Back to Journals » Cancer Management and Research » Volume 17
The Combination of D-TACE-HAIC, Lenvatinib, and PD-1 Inhibitors Shows Significant Clinical Efficacy in Patients with Unresectable Hepatocellular Carcinoma
Authors Wu Y, Zhu J, Zhang H, Xia N
Received 15 August 2024
Accepted for publication 9 January 2025
Published 6 February 2025 Volume 2025:17 Pages 239—247
DOI https://doi.org/10.2147/CMAR.S481242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yintao Wu,* Jianyong Zhu,* Hong Zhang, Nianxin Xia
Senior Department of Hepato-Pancreato-Biliary Surgery, the First Medical Center of PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nianxin Xia, Senior Department of Hepato-Pancreato-Biliary Surgery, the First Medical Center of PLA General Hospital, Beijing, 100853, People’s Republic of China, Email [email protected]
Purpose: This study was developed to compare the efficacy of combined D-TACE-HAIC + lenvatinib + PD-1 inhibitor treatment to that of TACE + sorafenib treatment for patients with intermediate and advanced HCC.
Patients and Methods: Here, a retrospective analysis of patients with unresectable HCC who underwent transarterial chemoembolization (TACE) from March 2018 to March 2022 at the our hospital was conducted. In total, 60 patients underwent treatment with drug-eluting beads-TACE-hepatic arterial infusion chemotherapy (D-TACE-HAIC) combined with lenvatinib and PD-1 inhibitors (Group A), while 21 underwent combined TACE and sorafenib treatment (Group B).
Results: In this study cohort, the rate of surgical conversion in Group A was significantly higher than that in Group B (33.3% vs 9.5%). As per the Revised Evaluation Criteria for Clinical Efficacy in Solid Tumors (mRECIST) criteria, the objective remission rate in Group A was significantly higher than that in Group B (86.6% vs 33.4%). Group A also exhibited significantly higher rates of overall adverse events including hypertension, abdominal pain, leukopenia, thrombocytopenia, and hypoproteinemia as compared to Group B, although the incidence of hand-foot syndrome in Group A was significantly reduced as compared to Group B (13.3% vs 42.8%). The median progression-free and overall survival (PFS and OS) of patients in Group A were 13.2 and 28.8 months, with both being significantly higher than the corresponding intervals in Group B (5.7 and 10.8 months, respectively). Cox multivariate analyses identified combination D-TACE-HAIC + lenvatinib+ PD-1 inhibitor treatment as being independently associated with patient PFS and OS.
Conclusion: In summary, D-TACE-HAIC + lenvatinib + PD-1 inhibitor treatment exhibits a favorable safety profile, outperforming TACE + sorafenib treatment for unresectable HCC patients while improving overall rates of translational efficacy, increasing rates of surgical conversion, prolonging patient survival, and conferring long-term survival benefits.
Keywords: unresectable hepatocellular carcinomas, drug-eluting beads-transarterial chemoembolization-hepatic arterial infusion chemotherapy, clinical efficacy, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third leading cause of tumor-related death worldwide. Based on the GLOBOCAN 2020 statistics, approximately 910,000 new cases are diagnosed with HCC annually and it is also claiming the lives of around 830,000 individuals each year.1 HCC remains the fourth most common and second deadliest malignancy in China, with the Chinese HCC incidence and mortality rates accounting for more than half of the global total, representing a major threat to public health.2 HCC tumors tend to be highly malignant, with an insidious onset lacking any signs or symptoms during the early stages of disease together with a tendency to progress rapidly. As a majority of patients are diagnosed with moderately advanced or advanced disease, just 20–30% are eligible for radical surgery. Patients may ultimately be unable to undergo surgery for reasons including the present or large lesions, multiple lesions, intrahepatic or extrahepatic metastases, hepatic insufficiency, a lack of sufficient residual liver volume, and/or vascular invasion.3–5 Per the HCC Diagnosis and Treatment Guidelines, various treatments, including interventional therapy, radiofrequency ablation therapy, radiotherapy, and systemic treatment can be deployed for patients with intermediate or advanced disease.6 Given the context of systemic therapy, patients are not only afforded extended survival advantages but also provided with the potential for down-staging conversion and the facilitation of sequential surgical procedures in the management of advanced hepatocellular carcinoma.
Transarterial chemoembolization (TACE) remains a standard approach to managing HCC in China, but in HCC patients exhibiting tumors more than 10 cm long, TACE tends to yield unsatisfactory efficacy as evidenced by a disease control rate of under 50% and a surgical conversion rate of roughly 10%.7–10 Hepatic arterial infusion chemotherapy (HAIC) together with an oxaliplatin plus fluorouracil and leucovorin (FOLFOX) regimen for HCC patients with locally advanced disease has been demonstrated to give rise to improved tumor response rates, including higher rates of conversion to surgery and a superior safety profile. Combining HAIC and targeted immunotherapy can thus be an efficacious and safe means of achieving high rates of surgical conversion in cases of locally advanced, potentially resectable HCC.11–15 At present, traditional monotherapeutic treatments tend to yield unsatisfactory efficacy such that a growing number of studies have been conducted in recent years highlighting the promising synergistic efficacy of various treatment combinations.
This study was developed to compare the efficacy of combined D-TACE-HAIC + lenvatinib + PD-1 inhibitor treatment to that of TACE + sorafenib treatment for patients with intermediate and advanced HCC. In the D-TACE-HAIC + lenvatinib + PD-1 inhibitor group, the rates of surgical conversion (33.3%) and objective remission (86.6%) were both higher than those in the TACE + sorafenib group, with patients in the former group exhibiting median PFS and OS intervals of 13.2 and 28.8 months, respectively. Cox multivariate analyses revealed that D-TACE-HAIC + lenvatinib + PD-1 inhibitor treatment was independently associated with patient OS and PFS.
Materials and Methods
Study Population
This study entailed the retrospective analysis of patients with unresectable HCC who underwent TACE between March 2018 and March 2022 at the senior Department of Hepato-Pancreato-Biliary Surgery, the First Medical Center of PLA General Hospital. In total, 60 patients underwent combination D-TACE-HAIC, lenvatinib, and PD-1 inhibitor treatment (Group A), while 21 underwent combination TACE and sorafenib treatment (Group B). The Medical Ethics Committee of the senior Department of Hepato-Pancreato-Biliary Surgery, the First Medical Center of PLA General Hospital approved this study, with all patients having provided written informed consent.
Patient Inclusion and Exclusion
Eligible patients were those individuals who (1) were diagnosed with HCC as per the Diagnostic and Therapeutic Guidelines for HCC of the China Healthcare Commission (2022 edition), or those with pathologically confirmed disease; (2) patients with stage Ib, IIa, or IIb disease who were not eligible for radical surgical treatment owing to severe cirrhosis or insufficient residual functional liver volume, or those with stage IIIa or IIIb disease; (3) patients with Child-Pugh classifications of A or B; (4) patients with an ECOG physical status of 0–1; and (5) patients with good organ function who were not receiving any antitumor targeted immunotherapies. Patients were excluded if they had received a pathologic diagnosis of fibrous platysmal or sarcomatoid HCC, exhibited disease with a cholangiocarcinoma component, had a Child-Pugh classification of C, were in poor general condition, exhibited serious cardiopulmonary disease, or were unable to tolerate targeted immunotherapy.
Therapy
Group A treatment: Patients < 60 kg and ≥ 60 kg were respectively treated with oral lenvatinib at doses of 8 mg/day and 12 mg/day. Patients were administered intravenous PD-1 inhibitors and underwent D-TACE-HAIC treatment every 3 weeks. D-TACE-HAIC was performed with the Seldinger technique, in which a percutaneous femoral artery was cannulated for abdominal arteriography, inserting a catheter to conduct arteriography in the celiac trunk and superior mesenteric artery. Based on the arterial blood supply for the target tumor, a microcatheter was inserted at the end of the tumor blood-supplying artery, with the peripheral and distal tumor blood supply then being embolized using drug-loaded microspheres that had been mixed with oxaliplatin (50 mg) without complete devascularization. After embolization, the microcatheter was allowed to remain in the main trunk of the tumor blood-supplying artery or the left/right hepatic artery, and aqueous heparin (10 mL; 1,000 U, diluted 1:1,000) was injected to protect against any microcatheter-associated coagulation. The area exposed to the catheter was covered using sterile medical gauze and secured to the skin of the groin and lower abdomen. This microcatheter was connected to a micro-pump which was used to continuously infuse oxaliplatin (85 mg/m2) for 2 h, calcium folinate (400 mg/m2) for 2h, 5-fluorouracil (400 mg/m2) for 15 min, and 5-fluorouracil (2400 mg/m2) for 46 h.
Group B treatment: Patients in Group B received oral sorafenib 400 mg twice per day and underwent TACE treatment every 3–4 weeks based on the physical condition and liver function of each patient. TACE was performed with the Seldinger technique, with percutaneous femoral artery puncture intubation to abdominal arteriography. After tumor vessels and blood-supplying arteries had been defined, pirorubicin (40 mg) emulsified with iodine oil (10–20 mL) was administered for vein chemoembolization.
Adverse Event Management
Drug-related adverse events (AEs) were assessed using the common terminology criteria for adverse events 5.0 (CTCAE 5.0). In cases where the side effects of lenvatinib or sorafenib were intolerable for patients, treatment was discontinued to await recovery, after which the corresponding drugs were administered as per relevant guidelines with delayed treatment or reduced dosages.16,17 Treatment was interrupted if patients exhibited tumor progression or experienced excessive toxic side effects.
Evaluation of Clinical Efficacy
Patients in both groups underwent routine blood testing, tumor marker analyses, and tests of liver and kidney function prior to each 3-week treatment cycle. Tumors were evaluated every two cycles via CT or MRI with enhancement. Portal and hepatic vein thrombosis staining was performed in accordance with the Japanese Liver Cancer Study Group.18 In cases where radical surgery or radiofrequency was feasible, PD-1 inhibitor treatment and lenvatinib treatment were respectively discontinued three and one weeks before surgery. Adjuvant therapy was initiated 2–3 weeks post-surgery, with the treatment regimen being selected as per the degree of pathological remission for a given patient. PD-1 inhibitor treatment alone was administered for 6 months when patients achieved a pathologic complete response (pCR). Treatment was continued for 12 months using the original regimen but discontinuing D-TACE-HAIC or TACE when patients did not achieve pCR. D-TACE-AIC or TACE were discontinued in cases of stenosis or occlusion of the artery supplying blood to the tumor as a result of repeated D-TACE-HAIC or TACE treatment, the loss of blood supply to the extrahepatic side branches, or loss of an intratumoral blood supply. Tumor responses were assessed as per the modified response evaluation criteria in solid tumor (mRECIST) criteria, and were classified into cases of complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). In addition, patient progression-free and overall survival (PFS and OS) as well as objective response rate (ORR) and disease control rate (DCR) were evaluated.19,20 Outpatient or telephone-based follow-up were performed through March 2023.
Statistical Analysis
Data were analyzed with SPSS 20.0. Categorical data are reported as cases (%) and were compared with chi-square and Fisher’s exact test. The Kaplan-Meier method was used for survival curve construction, while survival comparisons were made with the Log rank test. Cox regression models were used to identify factors associated with survival. P < 0.05 served as the significance threshold.
Results
Clinicopathological Characteristics
Of the patients enrolled in this study, 95.1% were HBsAg positive, while 66.7% exhibited alpha-fetoprotein (AFP) levels exceeding 400 ug/L (Table 1). Liver function tests indicated that the total bilirubin (TB) and glutamic pyruvic transaminase (ALT) levels were reduced in both groups (Table 1), with a significant decrease in albumin (ALB) levels. Imaging results suggested that portal vein tumor thrombosis was more common than hepatic vein tumor thrombosis in this study cohort.
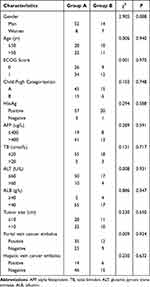 |
Table 1 The Clinicopathological Characteristics of the Enrolled Hepatocellular Carcinomas Patients |
Surgical Conversion
The median number of D-TACE-HAIC treatment cycles in Group A was 2 (2–40, while the median number of TACE cycles in Group B was 2 (1–3). Radical hepatectomy was successfully performed after treatment for 20 patients in Group A but just 2 patients in Group B. The rate of surgical conversion in Group A of 33.3% (20/60) was significantly higher than the Group B rate of 9.5% (2/21) (χ2=5.181, P < 0.05). Three patients in Group A and none in Group B achieved postoperative pathologic CR.
Clinical Efficacy
Per the mRECIST criteria, significantly higher PR and OR rates were observed for patients in Group A relative to Group B, whereas SD rates in Group A were significantly lower than in Group B (P < 0.05, Table 2). There was no significant difference between these groups in DCR.
 |
Table 2 Assessment of Clinical Efficacy of Hepatocellular Carcinoma Patients in Two Groups |
Adverse Reactions
With respect to overall AEs, rates of hypertension, abdominal pain, leukopenia, thrombocytopenia, and hypoproteinemia in Group A were significantly higher than those in Group B. With respect to Grade 3–4 AEs, abdominal pain, leukopenia, and thrombocytopenia rates in Group A were significantly higher than those in Group B (Table 3). There were no significant differences in the incidence rates for any other AEs between these groups, nor did either group exhibit any instances of treatment-associated death.
 |
Table 3 Adverse Reactions Statistics in Two Groups |
Prognosis
Enrolled patients were followed for a median 15.3 (range: 7.5–30.4) month interval. In total, 43 and 4 patients in Group A and Group B, respectively, survived. The median PFS in Group A was 13.2 months (95% CI: 10.32–15.34) as compared to 5.7 months (95% CI: 4.58–6.23) in Group B (Figure 1). The median OS of patients in Group A was 28.8 months (95% CI: 14.28–44.63) as compared to 10.8 months (95% CI: 9.32–12.37) in Group B (Figure 2). Significant differences were observed between these groups with respect to both PFS (χ2=31.324, P<0.05) and OS (χ2=67.349, P<0.05). In Cox multivariate analyses, combined treatment with D-TACE-HAIC, lenvatinib, and PD-1 inhibitors was independently associated with patient PFS and OS (HR=0.248, 0.487; p<0.05).
 |
Figure 1 HCC patient progression-free survival in the two study groups. |
 |
Figure 2 HCC patient overall survival in the two study groups. |
Discussion
Per current consensus guidelines, technically unresectable HCC (stage Ia, Ib, IIa) and oncologically unresectable HCC (stage IIb, IIIa) are classified as cases of junctional resectable HCC. Efforts to actively explore transformative preoperative treatments are encouraged for these patients with the aim of improving overall prognostic outcomes. In some patients with technically unresectable stage IIb and IIIa HCC, the combination of aggressive systemic and local treatment can improve overall surgical accessibility.21 Preoperative TACE and radiotherapy in cases of unresectable HCC can potentially lead to tumor downstaging, thereby providing patients with the opportunity to undergo surgery. In past studies, conventional TACE treatment strategies have been shown to be effective but suboptimal.22–25 The increasingly common application of targeted immunotherapies and combination D-TACE, HAIC, radiotherapy, and radiofrequency-based treatment strategies has contributed to increasingly encouraging treatment efficacy for HCC patients in the clinic.26,27 In HCC patients exhibiting a large tumor burden, an abundant tumor blood supply, and portal vein tumor thrombosis, D-TACE of the distal and peripheral tumor blood supply can provide an effective means of preserving the overall blood supply without complete devascularization. This approach, together with subsequent HAIC treatment, can significantly reduce the total chemotherapeutic drug doses used while prolonging the duration of efficacy for highly concentrated chemotherapeutic drugs, improving the overall therapeutic efficacy.28,29 Lenvatinib can also selectively bind to inhibit TACE-induced VEGF generation.30,31 Regulatory T cells (Tregs) are key immunosuppressive effectors, and TACE treatment can reduce the overall proportion of Tregs, thereby improving immune function and enhancing overall immunotherapeutic efficacy.9,32,33
The present results revealed that when treating cases of unresectable HCC, D-TACE-HAIC combined with lenvatinib and PD-1 significantly outperformed the combination of TACE and sorafenib with respect to the surgical conversion rate, median OS, PFS, and ORR of treated patients. In total, 20 patients from the combination D-TACE-HAIC group were able to undergo subsequent radical surgery, of whom 3 achieved postoperative pathologic CR. In contrast, just 2 of the patients from the combined TACE and sorafenib treatment group had the opportunity to undergo radical surgery, and neither achieved pathologic CR. Per the mRECIST criteria, the objective remission rate in the combination D-TACE-HAIC + lenvatinib + PD-1 inhibitor group was significantly higher than that in the TACE + sorafenib group (86.6% vs 33.4%). The D-TACE-HAIC + lenvatinib + PD-1 inhibitor regimen thus provides HCC patients with a greater potential for conversion to radical surgery. Even in cases when this combined regimen did not lead to radical surgical treatment, it did effectively arrest tumor progression, prolonging patient survival and increasing the odds of long-term survival such that the treated HCC patients can be regarded as having a chronic disease.
With respect to AEs, the combined D-TACE-HAIC + lenvatinib + PD-1 inhibitor regimen was associated with significantly higher rates of hypertension, abdominal pain, leukopenia, thrombocytopenia, and hypoproteinemia relative to TACE + sorafenib treatment, while the rate of hand-foot syndrome was significantly reduced relative to that in the TACE + sorafenib group. The D-TACE-HAIC + lenvatinib + PD-1 inhibitor group also presented with higher rates of grade 3–4 AEs including abdominal pain, leukopenia, and thrombocytopenia, while there were no significant differences between these two groups with respect to other grade 3–4 AEs. This difference is presumably related to the fact that the former regimen entails the combination of several different therapies, thereby incurring a greater risk of AE incidence. Fortunately, the majority of patients were able to tolerate these treatment-related AEs, with symptomatic treatment coinciding with significant reductions in tumor size and no instances of treatment-related mortality. The most common treatment-related AEs were leukopenia and thrombocytopenia, which were largely managed successfully through the administration of granulocyte-stimulating factor and thrombopoietin. In patients experiencing severe myelosuppression, the dose of chemotherapeutic drugs including oxaliplatin can be reduced as needed. Abdominal pain in both groups was primarily associated with vasospasm and necrosis following tumor embolization as a result of oxaliplatin infusion. These symptoms can be rapidly relieved by slowing or arresting the oxaliplatin infusion and administering appropriate analgesic or antispasmodic drugs. Rashes were primarily associated with oral lenvatinib and sorafenib administration, and were resolved in patients following dose reductions or suspension. Hypertension was primarily associated with oral lenvatinib administration and can be alleviated through symptomatic antihypertensive management. These results thus suggest that combining D-TACE-HAIC with lenvatinib and PD-1 inhibitors can effectively achieve high levels of translational efficacy without causing any serious immune-related AEs.
Conclusions
In summary, relative to combination TACE plus sorafenib treatment, the management of unresectable HCC using a combination of D-TACE-HAIC, lenvatinib, and PD-1 inhibitors is associated with a more favorable safety profile, shortening the cycle of translational therapy, improving overall translational therapeutic efficacy, increasing rates of surgical conversion, prolonging patient survival, and affording long-term survival benefits.
Ethical Statement
All patients submitted their informed consent before enrolment. This study was approved by the Medical Ethics Committee of the senior Department of Hepato-Pancreato-Biliary Surgery, the First Medical Center of PLA General Hospital. The research was performed following the World Medical Association Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Zheng RS, Zeng HM, Zhang SW, Chen WQ. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):66. doi:10.1186/s40880-017-0234-3
3. Li XH, Wei L. The comparison among the guidelines for the diagnosis and treatment of hepatocellular carcinoma in China, AASLD and EASL. Zhonghua Gan Zang Bing Za Zhi. 2019;27(3):236–240. doi:10.3760/cma.j.issn.1007-3418.2019.03.015
4. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308
5. Blanc JF, Debaillon-Vesque A, Roth G, et al. Hepatocellular carcinoma: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, AFEF, SIAD, SFR/FRI). Clin Res Hepatol Gastroenterol. 2021;45(2):101590. doi:10.1016/j.clinre.2020.101590
6. Zhou J, Sun HC, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
7. Liang L, Li C, Wang MD, et al. Development and validation of a novel online calculator for estimating survival benefit of adjuvant transcatheter arterial chemoembolization in patients undergoing surgery for hepatocellular carcinoma. J Hematol Oncol. 2021;14(1):165. doi:10.1186/s13045-021-01180-5
8. Xue TC, Le F, Chen RX, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32(3):64. doi:10.1007/s12032-015-0504-3
9. Ye TW, Wang DD, Lu WF, et al. Survival benefit of adjuvant transcatheter arterial chemoembolization for patients with hepatocellular carcinoma after anatomical hepatectomy. Expert Rev Gastroenterol Hepatol. 2023;17(4):395–403. doi:10.1080/17474124.2023.2192479
10. Yeh ML, Huang CI, Huang CF, et al. Neoadjuvant transcatheter arterial chemoembolization does not provide survival benefit compared to curative therapy alone in single hepatocellular carcinoma. Kaohsiung J Med Sci. 2015;31(2):77–82. doi:10.1016/j.kjms.2014.11.003
11. Guo JH, Liu SX, Gao S, et al. Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma. World J Gastroenterol. 2020;26(27):3975–3988. doi:10.3748/wjg.v26.i27.3975
12. Guo WB, Gao J, Zhuang WQ, Wu ZQ, Li B, Chen S. Efficacy and safety of hepatic arterial infusion chemotherapy combined with transarterial embolization for unresectable hepatocellular carcinoma: a propensity score-matching cohort study. JGH Open. 2020;4(3):477–483. doi:10.1002/jgh3.12285
13. Lyu N, Lin Y, Kong YN, et al. FOXAI: a Phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67(2):395–396. doi:10.1136/gutjnl-2017-314138
14. Lyu N, Kong YN, Mu LW, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
15. Chen SG, Zhang KZ, Liu WF, Yu WC. Hepatic arterial infusion of oxaliplatin plus raltitrexed in patients with intermediate and advanced stage hepatocellular carcinoma: a Phase II, single-arm, prospective study. Eur J Cancer. 2020;134:90–98. doi:10.1016/j.ejca.2020.03.032
16. Kudo M, Finn RS, Qin SK, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
17. Xie FH, Feng S, Sun LJ, Mao YL. The first-line treatment for unresectable hepatocellular carcinoma patients: lenvatinib versus sorafenib, or beyond? Hepatobiliary Surg Nutr. 2018;7(3):221–224. doi:10.21037/hbsn.2018.06.06
18. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi:10.1159/000327577
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
20. Takada J, Hidaka H, Nakazawa T, et al. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609. doi:10.1186/s13104-015-1565-2
21. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
22. Orlacchio A, Chegai F, Merolla S, et al. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol. 2015;7(12):1694–1700. doi:10.4254/wjh.v7.i12.1694
23. Nouso K, Miyahara K, Uchida D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Cancer. 2013;109(7):1904–1907. doi:10.1038/bjc.2013.542
24. Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi:10.1016/S2468-1253(18)30078-5
25. Kondo M, Morimoto M, Kobayashi S, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19(1):954. doi:10.1186/s12885-019-6198-8
26. Chong JU, Choi GH, Han DH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2018;25(11):3308–3315. doi:10.1245/s10434-018-6653-9
27. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. doi:10.1186/s40880-017-0251-2
28. He MK, Li QJ, Zou RH, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
29. He MK, Zou RH, Li QJ, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol. 2018;41(5):734–743. doi:10.1007/s00270-017-1874-z
30. Fu ZG, Li XW, Zhong JM, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi:10.1007/s12072-021-10184-9
31. Liao J, Xiao JW, Zhou YF, Liu ZL, Wang CH. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12(4):6065–6071. doi:10.3892/mmr.2015.4171
32. Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006;23(1):129–135. doi:10.1111/j.1365-2036.2006.02704.x
33. Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73(2):109–114. doi:10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

