Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
The Association Between Preoperative Triglyceride Glucose Index and Postoperative Adverse Cardiovascular Events in Non-Cardiac Surgery: A Single-Center Study From China
Authors Hao J, Qu L, Yang Y, Sun Y, Xu G
Received 23 January 2025
Accepted for publication 4 April 2025
Published 13 April 2025 Volume 2025:21 Pages 467—479
DOI https://doi.org/10.2147/TCRM.S518077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jiandong Hao,1 Li Qu,2 Yang Yang,1 Yun Sun,1 Guiping Xu2
1Graduate School of Xinjiang Medical University, Urumqi, People’s Republic of China; 2Department of Anesthesiology, People’s Hospital of Xinjiang Uygur Autonomous Region, Xinjiang Clinical Clinical Research Center for Anesthesia Management, Urumqi, People’s Republic of China
Correspondence: Guiping Xu, Department of Anesthesiology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, 830001, People’s Republic of China, Email [email protected]
Background: The incidence of postoperative adverse cardiovascular events (PACE) in non-cardiac surgery has significantly increased, severely affecting surgical outcomes and patient prognosis. This study investigates the relationship between preoperative triglyceride-glucose (TyG) index and PACE in patients who underwent non-cardiac surgery.
Methods: We conducted a single-center retrospective study, including adult patients (age ≥ 18 years) who underwent non-cardiac surgery. Univariate and multivariate logistic regression analyses assessed the relationship between the TyG index and PACE. Nonlinear correlations were investigated using restricted cubic splines (RCS). Additionally, subgroup analysis was performed to evaluate the relationship between the TyG index and PACE in different subsamples.
Results: 16,066 patients were studied, among which 1505 cases (9.37%) developed PACE, with a median TyG index of 8.61 (8.22, 9.07). Using the lowest quartile of the TyG index as a reference, the fully adjusted (ORs) (95% CIs) for PACE in the second, third, and fourth quartiles of the TyG index were 1.78 (1.49~2.11), 2.16 (1.81~2.59), and 2.30 (1.88~2.83), respectively. After adjusting for all confounding factors, we found that patients with the highest TyG index had a 68% increased risk of PACE (OR 1.68, 95% CI 1.50~1.90). The results of the subgroup analysis were similar to those of the primary analysis. The RCS model suggests a linear positive correlation between the TyG index and the risk of PACE occurrence. (P for overall < 0.001, P for nonlinear = 0.547).
Conclusion: This cohort study indicates that preoperative TyG index is linearly and positively correlated with an increased incidence of PACE in the non-cardiac surgery population. This finding suggests that intensifying the evaluation of the TyG index may provide a more convenient and effective tool for identifying individuals at risk of PACE during non-cardiac surgeries.
Keywords: postoperative adverse cardiovascular events, TyG index, non-cardiac surgery
Introduction
Globally, over 300 million major surgeries are performed annually, of which 85% are non-cardiac surgeries.1 In order to improve postoperative outcomes, it is crucial to promptly identify and optimally manage the main complications following non-cardiac surgery.2 The incidence of perioperative complications for non-cardiac surgery ranges from 7% to 11%, with a mortality rate between 0.8% and 1.5%, where cardiovascular complications account for up to 42%.3 Postoperative adverse cardiovascular events (PACE) encompass a range of cardiovascular incidents that pose significant threats to patients’ perioperative safety and can impact their long-term prognosis. These events include arrhythmias, myocardial infarction, new-onset atrial fibrillation during the perioperative period, myocardial injury after non-cardiac surgery, and acute heart failure. Not only do these events present significant immediate health challenges for patients, but they also have profound implications for their recovery process and quality of life. PACE encompasses perioperative cardiovascular incidents distinct from long-term major adverse cardiovascular events (MACE).
Insulin resistance (IR) is a pathophysiological state characterized by reduced sensitivity and responsiveness to insulin, ultimately leading to hyperglycemia.4 Perioperative IR can be observed in patients undergoing surgery, critically ill diabetic patients, as well as non-diabetic patients.5,6 IR-induced hyperglycemia leads to vascular dilation and impaired nitric oxide regeneration in endothelial cells, increased serum cytokine levels, neutrophil chemotaxis, and phagocytic function impairment, exacerbating the inflammatory response, increasing the risk of infection, and potentially leading to multiple organ dysfunction syndrome.7 IR has long been considered a risk factor for both microvascular and macrovascular diseases.8 In recent years, the triglyceride-glucose (TyG) index, calculated based on triglycerides and fasting glucose levels, has emerged as a potential simple, convenient, and cost-effective alternative for detecting IR.9 Compared to the hyperinsulinemic-euglycemic clamp technique, the TyG index stands out due to its cost-effectiveness and ready availability.10 Prior research has demonstrated a close association between the TyG index and cardiovascular disease,11 myocardial infarction,12 stent restenosis,13 the severity of coronary heart disease,14 ischemic stroke, atherosclerosis,15 acute decompensated heart failure16 and incident atrial fibrillation.17 Therefore, we hypothesize that there is a correlation between the levels of TyG index in patients undergoing non-cardiac surgery and the incidence of PACE, suggesting that TyG index may play a role in risk stratification for PACE.
Based on available and detailed clinical information, this study explores the association between the TyG index and the risk of PACE in patients undergoing non-cardiac surgery. The results could contribute to developing new strategies to improve patient outcomes in this population and provide important new insights into the role of TyG index in predicting patient prognosis.
Materials and Methods
Study Design and Participants
We conducted an observational cohort study using the perioperative retrospective database of a large hospital in Xinjiang, China. This study includes a cohort of adult patients (aged ≥18 years) who underwent non-cardiac surgery under general anesthesia at the People’s Hospital of Xinjiang Uyghur Autonomous Region from 2015 to 2024. The Hospital Institutional Review Board approved this study protocol, and informed consent was waived due to the retrospective use of de-identified data. No clinical treatment or personal privacy information was involved.
We only considered data from their first surgery for inclusion in our analysis. Based on this criterion, we initially identified all non-cardiac surgeries performed under general anesthesia and with patients aged ≥18 years. Subsequently, we excluded organ transplant surgeries, obstetric surgeries, surgeries with an ASA score of 5 or 6, and surgeries for which the TyG index could not be calculated. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.18
Outcome Ascertainment
The primary endpoint of this study is PACE, which primarily encompasses postoperative new-onset angina, arrhythmias, myocardial infarction, myocardial ischemia, heart failure, and cardiac arrest. A PACE event is recorded if any of the aforementioned complications occur between the end of surgery and discharge. A physician diagnoses each condition and classifies it according to ICD-10-CM coding standards.
Data Collection
Data were collected from the perioperative database of the People’s Hospital of Xinjiang Uygur Autonomous Region. Potential confounding factors influencing the occurrence of PACE are categorized into preoperative and intraoperative variables. Preoperative variables of interest include patient demographics, clinical history, medication history, and laboratory tests: (1) demographics, such as age, gender, ethnicity, body mass index (BMI), smoking history, and baseline blood pressure; (2) clinical history, such as hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), hyperlipidemia, rheumatic diseases; (3) medication history, such as, angiotensin-converting enzyme inhibitor(ACEI) /angiotensin receptor blocker (ARB), β-blockers, calcium channel blockers (CCB), diuretics, statins, anticoagulants; (4) preoperative laboratory data, such as fasting blood glucose (FBG), triglycerides (TG), glycosylated serum protein (GSP), hemoglobin (Hb), white blood cell (WBC) count, platelets (PLT), albumin (ALB), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), C-reactive protein (CRP). Intraoperative variables included ASA classification, type of surgery, duration of surgery, and blood loss. First, convert the units of FBG and TG from mmol/L to mg/dL, then calculate the TyG index as Ln[fasting TG (mg/dL) × FBG (mg/dL)/2]. Table S1 illustrates the extent of data missingness in this study. Although most variables exhibited only minor degrees of data incompleteness, multiple imputation was employed to preserve the largest possible sample size, thereby approximating the actual conditions more closely. Variables with a data missing rate exceeding 20% were excluded. For variables with a data missing rate below 20%, multiple imputation was performed using the “mice” package.19
Feature Screening
We utilized the Boruta algorithm to determine the most critical PACE features and construct the radiomics signatures. The Boruta algorithm is a feature selection and wrapping algorithm based on the random forest method. It identifies the importance of features by comparing the Z-scores of each feature with those of corresponding “shadow features”.20 Using Boruta (Version: 8.0.0) for feature selection, the algorithm iteratively compares the importance of each original variable with its shadow variable. It determines the significance of each variable after 100 iterations or until all variables stabilize. The default parameters used by the Boruta algorithm are “P value = 0.01” and “maxRuns = 100”, which represent the significance level for feature selection and the maximum number of iterations for the algorithm, respectively.21
Statistical Analysis
This study divided the participants into four groups (Q1-Q4) based on quartiles of the TyG index. Categorical variables are presented as frequencies (percentages), and differences between groups were assessed using the chi-square test. For continuous variables that passed the normality test, data are presented as mean ± standard deviation, and differences between groups were evaluated using analysis of variance. For continuous variables that did not pass the normality test, data are presented as median (interquartile range, IQR), and differences between groups were assessed using the Kruskal–Wallis rank sum test. Univariate and multivariate logistic regression analyses were employed to statistically infer the relationship between the TyG index (independent variable) and PACE risk (dependent variable). There were four models to control for confounding factors. In Model 1, no covariates were adjusted. Model 2 was adjusted for gender, age, and BMI. Model 3 included the variables in Model 2 plus smoking history, hypertension, diabetes, baseline blood pressure, and the use of ACEI/ARB, β-blockers, diuretics, statins, and anticoagulants. Model 4 was built based on Model 3 by adding TC, HDL, LDL, CRP, WBC, Hb, PLT, GSP, ALT, AST, ALB, ASA classification, intraoperative blood loss, and operation time. In Model 5, adjustments were made solely for FBG. The results were presented as odds ratio (OR) and their respective 95% confidence intervals (CI).
Additionally, restricted cubic spline (RCS) regression was employed to assess the potential nonlinear relationship between the TyG index and PACE risk. Subgroup analyses were also conducted based on age (<60 years or ≥60 years), gender (male or female), hypertension (presence or absence), diabetes (presence or absence), and smoking history (presence or absence) to determine whether the correlation between the TyG index and PACE differed among various subgroups. Interaction analysis was also performed to explore further the impact of subgroup factors on the predictive ability of the TyG index for PACE.
Using the data from Model 4, a sensitivity analysis was conducted by calculating E-values through the “EValue” R package.22 The E-value is primarily used to evaluate the impact of unmeasured confounding on the obtained results. Simply put, it refers to the minimum strength of association that an unmeasured confounder must have to overturn our current results, and the E-value represents this minimum required strength of association. The OR values of the confirmed PACE risk factors reported in the literature were compared with the E values from this study.23–25
All statistical analyses were conducted using R (version 4.3.2), with a significance threshold set at p < 0.05 (two-tailed).
Results
Basic Characteristics of the Study Population
Using inclusion and exclusion criteria, 16,066 non-cardiac surgical patients from the perioperative database were included in the study (Figure 1). This study included a total of 7322 female patients (46%) and 8744 male patients (54%). The median age of the participants was 60.00 years (interquartile range: 49.00–71.00), and the median TyG index was 8.61 (interquartile range: 8.22–9.07). The overall incidence of PACE among the study participants was 9.37%, with an increasing trend as the TyG index increased across quartiles (Quartile 1: 6.56%; Quartile 2: 9.31%; Quartile 3: 10.31%; Quartile 4: 11.38%). Baseline characteristics, as presented in Table 1 according to quartiles of TyG index, reveal that various factors, including age, gender, race, BMI, smoking history, baseline blood pressure, hypertension, diabetes, COPD, hyperlipidemia, β-blockers, CCBs, diuretics, statins, anticoagulants, GSP, Hb, WBC count, PLT, ALB, TC, LDL, HDL, ALT, AST, CRP, type of surgery, surgery time, and bleeding, showed significant differences (all p < 0.05) among the quartiles of the TyG index. Compared to those with the lowest TyG index, participants in the higher TyG index groups were significantly more likely to have diabetes, hypertension, and hyperlipidemia. They also had higher levels of GSP, Hb, WBC count, PLT, ALB, TC, LDL, ALT, AST, and CRP levels, lower HDL levels, and were more likely to be female smokers and undergo urgent surgery. No statistically significant differences were observed between the quartiles of the TyG index in terms of rheumatic diseases, ACEI/ARB, and ASA classification (all P > 0.05).
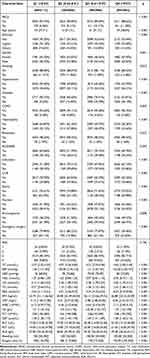 |
Table 1 Baseline Characterization and Comparison |
 |
Figure 1 Research flowchart. Abbreviations: PHXUAR, People’s Hospital of Xinjiang Uygur Autonomous Region; ASA, American society of anesthesiologists. |
Feature Selection
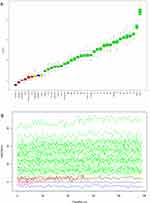
The Boruta algorithm was employed to identify the actual feature set by accurately estimating the importance of each feature. In the Boruta algorithm, variables in the green area are identified as important features, while those in the red area are deemed unimportant. The Boruta method confirmed 30 significant variables most related to PACE risk (Figure 2). These factors were selected for the final fully adjusted model when their z-scores were higher than shadow features in the Boruta analysis or when they had the maximum matching effect among a set of biomarkers (max, mean, and min) in terms of odds ratio or risk ratio upon addition to the model.
The Relationship Between TyG Index and Non-Cardiac Surgical PACE
To evaluate the relationship between the TyG index and PACE incidence in participants undergoing non-cardiac surgery, we established four logistic regression models (Table 2). Initially, Model 1 determined that for every one-unit increase in the TyG index, the risk of PACE increases by 39% (OR = 1.39, 95% CI: 1.28~1.50). In Model II, for every one-unit increase in the TyG index, the risk of PACE increases by 55% (OR = 1.55, 95% CI: 1.43~1.67). In Model III, for every one-unit increase in the TyG index, the risk of PACE increases by 75% (OR = 1.75, 95% CI: 1.60 ~ 1.91). In Model IV, for every one-unit increase in the TyG index, the risk of PACE increases by 68% (OR = 1.68, 95% CI: 1.50~1.90).
 |
Table 2 Association Between the TyG Index and the Risk of PACE |
To further elucidate the relationship between the TyG index and the incidence of PACE, the TyG index was divided into quartiles. In the fully adjusted model IV, compared to the first quartile (Q1), the ORs for Q2, Q3, and Q4 were 1.78 (95% CI: 1.49–2.11), 2.16 (95% CI: 1.81–2.59), and 2.30 (95% CI: 1.88–2.83), respectively. This indicates that, among non-cardiac surgery patients, participants in Q2, Q3, and Q4 had a 78%, 116%, and 130% higher risk of developing PACE compared to those in Q1.
To validate the independent effect of the TyG index, we established logistic regression Model 5 (Table S2). The model revealed that each 1-unit increase in TyG was associated with a 21% elevated risk of PACE (OR = 1.21, 95% CI: 1.10–1.33).
We employed RCS curves to assess the potential nonlinear relationship between the TyG index and PACE risk, as shown in Figure 3. Our results indicate that there is a linear relationship between the TyG index and the probability of PACE risk (P overall < 0.001, P nonlinear = 0.547). When the TyG index is 9.298, it differentiates the risk of PACE, with the OR value for the TyG index close to 1.
 |
Figure 3 The restricted cubic spline (RCS) analysis between the TyG index and the risk of PACE. |
Subgroup and Sensitivity Analyses
To further investigate the relationship between the TyG index and the incidence of PACE, subgroup and interaction analyses were conducted based on age (<60 years or ≥60 years), gender (male or female), hypertension (presence or absence), diabetes (presence or absence), and smoking (presence or absence). In the unadjusted model and adjusted model 4, the results of the stratified analysis (Figure 4) showed that the interaction was present only in the subgroup with hypertension (P for interaction = 0.004, 0.032), regardless of whether covariates were adjusted or not, while no interaction was observed in other subgroups (P for interaction > 0.05). The subgroup analysis was nearly consistent with the main study results.
Analysis of the E value using Model 4 data revealed that, under the control of measured confounders, unmeasured confounding effects would need to reach at least 2.75 to fully invalidate the observed OR in this study.26 The OR values of risk factors for PACE reported in other literature are all less than 2.75, indicating that the results of this study are robust.
Discussion
This single-center retrospective observational study evaluated the relationship between the TyG index and PACE. The results of the multivariate logistic regression indicate that a higher TyG index is independently associated with an increased risk of PACE. After adjusting for covariates, this finding remained consistent across subgroups stratified by age, gender, hypertension, and diabetes. There were no significant interactions between the baseline TyG index and the stratified variables, demonstrating the robustness of the study results. Furthermore, the RCS model based on the logistic regression model indicates that the TyG index has an approximately linear relationship with PACE, further validating the correctness of the aforementioned research findings. In conclusion, our study indicates that the TyG index serves as a valuable marker for PACE in non-cardiac surgery patients and may contribute to advancing preventive measures against PACE.
IR refers to the reduced ability of insulin-sensitive tissues, such as skeletal muscle and cardiac muscle, adipose tissue, and liver, to uptake glucose due to diminished biological effects of insulin.27 Extensive research has established that IR is an independent risk factor for cardiovascular events.4,28,29 The hyperinsulinemic-euglycemic clamp is the gold standard for assessing IR; however, its complex and costly technical requirements render it impractical for clinical settings.30 Therefore, it is crucial to establish a reliable surrogate marker for broader IR evaluation. Simental-Mendía et al proposed in 2008 the use of the TyG index to measure IR.31 Previous studies have shown that TyG is a low-cost, simple, and widely applicable method for identifying IR.32 Compared to the hyperinsulinemic-euglycemic clamp technique, it exhibits higher sensitivity (96.5%) and specificity (85.0%).33 In patients with stable cardiovascular disease, the TyG index is positively correlated with the future occurrence of adverse cardiovascular events, such as non-fatal myocardial infarction, stroke, and revascularization after discharge.34 Wu et al identified the TyG index as a predictor of major adverse cardiovascular events in patients with premature coronary artery disease.35 Additionally, in a retrospective study involving 1932 patients with Type 2 diabetes who experienced acute myocardial infarction, TyG was identified as an independent predictor of major adverse cardiovascular and cerebrovascular events.36 Although existing studies have clarified the association between high TyG index and cardiovascular events, no study has yet explored its relationship with postoperative common complications PACE. PACE may have a severe adverse effect on patient prognosis and is worthy of further investigation. Our study found a significant association between the TyG index and the incidence of PACE in patients undergoing non-cardiac surgery: specifically, for every one unit increase in the TyG index, there was a 68% increased risk of developing PACE. This suggests that further research into the potential role of the TyG index in preventing and managing PACE is essential. Participants in the fourth TyG quartile of non-cardiac surgery patients have approximately 2.3 times the risk of developing cardiovascular disease compared to those in the first quartile. More importantly, our research further confirmed that this relationship is linear. These findings help clarify the predictive value of the TyG index in non-cardiac surgery populations, thereby enabling a more accurate identification of high-risk individuals. Our study also found statistically significant interactions among hypertension subgroups, indicating that managing the TyG index in individuals with hypertension can significantly reduce the incidence of PACE compared to those without hypertension. To further validate the independence of the TyG index, we adjusted for FBG using a multivariate logistic regression model. The results demonstrated that the TyG index remained significantly associated with PACE even after controlling for FBG (OR = 1.21, 95% CI: 1.10–1.33). This finding indicates that the predictive value of TyG not only stems from glycemic levels but also reflects the specific role of TG in (IR. As a product function of TG and FBG, the TyG index provides a dual biomarker integrating both glycolipid metabolism parameters for preoperative risk assessment, holding significant clinical implications.
The potential mechanism by which the TyG index predicts future cardiovascular disease risk remains unclear, but it may be related to the following factors. The TyG index is an indicator composed of two cardiovascular disease risk factors; both lipid-related and glucose-related factors reflect IR in adults, which could be one explanation for this association.37 Research has indicated that IR triggers imbalances in glucose metabolism, leading to elevated blood glucose levels. This, in turn, further induces inflammatory responses and oxidative stress,38 which may contribute to the development of atherosclerosis.39 Secondly, IR can increase the production of glycosylation products and free radicals, leading to the inactivation of nitric oxide (NO)40 and impairing endothelial function by inducing excessive production of reactive oxygen species (ROS).41 This, in turn, may contribute to the development of cardiovascular diseases. Furthermore, IR promotes an increase in sympathetic excitability and an elevation in adrenaline secretion, leading to a vicious cycle of fluid activation. This cycle results in vasoconstriction and platelet aggregation. In severe cases, it can also lead to vascular narrowing.42 The aforementioned studies indicate that although the pathogenesis of cardiovascular diseases is complex, the IR index represented by the TyG index can explain some of the reasons and provide insights into the prevention and treatment of cardiovascular diseases.
Another finding of this study is that there is no interaction between the TyG index and PACE in individuals with or without diabetes. This indicates that IR can also be present in patients without diabetes, providing a pathophysiological basis for the transition from normal glucose tolerance (NGT) to prediabetes and diabetes.43 In patients with prediabetes, IR leads to relatively high blood glucose concentrations that do not meet the threshold for diabetes diagnosis. The results of a prospective cohort study in China indicate that an elevated TyG index can be used to predict the incidence of prediabetes.44 Previous studies on diabetic individuals have shown that a high TyG index is associated with major adverse cardiovascular and cerebrovascular events.45 However, few studies have been conducted in non-diabetic patients, especially among those undergoing non-cardiac surgery. Therefore, early preoperative screening using the TyG index could be utilized to identify patients at high risk for PACE and mitigate potential postoperative complications.
The strengths of this study are evident: Firstly, we focused on the correlation between preoperative IR status and PACE in non-cardiac surgery, an area with limited research. Secondly, we used multiple imputations to address missing covariate data, enhancing the statistical power and reliability of our analysis. Thirdly, we analyzed the TyG index as both a categorical and continuous variable to assess its association with PACE, thereby identifying risk differences across various TyG index levels and aligning with clinical practice. Fourthly, subgroup analysis revealed specific subpopulations within the non-cardiac surgery cohort where controlling the TyG index significantly reduced cardiovascular risks, and sensitivity analysis was conducted to evaluate the robustness of our findings.
However, the following limitations of this study remain. Firstly, we conducted a single-center retrospective study in China, which may have underestimated the incidence of PACE, and the generalizability of these findings may be limited. Secondly, we did not perform hyperinsulinic-euglycemic clamp tests on the participants as these are not routine clinical trials. However, the TyG index can be used to assess IR, and it is still necessary to use gold standards associated with IR in further prospective studies to elucidate the relationship between IR and PACE. Lastly, as an observational study focused on non-cardiac surgery patients, it is important to acknowledge the wide range of surgical procedures and patient characteristics involved. One potential limitation of this study lies in the extended timeframe of data collection (2015–2024), during which progressive refinements in surgical techniques and anesthetic management may have gradually influenced the incidence of PACE. Although we have adjusted for confounding factors including surgical category, ASA classification, and operative duration, the potential impact of temporal technological advancements on outcomes cannot be entirely excluded. It should be noted that no substantial modifications to the standard operating procedures (SOP) for surgical and anesthetic protocols were implemented at our institution during the study period. Future investigations employing multicenter designs or narrower temporal windows may further validate the robustness of these findings.
Conclusion
This retrospective cohort study demonstrates a linear positive correlation between preoperative TyG index and increased incidence of PACE in non-cardiac surgical patients. Enhanced preoperative TyG index evaluation may offer a practical clinical tool for early identification of high-risk populations.
Abbreviations
IR, Insulin resistance; TyG, Triglyceride-glucose; PACE, Postoperative adverse cardiovascular events; RCS, restricted cubic splines; OR, Odds ratio; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; TC, Total cholesterol; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HDL, High density lipoprotein; LDL, Low density lipoprotein; TG, Triglyceride; FBG, Fasting blood glucose; BMI, Body mass index; CRP, C-reactive protein; WBC, White blood cell; Hb, Hemoglobin; PLT, Platelets; GSP, Glycosylated serum protein; ALT, Alanine transaminase; AST, Aspartate aminotransferase; ALB, Albumin; ASA, American society of anesthesiologists; PHXUAR, People’s Hospital of Xinjiang Uygur Autonomous Region.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participation
The Research Ethics Committee of PHXUAR approved this study (approval reference no. KY2021031905). Due to the retrospective nature of the study, the requirement for written informed consent was waived, and patient information was anonymized before analysis. The present study was conducted in accordance with the ethical guidelines of the 2013 Declaration of Helsinki and its amendments.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Key Research and Development Program of Xinjiang Uygur Autonomous Region (No. 2022B03009-4).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022;43(39):3826–3924. doi:10.1093/eurheartj/ehac270
2. Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–1375. doi:10.1056/NEJMsa0903048
3. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–2431. doi:10.1093/eurheartj/ehu282
4. Hill MA, Yang Y, Zhang L, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolis. 2021;119:154766. doi:10.1016/j.metabol.2021.154766
5. Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97. doi:10.1097/SLA.0000000000000688
6. Meister KM, Hufford T, Tu C, et al. Clinical significance of perioperative hyperglycemia in bariatric surgery: evidence for better perioperative glucose management. Surg Obesity Related Dis. 2018;14(11):1725–1731. doi:10.1016/j.soard.2018.07.028
7. Lipshutz AKM, Gropper MA, Warner D, Warner M. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110(2):408–421. doi:10.1097/ALN.0b013e3181948a80
8. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi:10.2337/diabetes.54.6.1615
9. Kheirollahi A, Teimouri M, Karimi M, et al. Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in iranian polycystic ovary syndrome women. Lipids Health Dis. 2020;19(1):235. doi:10.1186/s12944-020-01410-8
10. Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med J Br Diabet Assoc. 2002;19(7):527–534. doi:10.1046/j.1464-5491.2002.00745.x
11. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi:10.1186/s12933-022-01511-x
12. Tian X, Zuo Y, Chen S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19. doi:10.1186/s12933-020-01210-5
13. Zhu Y, Liu K, Chen M, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137. doi:10.1186/s12933-021-01332-4
14. Su J, Li Z, Huang M, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):96. doi:10.1186/s12933-022-01523-7
15. Wang X, Ji X, Yu J, Wang F. Correlation between TyG index and coronary atherosclerosis assessed by CCTA in elderly male patients: a cross-sectional study. Diabetol Metab Syndr. 2023;15(1):176. doi:10.1186/s13098-023-01145-3
16. Huang R, Wang Z, Chen J, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. doi:10.1186/s12933-022-01507-7
17. Lee Y, Cha SJ, Park JH, et al. Association between insulin resistance and risk of atrial fibrillation in non-diabetics. Eur J Prev Cardiol. 2020;27(18):1934–1941. doi:10.1177/2047487320908706
18. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi:10.1136/bmj.39335.541782.AD
19. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi:10.1002/sim.4067
20. Kong C, Zhu Y, Xie X, Wu J, Qian M. Six potential biomarkers in septic shock: a deep bioinformatics and prospective observational study. Front Immunol. 2023;14:1184700. doi:10.3389/fimmu.2023.1184700
21. Degenhardt F, Seifert S, Szymczak S. Evaluation of variable selection methods for random forests and omics data sets. Briefings Bioinf. 2019;20(2):492–503. doi:10.1093/bib/bbx124
22. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45. doi:10.1097/EDE.0000000000000864
23. Gu Z, Sun C, Xiang D. Postoperative adverse cardiovascular events associated with leptin and adverse age after elective major non-cardiac surgery: an asian single-center study. Med Sci Monit. 2018;24:2119–2125. doi:10.12659/msm.906797
24. Roth S, M’Pembele R, Matute P, Kotfis K, Larmann J, Lurati Buse G. Cardiovascular-kidney-metabolic syndrome: association with adverse events after major noncardiac surgery. Anesth Analg. 2024;139(3):679–681. doi:10.1213/ANE.0000000000006975
25. Chan MTV, Wang CY, Seet E, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788–1798. doi:10.1001/jama.2019.4783
26. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi:10.1001/jama.2018.21554
27. Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. 2016;18(8):75. doi:10.1007/s11886-016-0755-4
28. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi:10.1186/s12933-018-0762-4
29. Wang C, Li F, Guo J, Li C, Xu D, Wang B. Insulin resistance, blood glucose and inflammatory cytokine levels are risk factors for cardiovascular events in diabetic patients complicated with coronary heart disease. Exp Ther Med. 2018;15(2):1515–1519. doi:10.3892/etm.2017.5584
30. Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obesity. 2022;30(8):1549–1563. doi:10.1002/oby.23503
31. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat D. 2008;6(4):299–304. doi:10.1089/met.2008.0034
32. Zhao J, Fan H, Wang T, et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol. 2022;21(1):123. doi:10.1186/s12933-022-01548-y
33. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi:10.1210/jc.2010-0288
34. Jin JL, Cao YX, Wu LG, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi:10.21037/jtd.2018.10.79
35. Wu Z, Liu L, Wang W, et al. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(1):142. doi:10.1186/s12933-022-01576-8
36. Zhang Y, Ding X, Hua B, et al. Predictive effect of triglyceride‑glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43. doi:10.1186/s12933-021-01236-3
37. Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74. doi:10.1186/s13098-018-0376-8
38. Jiang Y, Lai X. Clinical features of early-onset type 2 diabetes and its association with triglyceride glucose-body mass index: a cross-sectional study. Front Endocrinol. 2024;15:1356942. doi:10.3389/fendo.2024.1356942
39. Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev mol Cell Biol. 2018;19(10):654–672. doi:10.1038/s41580-018-0044-8
40. Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1):1. doi:10.1007/s11906-015-0615-4
41. Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a meta-analysis. Front Cardiovasc Med. 2021;8:644035. doi:10.3389/fcvm.2021.644035
42. Kelem A, Adane T, Shiferaw E. Insulin resistance-induced platelet hyperactivity and a potential biomarker role of platelet parameters: a narrative review. Diabetes, Metab Syndr Obes Targets Ther. 2023;16:2843–2853. doi:10.2147/DMSO.S425469
43. Gerich JE. Contributions of insulin-resistance and insulin-secretory defects to the pathogenesis of type 2 diabetes mellitus. Mayo Clin Proc. 2003;78(4):447–456. doi:10.4065/78.4.447
44. Wen J, Wang A, Liu G, et al. Elevated triglyceride-glucose (TyG) index predicts incidence of prediabetes: a prospective cohort study in China. Lipids Health Dis. 2020;19(1):226. doi:10.1186/s12944-020-01401-9
45. Guo Q, Feng X, Zhang B, et al. Influence of the triglyceride-glucose index on adverse cardiovascular and cerebrovascular events in prediabetic patients with acute coronary syndrome. Front Endocrinol. 2022;13:843072. doi:10.3389/fendo.2022.843072
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.