Back to Journals » International Journal of Nanomedicine » Volume 19
The Application of Nanotechnology for the Diagnosis and Treatment of Endocrine Disorders: A Review of Current Trends, Toxicology and Future Perspective
Received 27 June 2024
Accepted for publication 28 August 2024
Published 25 September 2024 Volume 2024:19 Pages 9921—9942
DOI https://doi.org/10.2147/IJN.S477835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Yan Yan, Hanqing Cai, Maoguang Yang
Department of Endocrinology, The Second Hospital of Jilin University, Changchun, 13000, People’s Republic of China
Correspondence: Maoguang Yang, Department of Endocrinology, The Second Hospital of Jilin University, Changchun, 13000, People’s Republic of China, Email [email protected]
Abstract: The endocrine system regulates many biological systems, and disruptions may result in disorders, such as diabetes, thyroid dysfunction, Cushing’s syndrome, and obesity. The total incidence of endocrine illnesses was found to be 47.4%, excluding type 2 diabetes mellitus, with a significant frequency of newly diagnosed endocrine disorders. Nanotechnology manipulates particles at the atomic and molecular levels, opening up new paths for studying disease etiology and therapeutic alternatives. The goal of using nanomaterials in the treatment of endocrine illnesses is to create endogenous nano-biosensors that can detect even modest changes in hormone levels and react spontaneously to restore normal function. The size and surface characteristics of nanoparticles enhances the sensitivity in nano-sensors and are functionalized for targeted drug delivery. Nano-sized carriers composed of lipids, polymers, carbon, or metals have been shown to work much better than standard drug delivery methods. Nanoparticles (NPs) offer various advantages over current methods for diagnosing and treating endocrine disorders, acting as hydrogels for insulin delivery and wound healing. Incorporating selenium NPs into inorganic nanoparticles enhances their bioactivity and targeted delivery. Gold NPs show a promising precise insulin delivery. Mesoporous silica NPs maintain glycemic level effectively and lipid and polymeric NPs protect drugs from degradation in the gastrointestinal tract. Carbon nanotubes (CNTs) have become popular in thyroid surgeries. These characteristics make nanoparticles valuable for developing effective diagnostic and therapeutic systems. NP-based medicines have been thoroughly researched in order to identify the beginning point for the creation of theranostics, which may function in two ways: as imaging agents or therapeutics. The study posits that nanotechnology bridges diagnostics and therapies, potentially revolutionizing endocrine disorder treatments. This review delves into nanotechnology techniques, emphasizing their applications in diagnosing and treating diabetes mellitus.
Keywords: nanomedicines, theranostics, endocrine disorders, diabetes, thyroid dysfunction
Introduction
The endocrine system is made up of several glands that secrete hormones that play an important role in regulating different physiological activities. These glands might exist as fully developed organs or as a smattering of tissues and cells. Endocrine malfunction may result in a variety of disorders, such as diabetes, thyroid dysfunction, Cushing’s syndrome, and obesity. While there is no treatment for these conditions, modern drugs may help to monitor and maintain hormone levels as near to natural levels as feasible, hence assisting in the preservation of bodily hemostasis.1
The overall incidence of endocrine diseases, excluding type 2 diabetes mellitus, was found to be 47.4%. Primary hypothyroidism was diagnosed in 18.1% of patients, whereas pituitary disease, Cushing syndrome, and other endocrine illnesses were found in 1.9%, 0.8%, and less than 1%, respectively. Remarkably, the study also revealed a high prevalence of newly diagnosed endocrine disorders, which were found in 16.3% of patients.2 According to the study conducted to check the prevalence of endocrine disorders in obese patients, it has been found that about 14% of obese had hypothyroidism; other 14.6% had milder form of hypothyroidism called subclinical hypothyroidism.3
The population of the United States is plagued by a wide variety of endocrine disorders, such as thyroiditis, diabetes, impaired insulin responsiveness, obesity, metabolic syndrome, osteoporosis, osteopenia, mild-to-moderate hypovitaminosis D, erectile dysfunction, and dyslipidemia. Some of these disorders are more prevalent in some demographics than others.4 Findings from the recent study also describe that individuals with Down syndrome in the United States face a greater prevalence of endocrine disorders compared to individuals of the same age and sex who do not have Down Syndrome.5 These diseases affect at least 5% of the adult population in the United States, resulting in substantial health and economic consequences.4
Over the last decade, nanotechnology has seen significant integration into biomedical applications, such as drug delivery, disease diagnosis, and diagnostic imaging. Nanotechnology is the manipulation of atomic and molecular particles with at least one dimension ranging from 1 to 100 nanometers which are called nanoparticles.6 These particles are more effective in localizing cells than bulkier materials because they have unique physical, chemical, biological, size distribution, form, and other features (Royal Society). Prior to recent developments, noble metal nanoparticles (NPs), magnetic nanoparticles (NPs), and quantum dots (QDs) were used in pharmaceutical and biomedical analysis and its application in drug delivery and detecting metal ions, proteins, and nucleic acids in biological markers.7
Nanomaterials are widely used in different fields, including biological systems. Endocrine dysfunction can result in well-established diseases like diabetes mellitus, thyroid and parathyroid disorders, infertility, and obesity. Keeping the gravity of the situation in sight, the researchers are working to find better treatment strategies for endocrine disorders. Nanotechnology offers a promising technique to address this issue.1 Despite the promising outcomes, research on the use of nanotechnology for managing endocrine disorders is still in the preclinical stage. The objective of incorporating nanomaterials in the treatment of endocrine diseases is to establish endogenous nano-biosensors capable of detecting even minor changes in hormonal levels and spontaneously responding in a coordinated manner to restore normal function. This internal monitoring system is referred to as nanonetworks, consisting of nanomachines that form units and communicate chemically with each other.2
Recent advances in nanotechnology provide vast opportunities to target and alter the behavior of the cells at the nanoscales and combine diagnosis and treatment on a single platform. In order to establish the foundation for theranostics, nano-therapy shows promising prognoses, preventing curable diseases from turning fatal sue to delayed diagnosis and treatment. Some nanoparticles have already been used as imaging agents and hold the potential to become theranostic in the future, serving dual purposes simultaneously.8 The word “theranostics” was created to characterize highly specialized and tailored treatments for a variety of disorders. Conventional methods of drug delivery have a number of drawbacks that are being addressed through the research and development of nanoscale carriers comprised lipids, polymers, carbon, or metals.9
Endocrine system plays a vital role in maintaining body homeostasis through hormone regulation. Disruption of this system can lead to various disorders, such as diabetes mellitus, thyroid and parathyroid disorders, Cushing’s syndrome, infertility, and obesity. There are currently no absolute treatments for these diseases. However, there have been active endeavors to realize superior regimen strategies for the amelioration of endocrine disorders. Nanotechnology has emerged as a promising approach to address these issues, particularly through targeted drug delivery. In this review, we focus nanotechnology techniques and their applications for tackling endocrine disorders, particularly focusing on diagnosis and treatment of diabetes mellitus.
Endocrine Disorders
Hormones are chemical substances that regulate many bodily functions; they are produced and secreted by the endocrine system that regulates a wide range of biological functions like development, metabolism, and reproduction. Endocrine diseases occur when the endocrine system fails to operate correctly, resulting in either excessive or insufficient hormone production. These conditions may have a substantial influence on a person’s health and quality of life.10
Diabetes is one of the most prevalent endocrine illnesses, and it arises when the pancreas fails to generate enough insulin, or the body becomes insulin resistant. Diabetes affects millions of individuals throughout the globe and may lead to major problems such as heart disease, kidney disease, and blindness.11 Research has shown that diabetes is growing more frequent in developing nations, with an estimated 70% of diabetics residing in these countries.12 Thyroid disorders, which affect the thyroid gland in the neck, are another prevalent endocrine disorder. Thyroid disorder may occur in either overproduction or underproduction of thyroid hormones, which control metabolism.13 Hypothyroidism, or a thyroid that is underactive, is more frequent than hyperthyroidism, or an overactive thyroid. Hypothyroidism symptoms include weariness, weight gain, and sadness, while hyperthyroidism symptoms include weight loss, anxiety, and palpitations. Thyroid diseases are more common in women than males.14 Adrenal disorders are another kind of endocrine illness that may have serious consequences for one’s health. The adrenal glands, which are positioned on top of the kidneys, create chemicals such as cortisol and adrenaline, which govern the body’s stress response. Adrenal gland dysfunction may occur in either overproduction or underproduction of these hormones. Cushing’s syndrome, for example, is a disorder in which the body generates excessive cortisol, leading to symptoms, such as weight gain and a round face. However, insufficient cortisol production by the adrenal glands is characteristic of Addison’s disease, which leads to fatigue and weakness.15 Nanotechnology has emerged as a promising approach to address the issues, particularly through targeted drug delivery to endocrine organs. Various nanocarriers are used for addressing this issue that are enlisted in Table 1:
 |
Table 1 Nano-Carriers Used for Endocrine Illness Discussed Below.16 |
Physicochemical Properties Impacting the Theranostics
It is now well established that the physicochemical properties of nanoparticles (NPs) are crucial in determining their interactions with cells. However, the properties including size, shape and surface groups orientation among them are vital.
Size
Nanoparticles, ranging from 1 nm to 100 nm, possess a high surface area-to-volume ratio, making them highly reactive and capable of infiltrating body tissues and fluids.17 Their size and surface area influence their endocytosis, distribution, retention, and elimination within biological systems. Nanoparticles under 200 nm are internalized by clathrin-coated vesicles, while larger ones use caveolae-mediated endocytosis. In immune cells, nanoparticles are phagocytosed, with smaller particles entering through the phagocytotic pathway.18 Liposomes can be engineered for optimal uptake by mammalian cells, enhancing drug activity. Intracellular localization of nanoparticles is size-dependent, with smaller particles reaching the nucleus and larger ones remaining in the cytoplasm.19 Polymeric nanoparticles (20–200 nm) are effective for brain targeting and drug penetration through the blood–brain barrier. PEGylated nanoparticles improve drug stability and circulation time due to their high surface area.20
Surface Chemistry
The surface chemistry of nanoparticles, including their charge and attached chemical groups, is crucial in determining their reactivity and function. Modifications to nanoparticle surfaces, such as coating rod-shaped gold nanoparticles (AuNPs) and DNA with lipid layers or conjugating DNA to cationic liposomes, enhance cellular uptake.21,22 Liposomes and micelles, with their lipid layers, facilitate the intracellular delivery of nanoparticles. Silicon nanoparticles (SiNPs), used in optoelectronics, require surface modification with silicon dioxide (SiO2) to improve their hydrophilicity for biomedical applications. Zinc oxide (ZnO2) nanoparticles, commonly used in sunscreen, have had their surface properties altered to reduce cytotoxicity while retaining UV protection. Liposomes, composed of amphiphilic phospholipids, mimic the plasma membrane, allowing for effective drug delivery through receptor-mediated endocytosis or membrane fusion.23,24
The pH of the delivery environment can significantly influence the functionality of nanoparticles, particularly through their surface chemistry. This principle is harnessed to initiate drug release within the acidic microenvironment of tumors. For example, AuNPs capped with carrageenan oligosaccharides have demonstrated the ability to release epirubicin under acidic conditions, leading to apoptosis in HCT-116 colorectal cancer cells. The surface characteristics of nanoparticles play a crucial role in their behavior within aqueous biological systems, affecting their reactivity and delivery efficiency. These attributes make nanoparticles suitable for applications in biomedical sensors, coatings for medical implants, and drug delivery systems. A pertinent example is the use of silver nanoparticle (AgNP) functionalized titanium implants, which prevent postoperative infections due to their antimicrobial properties.25
In the grand tapestry of medical innovation, liposomes emerge as a marvel of modern science. These vesicles, with their lipid bilayer encasing an aqueous core, serve as vessels for the transport of therapeutic agents in the battle against disease. The inclusion of cholesterol fortifies the phospholipid bilayer, ensuring stability and resilience of the nanoparticles, such as AuNPs, and traditional pharmaceuticals are often cloaked in lipid layers, enhancing their harmony with mammalian cell membranes and facilitating their journey into the cell’s interior. The true genius of liposomes lies in their phospholipid heads, which can be adorned with various compounds, enabling precise targeting. A notable example is the PEGylation of liposomes, a process that cloaks them from the vigilant eyes of phagocytes, thereby enhancing their bioavailability and efficacy. Thus, liposomes stand as a testament to human ingenuity, bridging the realms of biology and technology to deliver hope and healing.26 In the intricate manifestation of cellular targeting, the process permits the incorporation of active groups such as folate and monoclonal antibodies, each serving as a beacon for specific cells. Folate, with its affinity for cancer cells, exploits the high expression of folate receptors to zero in on malignant targets. Meanwhile, monoclonal antibodies, with their versatile nature, bind to unique receptors or surface antigens, offering a tailored approach to targeted delivery.27 Conjugating these agents to nanoparticle surfaces enhances the delivery of nanoparticles or drugs to tumor cells for selective eradication.
Shape
Nanomaterials possess not only tunable sizes but also controllable shapes during their synthesis. The final stage of synthesis allows for the modification of nanoparticle shapes, typically involving the nucleation process where nanoparticulate nuclei, or seeds, merge to form a template for crystal growth. The shape of a nanoparticle, akin to its size, is pivotal to its biological function and reactivity. Generally, spherical nanoparticles are more readily endocytosed compared to rod or tube-shaped ones. This is because the shape influences endocytosis, affecting how the membrane envelops the nanoparticle upon contact. The reduced endocytosis of rod-shaped or other non-spherical nanoparticles is likely due to the cell’s inability to initiate the necessary action-dependent membrane dynamics. This may explain why most pharmacologically active nanoparticles are spherical. However, recent studies have shown that nanoparticles of different shapes, such as long rods, can have prolonged bioavailability and encapsulate more particles compared to spherical and short-rod nanoparticles. Other shapes like nanoflowers and nano-prisms exist but may not be as active due to their unique structures.20
It turns out that the shape of NP has a paramount effect on the process of endocytosis. It is thought that both the clathrin contingent and clathrin devoid cascades partake in it. It is actually the shape that determines the mode via which the NP is supposed to be internalized and perform the function assigned to it, be it the diagnostic one or therapeutic. NPs with regular shapes, especially the spherical shaped, are internalized via the clathrin-dependent cascade. A fun fact about the process is that it is incredibly selective and that additional properties like size also have a say in the matter. In contrast, the clathrin-independent pathways have the advantage of low selectivity, and the irregular shaped particles are taken up in lieu of this mode of endocytosis.28 Interestingly, it has been observed that for certain shapes, the likes of bristle-like or rod-shaped NPs are unresponsive to either pathways and may not be subject to the process of internalization at all.20
Nanomaterial’s Based Diagnostic Tools
Imaging
Imaging organs and tissues while they are still living, or in vivo, are required for morphological and functional assessments. It can help identify and analyse a variety of disorders involving the pituitary, thyroid, adrenal, and reproductive glands, as well as tumors affecting the pancreas, skeletal system, and other endocrine glands.29 In ultrasonography, X-ray computed tomography, magnetic resonance imaging, inherent contrast or contrast agents are commonly used. The structural and physiological properties of glands or lesions can be seen using these imaging techniques. Furthermore, scintigraphy, positron emission tomography (PET), and single-photon emission computed tomography (SPECT) can be used to perform functional and molecular imaging of the disease. These imaging methods use radionuclide-based agents that are applied to the patient’s skin to evaluate biochemical and metabolic markers.30
In endocrinology, optical imaging employing visible or near-infrared wavelengths has emerged as a viable alternative to traditional imaging techniques. In endocrine surgery, fluorescence imaging using indocyanine green or parathyroid autofluorescence, for example, has been used. Fluorescence-guided adrenalectomy or thyroidectomy surgeries, in particular, have been undertaken, with the latter enabling for enhanced identification of the parathyroid glands, which are generally difficult to spot and are occasionally removed inadvertently with the thyroid.31 Label-free autofluorescence imaging is an excellent intraoperative approach for identifying parathyroid tissue. However, the technique has some limitations, such as a maximum penetration depth of 3mm and a lack of data on tissue perfusion and oxygenation, both of which are critical for the preservation of parathyroid glands during surgery.32
Spherical Nanomaterials (0D)
Spherical nanomaterials are commonly used in diagnostic tools for endocrine diseases as they can be attached to biomarkers that react only under specific pathological conditions. This multipurpose nanoparticle serves as a biological label to detect and track target analytes or diseases. Detection mechanisms include measuring light absorption, peak wavelength shifts, surface plasmon resonance, electrical or electrochemical changes, and electrical impedance spectroscopy.33
Gold nanoparticles (AuNPs) are a commonly used 0D nanomaterial in diagnostic tools due to their high bio-affinity, strong color variations based on their diameter or surface, and catalytic properties. These characteristics make AuNPs well-suited for use in both optical and electrochemical point-of-care (PoC) devices.34
AuNPs found in different sizes, ranging from 1nm to 8 µm, and they also exist in various shapes, such as nanospheres, nanorods, nano-shells, nano-cubes, nanocages, and branched forms. AuNPs can be easily synthesized in various sizes and shapes. Due to their large surface area and negatively charged surfaces, they can be functionalized with biological molecules like drugs, genes, and ligands. AuNPs have unique optical and electronic properties that depend on their shape, size, and aggregation status, particularly through plasmon resonance. This property allows them to be highly sensitive to optical detection techniques, making them ideal for bimolecular detection, chemical and biological sensing, protein and cell labelling, molecular imaging, and delivering drugs, gene, antigen, and antibody into cells.35
One Dimensional Nanomaterial (ID)
Single-walled carbon nanotubes are an example of a 1D nanomaterial whose growth occurs in a straight line, resulting in a thickness of just one nanometer. One-dimensional nanomaterials get their name from this development trend. The length of these nanomaterials can range from hundreds of micrometers to a million times longer, and their shape has a significant impact on their application, including their absorption wavelengths and mechanical strength. However, the growth of 1D nanomaterials is complex, requiring careful synthetic routes to ensure their homogeneity.
Mostafalu and Sonkusale developed an innovative approach for electrocardiogram monitoring using 1D nanomaterials in paper substrates. In their study, nanowires made of platinum, nickel, and copper were introduced into the paper substrates to create electrodes that operate in dry conditions without the need for an electrolytic gel. The enormous surface area of the nanowires allows for impedance readings between 100 and 1 K, enabling the electrodes to respond with great quality. This method has the benefit of avoiding the application of an electrolytic gel, which quickly dries out and degrades when transferred. In addition to its potential medical applications, the paper electrodes also functioned well as cathodes in an acidic battery.36
Darabi et al developed a novel application of carbon nanotubes (CNTs) by cleaning chewing gum with water and ethanol, followed by mixing it with a carbon nanotube solution. The resulting mixture was stretched and folded to align the CNTs in one direction, allowing for the detection of humidity through measuring the electrical resistance in the gum. This approach also demonstrated the potential of the device as a motion sensor, detecting body movements and even the user’s breath. This technology could be useful for point-of-care diagnostics, detecting biting problems, dry mouth, pulsations, and other chemical targets. However, the use of CNTs as a sensing material raises concerns, as CNTs are currently classified as cytotoxic nanomaterials and the device’s response is measured through electrical circuits.37
Carbon nanotubes (CNTs) are cylindrical nanomaterials composed of one or more layers of carbon atoms. They can reach lengths in the micrometer range, having diameters around 1–2nm. Due to their unique properties such as high Young’s modulus, tensile strength, and thermal conductivity, they are used in coatings, adhesives, fluorescent markers and drug delivery systems. The CNTs used either pristine or surface modified manufacturer, with containing pristine, hydroxyl (-OH), or carboxylic acid (-COOH) surfaces.38
Liposomes
Liposomes, vesicles, or lipids are spherical structures generated when one or more lipid bilayers enclose water molecules, as seen in Figure 1. These vesicles are made from safe, amphiphilic phospholipids and cholesterol, and they have been widely employed to encapsulate both lipophilic and hydrophilic medicinal compounds. Water-soluble medications are confined inside the inner phase of liposomes, whereas lipid-soluble substances are contained within the lipid bilayer.39 One of the main benefits of using liposomes is that they are composed of components that are present in human physiological membranes, making them FDA-approved. Moreover, liposomes are biocompatible, non-toxic, and highly effective in cellular internalization.40 Liposomes serve as carriers for various molecules including drugs, proteins, genes (for gene therapy), and nucleotides. They are engineered and processed in different sizes, compositions and their specific functions.41
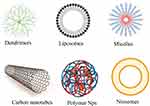 |
Figure 1 Nanomaterials known to exhibit potentials against metabolic disorders. |
Liposomes have received a lot of interest as drug carriers to boost the therapeutic effectiveness of chemotherapeutics.42 One example of their application is the encapsulation of doxorubicin (DOX) within PEGylated liposomes for the treatment of hepatocellular carcinoma (HCC). PEGylated liposomal DOX (PLD), characterized by improved hepatic absorption and preferential localization to the liver and spleen, has been demonstrated to be therapeutically useful in treating HCC cases.43 Furthermore, as compared to the pure medication, liposomal DOX was shown to have lower toxicity and similar anticancer activity, resulting in a greater therapeutic index and efficient dispensing of larger drug doses. Furthermore, the use of lyso-thermosensitive DOX liposomes in treating medium-to-large hepatic tumors has shown encouraging results, with a concentrated formulation at the tumor site and drug release at a specified temperature threshold, leading to tissue necrosis and lasting damage.44
Dendrimer(s)
Polymer nanoparticles (PN)-based vehicles are a promising noninvasive method for drug delivery across the blood–brain barrier.45 Polydisperse and hyperbranched polymers are thought to have more favorable size distribution properties.46 Among the PNs, the dendrimers hold significance in that they are shaped in a polymeric tree. The term “dendrimer” originates from the Greek “dendron”, meaning tree or branch. These polymers, with a hollow core and branching tendrils, were aptly named by Tomalia for their tree-like structure.47 Dendrimers’ ability to act as a biological scaffold for many ligands increases ligand concentration, the likelihood of statistical rebinding, and the likelihood of multivalent interactions. This is one of the primary advantages of architecture.48,49 Altering the surface properties of polymeric nanoparticles significantly changes their distribution in the body and enhances drug delivery.45
A greater variety of pharmaceuticals may be transported by dendrimers thanks to chemical synthesis and modification of the dendrimer nucleus, which can result in the manufacture of bigger dendrimer generations with a larger surface area. However, compared to other nano systems, dendrimers have limitations in controlling drug release, discriminating between normal and cancer cells, and purification.50 Highly branched structure of dendrimers is essential for their physical and biological properties. Their unique arrangement creates internal spaces where drugs can be stored, increasing their solubility and stability. Dendrimers are as low generation (G < 4) or high-generation (G ≥ 4) depending on their size. The physicochemical properties of dendrimers depend on branching units and surface functional groups. Their distinct characteristics such as the globular shape, three-dimensional structure, high functionality, presence of internal cavities and small size makes them ideal nano-carriers for drug delivery.51
Micelles
Micelles are amphiphilic surfactant molecules that self-assemble into spherical structures with a diameter of 10–100 nm. In an aqueous environment, these structures have a hydrophilic shell and a hydrophobic core, making them excellent for encapsulating hydrophobic medicinal compounds. The hydrophilic coating of the micelles limits drug loss and lowers the possibility of opsonization, a process that may result in the fast elimination of medicines from the body.52 Micelles serve as carriers for hydrophobic medicinal drugs, increasing their solubility and bioavailability.53 Polymeric micelles are crucial in reducing the toxicity of hydrophobic medicines, small-molecule medications, proteins, DNA, siRNA, peptides, and photosensitizers, which are all harmful.54 Micelle self-assembly can be influenced by a drug’s characteristics, concentration, and chemical make-up of the copolymer backbone.55 Micelles can have cylinder- or star-shaped structures, depending on the molecular weight.56 Polymeric micelles have been shown in studies to get through ocular barriers and prevent harmful pharmaceutical formulation adverse effects. Therapeutic compounds may be added to polymeric micelles by direct dissolution, dialysis, oil-in-water emulsion, solvent and co-solvent evaporations, and freeze-drying procedures.57
Metallic Nanomaterials
Metallic nanoparticles hold significance in the management of metabolic disorders.58 Quantum dots, a type of nanoparticle, exhibit luminescence when exposed to UV light. Upon targeting cancerous cells, these quantum dots illuminate, thereby identifying tumors. Recent studies have explored the conjugation of antibodies with magnetic poly- (D, L-lactide-co-glycolide) nanoparticles loaded with doxorubicin (DOX). These magnetic nanoparticles, integrated with DOX within PLGA nanoparticles, are designed to target malignant cells. Specifically, the antibody Herceptin has shown superior efficacy in targeting breast cancer cells.59 Nanoparticles play a significant role in managing infectious and inflammatory diseases. Hepatitis, a viral infection, is a chronic condition that persists throughout life. Nanoparticles are particularly useful in diagnosing this disease, with gold nanoparticles being especially effective. Gold nano-protein chips have been developed to detect hepatitis antibodies, proving highly efficient for diagnostic purposes. For treatment, DNA vaccines coated with SiO2 (LDH) nanoparticles are commonly used to induce an antibody response.60 Nanotechnology is also applied in treating bone inflammation, with metal nanoparticles proving highly effective in osteoblast formation by providing increased surface area. Titanium is commonly used for this purpose. Superparamagnetic iron oxide nanoparticles, combined with PLGA particles, are utilized for joint inflammation.61 Additionally, nanotechnology shows promise in treating skin infections. Nitric acid-coated nanoparticles are used for this, and iron oxide nanoparticles interact directly with thrombin, aiding tissue repair and enhancing the penetration of anti-inflammatory drugs into the skin.62 Gold nanoparticles (AuNPs) conjugated with multiple trastuzumab antibodies are designed to specifically target the human epidermal growth factor receptor (HER)-2 in SKBR-3 breast cancer cells. Upon binding to the HER-2 receptor, these gold-HER particles are internalized by the cells, leading to a two-fold increase in trastuzumab cytotoxicity.63
Table 2 highlights recent advances in nano-based diagnostic tools for the detection and treatment of diseases.
 |
Table 2 Recent Advances in Nano-Based Diagnostic Tools for Disease Detection and Treatment |
Toxicity of Nanomaterials on Endocrine System
The rapid growth of nanotechnology and large-scale use of nanomaterials have made engineered nanomaterials that are available to people through digestive tract, skin, and respiratory tract during diagnosis and treatment. Researchers found that nanomaterials can be distributed across various organs and induce toxicity in those organs. The endocrine system plays an important role in maintaining the body’s homeostasis through hormone regulation. Sometimes nanomaterials act as the Endocrine disrupting chemicals (EDCs) to disrupt normal hormonal function.72
Dendrimer Effect on Endocrine Treatment and Diagnosis
Polymeric nanoparticles (PNs) are a promising noninvasive method for drug delivery across the blood–brain barrier. Modifying the surface properties of PNs significantly changes their biodistribution and helps in drug delivery. To date, there is no evidence that PNs affect neuroendocrine or endocrine function. However, there is a hypothesis suggested that exposure to PNs might influence the estrogen feedback system in the hypothalamus. This hypothesis is primarily based on previous research showing the effects of endocrine-disrupting chemicals (EDCs) on hypothalamic centers that regulate the hypothalamic-Adrenal-pituitary-gonadal axis. In another study using a human placental perfusion model, researchers discovered that fluorescent polystyrene particles, with sizes up to 240nm, could pass through placental barriers without affecting the placental tissue viability. This shows that some dendrimers may have the ability to cross placenta, which emphasis on conducting more nanotoxicological studies on this organ system.45
Endocrine Disrupting NPs Impact on Insulin Action and Metabolism
The results of several toxicology and epidemiology studies have indicated that endocrine-disrupting chemicals (EDCs) are associated with metabolic disorders, including insulin resistance (IR), obesity, type 2 diabetes mellitus (T2DM), and polycystic ovary syndrome. These specific EDCs are also referred as metabolic disruptors. Recently, various in vitro and in vivo studies have examined the potential disruption of insulin signaling pathways and insulin production resulting from exposure to different metal-based nanoparticles.73
There have been studies dedicated to the exploration of the potential impact of TiO2 nanoparticles (TiO2-NPs) on metabolic and endocrine disorders such as obesity and insulin resistance (IR). They exposed Fao rat hepatoma cells to TiO2-NPs through direct interference with insulin-signaling pathways and indirect inflammatory activation of macrophages to find impaired insulin response and induced insulin resistance. In another in vitro study, pancreatic islets treated with CeO2 nanoparticles (CeO2-NPs) showed increased cell viability and insulin secretion in response to glucose stimuli. The proinflammatory action of Ti02-NPs and the antioxidant potential of Ce02-NPs likely exert effect on insulin cascade and release.74
Endocrine Disrupting NPs Affect Thyroid Function
Thyroid hormones act as regulators for various physiological processes like metabolism, remodeling, and cardiac function. Different metal-based NPs and QDs disrupt the thyroid hormonal pathways. A myriad of analyses have examined how silver nanoparticles (Ag-NPs), zinc oxide nanoparticles (ZnO-NPs), and quantum dots (QDs) affect thyroid hormone (TH) signaling in frog tissue. Results showed that Ag-NPs and QDs led to decrease in levels of transcripts encoding the TH-induced receptor β (TRβ) and TH-repressed Rana larval keratin type І (RLKІ).73
The chemical composition of nanomaterials determines their toxicity. Furthermore, size, charge, and surface modifications largely influence the degree of toxicity and need to be thoroughly evaluated in the assessment of nanomaterials endocrine toxicity. Standardized testing procedures and toxicity evaluation programs are needed before conducting risk assessments of nanomaterials, as the relationship between toxicity and nanomaterial properties is complex. Detailed information on the size, shape, surface, and optical properties of nanoparticles (NPs) should be provided to facilitate classification and standardization of toxicity results. Physicochemical characterization data help in establishing quantitative structure–activity relationships (QSAR), thereby reducing time and resources in risk assessment. Advances in omics technologies, such as transcriptomic and metabolomics, allow for the creation of databases of toxicological properties of nanomaterials in order to assess the risks of nanomaterials.75 Table 3 lists the toxic effects of various nanomaterials on endocrine system.
 |
Table 3 Toxicity of Nanomaterials on Endocrine System |
The Impact of Biocorona on NP Function
The biocorona influences NP function positively and negatively. It impacts significantly by altering cellular interactions, viability, and immune responses, crucial for safe biomedical applications.79 When nanoparticles (NPs) are introduced into a biological environment, a complex layer of biomolecules is coated on their surface, referred to as a biocorona (BC). This coating changes the physical properties of nanomaterials, affecting cellular viability, internalization, and immune responses. In order to safely use NPs in medical applications, it is important to comprehend how the BC effects cellular responses. It becomes difficult for nanoparticles to perform their function in the presence of BC because it alters the function and biodistribution and might increase toxicity. There is some controversy regarding the role of BC in biomedical applications. While some researchers view BC only as a barrier to the clinical use of NP therapeutics, others suggest that it could be modified and used as an advantage in NP-based biomedical applications.79 The formation of a biocorona influences the stability, reactivity, and dissolution of cobalt nanoparticles under physiological conditions, showing the importance of considering its impact on NP function.80 The biocorona formed on nanoparticles (NPs) plays a vital role in determining their role and determining their function in endocrine illnesses.
Influence of BC on NP-Endothelial Cell Interactions
The biocorona (BC) affects the interactions between nanoparticles (NPs) and endothelial cells, particularly in intravenous therapeutics. While BC can enhance the endothelial cell viability, it also poses challenges by disrupting targeting and triggering inflammatory responses through specific protein absorption and structural alterations. Moreover, BC alters NP interactions with cell surface receptors and intracellular processes, potentially leading to inflammatory diseases like atherosclerosis. Intravenously delivered NPs, such as single-walled CNTs, can influence vascular tone, causing adverse cardiovascular effects. However, there is limited evaluation of the BC’s impact on vascular tone regulation and its role in diseases like obesity, diabetes, and cancer. Further research is needed in this area to develop and use of NP therapeutic effectively, especially for intravenous application like imaging agents and cancer treatments.79
BC in Metabolic Disorders
BC significantly influences nanoparticle (NP) interactions with cells, impacting biological responses and nano-safety research. Ignoring BC in early NP assessments can lead to inconsistent experimental results and unexpected clinical outcomes, reducing therapeutic potential. The BC’s composition, including proteins and lipids, affects NP functionality, biodistribution, and toxicity. Current research is limited, focusing mainly on proteins, while the role of lipids and other biomolecules remains underexplored. Disease states and lifestyle factors like diet and exercise alter BC formation, affecting NP-cell interactions. Understanding these variations is crucial for translating in vitro findings into clinical applications and developing personalized medical treatments.79 An investigation by Li X et al reveals the role of the biocorona, a layer of proteins and biomolecules that forms on the surface of gold nanoparticles, in modulating the inflammatory response in human epidermal keratinocytes. The research demonstrates that the biocorona significantly affects cellular uptake and inflammatory pathways, highlighting the critical influence of nanoparticle surface chemistry on biological interactions. The findings may advance our understanding of nanomedicine and emphasize the importance of considering the broader biological implications of nanoparticle design.81 According to the study, these is a significant role of Nrf2 pathway in inducing the corresponding animal model, ie, the cascade responsible for the incidence and progression of metabolic disorders, the likes of diabetes.82 In 2022, a study details the implications of biocorona pertaining to the self-assembled nanoparticles in addressing the ever-elusive disorder of PCOS.This study investigates the development and evaluation of curcumin-encapsulated self-assembled nanoparticles as a potential treatment for PCOS in a female rat model. The results indicate that these nanoparticles significantly improved ovarian function, reduced cyst formation, and restored hormonal balance, suggesting a promising therapeutic approach for managing PCOS.83 However, herein lies the twist where there varied impacts of metabolic disorders on the BC composition as well, and the manuscript delves into how the protein corona (PC) composition on nanoparticles (NPs) changes in various disease states, impacting their targeting ability. The study concludes that diseases like cancer and diabetes significantly alter the PC, leading to different cellular interactions and responses. Specifically, it was found that these alterations can either enhance or hinder the NPs’ ability to target specific cells, depending on the disease state. This underscores the necessity of considering disease-induced variations in PC composition for the effective clinical application of NPs in precision medicine in addition to the fact that there are many avenues in need of exploration as far as the conception regarding the variation of coronal conformation in metabolic disorders, is concerned.84
Application of Nanomaterials in Endocrine Disorder
The applications of nanomaterials in the discipline of therapeutics are not in need of any introduction. From diabetes to cancer, metabolic disorders have been subject to testing against nanosized particles with variable success. However, as for the treatment or management of the disorders is concerned, one of the vital steps is the internalization of NPs prior to the performance of their actions. There are many mechanisms responsible among them the most significant are pinocytosis, micropinocytosis, phagocytosis, clathrin mediated endocytosis, caveolae contingent endocytosis and caveolae independent endocytosis.85
Diabetes and Nanotechnology
Diabetes mellitus is a common condition that affects people of all ages because of abnormalities in insulin activity and secretion. Hyperglycemia is a diabetes complication that can harm a number of essential organs and bodily systems, including the kidneys, eyes, heart, nerves, and blood vessels. Diabetes mellitus is characterized by insulin deficiency and insulin resistance caused by abnormalities in lipid, carbohydrate, and protein metabolism. These metabolic abnormalities are the result of an autoimmune process that damages pancreatic beta-cells. Diabetes mellitus is marked by insulin resistance and insufficiency. Long-term effective treatment is required as the condition progresses.86 According to recent projections, the number of adults with diabetes will exceed 280 million by 2030 and 400 million by 2050.87 The number of individuals living with diabetes mellitus (DM), an endocrine disease, is expected to rise to over 693 million by 2045, representing a 50% increase from 2017.88 Diabetes-related complications have caused 5.2 million deaths globally, with 252,806 deaths reported in the United States alone as of 2015. The prevalence of diabetes mellitus has resulted in significant morbidity, mortality, and healthcare costs.89
The field of nanotechnology has shown a connection to diabetes, both as unintended consequences of nano molecules that cause damage to glucose profiles and as potential applications, such as insulin delivery devices or nanoparticles acting as hydrogels that aid in wound healing through the use of bioactive molecules like proteins or growth factors.90 Due to the significant epidemiological impact of diabetes on the general population, transdisciplinary nanotechnology research involving diabetes is rapidly expanding. It can be utilized by employing both traditional diagnostic and therapeutic protocols, as well as advanced computer-assisted drug design (CADD) cheminformatics tools.91 The advancements made in glucose sensor technology have the potential to greatly benefit individuals with diabetes by providing more accurate readings and improving insulin dosing and overall diabetes management (Figure 2). Many critical questions remain unanswered in this field, including the specific relationship between endocrine disruptors and their effects on humans and animals, the amount of exposure time required to have a certain effect, and the interaction between a person’s genetic make-up and their exposure to the environment. Furthermore, there is a scarcity of adequate and high-quality evidence for specific anti-diabetic prescription regimens for type 2 diabetes patients of varying ages.
Many mechanisms are thought to be responsible for the efficiency of NPs in diabetes. Among them is the targeting of certain enzymes. Given that alpha-amylase and alpha-glucosidase are crucial enzymes in carbohydrate metabolism, their inhibition is a pivotal strategy for diabetes therapy. By preventing the breakdown of carbohydrates into monosaccharides, amylase and glucosidase inhibitors help manage blood glucose levels. An amylase inhibitor, when consumed with starchy foods, mitigates the typical rise in blood sugar levels. AgNPs have been identified as alpha-amylase inhibitors in numerous in vitro and in vivo studies.92
Selenium Nanoparticles (SeNPs) and Insulin Therapy
Incorporating selenium into inorganic nanoparticles has extended their established benefits of reduced toxicity, enhanced bioactivity, and improved targeting.93 While there are limits to selenium delivery, such as a short therapeutic window and potential toxicity, this vital trace element is required for the active core of several enzymes known as selenoproteins, which are predominantly linked with oxido-reductase activity. As a result, SeNPs are being researched for their potential use in diseases including diabetes and cancer linked to persistent oxidative stress.93 Furthermore, despite the fact that the precise mechanisms are not yet fully understood, SeNPs have the potential to be used as pharmacological carriers for a variety of medical conditions.94 The administration of oral insulin in type 1 and type 2 diabetes is one potential application. Insulin loaded SeNPs may overcome absorption barriers by delivering a regulated quantity of the hormone with good stability in the digestive environment, yielding around 9% bioavailability of subcutaneous insulin in mouse tests. Oral insulin has a poor bioavailability.95
Gold Nanoparticles and Delivery of Insulin
Owing to their decreased toxicity and increased surface area, gold nanoparticles have the potential to be used as drug delivery agents, especially in applications like the administration of growth factors and insulin for the treatment of diabetic wounds utilizing different nanotechnology-based devices.96 This type of nanoparticle, along with SeNPs, is considered a promising future insulin nanocarrier, alongside other options such as liposomes, dendrimers, niosomes, and micelles, which are currently being evaluated for their potential in the management of diabetes. In a 2020 study, researchers employed modified apple polysaccharide (MAP) to reduce chloroauric acid and deliver gold nanoparticles with insulin to diabetic rats. This nanotechnology-based insulin lowered glycemic levels 3.36-fold within 240 minutes, and after 28 days, it improved blood lipid levels and rat weight.97
Glucose-Responsive Insulin Delivery Devices
Systems that can regulate insulin release and maintain constant glycemia levels appear to be more promising for diabetes management than currently available options. One such system is based on mesoporous silica nanoparticles (MSNs) with self-regulation capabilities. In diabetic mice, insulin is adsorbed onto MSN channels and released intermittently over a 12-hour period.98 The MSM-insulin system benefits from the incorporation of real-time blood glucose sensors, such as Alizarin complexone, to guarantee proper release of the hypoglycemic hormone.99
Nanotechnology and Glucagon-Like Peptide (GLP) Analogues
In addition, other nanotechnology-based delivery systems have been developed, such as lipid nanoparticles and polymeric nanoparticles, which have shown promising results in improving the pharmacokinetics and pharmacodynamics of GLP-1 analogues. These nanoparticles can protect the drug from degradation in the gastrointestinal tract, improve its absorption and bioavailability, and allow sustained release over a longer period of time, resulting in a more effective glucose-lowering effect. Moreover, the use of nanoparticles can also minimize the frequency of administration of GLP-1 analogues, leading to increased patient compliance and better outcomes in diabetes management.100,101
Nanotechnology and the Treatment of Diabetic Wounds
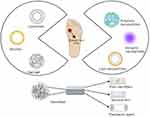
The microvascular and neuropathic effects of diabetes mellitus (DM) contribute to an already high risk of wounds, skin infections, and foot ulcers that heal poorly. Nanoparticles and hydrogels, in addition to bioactive medications, are the basis of nanotechnology-based wound-healing devices.90 Various types of nanocarriers employed for the treatment of chronic wounds, including self-assembled nanocarriers such as liposomes, micelles, and nanogels, as well as nanoparticles such as polymeric, inorganic, and lipid-based nanoparticles have been shown in Figure 3.
An example of the use of nanotechnology for diabetic wound healing comes from recent in vivo research that used chitosan hydrogel and silver ions to deliver epidermal growth factor that had antibacterial and cell growth benefits in diabetic mice. Among the favorable clinical outcomes seen during the trial were re-epithelialization of the ulcer area and increased collagen deposits in the ulcer-damaged area of the foot.100 The three components of the therapy system that can be utilized to treat chronic cutaneous diabetic lesions are hyaluronic acid, oxidized hydroxymethyl propyl cellulose, and oridonin-loaded alginate microspheres. This mechanism reduces inflammation by inhibiting chemicals like interleukin-6 and increases fibroblastogenesis and angiogenesis.101
Silicon Dioxide Nanoparticles (SiO2NPs) and Insulin Resistance
In a study using murine models, silica dioxide nanoparticles (SiO2NPs), which are commonly found in food industry-related environmental contaminants, have been found to induce insulin resistance. SiO2NPs have been found to be absorbed and function through interacting with glucose transporters and insulin receptors in organs involved in insulin action or secretion, such as the liver and pancreas. However, administration of SiO2NPs at doses calculated based on mouse body weight has been linked to hyperglycemia. The induction of ER stress, which occurs as a result of the overexpression of ROS-coding genes, is assumed to be the mechanism through which SiO2NPs cause injury. Increased ROS levels subsequently activate the NF-kB pathway, resulting in the generation of pro-inflammatory cytokines. Furthermore, SiO2NPs cause insulin resistance by phosphorylating insulin receptor substrate 1 (IRS1) at the serine residue.78
Monitoring of BGL with Nanometer
There has been substantial improvement in the technology of continuous glucose monitoring for detecting blood glucose levels, which incorporates glucose biosensor technology, during the last 50 years.100 In the third generation of glucose biosensors, the use of nanomaterials has enhanced the detection potency of glucose.. However, further development of this technology is still needed. The benefits of employing nanotechnology in glucose detection are numerous, including the small size of the sensor, which offers a greater surface area for catalytic activity. Additionally, the optical properties and longevity of the sensors are also improved. The incorporation of nanomaterials in measuring BGL is also advantageous because it allows for frequent measurements and is painless. Consequently, it can detect high fluctuations in BGL, which is significant in managing diabetes. Different nanomaterials have been utilized in the production of glucose biosensors, among which carbon nanotubes (CNTs) are a particularly promising type due to their high catalytic activity.102 While CNTs can be used as a single type of nanomaterial, other designs involve CNTs in conjunction with other nanomaterials to form nanocomposites.103
There are glucose biosensors that detect glucose without using enzymes, instead designed to directly detect glucose through the electrode, providing increased stability to the sensor. Nanoparticles have been utilized in “non-enzymatic sensors” to improve precision and accuracy. For example, nickel oxide nanoparticles were placed on glassy carbon electrodes with DNA modifications in glucose biosensors.104 Glucose-binding sensors are another method, and the intensity of their fluorescence indicates the concentration of glucose in the blood.105 Fluorescence-based sensors utilizing glucose-binding moieties, such as lectins, linked to carbon nanotubes have also shown promise and have reached clinical trial stages, although concerns remain about the toxicity of carbon nanoparticles in these sensors.106 Table 4 describes various nano-sensors used for endocrine illnesses.
 |
Table 4 Nano-Sensors Used for Endocrine Illness Discussed Below1 |
Role of Metal Oxide Nanoparticles for Diabetes Treatment
Various research studies have shown the importance of metal oxide in glucose metabolism and their connection with diabetes. According to the literature, vanadium, chromium, magnesium, silver and zinc are involved in diabetes treatment and contribute to blood sugar regulation. Zinc, an essential micronutrient, significantly influences various biological processes including aorta, gonad, and mesonephros (AGM). Zinc oxide (ZnO) enhances hepatic glycogenesis through insulin signaling, which improves glucose utilization. It reduces blood glucose levels by inhibiting intestinal glucose production and increasing glucose absorption in skeletal muscle and adipose tissue. Moreover, zinc oxide enhances the structural integrity of insulin, crucial for its generation, synthesis, and secretion. It also decreases gluconeogenesis and glycogenolysis while promoting glycogen secretion. On the other hand, magnesium participates in glucose and lipid metabolism, involved in insulin release, glucose transfer, and glucose oxidation. Vanadium compounds can help reduce inadequate insulin response in diabetes mellitus. While they cannot reverse the insulin deficiency seen in type 1 diabetes, they can lower the requirement for external insulin or potentially eliminate the need for oral hypoglycemic agents in type 2 diabetes. Gold nanoparticles combined with aqueous extracts improved the weight of diabetic rats more effectively than natural extract alone by reducing glucogenesis, muscle loss, and lowering blood urea and creatinine levels.107
Metal oxides are used for the electrochemical detection of glucose level. Copper oxide (CuO) based materials possess electrochemical sensing capabilities. These oxides enhance the sensing performance of electrodes in sensors. Iron oxide (FeO) exhibits magnetic properties, and their nanoparticles are biocompatible and less toxic, making them suitable for biosensor development for biomolecule analysis. Manganese oxide-based sensors are used for glucose detection. These metal oxides are promising potential substitutes for developing highly efficient, reliable, and stable non-enzymatic glucose sensors for early detection of glucose in diabetic patients.108
CN in Thyroid Cancer
In China, carbon nanoparticles (CNs) have become increasingly popular in thyroid surgeries in recent years. These nanoparticles have a diameter of 150 nm, allowing them to enter lymphatic capillaries, which have an intercellular space of 120–500 nm, but not blood capillaries, which have an intercellular space of 20–50 nm. Once injected into thyroid tissue, the CNs would stain the drainage lymph node black, thus facilitating lymph node dissection. Furthermore, CNs do not stain the parathyroid glands due to their different lymphatic system from the thyroid, and therefore can be used to identify and protect the parathyroid glands during thyroidectomies. Recent clinical research and systemic reviews have demonstrated that CNs can assist prevent inadvertent parathyroid gland removal, protect parathyroid glands, and lessen postoperative hypocalcemia and hypoparathyroidism (hypoPT).109 Furthermore, CNs can increase the number of lymph nodes that pathology detects.
Due to its potential advantages in enhancing parathyroid protection and assisting with lymph node dissection, the use of carbon nanoparticle solution (CNs) in thyroid operations has drawn interest. The increasing use of CNs in Chinese clinical practice in recent years has shown positive effects, according to a new meta-analysis. The research showed that using CNs resulted in an average 3.39 increases in lymph node retrieval per patient, which significantly improved cancer staging and treatment planning. Furthermore, CNs showed a significant reduction of 22%, 31%, and 24%, respectively, in the rates of accidental parathyroid removal, transient hypoparathyroidism, and permanent hypoparathyroidism. These data imply that after thyroid operations, CNs may protect the parathyroid glands.103
Carbon Nanoparticles in Lymph Node
Carbon nanoparticles (CNs) are a type of nanoparticle that have been found to be successful in lymph node dissection in various types of cancers, such as breast carcinoma and gastric carcinoma. This is because of their distinct physical characteristics, such as their bigger mean width of 150 nm than the capillary endothelial cell gap of (20–50 nm). This indicates that CNs can pass through the lymphatic capillary endothelial cell gap (120–500 nm) but cannot enter blood arteries. Once they reach the lymphatic system, macrophages may phagocytize them and cause them to build up in the lymph nodes, where they leave a black stain that makes them visible to the human eye.110
The fact that the parathyroid glands (PGs) remain unstained due to their potential lack of lymphatics is one of the most important benefits of employing CNs in lymph node detection. This process, termed as “negative development”, not only boosts the quantity of lymph nodes extracted during compartmental dissection but also makes it easier for the surgeon to tell PGs apart from lymph nodes and reduces the likelihood of accidentally removing PGs.109 This is crucial because the parathyroid glands play an important role in regulating calcium levels in the body, and their removal can cause hypocalcemia, which can lead to a range of health problems.
HDL Biomimetic NPs for Treating Obesity
Obesity increases a person’s chance of developing cardiovascular and cerebrovascular disorders since it is related to the free lipoprotein metabolism, which is disturbed in many diseases including atherosclerosis. Apolipoprotein B (ApoB) and apolipoprotein A1 (ApoA1) make up lipoproteins. While HDL, which contains ApoA1, removes excess lipid from the majority of tissues, LDL, VLDL, and chylomicrons, which are lipoproteins that contain ApoB, supply lipids to other tissues. The risk of atherosclerosis may be decreased by HDL by reducing cholesterol levels.111
Because there are not many ApoA1 sources, apolipoprotein mimetic peptide particles are used as replacements. In vitro, the 26-amino acid apolipoprotein mimetic peptide (ATI-5261), for example, operates similarly to ApoA1 and is capable of successfully delivering cholesterol to hepatocytes through scavenger receptor BI (SR-BI). Simulations of ApoA1 in RCTs give alternatives for controlling cholesterol development in arterial walls.112
The use of gold nanoparticles (Au NPs) in the production of high-density lipoprotein (HDL) biomimetic nanoparticles has shown tremendous promise due to the exceptional biocompatibility of these particles and the ease with which they may be modified. These HDL biomimetic nanoparticles are supported by Au NPs and were synthesized by employing 2-iminothiolane; they contain apolipoproteins such as ApoA1 and ApoA1-SH. ApoA1 is necessary for increased cholesterol efflux and a successful treatment of obesity because it enhances cellular cholesterol transport via the pathway of adenosine triphosphate binding cassette transporter A1. This pathway is mediated by the scavenger receptor BI (SR-BI), which is responsible for driving diffusion.113
AuNPs inhibit TNF production, reducing inflammation in adipose tissue and contributing to a reduced overall body weight. Citrate-modified gold nanoparticles (21 nm) lowered TNF levels and exhibited anti-inflammatory effects on retroperitoneal adipose tissue, resulting in a reduction in mouse body weight. Mice fed a high-fat diet with Au NPs exhibited more anti-inflammatory M2 macrophages and less pro-inflammatory M1 macrophages. As a result, the weights of the mice decreased.114,115 Mice fed a high-fat diet lost weight after being treated with Au NPs, owing to an increase in anti-inflammatory M2 macrophages and a decrease in pro-inflammatory M1 macrophages.116
Recent Clinical Studies Employing Nanoparticles in Endocrine Diagnosis and Treatment
Nano-Sensors for Diabetic Biomarker Detection in Human Bio Fluid
Gold nanoparticles and graphene nanosheets embedded nanomaterial known as Electrochemical have been developed for the detection of biomarkers like HbA1c. Additionally, a dual sensor called Microfluidic amperometric have been designed for separate detection of HbA1c and total Hb, offers more accurate means for point-of-care analysis. Further, nano-sensor called Impedance-based electrochemical has shown effective results to HbA1c in clinically relevant levels with reversible electrodes and same as noble and metal oxide nanoparticles entrapped in non-enzymatic amperometric glucose sensors have concluded as elevate glucose detection capabilities compared to enzymatic sensors.117
Nano-Sensors Acetone Gas Detection in Human Breath
A semiconductor metal oxide-based sensors have been used for detecting acetone, a certain marker for noninvasive diabetes diagnosis. Moreover, WO3 nanofilms and SnO2 nanotubes have been applied to enhance sensitivity and selectivity in detecting acetone in exhaled breath. The integration of a complex PdO entrapped ZnO nanocatalyst on hollow SnO2 nanotubes has further elevated acetone sensing performance. Bimetallic Pt-based nanoparticles and Pt-functionalized WO3 hemicubes as supported on mesoporous WO3 nanofibers have elaborated superior acetone sensing capabilities.118
Expected Role of Nanotechnology in Mitigating Endocrine Diseases
Nanotechnology plays an important role in mitigation by offering diagnostic capabilities that overpower those of small molecules or microscale tools. Molecular imaging, which integrates molecular biology with medical imaging, has seen elevated adoption of NP imaging for both diagnostic and therapeutic purposes. The term “theranostic” is explained as a technology with concurrent diagnostic and therapeutic capabilities. While NPs have been FDA-approved for clinical use as transport vehicles for years, their full theranostic potential is still being realized. NPs have demonstrated remarkable success in drug delivery and magnetic resonance imaging (MRI). Image-guided resection, optical/photoacoustic imaging in vivo, contrast-enhanced ultrasound, and thermoablative therapy are the emerging technologies.119 Nano-sensors can be used to regulate real-time levels of hormones in the body, giving valuable data for personalized treatment. These sensors can be used into wearable devices or implanted directly into the body for continuous observation.120 Theranostic nanoparticles include both diagnosis and therapy embed in single nanomaterial which is multifunctional and holds vital potential for cancer detection and treatment. The pros of theranostic nanoparticle; it helps in selective accumulation of diseased tissue, effective therapeutic action, and safety.121
Conclusion and Future Perspectives
The endocrine system is a vital component of the human body that regulates several bodily functions. The prevalence of endocrine disorders is high, and many of these disorders can be managed with current medications. However, the diagnosis and management of endocrine disorders necessitate a thorough examination to detect undiagnosed dysfunctions that necessitate specific therapy or may preclude surgical treatment. Nanotechnology has transformed medication research and development by opening up new perspectives on disease pathogenesis and therapy alternatives. Recent advances in nanotechnology have enabled therapy and diagnostics to be brought closer to patients. Nanomaterials-based diagnostic tools, including imaging techniques and various nanostructures like spherical nanoparticles, one-dimensional nanomaterials, liposomes, dendrimers, and micelles, are used as biosensors and drug delivery systems. Especially in endocrine disorders, nanoparticles play an important role in insulin delivery and wound healing, gold nanoparticles, and mesoporous silica nanoparticles and maintain constant glycemia levels that appear to be more promising for diabetes management than currently available options. The development of nano-sized carriers comprised lipids, polymers, carbon, or metals is addressing the limitations of traditional drug delivery strategies. Moreover, the toxicity of nanomaterials, particularly their endocrine disrupting effects shows that different metal-based nanoparticles can disrupt insulin signaling pathways and insulin production. Recent clinical studies have found the uses of nano-sensors for detecting diabetic biomarkers in human biofluids, which shows the potential of nanotechnology in endocrine disease diagnosis and treatments.
Future theranostic agents may be created by the synthesis of nano-sized carriers, which can function in two different ways. They are mostly used in disease management to address issues with traditional NP systems such as patient compliance and safety. Thus, the use of nanotechnology has promising outcomes in managing endocrine disorders, and ongoing research may lead to novel treatments that can improve patient outcomes.
With ongoing research and development, the utilization of nanotechnology for metabolic disorders holds promise and the future does not seem to be as bleak as one may have predicted. The development of endogenous nano-biosensors capable of detecting even minor hormone fluctuations and unprompted responding in a coordinated manner to restore normal function is a significant breakthrough in the field. The application of nanotechnology in the diagnosis and treatment of endocrine disorders has the potential to result in treatments that are more targeted and personalized for a wide range of illnesses. With the development of theranostics, nanotechnology has the potential to provide a personalized approach to treatment, which can improve patient outcomes. Moreover, research on nanotechnology and nanomaterials often needs expensive experiments. Focusing on the importance of nanomaterials (NMs), improvements to the design of NM structures, properties, adsorption, and catalysis by machine learning (ML) has developed. Machine Learning (ML) led to innovation in nanomaterial (NM) development and also ML used for sustainable nanomaterials. Intriguingly, NMs, the likes of liposomes, micelles and polymeric NPs, via efficient augmentation of targeted drug dissemination will reduce the API concentration thereby curtailing toxicity. Withal, with the advent of smart polymers, continuous glucose monitoring in addition to formulation of artificial pancreas seems an attainable reality. Thus, the future prospects of nanotechnology in managing endocrine disorders are promising, and ongoing research may lead to the development of novel treatments that can transform the field of endocrinology.
Data Sharing Statement
There are no supplemental data with this paper. All information is available in the manuscript.
Ethical Approval Statement
Ethics approval is not applicable as this is a review paper and does not involve direct research on animals or humans.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received no funding from any source.
Disclosure
All the authors declare that they have no competing interests in this work.
References
1. Al-Khater KM, Al-Suhaimi EA. Application of Nanomaterials in Treatment of Endocrine Diseases. Applic Nanomat Human Health. 2020;191–210.
2. Fierabracci P, Pinchera A, Martinelli S, et al. Prevalence of endocrine diseases in morbidly obese patients scheduled for bariatric surgery: beyond diabetes. Obesity Surgery. 2011;21:54–60. doi:10.1007/s11695-010-0297-6
3. Van Hulsteijn L, Pasquali R, Casanueva F, et al. Prevalence of endocrine disorders in obese patients: systematic review and meta-analysis. European j Endocrinology. 2020;182(1):11–21. doi:10.1530/EJE-19-0666
4. Golden SH, Robinson KA, Saldanha I, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853–1878. doi:10.1210/jc.2008-2291
5. Rivelli A, Fitzpatrick V, Wales D, et al. Prevalence of endocrine disorders among 6078 individuals with Down syndrome in the United States. J Patient-Centered Res Revi. 2022;9(1):70. doi:10.17294/2330-0698.1877
6. Barkalina N, Charalambous C, Jones C, et al. Nanotechnology in reproductive medicine: emerging applications of nanomaterials. Nanomed Nanotech Biol Med. 2014;10(5):e921–e938. doi:10.1016/j.nano.2014.01.001
7. Badıllı U, Mollarasouli F, Bakirhan NK, et al. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Anal Chemi. 2020;131:116013. doi:10.1016/j.trac.2020.116013
8. Contera S, Bernardino de la Serna J, Tetley TD. Biotechnology, Nanotechnology and Medicine. Portland Press Ltd; 2020:551–554.
9. Ahmed N, Fessi H, Elaissari A. Theranostic applications of nanoparticles in cancer. Drug Discovery Tod. 2012;17(17–18):928–934. doi:10.1016/j.drudis.2012.03.010
10. Kovacs WJ, Ojeda SR. Textbook of Endocrine Physiology. OUP USA; 2011.
11. Devendra D, Liu E, Eisenbarth GSJB. Type 1 diabetes: recent developments. BMJ. 2004;328(7442):750–754. doi:10.1136/bmj.328.7442.750
12. Huizinga MM, Rothman RLJIJOMR. Addressing the diabetes pandemic: a comprehensive approach. Medknow. 2006;481–484.
13. Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271(3):e21–e93. doi:10.1097/SLA.0000000000003580
14. Taylor PN. Global epidemiology of hyperthyroidism and hypothyroidism. Nature Reviews Endocrin. 2018;14(5):301–316.
15. Miller WL. The adrenal cortex and its disorders. Sperling Pediatric Endocri Elsevier. 425–490.
16. Vyas J, Shah I. Nanotherapeutics for insulin resistance and diabetes mellitus using metal nanocomposites. In: Metal Nanocomposites in Nanotherapeutics for Oxidative Stress-Induced Metabolic Disorders. CRC Press:108–133
17. Nel A, Xia T, Mädler L, et al. Toxic potential of materials at the nanolevel. science. 2006;311(5761):622–627. doi:10.1126/science.1114397
18. Rejman J, OBERLE V, ZUHORN IS, et al. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377(1):159–169. doi:10.1042/bj20031253
19. Gosangari SL, Watkin KL. Effect of preparation techniques on the properties of curcumin liposomes: characterization of size, release and cytotoxicity on a squamous oral carcinoma cell line. Pharm Develop Tech. 2012;17(1):103–109. doi:10.3109/10837450.2010.522583
20. Yusuf A, Awatif Rashed Z, Henidi H, et al. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. doi:10.3390/polym15071596
21. Chithrani DB, Dunne M, Stewart J, et al. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomed Nanotech Biol Med. 2010;6(1):161–169. doi:10.1016/j.nano.2009.04.009
22. Fillion P, Desjardins A, Sayasith K, et al. Encapsulation of DNA in negatively charged liposomes and inhibition of bacterial gene expression with fluid liposome-encapsulated antisense oligonucleotides. Biochimica et Biophysica Acta-Biomembranes. 2001;1515(1):44–54. doi:10.1016/S0005-2736(01)00392-3
23. Pan G-H, Barras A, Boussekey L, et al. Alkyl passivation and SiO 2 encapsulation of silicon nanoparticles: preparation, surface modification and luminescence properties. J Mater Chem C. 2013;1(34):5261–5271. doi:10.1039/c3tc30571f
24. Yin H, Casey PS, McCall MJ. Surface modifications of ZnO nanoparticles and their cytotoxicity. J Nanoscien Nanotech. 2010;10(11):7565–7570. doi:10.1166/jnn.2010.2833
25. Wang J, Li J, Guo G, et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: new insight into the antimicrobial action of silver. Sci Rep. 2016;6(1):32699. doi:10.1038/srep32699
26. Milla P, Dosio F, Cattel L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Current Drug Metabolism. 2012;13(1):105–119. doi:10.2174/138920012798356934
27. Carron PM, Crowley A, O’Shea D, et al. Targeting the folate receptor: improving efficacy in inorganic medicinal chemistry. Curr Med Chem. 2018;25(23):2675–2708. doi:10.2174/0929867325666180209143715
28. Wang Y, Gou K, Guo X, et al. Advances in regulating physicochemical properties of mesoporous silica nanocarriers to overcome biological barriers. Acta Biomater. 2021;123:72–92. doi:10.1016/j.actbio.2021.01.005
29. Karlas A, Pleitez MA, Aguirre J, et al. Optoacoustic imaging in endocrinology and metabolism. Nat Rev Endocrin. 2021;17(6):323–335. doi:10.1038/s41574-021-00482-5
30. Golden SH, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocr Metab 2009;94(6):1853–1878.
31. Kahramangil B, Berber EJ. The use of near‐infrared fluorescence imaging in endocrine surgical procedures. J Surgical Oncology. 2017;115(7):848–855. doi:10.1002/jso.24583
32. Liu J, Cui Y, Liu L, et al. Near-infrared auto-fluorescence spectroscopy combining with Fisher’s linear discriminant analysis improves intraoperative real-time identification of normal parathyroid in thyroidectomy. BMC surgery. 2020;20:1–7. doi:10.1016/j.omtn.2020.01.033
33. Perfézou M, Turner A, Merkoçi AJ. Cancer detection using nanoparticle-based sensors. Chemi Socie Reviews. 2012;41(7):2606–2622. doi:10.1039/c1cs15134g
34. Cordeiro M, Ferreira Carlos F, Pedrosa P, et al. Gold nanoparticles for diagnostics: advances towards points of care. Diagnostics. 2016;6(4):43. doi:10.3390/diagnostics6040043
35. Nejati K, et al. Biomedical applications of functionalized gold nanoparticles: a review. J Cluster Science. 2021;1–16.
36. Mostafalu P, Sonkusale SJRA, Xu L, Hou B, Chen H. A high-density nanowire electrode on paper for biomedical applications. RSC Advances. 2015;5(12):8680–8687. doi:10.1038/srep08680
37. Darabi MA. Gum sensor: a stretchable, wearable, and foldable sensor based on carbon nanotube/chewing gum membrane. ACS applied mater inter. 2015;7(47):26195–26205. doi:10.1021/acsami.5b08276
38. Solorio-Rodriguez SA, Williams A, Poulsen SS, et al. Single-walled vs. multi-walled carbon nanotubes: influence of physico-chemical properties on toxicogenomics responses in mouse lungs. Nanomaterials. 2023;13(6):1059. doi:10.3390/nano13061059
39. Jiang W, Kim BYS, Rutka JT, et al. Advances and challenges of nanotechnology-based drug delivery systems. Exp opin drug deliv. 2007;4(6):621–633. doi:10.1517/17425247.4.6.621
40. Hann IM, Prentice HGJIJOAA. Lipid-based amphotericin B: a review of the last 10 years of use. Intern J antimicrob agen. 2001;17(3):161–169. doi:10.1016/s0924-8579(00)00341-1
41. Vats S. Role of nanotechnology in theranostics and personalized medicines. J Health Res Rev. 2017;4(1):1–7.
42. Souto E. Lipid Nanocarriers in Cancer Diagnosis and Therapy. Smithers Rapra; 2011.
43. Lombardi G, Zustovich F, Farinati F, et al. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: results of a Phase 2 study. Cancer. 2011;117(1):125–133. doi:10.1002/cncr.25578
44. Poon RT, Borys NJEOOP. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance efficacy of thermal ablation of liver cancer. Ex opin pharm. 2009;10(2):333–343.
45. Lu X, Liu Y, Kong X, et al. Nanotoxicity: a growing need for study in the endocrine system. Small. 2013;9(9‐10):1654–1671. doi:10.1002/smll.201201517
46. Nazemi A, Gillies ERJBJOPS. Dendritic surface functionalization of nanomaterials: controlling properties and functions for biomedical applications. Brazilian Journal of Pharmaceutical Sciences. 2013;49:15–32.
47. Wang J, Li B, Qiu L, et al. Dendrimer-based drug delivery systems: history, challenges, and latest developments. J Biol Eng. 2022;16(1):18. doi:10.1186/s13036-022-00298-5
48. Aulenta F, Hayes W, Rannard SJEPJ. Dendrimers: a new class of nanoscopic containers and delivery devices. European Polymer Journal. 2003;39(9):1741–1771.
49. Parat A, Bordeianu C, Dib H, et al. Dendrimer–nanoparticle conjugates in nanomedicine. Nanomedicine. 2015;10(6):977–992. doi:10.2217/nnm.14.196
50. Maruyama-Tabata H, Harada Y, Matsumura T, et al. Effective suicide gene therapy in vivo by EBV-based plasmid vector coupled with polyamidoamine dendrimer. Gene Therapy. 2000;7(1):53–60. doi:10.1038/sj.gt.3301044
51. Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials. 2019;13(1):65. doi:10.3390/ma13010065
52. Shiraishi K, Sanada Y, Mochizuki S, et al. Determination of polymeric micelles’ structural characteristics, and effect of the characteristics on pharmacokinetic behaviors. J Cont Rel. 2015;203:77–84. doi:10.1016/j.jconrel.2015.02.017
53. Kedar U, Phutane P, Shidhaye S, et al. Nanomed. Nanotechnol. 2010;6:714. doi:10.1016/j.nano.2010.05.005
54. Gothwal A, Khan I, Gupta U. Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharmaceutical Research. 2016;33:18–39. doi:10.1007/s11095-015-1784-1
55. Deng C, Jiang Y, Cheng R, et al. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today. 2012;7(5):467–480. doi:10.1016/j.nantod.2012.08.005
56. Bisht R, Jaiswal JK, Chen Y-S, et al. Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opinion on Drug Delivery. 2016;13(7):953–962. doi:10.1517/17425247.2016.1163334
57. Aliabadi HM, Lavasanifar AJEOODD. Polymeric micelles for drug delivery. Current Pharmaceutical Design. 2006;3(1):139–162.
58. Mughal SS. Diagnosis and Treatment of Diseases by Using Metallic Nanoparticles-A Review. Authorea Preprints; 2022.
59. Nasimi P, Haidari M. Medical use of nanoparticles: drug delivery and diagnosis diseases. International Journal of Green Nanotechnology. 2013;1:1943089213506978. doi:10.1177/1943089213506978
60. Klippstein R, Pozo D. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomedicine: nanotechnology. Biol Med. 2010;6(4):523–529.
61. Baghaban-Eslaminejad M. The role of nanomedicine, nanotechnology, and nanostructures on oral bone healing, modeling, and remodeling. Nanostructures for Oral Medicine. 2017;777–832.
62. Ikoba U, Peng H, Li H, et al. Nanocarriers in therapy of infectious and inflammatory diseases. Nanoscale. 2015;7(10):4291–4305. doi:10.1039/C4NR07682F
63. Hanif MA, et al. An overview on ajwain (Trachyspermum Ammi) pharmacological effects: current and conventional. Technology. 2021;5(1):1–6.
64. Wang Z, Hu T, Liang R, et al. Application of zero-dimensional nanomaterials in biosensing. Fronti Chemistry. 2020;8:320. doi:10.3389/fchem.2020.00320
65. Abraham J, et al. One-dimensional (1D) nanomaterials: nanorods and nanowires; nanoscale processing. Nanoscale Proces Elsevier. 71–101.
66. Kalia S, et al. Two-dimensional layered molybdenum disulfide (MoS2)-reduced graphene oxide (rGO) heterostructures modified with Fe3O4 for electrochemical sensing of epinephrine. Materials Chem Physics. 2022;287:126274.
67. Gogulapati N, Manalan BV, Nadendla RRJAJPP. Poly (propylene imine) Dendrimer: synthesis, characterization and applications in various drug delivery. Asian J.Pharm. 2020;6:190–203.
68. Filipczak N, Pan J, Yalamarty SSK, et al. Recent advancements in liposome technology. Adv Drug Delivery Reviews. 2020;156:4–22. doi:10.1016/j.addr.2020.06.022
69. Vaishya RD, et al. Controlled ocular drug delivery with nanomicelles. Wiley Interdis Rev. 2014;6(5):422–437.
70. Chopra SS, Theis TLJESN. Comparative cradle-to-gate energy assessment of indium phosphide and cadmium selenide quantum dot displays Environmental Science. 2017;4(1):244–254.
71. Zhang M, Yudasaka M. Carbon nanohorns and their high potential in biological applications. Carbon Nanopart Nanostruct. 2016;77–107.
72. Wang Y, Nowack B. Environmental risk assessment of engineered nano‐SiO2, nano iron oxides, nano‐CeO2, nano‐Al2O3, and quantum dots. Environ Toxi Chem 2018;37(5):1387–1395. doi:10.1002/etc.4080
73. Iavicoli I, Fontana L, Leso V, et al. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14(8):16732–16801. doi:10.3390/ijms140816732
74. Gurevitch D, Shuster-Meiseles T, Nov O, et al. TiO 2 nanoparticles induce insulin resistance in liver-derived cells both directly and via macrophage activation. Nanotoxicology. 2012;6(8):804–812. doi:10.3109/17435390.2011.625128
75. Yao Y, Tang M. Advances in endocrine toxicity of nanomaterials and mechanism in hormone secretion disorders. J Appl Toxicol. 2022;42(7):1098–1120. doi:10.1002/jat.4266
76. Arisha AH, Ahmed MM, Kamel MA, et al. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood–testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ. Sci. Pollut. Res. 2019;26:28749–28762. doi:10.1007/s11356-019-06066-1
77. Ahangarpour A, Alboghobeish S, Oroojan AA, et al. Mice pancreatic islets protection from oxidative stress induced by single-walled carbon nanotubes through naringin. Hum Exp Toxicol. 2018;37(12):1268–1281. doi:10.1177/0960327118769704
78. Hu H, Fan X, Guo Q, et al. Silicon dioxide nanoparticles induce insulin resistance through endoplasmic reticulum stress and generation of reactive oxygen species. Particle Fibre Toxi. 2019;16:1–18.
79. Kobos L, Shannahan J. Biocorona‐induced modifications in engineered nanomaterial–cellular interactions impacting biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1608. doi:10.1002/wnan.1608
80. Mei N, Hedberg J, Odnevall Wallinder I, et al. Influence of biocorona formation on the transformation and dissolution of cobalt nanoparticles under physiological conditions. ACS omega. 2019;4(26):21778–21791. doi:10.1021/acsomega.9b02641
81. Li X, Li D, Zhang G, et al. Biocorona modulates the inflammatory response induced by gold nanoparticles in human epidermal keratinocytes. Toxicol Lett. 2022;369:34–42. doi:10.1016/j.toxlet.2022.08.009
82. Dodson M, Shakya A, Anandhan A, et al. NRF2 and Diabetes: the Good, the Bad, and the Complex. Diabetes. 2022;71(12):2463–2476. doi:10.2337/db22-0623
83. Raja MA, Maldonado M, Chen J, et al. Development and Evaluation of Curcumin Encapsulated Self-assembled Nanoparticles as Potential Remedial Treatment for PCOS in a Female Rat Model. Int J Nanomed. 2021;16:6231–6247. doi:10.2147/IJN.S302161
84. Xu W, Xu M, Xiao Y, et al. Changes in target ability of nanoparticles due to protein Corona composition and disease state. Asian J. Pharm. Sci. 2022;17(3):401–411. doi:10.1016/j.ajps.2022.03.002
85. Behzadi S, Serpooshan V, Tao W, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–4244. doi:10.1039/C6CS00636A
86. Jiang H, Xia C, Lin J, et al. Carbon nanomaterials: a growing tool for the diagnosis and treatment of diabetes mellitus. Environmental Research. 2023;221:115250. doi:10.1016/j.envres.2023.115250
87. Shaw JE, Sicree RA, Zimmet PZ, et al. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010;87(1):4–14. doi:10.1016/j.diabres.2009.10.007
88. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practic. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
89. Baynes H. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab. 2015;6(5):1–9.
90. Bai Q, Han K, Dong K, et al. Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomedi. 2020;9717–9743. doi:10.2147/IJN.S276001
91. Kaur P, Khatik GJCDT. An overview of computer-aided drug design tools and recent applications in designing of anti-diabetic agents. Current Drug Targets. 2021;22(10):1158–1182. doi:10.2174/1389450121666201119141525
92. Bagyalakshmi J, Haritha H. Green synthesis and characterization of silver nanoparticles using Pterocarpus marsupium and assessment of its in vitro Antidiabetic activity. Am J Adv Drug Del. 2017;5(3).
93. Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int j Nanomed. 2015;6741–6756. doi:10.2147/IJN.S91377
94. Khurana A, Tekula S, Saifi MA, et al. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi:10.1016/j.biopha.2018.12.146
95. Deng W, Xie Q, Wang H, et al. Selenium nanoparticles as versatile carriers for oral delivery of insulin: insight into the synergic antidiabetic effect and mechanism. Nanomedicine. 2017;13(6):1965–1974. doi:10.1016/j.nano.2017.05.002
96. Kumari S, Kamboj VK, Rajpoot D, et al. The Unprecedented role of gold nanomaterial in diabetes management. Recent Pat Drug Del Form. 2019;13(3):219–227. doi:10.2174/1871526518666181114165352
97. Kumari Y, Singh SK, Kumar R, et al. Modified apple polysaccharide capped gold nanoparticles for oral delivery of insulin. International Journal of Biological Macromolecules. 2020;149:976–988. doi:10.1016/j.ijbiomac.2020.01.302
98. Hou L, Zheng Y, Wang Y, et al. Self-regulated carboxyphenylboronic acid-modified mesoporous silica nanoparticles with “touch switch” releasing property for insulin delivery. ACS Appl. Mater Interfaces. 2018;10(26):21927–21938. doi:10.1021/acsami.8b06998
99. Zou Z, He D, Cai L, et al. Alizarin complexone functionalized mesoporous silica nanoparticles: a smart system integrating glucose-responsive double-drugs release and real-time monitoring capabilities. ACS Appl Mater Interfaces. 2016;8(13):8358–8366. doi:10.1021/acsami.5b12576
100. Lee Y-H, Hong Y-L, Wu T-L, et al. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng. 2021;118:111385. doi:10.1016/j.msec.2020.111385
101. Tang Y. ITS sequence analysis used for parent selection in Lilium lancifolium Thunb. cross-breeding. J Appl Res Med Aromat Plants. 2021;100362.
102. Same S, Samee GJCJMBS. Carbon nanotube biosensor for diabetes disease. Crescent J Med Biolog Science. 2018;5:1–6.
103. Wang Y, Wei W, Liu X, et al. Carbon nanotube/chitosan/gold nanoparticles-based glucose biosensor prepared by a layer-by-layer technique. Mater Sci Eng. 2009;29(1):50–54. doi:10.1016/j.msec.2008.05.005
104. Guo C, Wang Y, Zhao Y, et al. Non-enzymatic glucose sensor based on three dimensional nickel oxide for enhanced sensitivity. Anal Methods. 2013;5(7):1644–1647. doi:10.1039/c3ay00067b
105. Chen W. nanotechnology, Nanoparticle fluorescence based technology for biological applications. J Nanoscien Nanotech. 2008;8(3):1019–1051. doi:10.1166/jnn.2008.301
106. Mohsen AMJCDT. Nanotechnology advanced strategies for the management of diabetes mellitus. Current Drug Targets. 2019;20(10):995–1007. doi:10.2174/1389450120666190307101642
107. Gaikwad S, Vora S, Bansode A, et al. Green Synthesis of Metal Oxide Nanoparticles: a Novel Approach to Treat Diabetes Mellitus. BioNanoScience. 2023;13(4):1582–1592. doi:10.1007/s12668-023-01205-y
108. Naikoo GA, Salim H, Hassan IU, et al. Recent advances in non-enzymatic glucose sensors based on metal and metal oxide nanostructures for diabetes management-a review. Front Chem. 2021;9:748957. doi:10.3389/fchem.2021.748957
109. Liu Y, Li L, Yu J, et al. Carbon nanoparticle lymph node tracer improves the outcomes of surgical treatment in papillary thyroid cancer. Cancer Biomark. 2018;23(2):227–233. doi:10.3233/CBM-181386
110. Xu S, Li Z, Xu M, et al. The role of carbon nanoparticle in lymph node detection and parathyroid gland protection during thyroidectomy for non-anaplastic thyroid carcinoma-a meta-analysis. PLoS One. 2020;15(11):e0223627. doi:10.1371/journal.pone.0223627
111. Sviridov D, Remaley ATJBJ. High-density lipoprotein mimetics: promises and challenges. Biochem J. 2015;472(3):249–259. doi:10.1042/BJ20150832
112. Hafiane A, Bielicki JK, Johansson JO, et al. Apolipoprotein E derived HDL mimetic peptide ATI-5261 promotes nascent HDL formation and reverse cholesterol transport in vitro. Biochimica Et Biophysica Acta (BBA)-Molecular and Cell Biol Lipids. 2014;1841(10):1498–1512. doi:10.1016/j.bbalip.2014.07.018
113. Luthi AJ, Lyssenko NN, Quach D, et al. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J Lipid Res. 2015;56(5):972–985. doi:10.1194/jlr.M054635
114. Chen H, Dorrigan A, Saad S, et al. In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice. PLoS One. 2013;8(2):e58208. doi:10.1371/journal.pone.0058208
115. Chen H, Ng JPM, Tan Y, et al. Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J Nanobiotechnol. 2018;16(1):1–12. doi:10.1186/s12951-018-0338-1
116. Kang H, Zhang K, Wong DSH, et al. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials. 2018;178:681–696. doi:10.1016/j.biomaterials.2018.03.007
117. Liu Y, Zeng S, Ji W, et al. Emerging theranostic nanomaterials in diabetes and its complications. Adv Sci. 2022;9(3):2102466. doi:10.1002/advs.202102466
118. Dhiman A, Pareek A, Shukla R. Concept of Nanomedicine in Endocrine Hormone Cancer Treatment. In: Hormone Related Cancer Mechanistic and Nanomedicines: Challenges and Prospects. Springer; 2023:253–267.
119. Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res. 2011;44(10):1050–1060. doi:10.1021/ar200106e
120. Gao W, Emaminejad S, Nyein HYY, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529(7587):509–514. doi:10.1038/nature16521
121. Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nature Rev Mater. 2016;1(5):1–12. doi:10.1038/natrevmats.2016.14
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.