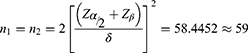Back to Journals » Journal of Inflammation Research » Volume 18
The Association Between Neonatal Respiratory Distress Syndrome and Plasma IgG N-Glycosylation: A Case-Control Study
Authors Wang Y, Shen Q, Yan R, Wang M, Xu M, Chen H, Li D
Received 21 February 2025
Accepted for publication 13 May 2025
Published 21 May 2025 Volume 2025:18 Pages 6439—6451
DOI https://doi.org/10.2147/JIR.S524188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yingjie Wang,1,2,* Qingqing Shen,3,* Ruxu Yan,1,2 Meng Wang,2,4 Min Xu,5 Hanxiang Chen,1 Dong Li2,6
1Department of Clinical Laboratory Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Medicine and Health Key Laboratory of Laboratory Medicine, Jinan, Shandong, People’s Republic of China; 2School of Public Health and Health Management, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 3Department of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong, People’s Republic of China; 4Jinshan District Center for Disease Control and Prevention, Shanghai, People’s Republic of China; 5Department of neonatology, Tai’an Maternal and Child Health Hospital, Tai’an, Shandong, People’s Republic of China; 6Jining Medical University, Jining, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hanxiang Chen, Email [email protected] Dong Li, Email [email protected]
Background: Neonatal respiratory distress syndrome (NRDS) is the leading cause of neonatal death. Changes in plasma immunoglobulin G (IgG) N-glycosylation have been demonstrated in a variety of diseases. However, its implications and clinical significance in NRDS remain to be clarified.
Methods: To determine the effect of IgG N-glycosylation on NRDS, we recruited 88 NRDS participants and 120 control participants from December 2021 to September 2022. Plasma was collected, IgG was isolated and purified, and the glycogram was analyzed by ultra performance liquid chromatography (UPLC) with fluorescence detector.
Results: The occurrence of premature rupture of membranes (PROM) [OR=9.043(1.036– 78.966), P=0.046] and the elevation of γ-glutamyltransferase (GGT) [OR=1.015(1.001– 1.029), P=0.032] were independent risk factors for the occurrence of NRDS. Furthermore, the area percentages of GP1, GP3, GP4, GP11, GP13, and GP24 were significantly higher in NRDS patients compared with control group. Conversely, GP14 was observed to be significantly lower. Furthermore, an increase in plasma IgG sialylation and core fucosylation was observed in NRDS, whereas the modification with galactosylation was decreased. The model constructed using GP1, GP13, GP14, PROM, and GGT as composite indices demonstrated robust predictive performance (AUC=0.902, 95% CI: 0.851– 0.953).
Conclusion: Patients with NRDS frequently exhibit alterations in the glycosylation of plasma IgG. These findings provide new insights into the diagnosis of NRDS and clinical treatment.
Keywords: neonatal respiratory distress syndrome, IgG N-glycosylation, ultra performance liquid chromatography, premature rupture of membranes
1.Introduction
Neonatal respiratory distress syndrome (NRDS) is a clinical condition that generally presents within 4 to 12 hours after birth, characterized by progressive dyspnea, grunting respirations, and cyanosis, and is predominantly observed in preterm infants.1 Despite advancements in medical technology reducing the incidence of NRDS, it remains a significant contributor to neonatal mortality and morbidity.2,3 Several studies have developed models to predict the incidence of NRDS;4 however, these studies predominantly focus on alterations in complete blood count indicators in pregnant women prior to delivery, while insufficiently addressing the variations in indicators among patients diagnosed with NRDS. A comprehensive exploration of NRDS pathogenesis is imperative to identify effective preventive and therapeutic strategies.
Protein glycosylation denotes the process of covalently attaching carbohydrates to protein functional groups, constituting a prevalent yet intricate post-translational modification. This process not only diversifies the proteome of organisms but also influences protein function and stability significantly.5,6 Immunoglobulin G (IgG) represents the predominant antibody subclass in the human circulatory system, pivotal in adaptive immune responses. IgG exhibits the highest N-glycosylation level among human serum proteins.7 Structurally, IgG comprises two identical light chains and two identical heavy chains interconnected by disulfide bonds, with Fab fragments facilitating antigen recognition and neutralization, and Fc fragments triggering phagocytosis, complement activation, and inflammatory responses. Alterations in Fc core fucose, galactose, N-acetylglucosamine, and sialic acid impact IgG’s affinity for diverse complement proteins and receptors, potentially eliciting the secretion of pro-inflammatory and anti-inflammatory cytokines.8 Consequently, modifications in IgG Fc N-glycosylation can disrupt immune homeostasis, predisposing individuals to various diseases.
Recent research has increasingly demonstrated the significant association between IgG Fc N-glycosylation modification and the pathogenesis of various diseases. Studies by Ivan Gudelj et al have highlighted alterations in serum IgG glycosylation profiles in patients with different diseases, particularly those with underlying inflammatory components, showing an increase in agalactosylated IgG abundance.9 This change has been linked to variations in inflammation and the expression of cardiometabolic markers, indicating a crucial role of IgG N-glycosylation in disease development. Additionally, Barbara Radovani et al observed distinct IgG N-glycosyl composition in patients with coronary artery disease (CAD), with a more pronounced difference noted in women, suggesting a potential influence of sex on IgG N-glycosyl composition. Furthermore, the presence of salivary N-glycan structure was found to be inversely correlated with CAD.10 Anna Birukov et al have also reported associations between changes in IgG N-glycosylation and cardiometabolic risk.11
While these findings underscore the intricate and diverse roles of IgG N-glycosylation in various diseases, the impact of IgG N-glycosylation profiles on NRDS remains unclear. Furthermore, current diagnostic approaches for NRDS are hindered by delayed diagnosis, absence of early biomarkers, and the potential risk of radiation exposure.12 Considering the emerging correlation between IgG N-glycosylation modifications and disease, we propose the hypothesis that IgG N-glycosylation modifications may undergo alterations in response to NRDS. This is based on the premise that the glycosylation profile has the potential to reflect disease subtypes and inform targeted therapeutic interventions. Accordingly, we conducted a comprehensive analysis of the alterations in clinical indicators among NRDS patients and investigated the modifications of plasma IgG N-glycosylation using Ultra Performance Liquid Chromatography (UPLC) technology. This investigation aims to establish a theoretical foundation for enhancing the understanding of the pathogenesis and clinical management of NRDS.
Materials And Methods
Subjects and Sample Collection
Based on our recently published research findings, the mean albumin-globulin ratio in the general population is 1.98±0.336. In contrast, neonatal patients diagnosed with hypoxic-ischemic encephalopathy exhibit an average albumin-globulin ratio of 2.19±0.425.13 Statistical analysis was performed with a significance level (α) of 0.05 and a statistical power (1-β) of 0.90. For the case-control study, the sample size for each group was calculated to compare the means of the two samples, resulting in a requirement of 59 participants per group.14 To mitigate potential issues such as information bias, data loss, and damage to blood samples, we increased the sample size by 10%. Consequently, the total number of participants across both groups was adjusted to 130.
In alignment with the case-control design of this retrospective study, a total of 88 patients diagnosed with NRDS and 120 control patients were recruited between December 2021 and September 2022 from Tai’an Maternal and Child Health Hospital and Shandong Provincial Hospital Affiliated to Shandong First Medical University. Of these, 21 NRDS patients and 53 concurrent controls were designated as the validation group, while the remaining participants constituted the training group. In order to be eligible for inclusion in the study, participants were required to meet the following criteria: 1. The diagnosis of NRDS was made by clinicians based on the patient’s clinical presentation (eg, dyspnea, moaning, tri specular signs within 6 hours after birth), arterial blood gas analysis and chest X-ray (eg, ground-glass changes, air bronchogram signs). The diagnosis also met the criteria set out in the 6th edition of the European Guidelines for the Management of NRDS.12 2.Newborns with informed consent of guardians. The exclusion criteria were as follows: 1. Severe congenital heart disease, pulmonary heart disease, vascular disease, malignancy and other diseases; 2. Maternal use of drugs that may affect glycosylation during pregnancy (such as immunosuppressants); 3. Incomplete outcome data or irrelevant clinical data. All subjects met the inclusion criteria, and their guardians provided informed consent. The study was approved by the Ethics Committee of Shandong First Medical University (R202410280375), and was conducted according to the Declaration of Helsinki principles.
Immunoglobulin G N-Glycans Analysis
The plasma samples underwent centrifugation and were subsequently combined with an extraction plate embedded with IgG-specific binding protein G. Following this, the samples were subjected to a series of washes, elution, and denaturation using 1.33% SDS and 4% gepal. Finally, the samples were digested and purified using PNGase. The resultant mixture was subsequently incubated in a water bath maintained at 37°C for a duration of 18 to 20 hours. N-glycans were labeled with fluorescence employing 2-aminobenzamide (2-AB). Quantitative and qualitative analysis was conducted utilizing an UPLC system equipped with a fluorescence detector.15 Each sample was injected with a volume of 10 microliters, and the chromatographic run time was set to 30 minutes per sample. Upon acquiring the chromatogram for each sample, the chromatogram was segmented into 24 initial Glycan peaks based on the reference Glycan peak map. The area of each peak was then normalized by dividing it by the total peak area, thereby yielding the 24 Glycan peak (GP) values for all samples. The composition and glycan profile of the IgG N-glycome were analyzed.
Statistical Analysis
For the statistical analysis, SPSS 26.0 and R 4.3.3 software were employed for data processing, and GraphPad Prism 10 was used for chart creation. The normality of the data distribution was evaluated using the Kolmogorov–Smirnov test. Continuous variables exhibiting a normal or approximately normal distribution were reported as mean ± standard deviation, whereas those with a skewed distribution were presented as median (interquartile range). Categorical variables were reported as n (%). Differences between two groups in continuous variables that followed a normal (or approximate normal) distribution were analyzed using an independent sample t-test. Non-normal distributed continuous variables’ differences between two groups were evaluated using Wilcoxon rank-sum test. Chi-square test was employed for categorical variables analysis. Variables that exhibited inter-group differences in the aforementioned analysis were incorporated into a multivariate logistic regression analysis to further identify independent risk factors. Based on the inclusion of independent risk factors as covariates, logistic regression analysis identified 24 initial glycans and 17 derivative indicators associated with NRDS. Hosmer-Lemeshow test (H-L test) was used for the fit degree of the model, and P > 0.05 was considered to be a good calibration degree. Additionally, the model underwent evaluation through K-fold cross-validation, with k set to 10.16 The significance level was set as α=0.05. All P-values<0.05 were considered significant.
Results
Baseline Information of Participants
We statistically analyzed the basic information of 134 participants (Figure 1). The study cohort consisted of 134 newborns, including 73 males and 61 females. None of the newborns had a gestational age of 42 weeks or more; therefore, gestational age was used as a binary variable to classify the newborns as preterm or term. Birth weight was also used as a binary variable to distinguish between low birth weight and normal birth weight. Preterm rupture of membranes (PROM) was defined as spontaneous rupture of membranes before delivery.17 The results of the chi-squared test are shown in Table 1. Statistically significant differences were found between the NRDS group and the control group in terms of gestational age in weeks, birth weight in grams, history of miscarriage and PROM. The findings indicate that gestational age, birth weight, history of miscarriage, and PROM may contribute to the pathogenesis of NRDS, corroborating the results of previous research.18,19 Furthermore, newborns with lower birth weight and earlier gestational age are at an increased risk, potentially due to compromised lung function, which may contribute to the development of NRDS.20
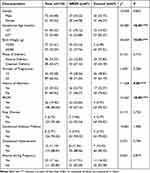 |
Table 1 Comparison of Basic Characteristics of 134 Neonatal NRDS Group and Control Group [n (%)] |
 |
Figure 1 A schematic diagram of the study participants. |
The Research Object of Clinical Indicators and Independent Risk Factors of NRDS
From the baseline data, we collected and analyzed the differences in clinical indicators between the two groups of neonates. The results of independent t-tests of the two samples showed that absolute neutrophil count (ANC), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), procalcitonin (PCT) and lactate dehydrogenase (LDH) levels were elevated in the NRDS group compared with the control group. In addition, the levels of α-hydroxybutyrate dehydrogenase (α-HBDH), alanine aminotransferase (ALT), total bilirubin (TBIL), serum albumin (ALB), prealbumin (PAB), total serum bile acids (STBA), triglyceride bile acids (CG) and cholinesterase (ChE) were also decreased (Table 2).
 |
Table 2 Comparison of Laboratory Examination Indexes Between NRDS Group and Control Group |
Incorporating the aforementioned baseline data and clinical indicators, along with between-group differences, into the logistic regression analysis revealed that both PROM and GGT are independent risk factors for NRDS. These factors remained statistically significant even after adjusting for the influence of other variables. (Table 3 and Figure S1). PROM is a frequent complication occurring in the mid to late stages of gestation, directly impacting maternal, fetal, and neonatal health, and is linked to the onset of NRDS21 Serum GGT, primarily originating from the hepatobiliary system, is recognized as a biomarker for liver damage, cancer, and low-grade chronic inflammation.22 This association may represent one of the potential mechanisms underlying its involvement in NRDS.
 |
Table 3 Analysis of Influencing Factors of Neonatal Respiratory Distress Syndrome Based on Multivariate Logistic Regression |
IgG N-Glycosylation Levels in Plasma of Populations
Subsequently, we conducted an extensive analysis to investigate the association between IgG N-glycosylation and the incidence of NRDS utilizing UPLC technology. The results of logistic regression analysis of primary glycans and their derived metrics with NRDS were shown in Figure 2 (see Table S1 for detailed data), and the levels of GP1, GP3, GP13, GP14, GP21, GP24, and their derived features, GPS, S1, S2, G0, G1, G2, F, and FG2, were different in the case group compared with the control group. Subsequent analysis of baseline sugar levels and their derived indices, using PROM and GGT as covariates, showed that the levels of GP1, GP3, GP4, GP11, GP13, GP14, and GP24 were significantly different in the NRDS compared to the control group. In contrast, the levels of GP21 were not statistically significantly different. In addition, derived indices such as GPS, S1, G0, G1, G2, F, BS, FG0, FG2 and Gal ratio were also statistically significantly different between the two groups (Figure 3 and Table S2). This suggests a correlation between the occurrence of NRDS and various modifications of plasma IgG, including sialylation, monosialylation, agalactosylation, monogalactosylation, bisgalactosylation, core fucosylation, agalactosylated core fucosylation, bisgalactosylated core fucosylation, and galactosylation. The sialylation of IgG plays a role in modulating inflammation, while an increase in core fucosylation of IgG can augment antibody-dependent cell-mediated cytotoxicity (ADCC). Additionally, the absence of galactosylation may influence IgG functionality.23 These findings suggest a potential mechanistic link between IgG N-glycosylation modifications and the development of NRDS.
Construction of NRDS Identification Model
In order to assess the clinical predictive value of alterations in these indicators for NRDS, a diagnostic model was constructed. Concurrently, to address the issue of multicollinearity among the independent variables (Figure S2), stepwise logistic regression was employed for variable selection (Table S3). We developed Model A and Model B based on clinical and glycosylation indicators, respectively. Model C was subsequently constructed by integrating the variables from both models to identify the predictive model with the optimal classification performance. The AUC for Model A, which was developed using PROM and GGT as combined biomarkers, was 0.785 (95% CI: 0.706–0.863). The H-L test indicated a χ² value of 1.840 with a P-value of 0.986. In contrast, Model B, utilizing GP1 and GP14 as predictors, demonstrated a lower predictive capability with an AUC of 0.743 (95% CI: 0.660–0.826), and the H-L test yielded a χ² value of 6.950 with a P-value of 0.542.The composite model C using GP1, GP13, GP14, PROM and GGT as composite indicators had an AUC of 0.902 (95% CI: 0.851–0.953), H-L test showed that χ2 value was 9.461, P= 0.305, which greatly exceeded the first two models (Figure 4). The calibration curves for each model within both the training and validation cohorts were generated (Figure 5). The proximity of the model’s calibration curve to the diagonal line is indicative of the model’s stability and the concordance between predicted and actual results. Among the models evaluated, only Model C demonstrated superior stability and fit across both the training and validation cohorts. To evaluate the internal stability of the models, a 10-fold cross-validation method was employed. The results of this cross-validation for the validation cohort revealed that Model C achieved an R-squared value of 0.417, whereas Models A and B attained R-squared values of 0.383 and 0.206, respectively (Table S4). Among the three models evaluated, only Model C exhibited superior fit in the validation cohort.
Discussion
Globally, approximately 15 million premature infants are born annually.24 NRDS remains the leading cause of mortality in neonates, particularly in preterm infants, and the leading cause of respiratory failure in this population.25 It is noteworthy that numerous studies have demonstrated a correlation between alterations in IgG N-glycosylation and the onset of various pathological conditions, but its role in NRDS has not been clarified.
The underlying cause of NRDS is the deficiency of pulmonary surfactant (PS) and the presence of immature lung tissue structure.26 The synthesis of PS is markedly affected by the presence of inflammatory mediators. Additionally, inflammatory markers hold substantial prognostic and predictive value in the context of NRDS. For instance, plateletcrit has been identified as a potential predictor for the occurrence of NRDS.27 Elevated PCT levels are correlated with an increased risk of developing NRDS.28 Furthermore, there is an elevated likelihood of lung infections in newborns with NRDS, especially subsequent to the administration of invasive respiratory support. IgG Glycosylation represents a pivotal post-translational modification, influencing both adaptive and innate FC-γR signaling pathways. This is achieved by modulating the structure of Fc segments, thereby activating a range of immune effects.29 Conversely, modifications in the glycosylation of the IgG Fc segment are dependent on the variations in inflammatory processes that transpire throughout the disease’s progression. Consequently, IgG glycosylation may serve both predictive and regulatory functions in the development of NRDS.
The current study demonstrated a significantly elevated level of IgG sialylation and fucosylation in the NRDS cohort compared to the control group. IgG sialylation may function as an anti-inflammatory modulator throughout all stages of the inflammatory response.23,30 Additionally, the observed increase in IgG fucosylation has the potential to markedly enhance the binding affinity between the monoclonal antibody Fc region and FcγRIIIα, thereby significantly augmenting ADCC.23,31 Consistent with our findings, in cases of fetal or neonatal alloimmune thrombocytopenia (FNAIT), there is a tendency for IgG Fc glycosylation against platelet and red cell antigens to decrease, while modifications in galactosylation and sialylation are increased.32 Furthermore, elevated IgG sialylation has also been observed in tumors such as renal cell carcinoma (RCC).33 Reduced sialylation has also been documented in other pathological conditions, including pediatric meningococcal septicemia and endometrial cancer (EC).34,35 It is postulated that these variations are predominantly linked to immune responses involved in the pathogenesis of various diseases. Analogously, the expression of core fucosylation exhibits variability across different disease states.
In contrast, the galactosylation process was observed to be diminished in neonates diagnosed with NRDS. Specifically, there was a notable reduction in the levels of agalactosylated IgG, monogalactosylated IgG, and bisgalactosylated IgG. The deficiency in galactosylation can not only impact IgG functionality but also precipitate excessive inflammatory responses, such as those mediated by Immunoglobulin A (IgA), under conditions of inflammatory signal regulation.23,36 Similarly, variations in IgG galactosylation exhibit distinct patterns across different disease states. Elevated levels of IgG galactosylation have been observed in acute Lyme disease (LD), early thyroid cancer (ETC), and Parkinson’s disease.37–39 Conversely, a deficiency in galactosylation has been reported in cases of urinary tract infections (UTIs), severe forms of coronavirus disease 2019 (COVID-19), and Guillain-Barré syndrome.33,40,41 These discrepancies in outcomes may be attributed to the specific characteristics of the participants, as well as the underlying mechanisms of the respective diseases.
Interestingly, in addition to illustrating the correlation between IgG N-glycosylation and NRDS, the model constructed with GP1, GP13, GP14, GGT, and PROM, following a stepwise logistic regression screening process, demonstrated considerable classification efficacy (AUC: 0.902, 95% CI: 0.851–0.953). This study further elucidates the potential clinical significance of IgG N-glycosylation in NRDS. Within this model, we examined the relevance of GGT and PROM in relation to demographic and clinical characteristics. GGT is an enzyme widely distributed throughout the human body, playing a significant role in both secretion and absorption processes. Its primary site of activity is the hepatobiliary system; nonetheless, it is also present in the lungs.42 Beyond its potential involvement in PS formation, GGT expression is elevated in inflammatory conditions and is associated with oxidative stress.22 The inflammatory response is crucial in the modification of IgG N-glycosylation, suggesting a potential link between GGT and NRDS. Furthermore, GGT levels have been shown to correlate with gestational age. Importantly, the gestational age of neonates in the NRDS group was significantly lower than that of the control group. It is plausible to suggest that the inclusion of GGT in the model was informed by these considerations. However, this does not eliminate the potential impact of sample size on the findings. Similarly, pregnant women with small pregnancy-age PROM are independent risk factors for NRDS, which supports our results.43
While this study has elucidated the relationship between IgG-N glycosylation modification and the onset of NRDS from an uncovered perspective, thereby potentially enhancing the understanding of NRDS pathogenesis, several limitations must be acknowledged: (1) the comprehensive evaluation and analysis of neonates in the postpartum period are relatively insufficient; (2) as a case-control study, the ability to establish a strong causal relationship is limited. Furthermore, additional investigation is required to bridge the gap between laboratory testing and clinical practice. This includes the standardization of operating procedures, the validation of larger sample sizes, and the reduction of associated costs, among other considerations. Future research should involve cohort studies with larger sample sizes, as well as cell or animal experiments, to further investigate the underlying mechanisms.
In summary, alterations in IgG N-glycosylation are linked to NRDS. Specifically, the levels of IgG core fucosylation and IgG sialylation are elevated in newborns with NRDS, contributing to anti-inflammatory processes. Conversely, the modifications with agalactosylation, single galactosylation, and double galactosylation are reduced in these neonates, potentially influenced by the underlying disease mechanisms, as well as genetic and environmental factors. The models of GGT, PROM, GP1, GP13, and GP14 developed on this basis exhibited satisfactory classification efficiency, indicating potential clinical value in the prediction and prognosis of NRDS.
Funding
This study was supported by the National Natural Science Foundation of China (82002755, 81973138), Natural Science Foundation of Shandong Province (ZR2020QH206), and the Youth Innovation Team Plan in Colleges and Universities of Shandong Province (2022KJ198).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hermansen CL, Lorah KN. Respiratory distress in the newborn. Am Family Phys. 2007;76(7):987–994.
2. Rubarth LB, Quinn J. Respiratory development and respiratory distress syndrome, neonatal network. NN. 2015;34(4):231–238. doi:10.1891/0730-0832.34.4.231
3. Li Y, Zhang C, Zhang D. Cesarean section and the risk of neonatal respiratory distress syndrome: a meta-analysis. Arch Gynecol Obstetrics. 2019;300(3):503–517. doi:10.1007/s00404-019-05208-7
4. Weng M, Wang J, Yin J, He L, Yang H, He H. Maternal prenatal systemic inflammation indexes predicts premature neonatal respiratory distress syndrome. Sci Rep. 2024;14(1):18129. doi:10.1038/s41598-024-68956-w
5. Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21(12):729–749. doi:10.1038/s41580-020-00294-x
6. Eichler J. Protein glycosylation, Current biology. CB. 2019;29(7):R229–R231. doi:10.1016/j.cub.2019.01.003
7. Trzos S, Link-Lenczowski P, Pocheć E. The role of N-glycosylation in B-cell biology and IgG activity. The aspects of autoimmunity and anti-inflammatory therapy. Front Immunol. 2023;14. 10.3389/fimmu.2023.1188838.
8. Kronimus Y, Dodel R, Galuska SP, Neumann S. IgG Fc N-glycosylation: alterations in neurologic diseases and potential therapeutic target? J Autoimmun. 2019;96:14–23. doi:10.1016/j.jaut.2018.10.006
9. Gudelj I, Lauc G, Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018;333:65–79. doi:10.1016/j.cellimm.2018.07.009
10. Radovani B, Vučković F, Maggioni AP, Ferrannini E, Lauc G, Gudelj I. IgG N-Glycosylation is altered in coronary artery disease. Biomolecules. 2023;13(2):375. doi:10.3390/biom13020375
11. Birukov A, Plavćsa B, Eichelmann F, et al. Immunoglobulin G N-glycosylation signatures in incident type 2 diabetes and cardiovascular disease. Diabetes Care. 2022;45(11):2729–2736. doi:10.2337/dc22-0833
12. Sweet DG, Carnielli VP, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023;120(1):3–23. doi:10.1159/000528914
13. Wang L, Lu X, Wang M, et al. The association between plasma IgG N-glycosylation and neonatal hypoxic–ischemic encephalopathy: a case-control study. Front Cell Neurosci. 2024;18. 10.3389/fncel.2024.1335688.
14. Woodward M. Epidemiology: study design and data analysis, third edition. Epidemiology. 2023;2023:1
15. Liu D, Xu X, Li Y, et al. Immunoglobulin G N-Glycan analysis by ultra-performance liquid chromatography. J Visualized Exp. 2020;155. 10.3791/60104.
16. García M-TJ, A SJ, Francisco H. Study on the impact of partition-induced dataset shift on k-fold cross-validation. IEEE Trans Neural Net Learn Syst. 2012;23(8):1304–1312. doi:10.1109/TNNLS.2012.2199516
17. Hay PE, Taylor-Robinson D, Lamont RF. Preterm premature rupture of membranes. Lancet. 1996;347(8995):203. doi:10.1016/S0140-6736(96)90390-6
18. Liu W, Xu P. The association of serum vitamin D level and neonatal respiratory distress syndrome. Italian J Pediatrics. 2023;49(1). doi:10.1186/s13052-023-01415-w
19. Wang CH, Chen JJ, Ge JJ, Ma XL, Shi LP. Risk factors and short-term prognosis of early pulmonary hypertension in preterm infants. Chin J Pediatr. 2022;60(7):682–687. doi:10.3760/cma.j.cn112140-20211222-01068
20. Weihong Y, Hong W, Feng C, Xinhong C, E XZ, Ya H. Risk factors and prediction score model for unplanned readmission among neonates with NRDS under one year of age: a retrospective cohort study. Front Pediatrics. 2022;10:964554. doi:10.3389/fped.2022.964554
21. Wu J, Liu J, Feng ZC, Huang JJ, Wu G. Influence of premature rupture of membranes on neonatal health. Chin J Pediatr. 2009;47(6):452–456. doi:10.3760/cma.j.issn.0578-1310.2009.06.013
22. Mitrić A, Castellano I. Targeting gamma-glutamyl transpeptidase: a pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic Biol Med. 2023;208:672–683. doi:10.1016/j.freeradbiomed.2023.09.020
23. Vattepu R, Sneed SL, Anthony RM. Sialylation as an important regulator of antibody function. Front Immunol. 2022;13. 10.3389/fimmu.2022.818736.
24. Crauciuc GA, Bănescu CV. Descriptive and functional genomics in neonatal respiratory distress syndrome: from lung development to targeted therapies. Int J Mol Sci. 2024;25(1). doi:10.3390/ijms25010649
25. Donda K, Vijayakanthi N, Dapaah-Siakwan F, Bhatt P, Rastogi D, Rastogi S. Trends in epidemiology and outcomes of respiratory distress syndrome in the United States. Pediatric Pulmonol. 2019;54(4):405–414. doi:10.1002/ppul.24241
26. Dani C, Reali MF, Bertini G, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Eur Respir J. 1999;14(1):155–159. doi:10.1034/j.1399-3003.1999.14a26.x
27. Dundar B, Dincgez Cakmak B, Ozgen G, Tasgoz FN, Guclu T, Ocakoglu G. Platelet indices in preterm premature rupture of membranes and their relation with adverse neonatal outcomes. J Obstetrics Gynaecol Res. 2018;44(1):67–73. doi:10.1111/jog.13484
28. Uçkan K, Başkıran Y, Çeleğen İ. Association of subclinical markers of inflammation with preterm premature rupture of membranes and adverse neonatal results: a case control study. Arch Gynecol Obstetrics. 2022;306(6):2063–2068. doi:10.1007/s00404-022-06756-1
29. Cobb BA. The history of IgG glycosylation and where we are now. Glycobiology. 2020;30(4):202–213. doi:10.1093/glycob/cwz065
30. Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. B-cell-independent sialylation of IgG. Proc Natl Acad Sci USA. 2016;113(26):7207–7212. doi:10.1073/pnas.1523968113
31. Ferrara C, Grau S, Jager C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108(31):12669–12674. doi:10.1073/pnas.1108455108
32. Sonneveld ME, Koeleman CAM, Holst S, et al. Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br J Haematol. 2016;174(2):310–320. doi:10.1111/bjh.14053
33. Iwamura H, Mizuno K, Akamatsu S, et al. Machine learning diagnosis by immunoglobulin N-glycan signatures for precision diagnosis of urological diseases. Cancer Sci. 2022;113(7):2434–2445. doi:10.1111/cas.15395
34. de Haan N, Boeddha NP, Ekinci E, et al. Differences in IgG Fc glycosylation are associated with outcome of pediatric meningococcal sepsis. mBio. 2018;9(3). doi:10.1128/mBio.00546-18
35. Lin S, Wang Y, Wang X, Yan B, Lou W, Di W. Serum immunoglobulin G N-glycome: a potential biomarker in endometrial cancer. Ann translat Med. 2020;8(12). doi:10.21037/atm-20-3504
36. Hodoniczky J, Yuan ZZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Progr. 2005;21(6):1644–1652. doi:10.1021/bp050228w
37. Haslund-Gourley BS, Grauzam S, Mehta AS, Wigdahl B, Comunale MA. Acute lyme disease IgG N-linked glycans contrast the canonical inflammatory signature. Front Immunol. 2022;13. 10.3389/fimmu.2022.949118.
38. Zhang Z, Wu J, Liu P, Kang L, Xu X. Diagnostic potential of plasma IgG N-glycans in discriminating thyroid cancer from benign thyroid nodules and healthy controls. Front Oncol. 2021;11. 10.3389/fonc.2021.658223.
39. Russell AC, Novokmet M, Wang Y, et al. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson’s disease. Glycobiology. 2017;27(5):501–510. doi:10.1093/glycob/cwx022
40. R FW-J, J SMH, R DJ, et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res. 2014;13(3):1722–1730. doi:10.1021/pr401213z
41. Marina KB, Tea P, Frano V, et al. Differences in immunoglobulin G glycosylation between influenza and COVID-19 patients. Engineering. 2022. doi:10.1016/j.eng.2022.08.007
42. Cabrera-Abreu JC, Green A. γ-glutamyltransferase: value of its measurement in paediatrics. Ann Clin Biochem. 2002;39(1):22–25. doi:10.1258/0004563021901685
43. Zhang X, Hu Z, Li J, Luo B, Chen L, Xu M. The relationship between different delivery timing and the outcome of premature rupture of membranes in primiparous women. Altern Therap Health Med. 2024;2024:AT10237.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.