Back to Journals » ClinicoEconomics and Outcomes Research » Volume 17
The Budget Impact of Cangrelor in the UK for the Treatment of Out-of-Hospital Cardiac Arrest Patients Who Require Percutaneous Coronary Intervention
Authors Modi B , Cain R, Stork R , Barwood C, Tarpey G, Colucciello A
Received 14 June 2024
Accepted for publication 25 January 2025
Published 15 March 2025 Volume 2025:17 Pages 189—197
DOI https://doi.org/10.2147/CEOR.S475503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Bhavik Modi,1 Rob Cain,2 Richard Stork,2 Caroline Barwood,3 Gina Tarpey,2 Alessia Colucciello4
1Cardiac Surgery Department, Glenfield Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK; 2Chiesi Ltd, Manchester, UK; 3FIECON Ltd, London, UK; 4Chiesi Farmaceutici S.p.A., Parma, Italy
Correspondence: Richard Stork, Chiesi Ltd., 333 Styal Road, Manchester, M22 5LG, UK, Email [email protected]
Background: Cangrelor is an intravenous, reversible P2Y12 inhibitor indicated for the reduction of thrombotic cardiovascular events in patients undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure, and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable (for example, in the out-of-hospital cardiac arrest [OHCA] population).
Objective: This study aimed to estimate the affordability and budget impact, in the United Kingdom, of introducing cangrelor within the licenced OHCA population.
Methods: A budget impact model was developed to estimate the impact of introducing cangrelor to hospitals over 5 years. Efficacy (thrombotic events) and safety (bleeding events) data were based on clinical trials, cost data (2021/22 GBP), literature, NHS reference costs and British National Formulary data. Comparators were glycoprotein IIb/IIIa inhibitors and aspirin in combination with heparin, reflecting current treatments used in UK centres for the target population. Cangrelor uptake was estimated as 50% in Year 1, 75% in Year 2, and 100% in Years 3– 5. The OHCA population was estimated from the British Cardiovascular Intervention Society National Audit 2021/22.
Results: Over 5 years, cangrelor leads to modelled cost savings of £ 2,709,853 (− 9.84%), varying from £ 322,218 in Year 1 (− 5.85%) to £ 636,150 (− 11.55%) in Year 5). This is driven by approximately 6,882 hospital days being avoided over 5 years due to fewer bleeding events.
Conclusion: Cangrelor for OHCA patients who cannot take oral P2Y12 inhibitors may lead to cost savings in the UK.
Keywords: glycoprotein inhibitor, P2Y12 inhibitor, cardiovascular disease, out-of-hospital cardiac arrest, antiplatelet therapy
Introduction
In the UK, cardiovascular disease (CVD), including ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) and unstable angina, is one of the leading causes of morbidity and mortality, and of increased healthcare costs.1 Healthcare costs relating to heart and circulatory diseases are estimated at £9 billion each year, and there are approximately 7.6 million people living with CVD in the UK.1 Of the 100,000 procedures per year in the UK, there are approximately 3,000 PCI procedures (3%) for out-of-hospital cardiac arrest (OHCA) patients, which presents a more challenging clinical presentation and great morbidity and mortality to the receiving PCI centres.2,3
Patients undergoing PCI require antiplatelet therapies to reduce the risk of ischaemic events, particularly stent thrombosis. These antiplatelet therapies are usually administered orally, including clopidogrel, as well as prasugrel and ticagrelor, which are newer and more potent therapies than clopidogrel.4 However, patients experiencing an OHCA are unsuitable for oral antiplatelet therapies because they are often unconscious and intubated. Patients are therefore either given intravenous (IV) glycoprotein IIb/IIIa inhibitors (GPIs) (eptifibatide and tirofiban), or rectal aspirin and IV heparin in combination.3 OHCA patients represent an ideal population for cangrelor use as they are both ineligible for oral medications as well as being candidates for increased thrombotic risk.5
Cangrelor (brand name Kengrexal®) is an IV, fast-acting, potent, and direct-acting platelet adenosine diphosphate (ADP) P2Y12 inhibitor that has rapidly reversible effects.4 Cangrelor quickly inhibits platelet function when given as a bolus plus infusion with near-normal function restored within one hour after discontinuation of the infusion.6 Cangrelor, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing PCI who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable.7 Cangrelor is referenced as a treatment option in the 2023 ESC Guidelines for the management of acute coronary syndromes.8
The group of clinical trials demonstrating the safety and efficacy of cangrelor are known as the CHAMPION programme. They are CHAMPION-PCI, CHAMPION-PLATFORM and CHAMPION-PHOENIX.4,9,10 The CHAMPION-PCI trial compared cangrelor with clopidogrel administered before PCI; the CHAMPION-PLATFORM trial compared cangrelor with clopidogrel administered at the time of PCI; and the CHAMPION-PHOENIX trial compared cangrelor with clopidogrel administered at the start or end of PCI.11 The largest of these studies, CHAMPION-PHOENIX, showed cangrelor significantly reduced the rate of ischaemic events, including stent thrombosis during PCI, with no significant increase in severe bleeding, when compared to oral clopidogrel.12 In addition, a pooled analysis of patient-level data from the CHAMPION programme showed that cangrelor reduced the odds of a composite of death, myocardial infarction (MI), ischaemia-driven revascularisation (IDR), or stent thrombosis (ST) at 48 hours by 19% (3.8% for cangrelor vs 4.7% for control; odds ratio [OR] 0.81, 95% CI 0.71–0.91, p = 0.0007) compared with clopidogrel. This pooled analysis showed that there was no statistically significant difference between cangrelor and clopidogrel in the primary safety outcome of severe bleeding not related to coronary artery bypass graft (CABG) according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) criteria at 48 hours (0.2% in both groups), in GUSTO moderate bleeding (0.6% vs 0.4%), or in transfusion (0.7% vs 0.6%), but cangrelor increased GUSTO mild bleeding (16.8% vs 13.0%, p < 0.0001).11
Importantly, these studies showed the efficacy and safety of IV cangrelor in a conventional PCI population but did not focus on the OHCA population where cangrelor has a growing use and unique advantage given its intravenous administration. We developed a budget impact model to calculate the impact of introducing cangrelor to UK hospitals over 5 years in OHCA patients in whom oral inhibitors are not feasible or desirable. This group of patients is a subpopulation of the licenced population for cangrelor.
Material and Methods
A budget impact model was built to calculate the difference in costs of two hypothetical scenarios over 5 years: one scenario considering patients receiving GPIs or aspirin and heparin in combination (market without cangrelor) and a second scenario considering a proposed management with patients treated with GPIs, aspirin and heparin in combination, or cangrelor (market with cangrelor). An overview of the budget impact model framework including the cost categories included is presented in Figure 1. The model adopts a UK perspective, specifically an NHS and PSS (Personal Social Services) perspective.
Inputs and Data Sources
A targeted literature review was conducted to identify the most relevant and appropriate data, which best reflected usual clinical practice in the UK. Assumptions were made in the absence of data and a consultant interventional cardiologist gave their expert opinion and validated sources, inputs, assumptions, and calculations.
Target Population
The British Cardiovascular Intervention Society (BCIS) National Audit of Adult Interventional Procedures 2021/22 was used to estimate the eligible population.2 This showed that in 2021/22, 3.1% of patients treated by PCI experienced an OHCA.2 There were 97,765 PCIs carried out in the UK in 2021/22, therefore approximately 3,031 patients were treated by PCI for an OHCA.2 There was assumed to be no increase in eligible population year-on-year due to a generally consistent number of PCIs being carried out each year in the UK (100,294 PCIs carried out in 2018/19, 100,112 in 2019/20, 90,708 in 2020/21 [impacted by the COVID-19 pandemic] and 97,765 in 2021/222,12–14). Similarly, the proportion of patients experiencing an OHCA has remained generally consistent at 3% of the total population who undergo PCI in the UK.
Expert clinical opinion was used to estimate the current market share of antiplatelet therapies in OHCA patients specifically and the expected market uptake of cangrelor in the UK over the next 5 years (Table 1).
 |
Table 1 Current Market Share and Market Uptake of Antiplatelet Therapies |
Efficacy and Safety Data
Efficacy and safety data for cangrelor, eptifibatide and tirofiban were sourced from a pooled analysis of the CHAMPION clinical trials programme, which included patients assigned to cangrelor but not receiving GPIs, and patients assigned to clopidogrel or placebo and receiving routine GPIs. Propensity score matching based on 16 baseline clinical variables was used to yield 1,021 unique matched pairs.15 Results showed that there was a non-significant trend toward reduced rates of GUSTO severe bleeding with cangrelor compared to GPIs (0.3% vs 0.7%; OR, 0.43; 95% CI, 0.11–1.66, p = 0.22) and significantly lower rates of Thrombolysis In Myocardial Infarction (TIMI) major or minor bleeding in patients treated with cangrelor (0.7% vs 2.4%; OR, 0.29; 95% CI, 0.13–0.68, p = 0.004).15 Efficacy and safety data for aspirin and heparin in combination was conservatively assumed to be equal to clopidogrel since efficacy data for aspirin and heparin alone is limited. Clopidogrel efficacy and safety data were sourced from a pooled analysis of the CHAMPION clinical trials programme comparing cangrelor and clopidogrel.11 See Table 2 for full efficacy and safety data used in the model.
 |
Table 2 Efficacy and Safety Data Used in the Model |
Length of Stay Data
Length of stay (LOS) data for PCI using comparator therapies was sourced from Hospital Episode Statistics (HES) data.16 Post-operative stay for comparators was calculated as the mean LOS for non-elective PCI minus the mean pre-operative LOS. Post-operative LOS data for cangrelor was calculated by applying the percentage reduction in LOS from a US-based study of cangrelor versus GPIs during PCI (GPI LOS: 4.3 days, cangrelor: 3.5 days, 19% reduction).17 This reduction in LOS may be due to significantly lower major bleeding rates with cangrelor use compared to GPI use during PCI (event rate: 1.7% vs 5.1%, p = 0.001; regression analysis: OR 0.23; 95% CI, 0.09–0.59, p=0.002) and a reduction in rate of any vascular complication with cangrelor compared to GPI (event rate: 1.5% vs 3.2%, p = 0.04).17 It should be noted that this was a single centre US study in a general PCI population, and not specifically in the OHCA subpopulation; however, we expect the results to be applicable in this subpopulation. The applicability of this US-based study to a UK population was validated by expert clinicians who agreed that bleeding events lead to a longer LOS in hospital. LOS for PCI using comparator therapies was estimated at 3.59 days based on HES data. A 19% reduction was then applied to this based on the US-based study of cangrelor versus GPIs to give a LOS for PCI using cangrelor of 2.92 days.
Bailout GPI Use
Bailout GPI use was assumed to decrease from 6.5% of patients to zero upon the introduction of cangrelor, based on Lizano-Díez & Paz Ruiz (2021) and confirmed by clinical expert opinion.18 Bailout GPIs are defined as the use of GPI when the PCI operator has not intended to use GPI from the outset but considers that clinical or angiographic features (such as worsening or persistent thrombus burden) have changed during the course of the procedure, such that there may be benefit to giving the patient GPI.3
Cost Data
Treatment costs were sourced from the British National Formulary (BNF) (Table 3). Clinical event costs were sourced from the literature and the cost of an additional stay in hospital was sourced from the NHS reference costs (Table 3). Costs were updated to 2021/22 GBP based on the Unit Costs of Health and Social Care 2022 Manual and costs remain unchanged from Year 1 onwards based on the ISPOR budget impact analysis guidelines principles of good practice.19,20
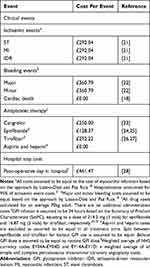 |
Table 3 Cost Data Used in the Model |
Sensitivity Analysis
A one-way sensitivity analysis was conducted to assess the impact of different parameters on the budget impact results. Parameters were varied by ±20%. The ten parameters with the largest effect on the budget impact were included.
Results
The number of OHCA PCI patients eligible for cangrelor treatment reaches 15,154 over 5 years, with an average of 3,031 patients per year. A total of 12,881 patients are predicted to be treated with cangrelor over 5 years, with an average of 2,576 patients treated per year. Over 5 years, the total costs incurred by the NHS and PSS in the scenario without cangrelor are £27,548,187. The total costs incurred by the NHS and PSS in the scenario with cangrelor introduced are £24,838,334. Cangrelor leads to a cost saving of £2,709,853 over 5 years (−9.84%), varying from £322,218 in Year 1 (−5.85%) to £636,150 (−11.55%) in Year 5. Over 5 years, approximately 6,882 hospital days and 408 clinical events are predicted to be avoided through the use of cangrelor, including approximately 61 major bleeding events. As well as major bleeding events, these clinical events included ST, MI, IDR, death, and minor bleeding. Full results are available in Table 4.
 |
Table 4 Base Case Budget Impact Summary Results |
The results of the one-way sensitivity analysis suggested that the model was most sensitive to additional days in hospital for GPIs and additional days in hospital for cangrelor and aspirin in combination with heparin, since varying these by ±20% had the biggest effect on the budget impact. Varying the additional days in hospital for GPIs by −20% led to a budget impact of £3,313,512, compared to a cost saving of £2,709,853 cost saving in the base case, a £6,023,365 difference. Varying this parameter by +20% led to a cost saving of £10,022,912, a £7,313,059 difference. This indicates that hospital LOS is a major driver of the model that supports a cost saving with cangrelor. The third most sensitive parameter was the cost of cangrelor. See Table 5 and Figure 2 for full results of the one-way sensitivity analysis.
 |
Table 5 One-Way Sensitivity Analysis Results |
Discussion
This model demonstrates that introducing cangrelor in the UK for the treatment of OHCA PCI patients over 5 years, in whom the use of oral P2Y12 inhibitors is not feasible or desirable, is likely to lead to a reduction in hospital stays and cost savings.
Cangrelor is an innovative IV antiplatelet strategy that delivers an immediate, potent, and rapidly reversible effect in OHCA patients undergoing PCI when use of oral antiplatelet agents is not possible. Cangrelor is cost saving in the base case and the one-way sensitivity analysis. A common concern with cangrelor is the treatment acquisition cost, which we have demonstrated in this study to be offset by a decrease in clinical event costs and a decreased LOS in hospital post-PCI. Hospital LOS is a major driver in the model as demonstrated by the one-way sensitivity analysis. In addition to this study, the benefits of cangrelor have been demonstrated in real-world studies to show a reduced length of stay, potentially driven by fewer bleeding events.17
Limitations
- The OHCA STEMI patient population is heterogeneous, so natural variation in results is expected on an individual patient basis. Hospital LOS is expected to be dependent on the underlying anatomy and complexity of a patient. While the model tests this heterogeneity in sensitivity analyses, it is challenging to fully explore the heterogeneity of the UK patient population.
- The assumption that the efficacy of aspirin and heparin was considered to be equal to clopidogrel.29 However, this is likely to be a conservative assumption that favours aspirin and heparin.
- Market share of cangrelor and its comparators may differ from the model predictions in years 2–5. If cangrelor uptake is lower than estimated here, cangrelor may still be cost saving. The same cost for major bleeding events and minor bleeding events was used due to lack of data. Therefore, bleeding costs may have been overestimated. However, in the one-way sensitivity analysis, which varied the bleeding costs by ±20%, cangrelor remained cost saving in all scenarios.
- The cost for ST and IDR is assumed to be the same as MI due to lack of data availability. It is anticipated that a ST or IDR would lead to an additional PCI procedure.
It should also be noted that this model does not consider societal losses or impact on patients’ quality of life (QoL). OHCA patients undergoing PCI are generally classified as seriously unwell and therefore an additional comorbidity of a bleeding event or other vascular event can be extremely detrimental to their QoL. Cangrelor significantly reduces bleeding events and hospital LOS, which is likely to have a large impact on patients’ wellbeing. A reduced LOS also means a reduction in productivity losses due to short-term and long-term inability to work.
Building upon the findings of this model, future research could investigate the budget impact of introducing cangrelor to a wider subpopulation. This could include a subset of STEMI patients with underlying gastric absorption issues, rendering oral drug use ineffective (ie an intravenous drug such as cangrelor is required).
Despite limitations, this budget impact analysis provides an estimate of the cost of introducing a novel antiplatelet IV therapy in patients undergoing PCI for OHCA, in whom oral P2Y12 inhibition is not feasible or desirable and suggests that cangrelor may be cost saving in this eligible population.
Conclusion
Cangrelor is a fast-acting and direct-acting intravenous P2Y12 inhibitor that has rapidly reversible effects. Cangrelor represents an alternative therapy for the treatment of the OHCA population in whom oral P2Y12 inhibitors are not feasible or desirable. To date, there has been concern about the cost implications and hence the need for this budget modelling.
Introducing cangrelor for OHCA patients subsequently undergoing PCI, where the established use of oral therapies is not possible, is effective, and this study shows it may lead to cost savings in the UK health system, driven predominately by a reduction in post-operative hospital LOS, due to a reduction in bleeding events.
Acknowledgments
This research was funded by Chiesi Ltd.
Disclosure
Rob Cain, Richard Stork and Gina Tarpey are employees of Chiesi Ltd. Dr Bhavik Modi has received consultancy fees from Chiesi Ltd. Caroline Barwood is an employee of FIECON Ltd who were contracted by Chiesi to build the model for this manuscript. The authors report no other conflicts of interest in this work.
References
1. British Heart Foundation. UK factsheet 2023. British Heart Foundation. 2023. Available from: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf.
2. Ludman PF. BCIS National Audit Adult Interventional Procedures - 2021-22. British Cardiovascular Intervention Society, 2023. Available from: https://www.bcis.org.uk/audit-results/.
3. National Institute for Health and Care Excellence. Acute coronary syndromes. 2020. Available from: https://www.nice.org.uk/guidance/ng185/chapter/Recommendations.
4. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. doi:10.1056/NEJMoa1300815
5. Erlinge D. Cangrelor for ST-segment–elevation myocardial infarction. Circulation. 2019;139(14):1671–1673. doi:10.1161/CIRCULATIONAHA.119.039253
6. Angiolillo DJ, Schneider DJ, Bhatt DL, et al. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J Thromb Thrombolysis. 2012;34(1):44–55. doi:10.1007/s11239-012-0737-3
7. European Medicines Agency. Kengrexal. European Medicines Agency 2018. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kengrexal.
8. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European society of cardiology (ESC). Eur Heart J. 2023;ehad191.
9. Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318–2329. doi:10.1056/NEJMoa0908628
10. Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330–2341. doi:10.1056/NEJMoa0908629
11. Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet Lond Engl. 2013;382(9909):1981–1992. doi:10.1016/S0140-6736(13)61615-3
12. The National Institute for Cardiovascular Outcomes Research (NICOR). Percutaneous coronary intervention (PCI) – 2020 summary report. HQIP 2020. Available from: https://www.hqip.org.uk/resource/percutaneous-coronary-intervention-pci-2020-summary-report/.
13. The National Institute for Cardiovascular Outcomes Research (NICOR). National audit of percutaneous coronary intervention: 2021 summary report. 2021. Available from: https://www.hqip.org.uk/resource/national-audit-of-percutaneous-coronary-intervention-2021-summary-report/.
14. The National Institute for Cardiovascular Outcomes Research (NICOR). Percutaneous coronary intervention (PCI) - 2022 summary report. 2022. Available from: https://www.nicor.org.uk/wp-content/uploads/2022/06/NAPCI-Domain-Report_2022-FINAL.pdf.
15. Vaduganathan M, Harrington RA, Stone GW, et al. Evaluation of ischemic and bleeding risks associated with 2 parenteral antiplatelet strategies comparing cangrelor with glycoprotein IIb/IIIa inhibitors: an exploratory analysis from the champion trials. JAMA Cardiol. 2017;2(2):127–135. doi:10.1001/jamacardio.2016.4556
16. NHS Digital. Hospital Episode Statistics (HES) to map Percutaneous Coronary Intervention. 2021. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics.
17. Yerasi C, Case BC, Chezar-Azerrad C, et al. Cangrelor vs. glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention. Am Heart J. 2021;238:59–65. doi:10.1016/j.ahj.2021.04.013
18. Lizano-Díez I, Paz Ruiz S. Analysis of the financial impact of using cangrelor on the safety and efficacy outcomes in patients undergoing percutaneous coronary intervention in whom oral therapy with P2Y12 inhibitors is not feasible or desirable, in Spain. Clin Outcomes Res. 2021;13:77–87.
19. Jones KC, Weatherly H, Birch S, et al. Unit Costs of Health and Social Care 2022 Manual (PSSRU). 2023. doi:10.22024/UniKent/01.02.100519
20. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
21. Danese MD, Gleeson M, Kutikova L, et al. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open. 2016;6(8):e011805. doi:10.1136/bmjopen-2016-011805
22. Mamas MA, Tosh J, Hulme W, et al. Health economic analysis of access site practice in England during changes in practice. Circ Cardiovasc Qual Outcomes. 2018;11(5):e004482. doi:10.1161/CIRCOUTCOMES.117.004482
23. National Institute for Health and Care Excellence. Cangrelor - BNF. Available from: https://bnf.nice.org.uk/drug/cangrelor.html.
24. National Institute for Health and Care Excellence. Eptifibatide - BNF. Available from: https://bnf.nice.org.uk/drug/eptifibatide.html.
25. GlaxoSmithKline UK. INTEGRILIN 0.75 mg/mL solution for infusion - summary of product characteristics (SmPC) - (emc). 2021. Available from: https://www.medicines.org.uk/emc/product/3889/smpc.
26. National Institute for Health and Care Excellence. Tirofiban - BNF. Available from: https://bnf.nice.org.uk/drug/tirofiban.html.
27. ADVANZ Pharma. Aggrastat 50 mcg/mL solution for infusion - summary of product characteristics (SmPC) - (emc). 2018. Available from: https://www.medicines.org.uk/emc/product/566/smpc.
28. NHS Improvement. 2017/18 reference costs | archived reference costs 2020. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/.
29. Tan BE-X, Wong PY, Baibhav B, et al. Clopidogrel Vs aspirin monotherapy following dual antiplatelet therapy after percutaneous coronary intervention: a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48(8):101174. doi:10.1016/j.cpcardiol.2022.101174
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Personalized Approaches to Antiplatelet Treatment for Cardiovascular Diseases: An Umbrella Review
Oliva A, Cao D, Spirito A, Nicolas J, Pileggi B, Kamaleldin K, Vogel B, Mehran R
Pharmacogenomics and Personalized Medicine 2023, 16:973-990
Published Date: 3 November 2023



