Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Association Between Antioxidant Enzyme Polymorphisms with Type 2 Diabetes Mellitus in Jazan Province
Authors Habibullah MM , Hurubi A, Hakamy AO, Alwadani AAJ, Atti IM, Alothaid H , Shamlan G, Aldughaim M, Kaabi YA, Alhazmi A, Hamali H
Received 28 August 2024
Accepted for publication 22 November 2024
Published 30 November 2024 Volume 2024:17 Pages 4593—4598
DOI https://doi.org/10.2147/DMSO.S493459
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Konstantinos Tziomalos
Mahmoud M Habibullah,1 Ali Hurubi,1 Ali O Hakamy,2 Abbas Ali Jaber Alwadani,3 Ibrahim Mohammed Atti,3 Hani Alothaid,4 Ghalia Shamlan,5 Mohammed Aldughaim,6 Yahia A Kaabi,1 Alaa Alhazmi,1 Hassan Hamali1
1Department of Medical Laboratory Technology, College of Nursing and Health Sciences, Jazan University, Jazan, Saudi Arabia; 2Nursing Department, College of Nursing and Health Sciences, Jazan University, Jazan, Saudi Arabia; 3Department of Laboratory and Blood Bank, Prince Mohammed Bin Nasser Hospital, Ministry of Health, Jazan, Saudi Arabia; 4Department of Basic Medical Science, Faculty of Applied Medical Sciences, Al-Baha University, Al-Baha, Saudi Arabia; 5Department of Food Science and Nutrition, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia; 6Research Centre, King Fahad Medical City, Riyadh, Saudi Arabia
Correspondence: Mahmoud M Habibullah, Department of Medical Laboratory Technology, College of Nursing and Health Sciences, Jazan University, 6514 Makkah Al Mukarrama Road, Jazan, 82822, Saudi Arabia, Email [email protected]
Introduction: Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by persistent hyperglycemia, which results in initiates oxidative stress and disrupts various cellular pathways. In this study, we examined the relationship between polymorphisms in antioxidant enzymes, specifically glutathione peroxidase 1 (GPx1) and catalase (CAT), and the susceptibility to T2DM in a Saudi population from the Jazan Province.
Methods: A total of 419 participants were evaluated, including 247 T2DM patients and 172 controls. They were genotyped for the GPx1 Pro198Leu and CAT-262C/T polymorphisms by a PCR-based method.
Results: The results indicated that individuals with the CAT T/T genotype had a 60% lower likelihood of developing T2DM compared with those harboring the C/C genotype (uOD 0.4; 95% CI 0.2– 0.8, p = 0.04); however, no significant association was observed between the GPx1 polymorphism and T2DM.
Discussion: The results suggest that CAT polymorphism may confer a protective effect against T2DM, whereas the GPx1 polymorphism appears not to be a determinant of T2DM susceptibility in this population. Further studies including larger and more diverse cohorts are necessary to validate these results and elucidate the underlying mechanisms. Understanding the genetic factors that contribute to T2DM is essential for developing targeted preventive and therapeutic strategies.
Keywords: type 2 diabetes mellitus, antioxidant enzymes, glutathione peroxidase 1, catalase, polymorphism, Saudi population
Introduction
Oxidative stress is a major contributor to the development of type 2 diabetes mellitus (T2DM) through various molecular mechanisms. The chronic hyperglycemia characteristic of T2DM results in the generation of free radicals and increased oxidative stress, which affects multiple cellular pathways and molecular mechanisms.1–3 Oxidative stress refers to an imbalance between free radical production and the antioxidant system, which results in a reduction of peripheral insulin sensitivity.4 Oxidative stress has been implicated in the reduction of peripheral insulin sensitivity, which is a key factor in the development of T2DM.4 In addition, altered glucose metabolism, oxidative damage to pancreatic β-cells, and endothelial dysfunction are among the major pathways involved.5
Increased oxidative stress and/or impaired antioxidant defense associated with diabetes may also contribute to its development and progression; however, data for this association remains scarce. Mitochondrial function may be affected as a result of chronic hyperglycemia, which leads to an increase in reactive oxygen species (ROS) production.6 Glutathione peroxidase 1 (GPX1) and catalase (CAT) polymorphisms have been actively investigated as potential genetic factors that contribute to T2DM susceptibility.
The Pro198Leu polymorphism in Gpx1 involves a substitution of proline with leucine at position 198. Some studies suggest that the Leu allele may be associated with reduced GPx1 activity, resulting in decreased antioxidant defense against oxidative stress in the GPx1 protein.7 Polymorphisms in the glutathione peroxidase 1 gene may affect selenium status and GPx activity in response to dietary intake. This variability in GPx activity resulting from the Leu allele suggests a potential impact on antioxidant defense mechanisms in individuals with chronic conditions.8 Another study found that a CAT gene polymorphism was associated with a decreased risk of T2DM.9,10
In the present study, we determined the associations between single nucleotide polymorphisms (SNPs) of GPx1 and CAT with T2DM. We studied a Saudi cohort with T2DM from the southwest part of the country.
Material and Methods
Patients and Data Collection
All participants voluntarily provided written informed consent before they participated in this study. The study included 419 male and female subjects carefully matched for age. These subjects were recruited from various hospitals in the Jazan Province. The control group consisted of blood donors, whereas the diabetic patient group was selected from the Diabetes Centers, Jazan.
These individuals were randomly selected from the Saudi population, predominantly from the Jazan region. Sample collection took place between March 2021 and September 2021. Rigorous inclusion and exclusion criteria were applied during selection.
The inclusion criteria for the control group included healthy individuals without a family history of allergies, infections, or inflammatory diseases. In addition, fasting blood glucose (FPG), postprandial glucose (PPG), and body mass index had to fall within the normal range. Diabetic patients were included based on the criteria established by the American Diabetes Association (ADA)11 for T2DM as outlined in the 2020 ADA guidelines. For both groups exclusion criteria included pregnant or nursing mothers, patients with psychotic disorders, metabolic disorders, heart disease, or other types of diabetes.
Ethical Approval
The Jazan Research Ethics Committee of the General Directorate of Health Affairs (Jazan), Ministry of Health, Saudi Arabia, approved the current study, which complied with the Declaration of Helsinki (approval no. 2038).
DNA Extraction and Genetic Analysis
The specimens were collected into ethylenediaminetetraacetate (EDTA)-containing tubes. Genomic DNA extraction was performed utilizing the GeneJET Whole Blood Genomic DNA Purification Mini Kit from (Thermo Fisher, Paisley, UK) based on the manufacturer’s instructions. The quality and quantity of the purified DNA were determined using a NanoDrop 200 spectrophotometer (Thermo Fisher, Paisley, UK). A standard polymerase chain reaction (PCR) program was employed.12,13
Two SNPs were selected based on previous studies.14,15 The digested products exhibited two fragments of 170 and 52 bp for the 198Pro wild-type homozygous sequence, 3 fragments of 222, 170, and 52 for the 198Pro/Leu (CT) heterozygote, and one 222 bp fragment for the 198Leu (T) mutated homozygous sequence (Table 1). The PCR samples for were also sent to Macrogen (Seoul, South Korea) for C242T variation sequencing. The MacVector Software, Version 12.7 (MacVector, Inc., Apex, NC, USA) was used to visualize the sequencing electropherograms (Figure 1).16
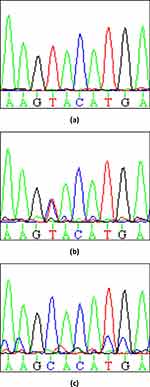 |
Figure 1 The figure shows the frequency of three genotypic variations: (a)T/T, (b)T/C, and (c) C/C, represented across the sample sequencing electropherograms. |
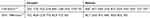 |
Table 1 Primers Used for PCR and Sequencing |
Statistical Analysis
Statistical analyses were performed using Stata version 13 software (StataCorp LP, College Station, TX, USA). Univariate logistic regression analysis was done using the crude odds ratio with a 95% confidence interval for the association study. A p-value of <0.05 was considered statistically significant to assess the association between the polymorphisms and the likelihood of developing diabetes.
Results
The mean age for all participants was 46 years (SD ± 14). The CAT-262C/T and GPX1 198Pro/Leu polymorphisms were examined in 419 participants. Of these, 247 (59%) were diagnosed with diabetic mellitus. All allele and genotype frequencies were consistent with the Hardy–Weinberg Equilibrium principle. Among the participants, 112 (46%) exhibited wild-type GPX1 (C/C) and the same number of cases were CAT (C/C). Subjects with mutated CAT (T/T) were 60% less likely to have DM compared with those harboring the CAT C/C polymorphism (uOD 0.4; 95% CI 0.2–0.8, p = 0.04). Moreover, the logistic regression analysis for the double combinations showed 53 (22%) participants with DM containing the GPX1 C/C and CAT C/T polymorphisms. (Table 2)
 |
Table 2 Genotype and Allele Distribution in Control and Type 2 Diabetes Mellitus (T2DM) Groups |
Discussion
Understanding the relationship between antioxidant enzymes and T2DM is an active area of investigation. The etiopathogenesis of this disease remains complex, with genetic, environmental, and lifestyle aspects considered to be involved. GPx1 and CAT are two important antioxidant enzymes associated with ROS detoxification, regulating ROS levels generated in the mitochondria. However, it is debatable as to whether these enzymes contribute to T2DM and its complications. For example, one study suggested that the GPx1 198Pro/Leu polymorphism is involved in peripheral neuropathy in T2DM, but not cardiovascular complications.17 A study on Polish patients contradicted the correlation between GPx1 and CAT polymorphisms.18 Therefore, in the present study, we examined the association of GPx1 and CAT polymorphisms in a T2DM cohort from the Saudi population.
Our findings suggest an association between CAT polymorphism and T2DM, in which patients carrying the wild-type genotype are more likely to develop T2DM. In contrast, patients with a CAT T/T genotype are at a lower risk. Neither the GPX1 results nor the double combination alleles were significantly associated with our study population. Our findings for GPX1 are consistent with those reported for a Tunisian19 and Chinese20 population.
A polymorphism in the catalase gene (CAT) has been associated with a decreased risk for T2DM in our cohort. One study indicated that individuals with this polymorphism have a significantly lower risk of developing severe respiratory syncytial virus bronchiolitis, suggesting a protective effect of this CAT polymorphism against disease severity, which may extend to other oxidative stress-related conditions, such as T2DM.21 Another study found that the CAT polymorphism rs7943316 may alter the risk of developing T2DM. Thus, variations in CAT may influence antioxidant defense mechanisms, potentially reducing the risk of T2DM.9
In the present study, associations between GPx enzyme polymorphisms and T2DM were observed; however, additional studies are needed to validate these findings and to determine the underlying mechanisms for these associations. Larger and more diverse study populations as well as functional studies to assess enzyme activity will be important to obtain a comprehensive understanding of the relationship of these variants through gene-environment interactions, ethnicity, and other environmental factors.
Conclusion
In this study, we examined the association between polymorphisms in the GPx1 and CAT genes with susceptibility to T2DM in a Saudi cohort from the Jazan Province. Our findings suggest that the CAT-262C/T polymorphism, particularly the T/T genotype, is significantly associated with a reduced risk of developing T2DM. This suggests that the CAT gene may have a protective role against oxidative stress-induced damage, which may lead to the onset and progression of T2DM.
Conversely, no significant association was observed between the GPx1 Pro198Leu polymorphism and T2DM. This lack of association is consistent with previous studies, but contrasts with others, which highlights the complexity of the genetic factors involved in T2DM and the potential influence of population-specific factors, such as gene-environment interactions.
Our results highlight the importance of considering genetic diversity and population-specific factors when studying the etiology of T2DM. Future studies should focus on larger, more diverse populations, and include functional studies to better understand the mechanisms by which these polymorphisms contribute to T2DM risk. In addition, exploring the interplay between genetic predisposition, lifestyle, and environmental factors will be necessary for developing more effective, preventive, and therapeutic strategies for T2DM.
Acknowledgments
The authors express deepest gratitude to all those who have contributed to the completion of this research including patients, donors and the diabetic center in Jazan. A Special thanks to Dr Yahia Solan, whose assistance was crucial to the success of this study.
Funding
This research was supported by the Deanship of Scientific Research, Jazan University, Jazan, Saudi Arabia, under grant number Waied (W41-40).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Caturano A, D’Angelo M, Mormone A, et al. Oxidative Stress in Type 2 Diabetes: impacts from Pathogenesis to Lifestyle Modifications. Current Issues in Molecular Biology. 2023;45(8):6651–6666.
2. Gorini F, Sabatino L, Gaggini M, Chatzianagnostou K, Vassalle C. Oxidative Stress Biomarkers in the Relationship between Type 2 Diabetes and Air Pollution. Antioxidants. 2021;10(8):1234.
3. González P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: an Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int J Mol Sci. 2023;24(11):9352.
4. Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020:8609213. doi:10.1155/2020/8609213
5. Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative Stress: pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull Exp Biol Med. 2021;171(2):179–189. doi:10.1007/s10517-021-05191-7
6. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 414(6865):813. doi:10.1038/414813a
7. Zaidan AaHMAHA-SHK. GPx1-rs1050450 Gene Polymorphism Association with End Stage Renal Disease in Type2 Diabetic Patients of Babylon Province/Iraq. Indian J Med Forensic Med Toxicol. 2021;15(2):2017–2021. doi:10.37506/ijfmt.v15i2.14660
8. Cardoso BR, Busse AL, Hare DJ, et al. Pro198Leu polymorphism affects the selenium status and GPx activity in response to Brazil nut intake. 10.1039/C5FO01270H. Food Funct. 2016;7(2):825–833. doi:10.1039/C5FO01270H
9. Saravani S, Miri HR, Saravani R, Yari D, Nakhaee A, Mahjoubifard M. Association of catalase (rs7943316) and glutathione peroxidase-1 (rs1050450) polymorphisms with the risk of type 2 diabetes (T2DM). Mo GeneMicrobi Virol. 2015;30(4):216–220. doi:10.3103/S0891416815040096
10. Banerjee M, Vats P, Kushwah AS, Srivastava N. Interaction of antioxidant gene variants and susceptibility to type 2 diabetes mellitus. British J Biomedi Sci. 2019;76(4):166–171. doi:10.1080/09674845.2019.1595869
11. Association AD. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(1):S15–S33. doi:10.2337/dc21-S002
12. Habibullah MM, Hakamy A, Mansor AS, Atti IM, Alwadani AAJ, Kaabi YA. The Association of UCP2-866 G/A Genotype with Autoimmune Hypothyroidism in the Southwestern Saudi Arabia Population. Int J Gene Med. 2023;16(null):875–879. doi:10.2147/IJGM.S400424
13. Habibullah M, Akter F, Qin X, Lohani M, Aldughaim MS, Al-Kaabi Y. Association between Angiotensin-Converting Enzyme- Insertion/Deletion Polymorphism and Diabetes Mellitus-2 in Saudi Population. Asian Pac J Cancer Prev. 2021;22(1):119–123. doi:10.31557/apjcp.2021.22.1.119
14. Silva PX, Aguiar L, Gaspar M, et al. Analysis of Genes Involved in Oxidative Stress and Iron Metabolism in Heart Failure: a Step Forward in Risk Stratification. Cureus. 2024;16(5):e60707. doi:10.7759/cureus.60707
15. Albadawi N, Tarazawi E, Ismail A, Altoum S, Bakheet K. Association 0f Gpx-198 C/T gene polymorphism (rs1050450-198 C/T) in Sudanese with Diabetic Retinopathy. J Blood Lymph. 2017;7(162):2.
16. Halawani AJ, Mansor AS, Assaggaf HM, et al. Investigation of Dombrock Blood Group Alleles and Genotypes among Saudi Blood Donors in Southwestern Saudi Arabia. Genes. 2022;13(6):1079.
17. Buraczynska M, Buraczynska K, Dragan M, Ksiazek A. Pro198Leu Polymorphism in the Glutathione Peroxidase 1 Gene Contributes to Diabetic Peripheral Neuropathy in Type 2 Diabetes Patients. Neuromolecular Med. 2017;19(1):147–153. doi:10.1007/s12017-016-8438-2
18. Kasznicki J, Sliwinska A, Kosmalski M, Merecz A, Majsterek I, Drzewoski J. Genetic polymorphisms (Pro197Leu of Gpx1, +35A/C of SOD1, −262C/T of CAT), the level of antioxidant proteins (GPx1, SOD1, CAT) and the risk of distal symmetric polyneuropathy in Polish patients with type 2 diabetes mellitus. Adv Med Sci. 2016;61(1):123–129. doi:10.1016/j.advms.2015.10.006
19. Souiden Y, Mallouli H, Meskhi S, et al. MnSOD and GPx1 polymorphism relationship with coronary heart disease risk and severity. Biol Res. 2016;49:22. doi:10.1186/s40659-016-0083-6
20. Yeh HL, Kuo LT, Sung FC, Yeh CC. Association between Polymorphisms of Antioxidant Gene (MnSOD, CAT, and GPx1) and Risk of Coronary Artery Disease. Biomed Res Int. 2018;2018:5086869. doi:10.1155/2018/5086869
21. Chambliss JM, Ansar M, Kelley JP, Spratt H, Garofalo RP, Casola A. A Polymorphism in the Catalase Gene Promoter Confers Protection against Severe RSV Bronchiolitis. Viruses. 2020;12(1):57.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

