Back to Journals » Drug Design, Development and Therapy » Volume 19
The Changing Landscape of Heart Failure Drug Clinical Trials in China, 2013–2023
Authors Zhang W, Zhang Y, Tang J, Wang X, Meng C, Wu J, Li J
Received 20 December 2024
Accepted for publication 27 March 2025
Published 3 April 2025 Volume 2025:19 Pages 2597—2608
DOI https://doi.org/10.2147/DDDT.S511608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Wenjie Zhang,1,2,* Yinming Zhang,3,* Jiawei Tang,4,* Xuejiao Wang,1 Chao Meng,1,2 Ji Wu,1 Jun Li1
1Department of Cardiology, Guang’Anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, People’s Republic of China; 2Graduate School, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China; 3Department of Emergency, Yankuang New Journey General Hospital, Zoucheng, Shandong Province, 273500, People’s Republic of China; 4School of Computer Science, Beijing University of Posts and Telecommunications, Beijing, 100876, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ji Wu, Email [email protected] Jun Li, Email [email protected]
Objective: This review aimed to delineate the changing landscape of heart failure (HF) drug clinical trials conducted in China during 2013– 2023.
Methods: Detailed information on HF drug trials registered on the National Medical Products Administration Clinical Trial Information Disclosure Platform from January 1, 2013, to December 31, 2023, was collected. The characteristics, drug mechanisms, data safety, participant protection, geographical locations, and scales of HF drug clinical trials were analyzed.
Results: China initiated 354 hF drug clinical trials during 2013– 2023, encompassing eight acute heart failure (AHF) trials and 346 trials for chronic heart failure (CHF). The overall number of HF trials continued to increase, whereas the number of AHF trials remained consistently low. Significant differences were observed between AHF and CHF trials regarding trial phases, drug types, trial designs, blinding methods, and geographical coverage. 85.8% CHF trials were bioequivalence studies, whereas AHF trials were exclusively Phase I–III studies. Most trial drugs were chemical drugs, with renin-angiotensin-aldosterone system inhibitors accounting for the highest proportion. Sixteen new drug studies involved 13 different new drugs. The proportion of studies establishing independent data monitoring committees annually remained generally low, whereas the proportion of studies purchasing clinical trial insurance for participants annually exhibited an overall upward trend. The 354 trials were led by principal investigators from 27 provinces, autonomous regions, or municipalities in China. 47.2% leading units for these studies were distributed in the eastern coastal regions of China. There were 30 drug clinical trials with more than ten participating centers and 16 drug clinical trials with a target number of participants of over 1000 individuals.
Conclusion: Over the past decade, China has experienced rapid development in HF drug trials, particularly in drug consistency evaluations. All stakeholders involved in drug trials should carefully consider the inadequate innovations in first-in-class drugs.
Keywords: heart failure, China, drug clinical trial, changing landscape
Introduction
Heart failure (HF) can be categorized into acute heart failure (AHF) and chronic heart failure (CHF) based on the basis of its onset duration and progression velocity.1 In China, the standardized prevalence rates of HF among populations aged 25–64, 65–79, and 80 years and older are 0.57%, 3.86%, and 7.55%, respectively.2 Owing to population growth, the intensification of aging, and an elevated prevalence of comorbidities, the incidence of HF in China has exhibited a persistent upward trend.3–5 Although there has been a notable improvement in the survival rates of HF patients, the hospitalization rates and quality of life impacted by HF remain suboptimal.6,7 Whether it is AHF or CHF, pharmacological intervention represents a pivotal treatment approach. Pharmacotherapy for HF is highly important for controlling etiology, managing volume status, alleviating symptoms, and enhancing patient prognosis and quality of life.
Vaduganathan et al reported that the proportion of global clinical trials for HF increased from 2001–2016. Specifically, in Asia, the percentage of HF clinical trials increased from 2.1% to 13.4% between 2009–2012 and 2013–2016.8 As the most populous country in Asia, China’s clinical trials for HF have had a significant impact on the overall situation of HF clinical trials in Asia. In 2013, the National Medical Products Administration (NMPA) of China enacted a mandate requiring the registration and announcement of all approved drug clinical trials on the NMPA platform.9,10 Between 2013 and 2017, the Chinese government introduced a series of policies aimed at fostering innovative drugs and clinical trials.11 Drug trials for HF in China are pertinent to tens of millions of patients. Over the past decade, advancements in drug development and approval policies in China have facilitated substantial progress in HF drug trials.12,13 Chinese researchers have conducted comprehensive summaries and reviews of the evolving landscape of drug trials in China for cancers, pediatrics, and thyroid diseases.14–16 However, to date, no review has provided an analysis of the trends and characteristics of HF drug clinical trials in China. This review aimed to analyze the shifts in HF drug clinical trials in China from 2013–2023, offering valuable insights for researchers, physicians, pharmaceutical enterprises, policymakers, and other pertinent stakeholders.
Methods
Data Sources
We searched the NMPA Registration and Information Disclosure Platform for Drug Clinical Studies website (http://www.chinadrugtrials.org.cn accessed on April 11, 2024) via the term “heart failure” in the “Indication” search bar of the advanced query. We screened trials that met the time requirements using the “first public information date” in the basic information of the trials.
Search Strategy and Selection Criteria
The trials included in this study were required to meet the following criteria: (1) The clinical indication must be HF. (2) The trial registration period should be between January 1, 2013, and December 31, 2023. (3) The study location should be within China not a multi-center trial with a major research institution outside China. (4) The drug clinical trial information that needed to be collected included the drug name, drug type (based on registration statements, including traditional Chinese/natural medicines, chemical drugs, and biological products), trial status (based on registration statements, including proceeding, completed, suspended, and terminated), registration date, trial phase (based on registration statements, including bioequivalence, Phase I, Phase II, Phase III, and phase IV), study design (based on registration statements, including single-arm, crossover, and parallel-group), randomization status, blinding status (based on registration statements, including open-label, single-blind, and double-blind), trial coverage (based on registration statements, including international multi-center and domestic), geographical location of the principal investigators’ (PIs’) leading units, number of participating centers, target number of participants, independent data monitoring committee (IDMC) status, and purchase of clinical trial insurance. Trials lacking registration information were excluded. Data selection and extraction were conducted by two researchers (WJZ and YMZ), and any disagreements were resolved by a third researcher (JL), who served as an arbitrator. Ultimately, we included 354 trials that met the inclusion criteria (Figure 1).
 |
Figure 1 PRISMA flow diagram of the trial selection process. Note: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.17 |
Statistical Analysis
In the included trials, the target population described in the “Indications” and/or “Clinical Trial Information” was divided into AHF and CHF. The 2023 ESC Guidelines categorize HF into AHF and CHF on the basis of the onset duration and progression velocity.1 Therefore, we divided the drug clinical trials into two categories, AHF and CHF, according to the descriptions in “Indications” and/or “Clinical Trial Information”. We analyzed the characteristics, trends, drug mechanisms, data safety, participant protection, geographical location, and scale of these drug clinical trials. Drug mechanisms were classified on the basis of the drug leaflet and the 2023 ESC guidelines.1 For drugs in multiple categories, such as spironolactone and eplerenone, which are both RAASIs and diuretics, we counted these two drugs as two statistical units, thereby expanding the statistical basis. Quantitative variables are presented as numbers and percentages (%). Statistical significance was defined as a two-sided p-value of< 0.05. All the statistical analyses were performed using GraphPad Prism software, version 10.1.2.
Results
Time Trends of Initiated Trials
A total of 354 drug clinical trials for HF were registered on the NMPA platform, including eight trials for AHF and 346 trials for CHF (Figure 2). The number of registered trials for both AHF and CHF was low between 2013 and 2016. However, from 2017 to 2023, there was a significant increase in the number of trials for CHF, whereas the number for AHF remained consistently low. Overall, the overall number of HF trials continued to increase from 2013 to 2023.
 |
Figure 2 Annual numbers of initiated heart failure drug clinical trials by onset duration and progression velocity in China, 2013–2023. |
Characteristics and Drug Mechanisms Distribution of Clinical Trials
Significant differences exist between clinical trials for AHF and CHF in terms of trial phase, drug type, trial design, blinding method, and geographical coverage (Table 1). In terms of status, trials for both AHF and CHF were primarily in the completed or ongoing stages. The completion rate of clinical trials for HF has consistently increased from 2013 to 2023 (Figure 3). Specifically, the completion rate increased from 62.5% to 84.9% between 2016 and 2018, and remained between 80.9% and 95.5% from 2018 to 2023. Bioequivalence (BE) studies accounted for 85.8% of CHF trials, whereas in AHF trials, the proportion of BE studies was 0%, followed by 25.0% in phase I, 25.0% in phase II, and 50.0% in Phase III. In terms of study design, 85.3% of the CHF trials were crossover studies, and 87.5% of the AHF trials were parallel-group studies. In terms of blinding methods, 87.0% of the CHF trials were open-label studies, whereas 87.5% of the AHF trials were double-blind studies. In terms of geographical coverage, 94.8% of the CHF trials were conducted domestically, whereas 62.5% of the AHF trials were international multi-center studies.
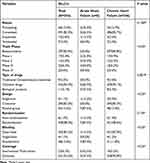 |
Table 1 Characteristics of Heart Failure Drug Clinical Trials in China (N=354) |
 |
Figure 3 Annual trial status proportions of heart failure in China, 2013–2023. |
Both the AHF and CHF trials predominantly involved chemical drugs. A total of 334 chemical drug trials included 52 distinct chemical drugs. The AHF trials involved three chemical drugs, primarily empagliflozin, along with two new drugs (Istaroxime and GNP). CHF trials covered all 52 chemical drugs.
We categorized these 52 chemical drugs on the basis of their pharmacological mechanisms (Figure 4, Table 2). Since spironolactone (n=4) and eplerenone (n=3) are both RAASIs and diuretics, we expanded the statistical analysis base for chemical drug clinical trials from 334 to 341. The highest proportion was observed for RAASIs (39.9% [136/341]), followed by anticoagulants (18.0% [63/341]) and adrenoceptor antagonists (11.0% [37/341]).
 |
Table 2 Drug Trials for Heart Failure by Drug Class |
 |
Figure 4 The distribution of drug mechanisms for heart failure drug clinical trials. |
Further categorization of the 52 chemical drugs revealed that sacubitril/valsartan (n=49), an angiotensin receptor neprilysin inhibitor (ARNI), and rivaroxaban (n=42), a factor Xa inhibitor, were the most studied, followed by captopril (n=25), and an angiotensin-converting enzyme inhibitor (ACEI). The 16 new drug studies collectively involved 13 different new drugs, with only the Phase I study of neladenoson bialanate being voluntarily terminated.
Data Safety and Participant Protection
A total of 21 clinical drug trials established IDMCs and 214 clinical drug trials purchased clinical trial insurance for participants. From 2013–2023, the proportion of studies establishing IDMCs annually remained generally low, whereas the proportion of studies purchasing clinical trial insurance for participants annually exhibited an overall upward trend (Figure 5A). In the AHF trials, the proportions of establishing IDMCs and purchasing clinical trial insurance for participants were 75.0% and 100.0%, respectively, which were significantly higher than those in the CHF trials (4.3% and 59.5%, respectively) (Figure 5B).
Geographical Distribution of Trial Leading Units in China
The PIs’ leading units for the 354 drug clinical trials originated from 27 provinces, autonomous regions, or municipalities in China (Figure 6; Supplementary Table 1). Hunan had the highest number of leading units (n=50), followed by Beijing (n=47) and Jiangsu (n=34). A total of 47.2% of the leading units for these studies were distributed across 10 provinces, autonomous regions, or municipalities in the eastern coastal regions of China (including Beijing, Jiangsu, Zhejiang, Shanghai, Guangdong, Hebei, Tianjin, Shandong, Fujian, and Hainan). The distribution of leading units in the AHF trials was limited and was primarily concentrated in Beijing. In contrast, the distribution of leading units for CHF trials was broader and was located mainly in the eastern and central regions of China.
 |
Figure 6 Geographical distribution of leading units of heart failure drug clinical trials in China, 2013–2023. |
Sizes of Clinical Trials
We analyzed the size of the clinical trials on the basis of the number of participating centers and the target number of participants. The maximum number of centers participating in drug clinical trials was 1042 (Figure 7A). There were 30 drug clinical trials with more than ten participating centers, among which 26 trials were related to CHF. The maximum target number of participants for drug clinical trials was 8400 individuals (Figure 7B). There were 16 drug clinical trials with a target number of participants of over 1000 individuals, 13 of which were related to CHF.
Discussion
Drug Trials and Informed Consent
We analyzed 354 trials in China from 2013–2023, including eight trials for AHF and 346 for CHF. Compared with the rapid development of drug trials for CHF, trials for AHF have progressed slowly. A survey on the willingness of individuals from developing countries to participate in clinical trials revealed a low consent rate for volunteers to engage in life-threatening medical research.18 This finding is associated with safety concerns arising from the vulnerability of critically ill patients, which subsequently increases the difficulty of obtaining informed consent from these volunteers. AHF, a life-threatening condition, has undergoing limited research progress, and few have been conducted studies over the years, which could be attributed to the aforementioned reasons. This underscores the importance of researchers providing more general information to volunteers to gain trust, especially in the context of AHF studies.
Chinese Policies and New Drug Research and Development
Owing to the retarded progression of new drug research and development (R&D) endeavors in China, the Chinese government has implemented a suite of policies aimed at fostering advancements in new drug R&D since 2015.19 In the subsequent year of 2016, the government enacted policies that incentivized Chinese pharmaceutical enterprises to undertake BE studies.20 Supported by these policy initiatives, there has been a noticeable upward trend in the total number of CHF trials focusing on BE studies since 2016. Additionally, HF clinical trials maintained a high completion rate from 2018 to 2023, indicating that the government’s supportive policies have positively impacted HF clinical trials both in the short and long term. Nevertheless, Phase I–IV studies comprising CHF trials constituted only 14.2% of the total, suggesting an inadequate investment in new drug R&D for CHF in China. Although the overall number of AHF trials remained relatively low, a significant proportion of these trials were categorized into phases I–III. The substantial allocation of new drug R&D for AHF holds considerable importance for advancing therapeutic interventions for AHF.
Generic and Novel Drugs
The field of HF drug trials has focused predominantly on chemical drugs. Chinese pharmaceutical enterprises have conducted extensive consistency evaluations of commonly prescribed chemical drugs, such as sacubitril/valsartan. Leveraging existing therapeutic targets, these enterprises have embarked on a series of independent research endeavors to develop novel drugs. Examples include BTP0611, which shares similarities with ivabradine; HEC95468, which is analogous to vericiguat;21 and another variant, S086, which mimics the effects of sacubitril/valsartan. Chinese pharmaceutical enterprises have also developed the highly potent and selective phosphodiesterase 9 (PDE9) inhibitor TT-00920 and the diuretic HRS-9057, both of which have secured clinical trial approval. Notably, influenced by traditional Chinese medicine, Chinese pharmaceutical enterprises have isolated a component from Aconitum carmichaelii Debx. that safeguards the structural and functional integrity of myocardial mitochondria and is referred to as GD-N1702. Currently, this drug has successfully completed phase I clinical trials.
Furthermore, novel drugs developed by non-Chinese pharmaceutical enterprises, such as BAY 1753011, OPC-61815, and neladenoson bialanate, have undergone clinical trials in China. However, the development of new drugs is fraught with high risk and low success rates. A phase IIb clinical trial conducted across 76 centers, including the United States, revealed that the adenosine receptor agonist neladenoson bialanate failed to augment cardiac function in patients with HF with either preserved or reduced ejection fraction. Consequently, the researchers have voluntarily discontinued the phase I studies of this drug in China.22,23
Protection of Participants’ Rights and Interests
The establishment of IDMCs holds paramount significance in assessing the risk-benefit ratio for participants, ensuring the unmatchable scientific integrity of research data, and facilitating well-informed decision-making in research endeavors.24 Consequently, PIs should consider establishing IDMCs when conducting clinical trials of drugs. Given that the risk of AHF is greater than that of CHF and that the condition of AHF patients can deteriorate more rapidly than that of CHF patients can, the proportion of AHF trials that establish an IDMC is significantly greater than that of CHF trials. From 1999 to 2021, the NMPA reported an overall increasing trend in adverse drug reactions.25 With the progressive increase in the volume of drug clinical trials, the participant population has correspondingly expanded, thereby rendering the protection of participants’ rights and interests a matter of increasing importance. In 2020, the NMPA further clarified the regulations on the protection of participants’ rights and interests, building upon the policies established in 2003.26 Between 2013 and 2023, the proportion of HF trials that have procured clinical trial insurance for participants has exhibited a general upward trajectory, suggesting that PIs in China place greater emphasis on safeguarding participants’ rights and interests.
The establishment of an IDMC and the purchase of clinical trial insurance both serve to protect the rights and interests of the participants. The IDMC is responsible for the scientific monitoring of drug trials to prevent risks, and it is currently more commonly used in high-risk studies.24 On the other hand, purchasing trial insurance provides economic compensation after harm occurs during a trial and does not involve managing the trial process; therefore, it cannot prevent the occurrence of harm. Given the increasing number of reports of adverse drug reactions and the contradictory situation in which the proportion of established IDMCs in current HF drug trials in China is relatively low, despite the continuous increase in the proportion of purchasing trial insurance, there are still deficiencies in scientifically predicting trial risks and preventing adverse drug reactions. Therefore, PIs should emphasize on establishing IDMCs and purchasing trial insurance to ensure the protection of participants’ rights and interests.
Geographical Distribution of Trial Leading Units and HF Patients
Chinese scholars have discovered that compared with eastern China and central China, patients with HF in western China have a higher hospitalization rate and poorer cardiac function.27 High-quality medical resources are concentrated in the eastern and central China, while the scarcity of such resources in western China contributes to the increasing number of HF cases and the severity of the condition.28 Our findings indicate that the PIs conducting HF drug trials are predominantly located in the eastern and central China, which aligns with the distribution of high-quality medical resources in the country. Owing to the uneven distribution of medical resources, a larger proportion of HF patients in western China are unable to participate in drug trials. The uneven geographical distribution of participants may have resulted in incomplete drug trial results. Furthermore, the geographical distribution of AHF drug trials was more limited than that of CHF drug trials, which are primarily concentrated in Beijing. This is associated with the more critical condition of AHF and the denser availability of high-quality medical resources in Beijing.28 However, this restricts the promotion of AHF drug trials. Therefore, China should strive to distribute high-quality medical resources more evenly to address the uneven geographical distribution of drug trials.
Challenges and Suggestions
Although significant progress has been made in HF drug trials in China between 2013 and 2023, some notable deficiencies still deserve attention. First, the number of international multicenter trials led by Chinese pharmaceutical enterprises remains limited, and the scale of these trials is generally small. Factors such as insufficient clinical efficacy of drugs, poor drug specificity, uncontrollable toxicity, and weak business strategies have contributed to the limited international influence of Chinese pharmaceutical enterprises.29 This situation not only hinders the ability of Chinese pharmaceutical enterprises to conduct large-scale international multicenter trials but also obstructs the global promotion of their drugs. Second, the development of HF drugs suffered from a scarcity of first-in-class drugs. The R&D of new drugs in China faces numerous challenges in terms of policy, funding, and research capabilities. These challenges include insufficient R&D expenditure due to reduced industry profits caused by centralized drug procurement, a lack of research funding resulting from decreased investment in pharmaceutical R&D by the Chinese government and enterprises, prolonged drug promotion cycles caused by restrictive market access and medical insurance policies, and a shortage of talent in drug R&D.29–31 Third, the establishment of IDMCs in trials was infrequent, posing a threat to the protection of participants’ rights and the assurance of research data reliability. Fourth, the distribution of leading institutions conducting the trials did not align with the geographical distribution of HF patients in China, which may have resulted in incomplete drug trial outcomes.
Other low- and middle-income countries also face similar challenges, which affect the completion rate of drug trials and the speed of new drug R&D.32 Developed countries such as the United States have a fast pace of new drug R&D and a large number of first-in-class drugs.33 This is attributed to favorable policies, abundant funding, strong R&D capabilities, and innovative drug trial methodologies.33,34 In response to the current situation in China and other low- and middle-income countries, PIs can learn from innovative drug trial methodologies to reduce the time and cost of drug trials, thereby advancing the progress of drug development. For example, adaptive trial designs can decrease the duration, cost, and sample size of drug trials while increasing success rates.35 Decentralized and digitalized clinical trials, which rely on digital technology to decentralize clinical trials, can increase participant convenience, lower drug trial costs, and address the issue of uneven geographical distribution in drug trials.36
Conclusions
This study conducted an exhaustive analysis of drug trials for HF in China over the past decade. The significant surge in the number of trials underscores the advancements in clinical trial capabilities dedicated to the development of novel HF drugs. Despite the abundance of drug consistency evaluation trials, there is a notable scarcity of first-in-class drugs. The proportion of trials that have established IDMCs was comparatively low, highlighting the urgent need for PIs to increase their awareness regarding the protection of participants’ rights and the reliability of research data. Given the increasing prevalence of HF in China, it is imperative for relevant policymakers, researchers, pharmaceutical enterprises, and hospital clinical trial institutions to continuously and collaboratively develop and optimize innovative HF drugs. Furthermore, the NMPA platform requires pharmaceutical enterprises to publicly disclose drug trial information within 1–3 years of obtaining drug trial approval.37 The data included in this review may have delayed updates, which could potentially impact the conclusions drawn in this review. Continuous data updates are necessary to address the limitations of this review.
Data sharing statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (2022YFC3500102) and the High Level Chinese Medical Hospital Promotion Project (HLCMHPP2023065). The funding source of this study does not influence or involve in study design; collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Supplementary materials
Supplementary material associated with this article can be found in the supplementary information files.
Disclosure
All authors declare no competing interests.
References
1. McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–3639. doi:10.1093/eurheartj/ehad195.
2. Wang H, Chai K, Du M, et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. 2021;14(10):e008406. doi:10.1161/CIRCHEARTFAILURE.121.008406
3. Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–3287. doi:10.1093/cvr/cvac013
4. Shahim B, Kapelios CJ, Savarese G, et al. Global public health burden of heart failure: an updated review. Card Fail Rev. 2023;9. doi:10.15420/cfr.2023.05
5. Chinese Society of Cardiology; Chinese Medical Doctor Association, Cardiovascular Physicians Branch; Heart Failure Professional Committee of Chinese Medical Doctor Association; et al. Chinese guidelines for the diagnosis and treatment of heart failure 2024. Zhonghua Xin Xue Guan Bing Za Zhi. 2024;52(3):235–275. doi: 10.3760/cma.j.cn112148-20231101-00405
6. Salah HM, Minhas AMK, Khan MS, et al. Causes of hospitalization in the USA between 2005 and 2018. European Heart Journal Open. 2021;1(1):oeab001. doi:10.1093/ehjopen/oeab001
7. Khan MS, Shahid I, Bennis A, et al. Global epidemiology of heart failure. Nat Rev Cardiol. 2024;21(10):717–734. doi:10.1038/s41569-024-01046-6
8. Vaduganathan M, Samman Tahhan A, Greene SJ, et al. Globalization of heart failure clinical trials: a systematic review of 305 trials conducted over 16 years. Eur J Heart Fail. 2018;20(6):1068–1071. doi:10.1002/ejhf.1130
9. Huang Q, Wang Y, Fan Y, et al. Introducing the platform for registry and publicity of drug clinical trials and analyzing the common questions in trial registry. Chin J New Drugs. 2014;23:2721–2724.
10. Wang Y, Wang P, Shi J, et al. Building the platform for registry and publicity of drug clinical trials to safeguard the interests of human subject. Chin J New Drugs. 2015;24:496–498.
11. Su X, Wang H, Zhao N, et al. Trends in innovative drug development in China. Nat Rev Drug Discov. 2022;21(10):709–710. doi:10.1038/d41573-022-00077-3
12. Wang J, Chen J, Zhao J, et al. Establishment of RWS guidance reflecting contributions of China to regulatory science. journal of Biopharmaceutical Statistics. 2024;34(6):864–872. doi:10.1080/10543406.2024.2330208
13. Tang W, Huang Y, Zhou D, et al. Evolving drug regulatory landscape in China: a clinical pharmacology perspective. Clin Transl Sci. 2021;14(4):1222–1230. doi:10.1111/cts.12987
14. Zhong Q, Tao Y, Chen H, et al. The changing landscape of anti-lung cancer drug clinical trials in mainland China from 2005 to 2020. Lancet Reg Health West Pac. 2021;11:100151. doi:10.1016/j.lanwpc.2021.100151
15. Wu WW, Ji X, Wang H, et al. Pediatric clinical trials in mainland china over the past decade (From 2009 to 2020). Front Med Lausanne. 2021;8:745676. doi:10.3389/fmed.2021.745676
16. Li C, Hao J, Wang C, et al. Changes in drug clinical trials of thyroid diseases in China, 2009-2022. Drug Des Devel Ther. 2023;17:2315–2324. doi:10.2147/DDDT.S409617
17. Liberati A, Altman DG and Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1). doi:10.1136/bmj.b2700), b2700–b2700.
18. Bouida W, Grissa MH, Zorgati A, et al. Willingness to participate in health research. Tunisian Survey BMC Med Ethics. 2016;17(1):47. doi:10.1186/s12910-016-0131-3
19. Han Y, Jiang R, Li J, et al. The impact of regulatory reforms in China on drug lag: the role of clinical development strategies. Clin Pharmacol Ther. 2024;115(6):1400–1407. doi:10.1002/cpt.3227
20. Opinions on the evaluation of consistency of quality and efficacy of generic drugs. 2016. Available from: https://www.gov.cn/zhengce/content/2016-03/05/content_5049364.htm.
21. Gui Y-Z, Wang W, Wu Q-Q, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a soluble guanylate cyclase stimulator, HEC95468, in healthy volunteers: a randomized, double-blinded, placebo-controlled Phase 1 trial. Front Pharmacol. 2024;15:1359939. doi:10.3389/fphar.2024.1359939
22. Shah SJ, Voors AA, McMurray JJV, et al. Effect of Neladenoson Bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2019;321(21):2101–2112. doi:10.1001/jama.2019.6717
23. Voors AA, Bax JJ, Hernandez AF, et al. Safety and efficacy of the partial adenosine A1 receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced ejection fraction: a phase IIb, randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2019;21(11):1426–1433. doi:10.1002/ejhf.1591
24. Schöffski P. Importance and role of independent data monitoring committees (IDMCs) in oncology clinical trials. BMJ Open. 2021;11(10):e047294. doi:10.1136/bmjopen-2020-047294
25. National medical products administration: released the national adverse drug reaction surveillance annual report. 2021. Available from: http://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20220329161925106.html.
26. National medical products administration: announcement on the issuance of good management practices for drug clinical trials. Available from: https://www.gov.cn/gongbao/content/2020/content_5525106.htm.
27. Liu HM, Zhang LH. Clinical characteristics, management, and one-year outcome of patients hospitalized for acute heart failure in different regions of China. Chin Circul J. 2024;39(06):592–598. doi:10.3969/j.issn.1000-3614.2024.06.010
28. Zhou Y, Zhao K, Han J, et al. Geographical pattern evolution of health resources in China: spatio-temporal dynamics and spatial mismatch. Trop Med Infect Dis. 2022;7(10):292. doi:10.3390/tropicalmed7100292
29. Han W, Zhou Q, Wang MW. Current challenges and future perspectives of drug discovery in China. Expert Opin Drug Discov. 2025;19:1–10. doi:10.1080/17460441.2025.246829
30. Zhang Y, Xu SY, Tan GM. Unraveling the effects of DIP payment reform on inpatient healthcare: insights into impacts and challenges. BMC. Health Serv Res. 2024;24(1):887. doi:10.1186/s12913-024-11363-8
31. Sun D, Gao W, Hu H, et al. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B. 2022;12(7):3049–3062. doi:10.1016/j.apsb.2022.02.002
32. Alipour S, Nadimi Parashkouhi S, Mojahedian M, et al. Assessing drug lag in new drug approvals by the Iran food and drug administration compared to the U.S. FDA, EMA, and PMDA: a 20-year analysis (2001-2021). Medicine. 2024;103(25):e38142. doi:10.1097/MD.0000000000038142
33. Li QH, Chen YF. The operational mechanism of innovative drug policies in the United States and its implications for China. J Guangdong Pharm Univ. 2019;35(02):274–278+284. doi:10.16809/j.cnki.2096-3653.2018122607
34. Michaeli DT, Michaeli T, Albers S, et al. Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy. Eur. J Health Econ. 2024;25(6):979–997. doi:10.1007/s10198-023-01639-x
35. Lee H, Hwang S, Jang IJ, et al. Adaptive design clinical trials: current status by disease and trial phase in various perspectives. Transl Clin Pharmacol. 2023;31(4):202–216. doi:10.12793/tcp.2023.31.e21
36. Chen J, Di J, Daizadeh N, et al. Decentralized clinical trials in the era of real-world evidence: a statistical perspective. Clin Transl Sci. 2025;18(2):e70117. doi:10.1111/cts.70117
37. The China food and drug administration: announcement regarding the drug clinical trial information platform. Available from: https://www.jiangxi.gov.cn/art/2014/9/28/art_5292_324716.html.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



