Back to Journals » Journal of Multidisciplinary Healthcare » Volume 18
The Association Between Lifestyle and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: A 3-year Prospective, Observational Study
Authors Zhang L, Zhang S, Tang X
Received 30 October 2024
Accepted for publication 8 January 2025
Published 20 March 2025 Volume 2025:18 Pages 1721—1729
DOI https://doi.org/10.2147/JMDH.S503669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Charles V Pollack
Lu Zhang, Sumei Zhang, Xuanbing Tang
School of Nursing and Rehabilitation, Xi’an Medical University, Xi’an, Shaanxi, People’s Republic of China
Correspondence: Lu Zhang, School of Nursing and Rehabilitation, Xi’an Medical University, Xi’an, Shaanxi, 710021, People’s Republic of China, Tel +8613891432973, Email [email protected]
Purpose: Lifestyle is one of the important factors affecting health. There are few studies that comprehensively analyze the impact of a combination of lifestyle factors on mortality in patients undergoing maintenance hemodialysis. So, to measure the association between lifestyle factors and mortality for patients undergoing maintenance hemodialysis.
Patients and Methods: A prospective, observational study design was employed. Through convenience sampling, the patients who are over 18 years old and have undergone dialysis for at least 3 months, from the hemodialysis center outpatient in two hospitals of Xi’an were selected. The questionnaires of this study include sociodemographic characteristics and lifestyle-related scales, such as nutrition, sleep and self-management scales. The differences between the deceased group and the surviving group were analyzed using the t-test or Mann–Whitney tests and chi-square tests. Logistic regression analysis was applied to identify the association between lifestyle factors and mortality.
Results: In this study, 286 patients who completed the questionnaire were screened. During the follow-up of this 3 years, patients who transferred to another hospital (n=31), kidney transplantation (n=6) and termination of dialysis (n=13) were excluded. Finally, 236 participants were tracked to the final outcome. Of these 236 patients, 66.95% were men. The proportion of patients under 60 years old is slightly higher than that of patients over 60 years old. More than half (64.83%) of the patients have a lower education level. And the main primary disease of ESRD was diabetic nephropathy (39.83%). Through a 3-year follow-up study, 73 patients died, accounting for 30.93%. The results showed that compared with surviving patients, deceased patients had significantly lower scores of self-management (Z=− 2.09, P=0.036) and higher scores of malnutrition-inflammation score (Z=− 2.31, P=0.021). Moreover, deceased patients had a significantly higher proportion of poor sleepers (χ2=4.38, P=0.036) and No exercise (χ2=5.16, P=0.023). However, there were no statistically significant differences in BMI, smoking history and drinking history between the two groups. In logistic analyses, age (χ2=19.63, P< 0.001, OR=0.26, 95% CI=0.14~0.47) and self-management score (χ2=3.82, P=0.051, OR=1.03, 95% CI=1.00~1.06) were major factors related to mortality.
Conclusion: Self-management and age are closely related to the mortality rate of patients. And our study showed that the relationship between self-management and mortality is strongest, so doctors and nurses at dialysis centers should pay more attention to and actively improve self-management level of patients undergoing maintenance hemodialysis.
Keywords: renal dialysis, life style, self-management, mortality
Introduction
End-stage renal disease (ESRD) is a rapidly increasing global health and health-care burden. According to the data of the survey conducted by the International Society of Nephrology (ISN) in 2019 among 79 countries, on average, there are 144 newly diagnosed patients with ESRD per million general population.1 Hemodialysis is an important treatment method for prolonging the lifespan of patients with ESRD.2,3 For example, in the United States, nearly 90% of ESRD patients receive hemodialysis treatment based on dialysis centers.2 In China, the data from the Chinese National Renal Data System (CNRDS) revealed that the number of patients undergoing MHD in China increased from 0.235 million in 2011 to 0.447 million in 2016 (90.2% within 5 years), and the prevalence increased from 174 cases per million in 2011 to 298 per million in 2016 (71.3% within 5 years).4 Although there have been considerable improvements in the overall survival rate, the prognosis of patients on dialysis remains very poor, the mortality rates were 166 for hemodialysis patients per 1,000 patient-yeasignificantly higher than that of the general population, especially for patients over 65 years old and those who have been on dialysis for a long time.5
According to the definition of Medical Subject Headings (MeSH), lifestyle is a typical way of life or manner of living characteristic of an individual or group. Lifestyle is one of the important factors affecting health. Healthy lifestyle is a pattern of behavior involving life style choices that ensure optimum health. Examples include eating right, maintaining physical, emotional, and spiritual wellness, and taking preemptive steps against communicable diseases. Healthy lifestyle can promote health. On the contrary, unhealthy lifestyle can lead to the occurrence of lifestyle-related diseases, such as hypertension, diabetes, and dyslipidemia, which can lead to the occurrence of chronic kidney diseases such as hypertensive nephropathy and diabetic nephropathy, and thus further increase the number of patients on maintenance hemodialysis (MHD).6 The American Heart Association (AHA) has a number of lifestyle recommendations including avoiding smoking, engaging in regular physical activity, keeping an appropriate body mass index (BMI), adhering to a healthy diet (rich in fruits, vegetables, and fish and low in salt and sugar), and maintaining blood pressure (BP), cholesterol, and glucose within recommended targets.7
There are studies investigating lifestyle and survival in patients with maintenance hemodialysis (MHD) and chronic kidney disease (CKD). Healthy lifestyle can reduce the risk of all-cause mortality and symptoms and psychiatric disease burden in patients with mild to moderate CKD.8 Similar research findings adherence to a healthy lifestyle (non-smoking, regular physical activity, appropriate BMI, and healthy diet) can reduce the risk of death in patients with CKD stages 1–4 by 20% to 70%.9 A 4-year prospective study on maintenance hemodialysis patients found that a healthy lifestyle can reduce all-cause mortality and cardiovascular mortality by 35% and 36%, respectively.10 Therefore, in long-term dialysis treatment, maintaining a healthy lifestyle is an important task for both patients and healthcare workers, which is an important measure to improve patient prognosis.10
At present, most studies on the relationship between lifestyle and prognosis of patients on MHD, only choose a single factor, such as nutrition or physical exercise, with mortality. There are few studies that comprehensively analyze the impact of a combination of lifestyle factors on mortality in patients undergoing MHD. According to the lifestyle recommendations of AHA, our study conducted a 3-year follow-up study to comprehensively evaluate the relationship between multiple lifestyle factors and all-cause mortality in patients undergoing MHD: nutrition, physical activity, self-management, BMI, sleep, smoking history and drinking history, in order to provide a theoretical basis for improving the prognosis of these patients.
Materials and Methods
Design
Through a 3-year follow-up investigation, we aimed to determine the effects of lifestyle on all-cause mortality in patients undergoing maintenance hemodialysis. We investigated the lifestyle status by investigating nutrition, physical activity, self-management, BMI, sleep, smoking history and drinking history.
This prospective, observational study enrolled participants in maintenance hemodialysis center outpatients in two hospitals. The baseline study is from April to May 2021. The primary outcome was all-cause mortality. The last follow-up visit time was April 30, 2024.
Study Setting and Sampling
This study used a convenience sampling method.
According to the empirical method, the sample size is 10 to 15 times the number of covariates.11 This study involves 18 covariates. After calculation, the required sample size for this study is between 180 ~ 270.
Inclusion and Exclusion Criteria
The inclusion criteria include the following : (1) MHD duration exceeds 3 months; (2) over 18 years old. The exclusion criteria include the following : (1) any acute medical or surgical condition requiring hospitalization; (2) patients with dementia or any psychotic disorder.
Instrument
The research data were collected through survey questionnaires. The questionnaires include sociodemographic characteristics and lifestyle-related scales, such as nutrition, sleep and self-management scales.
Sociodemographic and Clinical Characteristics
Sociodemographic characteristics included age, gender, marriage, primary renal diagnosis, smoking history, drinking history, and so on. Blood pressure (BP) was measured before dialysis. Laboratory data for calcium (Ca) and phosphate (P) were collected upon patients’ entry into this study.
Evaluation of Sleep Quality
Patients’ sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) questionnaire. This scale consists of 19 questions, including 7 dimensions: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction. Respondents were required to recall their sleep status for the past one month and choose corresponding options based on the actual situation. The total score of the scale is between 0 and 21 points, with a higher score indicating poorer sleep quality. If the PSQI score >5, it indicates poorer sleep quality, and if the score is ≤5, indicating good sleep quality.12
Evaluation of Nutrition
Patients’ nutrition was assessed using the Malnutrition-Inflammation Score (MIS). MIS consists of four main parts, including medical history, physical examination, BMI and patient’s laboratory examination. The medical history includes weight changes, dietary intake, gastrointestinal symptoms, functional capacity, dialysis time and complications. Physical examination includes the reduction of subcutaneous fat and signs of muscle consumption. Laboratory parameters include serum albumin and total iron-binding capacity (or transferrin). These 10 items all have 4 evaluation indicators ranging from 0 (normal) to 3 (severely abnormal). The higher the MIS total score, the more severe the malnutrition.13
In the investigation content, dietary intake, gastrointestinal symptoms, and functional capacity are filled in based on the patient’s symptoms, weight changes are filled in based on weight measurement results, serum albumin and total iron-binding capacity (or transferrin) are filled in based on the patient’s recent laboratory examination results in the past 3–6 months, and subcutaneous fat reduction is judged based on the thickness of the triceps brachii skin fold (TSF) measured by the skin fold thickness meter.14 Mid upper arm circumference (MAC) can better reflect the consumption of fat-free muscles.13 The MAC was calculated by measuring the length of a circle of the part between the acromion of the scapula and the olecranon of the ulna with a non-elastic soft tape.14
Evaluation of Self-Management
The Hemodialysis Self-Management Instrument (HDSMI) is used to evaluate patients’ self-management level, which includes four dimensions: problem solving, emotional management, self-care, and partnership. This scale has a total of 20 items, with each item’s options ranging from 1 point (never) to 4 points (always). The total score range is 20–80, and the higher the total score, the higher the patient’s self-management level. The internal consistency reliability of the scale is 0.81.15
Data Collection
Data were collected through face-to-face interviews, measurements of body indicators and electronic medical records.
Statistical Analyses
Statistical descriptions of continuous data were expressed as the mean ± standard deviation (SD), while categorical data is expressed as a percentage. Continuous variables were compared using t tests or Mann–Whitney tests as appropriate. Categorical variables were compared using the 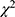 test. The meaningful variables from the above single factor analysis are included in the logistic regression. Logistic regression corrected for confounding factors to better identify lifestyle factors that relate to mortality. In logistic regression, the step probability entering is 0.05, excluding is 0.10. The results are presented with Odds Ratio (OR) and 95% confidence intervals (CI).
test. The meaningful variables from the above single factor analysis are included in the logistic regression. Logistic regression corrected for confounding factors to better identify lifestyle factors that relate to mortality. In logistic regression, the step probability entering is 0.05, excluding is 0.10. The results are presented with Odds Ratio (OR) and 95% confidence intervals (CI).
Ethical Consideration
In our study, human participants were investigated. All procedures involving human investigators met ethical requirements and the 1964 helsinki Declaration as well as relevant ethical standards.
All information was obtained based on the patient’s informed consent. The respondents voluntarily participated in this study. All the identifying details of the participants that were studied should not be published in publication. The study was approved by the Medical Ethics Review Committee of Xi’an Medical University (XYLS2024220).
Results
Participants
In this study, 286 patients who completed the questionnaire were screened at the baseline survey.
Outcome Data
During the follow-up of this 3 years, patients who transferred to another hospital (n=31), kidney transplantation (n=6) and termination of dialysis (n=13) were excluded. Finally, 236 participants were tracked to the final outcome. Through a 3-year follow-up study, 73 patients died, accounting for 30.93%.
Descriptive Data
We studied 236 MHD patients, of which 66.95% (n=158) were men. The proportion of patients under 60 years old is slightly higher than that of patients over 60 years old. More than half (64.83%) of the patients have a lower education level. And the main primary disease of ESRD was diabetic nephropathy (n=94, 39.83%). Other causes were hypertensive nephropathy (n=54, 22.88%), glomerulonephritis (n=47, 19.92%), and other kidney diseases (n=41, 17.37%). More than half (69.07%) of the patients had autologous arteriovenous fistula as their vascular access. The characteristics are shown in Table 1.
 |
Table 1 Comparison of General Information Between of the Two Groups |
Main results
Comparison of Basic General Characteristics Between of the Two Groups
In the general information, there were no significant differences in gender, marriage, blood pressure, calcium (Ca) and phosphate (P) between the two groups. Compared with surviving patients, deceased patients were significantly older (χ2=21.28, P<0.001), lower education level (χ2=3.87, P=0.049), and more retired (χ2=12.13, P=0.002). Diabetes nephropathy accounted for a higher proportion of primary renal diagnosis in deceased patients (χ2=16.52, P=0.001). There is a higher proportion of deep vein catheterization patients in the deceased group (χ2=3.83, P=0.05) (Table 1).
Comparison of Basic Lifestyle Differences Between of the Two Groups
The median MIS, BMI and self-management score were (8.38±2.79), (21.87±3.59) and (55.16±11.18), respectively.
In lifestyle differences, there were no significant differences in BMI, smoking history and drinking history between the two groups. Compared with surviving patients, deceased patients had significantly lower score of self-management (Z=−2.09, P=0.036) and higher scores of MIS (Z=−2.31, P=0.021). Moreover, deceased patients had a significantly higher proportion of poor sleepers (χ2=4.38, P=0.036) and No exercise (χ2=5.16, P=0.023) (Table 2).
 |
Table 2 Comparison of Lifestyle Differences Between of the Two Groups |
Multiple Factors Logistic Regression
Wald logistic regression was used, with deceased or surviving patients grouping as dependent variable y and meaningful variables of univariate analysis as independent variable x. Significant variables in univariate analysis include age, education, employment, primary renal diagnosis, vascular access, MIS, self-management score, PSQI and exercise status. These variables enter the multivariate logistic regression analysis as X. Among them, according to the results in Table 2, lifestyle factors include MIS, self-management score, PSQI and exercise status. In multivariate logistic analyses, age (χ2=19.63, P<0.001, OR=0.26, 95% CI=0.14~0.47) and self-management score (χ2=3.82, P=0.051, OR=1.03, 95% CI=1.00~1.06) were major factors related to mortality (Table 3). After multiple logistic regression analysis, only the self-management score remained among the lifestyle factors. Therefore, self-management level is an important factor related to mortality in MHD patients.
 |
Table 3 Multiple Factors Logistic Regression on Mortality |
Discussion
Patient-oriented self-management is the cornerstone of chronic disease management, and optimizing self-management is the foundation for controlling disease risks and improving disease management. Strengthening self-management is to better optimize patient behavior, rather than simply providing health education to patients.16 Self-management requires patients to be better responsible for their own illness, rather than passively receiving education.17 Patients are no longer passive recipients of education; they are active determiners of their health.
Our study showed that though logistic regression, age and HDSMI score were major factors related to mortality. Self-management behaviors in chronic diseases are the patient’s proactive engagement in healthcare activities to learn to solve problems, control their disease, and adjust their way of living to coexist with their chronic disease in everyday life; effective self-management includes the ability to monitor the individual’s own condition and emotions to maintain a satisfying quality of life and reduce medical costs.18 For patients with CKD, self-management intervention can reduce the modifiable risk of kidney disease progression (such as proteinuria, blood pressure, blood glucose, and exercise ability), thereby improving the prognosis of patients with CKD, avoiding progression to ESRD, and thus increasing survival rates.19
Since ESRD is irreversible and patients need lifelong dialysis therapy, their self-management behaviors play a critical role in determining their quality of life and outcomes. Our study proved that self-management is a very important factor affecting the prognosis of dialysis patients. Dialysis patients must manage themselves well, such as fluid intake, diet, medication, scheduling dialysis, balancing life and health needs. Good health education and a correct understanding of dialysis treatment can improve patients’ treatment compliance, the self-management level of patients can also be improved accordingly, patients can become an active participant in their treatment plans to suit the patient’s metabolic needs and personal lifestyle preferences to improve their treatment effectiveness and quality of life, and ultimately improving their prognosis.20
The quality of health and survival depends on how hemodialysis patients manage their chronic illness, dialysis, and everyday lifestyle throughout their lives.21 Actively conducting home-based self-observation and disease self-management has become a key measure to improve the quality of life and prognosis of dialysis patients.20 Each patient is the primary manager of their own disease and treatment, and must apply the nursing knowledge learned from medical staff to active self-management, including regular blood glucose and blood pressure measuring, etc.22
The goal of self-management is to help patients identify strategies for disease management, including goal setting, problem-solving, symptom management, and shared decision-making, in order to enable patients to live a positive and effective life.23 Possible measures include better preparing patients for dialysis through pre-dialysis educational programs,24 pro-actively anticipating and managing common problems faced by dialysis starters.25
In lifestyle differences, there were significant differences in MIS, self-management score, PSQI and exercise status between two groups. However, in logistic regression, only self-management is the major factor related to mortality. The possible reason is that compared to indicators such as nutrition, exercise, and sleep, self-management is a more comprehensive indicator that requires patients to be able to comprehensively control diseases, adjust their lifestyle, and better coexist with chronic diseases. Therefore, the implication of this study is to advocate more comprehensive self - management of patients from aspects such as diet, exercise, and sleep, thereby comprehensively improving lifestyle to better improve patients’ prognoses.
Additionally, our study once again confirmed the poor prognosis and high mortality rate of elderly dialysis patients. Due to aging ESRD populations, the increased risk of geriatric disease, progressive decline of physiological function and poor tolerance of related symptoms and psychiatric disease burden, older patients often have a poor prognosis.26 The Dialysis Outcomes and Practice Patterns Study (DOPPS) suggested that mortality of patients aged 65 and above is higher than that of the general dialysis population, with approximately 40 deaths per 100 people-years.27 Therefore, we still need to focus on the prognosis of elderly dialysis patients.
The strengths of our study include a prospective design to determine the causal effects of lifestyle on outcomes in patients undergoing MHD. The prospective design prevents recall bias with respect to the outcomes. Second, we comprehensively analyzed the impact of multiple lifestyle factors on the outcome, and identified the most influential factors. In addition, there are few studies analyzing the impact of self-management on all-cause mortality in patients. In future research, we can conduct the study of self-management interventions to help hemodialysis patients better clarify their goals, improve their own skills and techniques, and enhance self-management of their chronic conditions.
This study had some limitations as well. First, due to the time limit of this fund project, we only tracked the survival time of 3 years, and we will continue to track the prognosis of these dialysis patients in the future. Second, this study enrolled only hemodialysis patients, and the results cannot easily be generalized to peritoneal dialysis patients.
Conclusion
Our study indicates that the relationship between self-management and mortality is strongest, so doctors and nurses at dialysis centers should pay more attention to and actively improve self-management level of patients undergoing MHD. In addition, considering the differences in other lifestyle factors between deceased and surviving patients, doctors and nurses still need to pay attention to the patient’s nutrition, exercise, sleep and other aspects to better improve the prognosis of patients undergoing maintenance hemodialysis.
Acknowledgments
We thank the staff from Nephrotic Hemodialysis Center, Shanxi Provincial People's Hospital and Hemodialysis Center, Department of Nephrology and Endocrinology, the Second Affiliated Hospital of Xi’an Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the Journal of Multidisciplinary Healthcare; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Basic Research Program of Shaanxi Province (funding recipient: ZL, grant number: 2021JM-498); Innovation Team Support Plan for SanQin Scholars ([2020]45) Shaanxi Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thurlow JS, Joshi M, Yan G, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52(2):98–107. doi:10.1159/000514550
2. Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2021;77(4 Suppl 1):A7–a8. doi:10.1053/j.ajkd.2021.01.002
3. Verberne WR, Geers AB, Jellema WT, Vincent HH, van Delden JJ, Bos WJ. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol. 2016;11(4):633–640. doi:10.2215/cjn.07510715
4. Meng Y, Wu HT, Niu JL, et al. Prevalence of depression and anxiety and their predictors among patients undergoing maintenance hemodialysis in Northern China: a cross-sectional study. Ren Fail. 2022;44(1):933–944. doi:10.1080/0886022x.2022.2077761
5. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3 Suppl 1):A7–A8. doi:10.1053/j.ajkd.2019.01.001
6. Hara A, Hirata T, Okamura T, Kimura S, Urushihara H. Lifestyle behaviors associated with the initiation of renal replacement therapy in Japanese patients with chronic kidney disease: a retrospective cohort study using a claims database linked with specific health checkup results. Environ Health Prevent Med. 2021;26(1):102. doi:10.1186/s12199-021-01022-3
7. Uijl A, Koudstaal S, Vaartjes I, et al. Risk for Heart Failure: the Opportunity for Prevention With the American Heart Association’s Life’s Simple 7. JACC Heart Fail. 2019;7(8):637–647. doi:10.1016/j.jchf.2019.03.009
8. Schrauben SJ, Hsu JY, Amaral S, Anderson AH, Feldman HI, Dember LM. Effect of kidney function on relationships between lifestyle behaviors and mortality or cardiovascular outcomes: a Pooled Cohort Analysis. J Am Soc Nephrol. 2021;32(3):663–675. doi:10.1681/asn.2020040394
9. Hu EA, Coresh J, Anderson CAM, et al. Adherence to healthy dietary patterns and risk of CKD progression and all-cause mortality: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2021;77(2):235–244. doi:10.1053/j.ajkd.2020.04.019
10. Su G, Saglimbene V, Wong G, et al. Healthy lifestyle and mortality among adults receiving hemodialysis: the DIET-HD Study. Am J Kidney Dis. 2022;79(5):688–698.e1. doi:10.1053/j.ajkd.2021.07.022
11. van Smeden M, Moons KG, de Groot JA, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res. 2019;28(8):2455–2474. doi:10.1177/0962280218784726
12. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
13. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–1263. doi:10.1053/ajkd.2001.29222
14. Sedhain A, Hada R, Agrawal RK, Bhattarai GR, Baral A. Assessment of Nutritional Status of Nepalese Hemodialysis patients by anthropometric examinations and modified quantitative subjective global assessment. Nutr Metabol Insights. 2015;8:21–27. doi:10.4137/NMi.S27640
15. Li H, Jiang YF, Lin CC. Factors associated with self-management by people undergoing hemodialysis: a descriptive study. Int J Nurs Studies. 2014;51(2):208–216. doi:10.1016/j.ijnurstu.2013.05.012
16. Jones F, Riazi A. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil. 2011;33(10):797–810. doi:10.3109/09638288.2010.511415
17. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi:10.1207/s15324796abm2601_01
18. Wang SL, Kung LF, Chen TH, Hsiao SM, Hsiao PN, Chiou CJ. Construction and validation of a chronic kidney disease self-care scale. Hu Li Za Zhi. 2016;63(4):90–99. Polish. doi:10.6224/jn.63.4.90
19. Peng S, He J, Huang J, et al. Self-management interventions for chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2019;20(1):142. doi:10.1186/s12882-019-1309-y
20. Ma LC, Liu YM, Lin YC, et al. Factors influencing self-management behaviors among hemodialysis patients. J Pers Med. 2022;12(11):1816. doi:10.3390/jpm12111816
21. Kim S, Kim E, Ryu E. Illness perceptions, self-care management, and clinical outcomes according to age-group in Korean hemodialysis patients. Int J Environ Res Public Health. 2019;16(22):4459. doi:10.3390/ijerph16224459
22. Stevenson J, Tong A, Campbell KL, Craig JC, Lee VW. Perspectives of healthcare providers on the nutritional management of patients on haemodialysis in Australia: an interview study. BMJ Open. 2018;8(3):e020023. doi:10.1136/bmjopen-2017-020023
23. Institute of Medicine Committee on Identifying Priority Areas for Quality I. In: Adams K, Corrigan JM, editors. Priority Areas for National Action: Transforming Health Care Quality. National Academies Press (US); 2003.
24. Lacson E, Wang W, DeVries C, et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis. 2011;58(2):235–242. doi:10.1053/j.ajkd.2011.04.015
25. Wingard RL, Chan KE, Lazarus JM, Hakim RM. The “right” of passage: surviving the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S114–20. doi:10.2215/cjn.04360709
26. Wongrakpanich S, Susantitaphong P, Isaranuwatchai S, Chenbhanich J, Eiam-Ong S, Jaber BL. Dialysis therapy and conservative management of advanced chronic kidney disease in the elderly: a systematic review. Nephron. 2017;137(3):178–189. doi:10.1159/000477361
27. Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi:10.1038/ki.2013.252
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Development and Validation of Prediction Models for All-Cause Mortality and Cardiovascular Mortality in Patients on Hemodialysis: A Retrospective Cohort Study in China
Yang M, Yang Y, Xu Y, Wu Y, Lin J, Mai J, Fang K, Ma X, Zou C, Lin Q
Clinical Interventions in Aging 2023, 18:1175-1190
Published Date: 28 July 2023
Current Knowledge of Beta-Blockers in Chronic Hemodialysis Patients
Haddiya I, Valoti S
International Journal of Nephrology and Renovascular Disease 2023, 16:223-230
Published Date: 12 October 2023
The Relationship Between Fracture and Mortality in a Chinese Maintenance Hemodialysis Patients Cohort
Liu X, Liu Z, Niu Y, Zhang K, Zhang X, Yu C
Journal of Multidisciplinary Healthcare 2024, 17:2031-2038
Published Date: 1 May 2024

