efforts to achieve reproductive health goals, there is still a large gap between the reproductive health technologies available in Brazil and those specialised in infertility, including ART.40,41 In addition, access to ART for infertile couples is limited in many ways due to inadequate services and a lack of political commitment to support existing and new services.39 Furthermore, Brazil is one of the world’s largest consumers of pesticides, and Brazilian agricultural producers use large amounts of pesticides in the production of their products to achieve higher yields,42 and a number of diseases, including male infertility and testicular cancer, are thought to be closely linked to high levels of pesticide use.43 As a result of these factors, the management of male infertility in Brazil has been in a difficult situation for the past 30 years.
Decomposition analysis shows that ageing is the main contributor to the growing burden of male infertility in China. Due to strict family planning policies over the past 30 years,44 China is now considered one of the fastest ageing countries in the world.45 Research shows that by 2019, 11.5% of China’s population will be aged 65 and over, and the United Nations estimates that this will rise to 16.9% by 2030.46 In addition, China experienced a wave of fertility peaks in the 1970s,47 a cohort that not only witnessed the country’s rapid economic growth between 1990 and 2010, but also happened to be in the middle of a full fertility cycle. In addition, China’s rapid industrialisation in the early 1990s led to increased pollution. And studies have shown that exposure to heavy metals, pesticides and chemicals is strongly associated with reduced male fertility.48,49 On the other hand, the Chinese government has continued to invest in healthcare as its economy has boomed, launching the largely state-funded New Rural Cooperative Medical Scheme (NRCMS) and Urban Resident Basic Health Insurance (URBHI) in 2003 and 2007 respectively.50,51 With continued progress in public health policies and improvements in medical facilities and technology, male infertility patients can be detected earlier and treated in a more timely manner. As a result, the increasing trend of the disease has been reversed. Furthermore, with the rapid development of Chinese society, public health awareness is increasing and men’s lifestyles (eg smoking, alcohol consumption and dietary habits) are gradually improving, which has also had a positive impact on the disease burden of male infertility.
The dissolution of the Soviet Union in the early 1990s not only led to dramatic social changes, but also had a significant impact on the lives of the Russian people.52 The social upheaval not only hampered Russia’s economic development, but also had a serious impact on the size and structure of the country’s population.53 As the only BRICS country with negative growth in male infertility, Russia’s improved disease burden is not due to proper management of male infertility, but is largely due to its declining population size. In addition, Russia’s unique geography (long, cold winters with frequent heavy snowfalls and strong winds) and social unrest have led to a rapid increase in alcohol consumption,54 the use of which has been shown to have a significant impact on the development of male infertility.55
The burden of male infertility in South Africa has seen significant ups and downs over the past 30 years and is expected to increase again over the next 15 years. Several factors have contributed to this phenomenon. Firstly, South Africa’s public health system faces serious challenges, not only in terms of a severe shortage of health professionals, but also in terms of a very unequal distribution of its limited health resources, reflected in the inequitable distribution of resources between sectors and geographical areas.37 This has resulted in a lack of equitable access to effective health care for the South African population, especially the poor.56 Secondly, South Africa has one of the highest unemployment rates in the world, with a youth unemployment rate of 66.5%.57 In the face of such poor economic conditions, South African men find it difficult to generate sufficient income and medical coverage to assess and treat male infertility, which affects them as individuals.
Even though we have chosen to use a variety of models to analyse the data in the latest GBD 2021 database, there are still some limitations to this study for the following reasons. First, the data included in the GBD 2021 database are estimates rather than actual observations. Therefore, the results obtained from modelling these data may be biased. In addition, this study lacks a more disaggregated analysis to capture local variations across regions within the BRICS countries. Further analysis of health care resources and their health problems, which vary from region to region within countries, would have provided more valuable information on the prevention and treatment of male infertility in the BRICS countries and globally.
Conclusion
This comprehensive analysis from GBD2021 provides a new interpretation of the changing burden of male infertility in the BRICS countries over the past three decades. Our study provides valuable insights for policy makers and healthcare providers by providing a careful analysis and scientific assessment of the prevalence of male infertility through multiple statistical analysis models. In addition, BRICS countries should develop targeted prevention strategies for potentially affected populations, as well as effective treatments for affected populations, based on national contexts and global trends.
Data availability statement
The data that support the findings of this study are available from the Global Burden of Disease 2021 database, which is publicly available. Requests to access the database should be directed to https://ghdx.healthdata.org/gbd-2021.
Ethics Statement
Ethical approval for this study, including the use of publicly available human data, was obtained from the Pingyang Hospital of Wenzhou Medical University Ethics Committee.
Acknowledgments
We thank all the participants and investigators of the GBD 2021.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no financial support was received for the research.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
2. Diaz P, Dullea A, Chu KY, et al. Future of male infertility evaluation and treatment: brief review of emerging technology. Urology. 2022;169:9–16. doi:10.1016/j.urology.2022.06.036
3. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi:10.1016/j.clinbiochem.2018.03.012
4. Huang B, Wang Z, Kong Y, Jin M, Ma L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: an analysis of global burden of disease study. BMC Public Health. 2023;23(1):2195. doi:10.1186/s12889-023-16793-3
5. Global fertility in 204 countries and territories, 1950-2021, with forecasts to 2100: a comprehensive demographic analysis for the global burden of disease study 2021. Lancet. 2024;403(10440):2057–2099. doi:10.1016/S0140-6736(24)00550-6
6. Öztekin Ü, Hacimusalar Y, Gürel A, Karaaslan O. The relationship of male infertility with somatosensory amplification, health anxiety and depression levels. Psychiatry Invest. 2020;17(4):350–355. doi:10.30773/pi.2019.0248
7. Ahmadi H, Montaser-Kouhsari L, Nowroozi MR, Bazargan-Hejazi S. Male infertility and depression: a neglected problem in the middle East. J Sex Med. 2011;8(3):824–830. doi:10.1111/j.1743-6109.2010.02155.x
8. Salie M, Roomaney R, Andipatin M, Volks C. Scoping review of the psychosocial aspects of infertility in developing countries: protocol. BMJ Open. 2021;11(5):e044003. doi:10.1136/bmjopen-2020-044003
9. Leeners B, Tschudin S, Wischmann T, Kalaitzopoulos DR. Sexual dysfunction and disorders as a consequence of infertility: a systematic review and meta-analysis. Hum Reprod Update. 2023;29(1):95–125. doi:10.1093/humupd/dmac030
10. Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology. 2012;80(4):822–825. doi:10.1016/j.urology.2012.07.002
11. Ricci E, Al Beitawi S, Cipriani S, et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34(1):38–47. doi:10.1016/j.rbmo.2016.09.012
12. Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. doi:10.1093/humrep/det428
13. Ma Y, He X, Qi K, et al. Effects of environmental contaminants on fertility and reproductive health. J Environ Sci. 2019;77:210–217. doi:10.1016/j.jes.2018.07.015
14. Crocetto F, Risolo R, Colapietro R, et al. Heavy metal pollution and male fertility: an overview on adverse biological effects and socio-economic implications. Endocr Metab Immune Disord Drug Targets. 2023;23(2):129–146. doi:10.2174/1871530322666220627141651
15. Ohlander SJ, Lindgren MC, Lipshultz LI. Testosterone and male infertility. Urol Clin North Am. 2016;43(2):195–202. doi:10.1016/j.ucl.2016.01.006
16. Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin Reprod Med. 2009;27(2):149–158. doi:10.1055/s-0029-1202303
17. De Jonge CJ, Barratt CLR, Aitken RJ, et al. Current global status of male reproductive health. Hum Reprod Open. 2024;2024(2):hoae017. doi:10.1093/hropen/hoae017
18. Mazzilli R, Rucci C, Vaiarelli A, et al. Male factor infertility and assisted reproductive technologies: indications, minimum access criteria and outcomes. J Endocrinol Invest. 2023;46(6):1079–1085. doi:10.1007/s40618-022-02000-4
19. Hanson BM, Kaser DJ, Franasiak JM. Male infertility and the future of in vitro fertilization. Urol Clin North Am. 2020;47(2):257–270. doi:10.1016/j.ucl.2019.12.012
20. Jakovljevic M, Lamnisos D, Westerman R, Chattu VK, Cerda A. Future health spending forecast in leading emerging BRICS markets in 2030: health policy implications. Health Res Policy Syst. 2022;20(1):23. doi:10.1186/s12961-022-00822-5
21. Jakovljevic M, Timofeyev Y, Ekkert NV, et al. The impact of health expenditures on public health in BRICS nations. J Sport Health Sci. 2019;8(6):516–519. doi:10.1016/j.jshs.2019.09.002
22. Xu R, Wang XJ, Lin QC, et al. Trends and projections of the burden of disease for male infertility in China from 1990 to 2021: an analysis from the global burden of disease 2021 study. Front Reprod Health. 2024;6:1501675. doi:10.3389/frph.2024.1501675
23. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi:10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
24. Kim HJ, Chen HS, Byrne J, Wheeler B, Feuer EJ. Twenty years since Joinpoint 1.0: two major enhancements, their justification, and impact. Stat Med. 2022;41(16):3102–3130. doi:10.1002/sim.9407
25. Kim S, Lee S, Choi JI, Cho H. Binary genetic algorithm for optimal joinpoint detection: application to cancer trend analysis. Stat Med. 2021;40(3):799–822. doi:10.1002/sim.8803
26. Bai Z, Han J, An J, et al. The global, regional, and national patterns of change in the burden of congenital birth defects, 1990-2021: an analysis of the global burden of disease study 2021 and forecast to 2040. EClinicalMedicine. 2024;77:102873. doi:10.1016/j.eclinm.2024.102873
27. Gupta PD, Habeebullah CM, Gupta PD. Standardization and decomposition of rates: a users’s manual. Washington d. Biochem Cell Biol. 1993;71(5–6):241–247. doi:10.1139/o93-037
28. ArunKumar KE, Kalaga DV, Sai Kumar CM, Chilkoor G, Kawaji M, Brenza TM. Forecasting the dynamics of cumulative COVID-19 cases (confirmed, recovered and deaths) for top-16 countries using statistical machine learning models: auto-regressive integrated moving average (ARIMA) and seasonal auto-regressive integrated moving average (SARIMA). Appl Soft Comput. 2021;103:107161. doi:10.1016/j.asoc.2021.107161
29. Wang YW, Shen ZZ, Jiang Y. Comparison of ARIMA and GM(1,1) models for prediction of hepatitis B in China. PLoS One. 2018;13(9):e0201987. doi:10.1371/journal.pone.0201987
30. Jian Y, Zhu D, Zhou D, et al. ARIMA model for predicting chronic kidney disease and estimating its economic burden in China. BMC Public Health. 2022;22(1):2456. doi:10.1186/s12889-022-14959-z
31. Newbold P. ARIMA model building and the time series analysis approach to forecasting. J Forecasting. 2010;2(1):23–35. doi:10.1002/for.3980020104
32. Kc S, Wurzer M, Speringer M, Lutz W. Future population and human capital in heterogeneous India. Proc Natl Acad Sci USA. 2018;115(33):8328–8333. doi:10.1073/pnas.1722359115
33. Ga R, Muvvala SPR. Access to infertility care and ART treatment in India: a clinician’s perspective. Best Pract Res Clin Obstet Gynaecol. 2023;86:102302. doi:10.1016/j.bpobgyn.2022.102302
34. Kaushal R, Gurnani N, Kumar M, Dada R, Kumar R. Male infertility in India: demographics, aetiology and outcomes of standard clinical practice. Natl Med J India. 2020;33(6):340–343. doi:10.4103/0970-258X.321136
35. Amodini KN, Chaudhuri S. Infertility Management in India: issues and Potential Solutions. J Obstet Gynaecol India. 2023;73(4):368–369. doi:10.1007/s13224-023-01814-3
36. Singh N, Shukla R, Acharya S, Shukla S. The past, present, and future of health economics in India. J Family Med Prim Care. 2022;11(12):7513–7516. doi:10.4103/jfmpc.jfmpc_2266_21
37. Marten R, McIntyre D, Travassos C, et al. An assessment of progress towards universal health coverage in Brazil, Russia, India, China, and South Africa (BRICS). Lancet. 2014;384(9960):2164–2171. doi:10.1016/S0140-6736(14)60075-1
38. Lancet T. The struggle for universal health coverage. Lancet. 2012;380(9845):859. doi:10.1016/S0140-6736(12)61485-8
39. Makuch MY, Bahamondes L. Barriers to access to infertility care and assisted reproductive technology within the public health sector in Brazil. Facts Views Vis Obgyn. 2012;4(4):221–226.
40. Makuch MY, Petta CA, Osis MJ, Bahamondes L. Low priority level for infertility services within the public health sector: a Brazilian case study. Hum Reprod. 2010;25(2):430–435. doi:10.1093/humrep/dep405
41. Makuch MY, Simônia de Padua K, Petta CA, Duarte Osis MJ, Bahamondes L. Inequitable access to assisted reproductive technology for the low-income Brazilian population: a qualitative study. Hum Reprod. 2011;26(8):2054–2060. doi:10.1093/humrep/der158
42. Panis C, Kawassaki ACB, Crestani APJ, et al. Evidence on human exposure to pesticides and the occurrence of health hazards in the Brazilian population: a systematic review. Front Public Health. 2021;9:787438. doi:10.3389/fpubh.2021.787438
43. Franco APS, Lima Figueiredo ER, Melo GS, et al. Predictors of testicular cancer mortality in brazil: a 20-year ecological study. Cancers 2023;15(16):4149. doi:10.3390/cancers15164149
44. Bu L, Fee E. Family planning and economic development in China. Am J Public Health. 2012;102(10):1858–1859. doi:10.2105/AJPH.2012.300731
45. Wu X, Li X, Xu M, Zhang Z, He L, Li Y. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS One. 2021;16(3):e0247617. doi:10.1371/journal.pone.0247617
46. Man W, Wang S, Yang H. Exploring the spatial-temporal distribution and evolution of population aging and social-economic indicators in China. BMC Public Health. 2021;21(1):966. doi:10.1186/s12889-021-11032-z
47. Stycos JM. The second great wall of China: evolution of a successful policy of population control. NPG Forum Ser. 1989;1–5.
48. Baszyński J, Kamiński P, Mroczkowski S, et al. Do aluminum, boron, arsenic, cadmium, lipoperoxidation, and genetic polymorphism determine male fertility? Ecotoxicol Environ Saf. 2024;284:116919. doi:10.1016/j.ecoenv.2024.116919
49. Uwamahoro C, Jo JH, Jang SI, et al. Assessing the risks of pesticide exposure: implications for endocrine disruption and male fertility. Int J Mol Sci. 2024;25(13):6945. doi:10.3390/ijms25136945
50. Liu Q, Liu J, Sui S. Public medical insurance and healthcare utilization and expenditures of older with chronic diseases in rural China: evidence from NRCMS. Int J Environ Res Public Health. 2020;17(20):7683. doi:10.3390/ijerph17207683
51. Xie Z, Chen S, He C, et al. Trends and age-period-cohort effect on the incidence of falls from 1990 to 2019 in BRICS. Heliyon. 2024;10(5):e26771. doi:10.1016/j.heliyon.2024.e26771
52. Brainerd E. Mortality in Russia since the fall of the soviet union. Comp Econ Stud. 2021;63(4):557–576. doi:10.1057/s41294-021-00169-w
53. Baker TD, Baker SP, Haack SA. Trauma in the Russian Federation: then and now. J Trauma. 2011;70(4):991–995. doi:10.1097/TA.0b013e31820e27c6
54. Leon DA, Shkolnikov VM, McKee M. Alcohol and Russian mortality: a continuing crisis. Addiction. 2009;104(10):1630–1636. doi:10.1111/j.1360-0443.2009.02655.x
55. Basic M, Mitic D, Krstic M, Cvetkovic J. Tobacco and alcohol as factors for male infertility-a public health approach. J Public Health. 2023;45(2):e241–e9. doi:10.1093/pubmed/fdac042
56. Ataguba JE, McIntyre D. Who benefits from health services in South Africa? Health Econ Policy Law. 2013;8(1):21–46. doi:10.1017/S1744133112000060
57. Geza W, Ngidi MSC, Slotow R, Mabhaudhi T. The dynamics of youth employment and empowerment in agriculture and rural development in South Africa: a scoping review. Sustainability. 2022;14(9):5041. doi:10.3390/su14095041
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





