Back to Journals » Journal of Pain Research » Volume 18
The CXCL12/CXCR4 Axis: An Emerging Therapeutic Target for Chronic Pain
Authors Chen Z, Xia Y, Liu B , Fang J, Hu Q
Received 13 December 2024
Accepted for publication 2 April 2025
Published 21 May 2025 Volume 2025:18 Pages 2583—2603
DOI https://doi.org/10.2147/JPR.S509541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor King Hei Stanley Lam
Zhangwei Chen, Yunfan Xia, Boyi Liu, Jianqiao Fang, Qimiao Hu
The Third School of Clinical Medicine (School of Rehabilitation Medicine), Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
Correspondence: Qimiao Hu, The Third School of Clinical Medicine (School of Rehabilitation Medicine), Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou, 310053, People’s Republic of China, Email [email protected]
Abstract: Chronic pain greatly affects patients’ quality of life and poses significant challenges for the healthcare system. Conventional medication is generally inadequate for managing chronic pain and frequently leads to numerous adverse effects. The chemokine C-X-C motif ligand 12 (CXCL12) and its receptor, the chemokine C-X-C motif receptor 4 (CXCR4), are emerging as significant neuromodulators within the nervous system. A growing body of evidence has underscored the critical roles of this chemokine axis in the development and persistence of pathological pain. In this review, we aim to synthesize recent findings that highlight the role and mechanisms of the CXCL12/CXCR4 axis in the etiology of chronic pain conditions. We focus on chronic pain stemming from sciatic nerve injury, diabetic neuropathy, spinal cord injury, bone cancer, opioid tolerance, and opioid-induced hyperalgesia. These conditions represent a diverse range of pathologies that underscore the broad impact of the CXCL12/CXCR4 axis in pain management. Furthermore, we discuss the potential for targeting the CXCL12/CXCR4 axis as a comprehensive therapeutic strategy for chronic pain. In this review, we aim to summarize emerging evidence on the critical role of the CXCL12/CXCR4 signaling in mediating chronic pain pathogenesis and its potential contributions to neurological disorders.
Keywords: CXCL12, CXCR4, chemokine, pain
Introduction
Chronic pain, including neuropathic, inflammatory, and cancer-related pain, is increasingly understood as a manifestation of neural plasticity, which occurs both in the peripheral nervous system (PNS), through peripheral sensitization, and in the central nervous system (CNS), through central sensitization. Recent studies have shown that chemokines and their receptors play a crucial role in the development of chronic pain by influencing glial activation and neural plasticity.1–3 Chemokines, a group of tiny cytokines, serve as attractors for the movement and placement of leukocytes by attaching to receptors found on the surface of specific cells.4–6 As of now, a total of 43 chemokines have been discovered, each attaching to a distinct receptor. The chemokine C-X-C motif ligand 12 (CXCL12), a chemokine belonging to the C-X-C subfamily, functions by binding to its specific receptor, the C-X-C motif receptor 4 (CXCR4). Initially identified and isolated from bone marrow stromal cells, CXCL12 was historically referred to as stromal cell-derived factor-1 (SDF-1). This chemokine plays a pivotal role in various physiological and pathological processes, including immune cell trafficking, tissue repair, and cancer metastasis.7 CXCR4, a G protein-coupled receptor (GPCR), is structurally similar to rhodopsin and has a unique affinity for CXCL12, meaning it exclusively binds to this chemokine. The high degree of homology between human and mouse CXCL12 at both the genomic and protein levels indeed makes mice an appropriate model for studying the role of CXCL12 in various pathological conditions.8 This homology ensures that the findings from mouse studies are likely to be relevant to human biology and disease. CXCL12 plays a multifaceted role in the nervous system. Under normal conditions, it is involved in neuromodulation and the intricate interactions between neurons and glial cells, which are crucial for maintaining neural health and function. Moreover, CXCL12’s involvement extends to the context of neurological disorders, particularly those associated with human immunodeficiency virus (HIV) infection.9–11 Over the past decade, pain research has experienced a substantial rise in publications focusing on the CXCL12/CXCR4 axis, highlighting its emerging role as a neuromodulator in chronic pain. Chemokines play an important role in central and peripheral sensitization by exerting chemotactic effect of neurons and glial cells. Numerous prestigious journals have featured reviews on this subject.12,13 After reading a lot of articles that passed the keyword search of (CXCL12) AND (pain), we made an outline of their contents. Then, we conducted a keyword search for different contents in each chapter, such as (CXCL12) AND (neuropathic pain), (CXCR4) AND (neuropathic pain); (CXCL12) AND (CFA), (CXCR4) AND (CFA). PubMed database was used to access relevant literature in the last 15 years (2009–2024). However, we also cite, for example, SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons from 2008, which describes in detail the mechanism of action of the CXCL12/CXCR4 signal axis, when beyond this time frame we feel that it is still constructive after careful reading. This makes an indelible contribution to our understanding of CXCL12/CXCR4 axis in the treatment of chronic pain. Consequently, this analysis delves into the healing possibilities of the CXCL12/CXCR4 pathway and the shift from acute to chronic pain studies, providing an extensive and up-to-date summary.
CXCL12/CXCR4 Axis and Its Activation Mechanism
CXCL12 has been acknowledged for its role in maintaining homeostasis, a function attributed to its engagement in key physiological activities. These activities include the development of the nervous system, the formation of the heart, the production of blood cells, the navigation of leukocytes to specific tissues, and the creation of new blood vessels. This chemokine plays a critical part in the directed movement and stimulation of hematopoietic stem and progenitor cells, the cells that line the blood vessels, and a diverse range of leukocytes.1 Unlike the majority of receptors that bind to inflammatory chemokines, the CXCR4 receptor is unique in that it exclusively pairs with the CXCL12 ligand. The CXCR4 was first identified due to its role in the entry mechanism of HIV and in the trafficking of leukocytes. It was later characterized as a seven-transmembrane domain receptor derived from leukocytes, also known as LESTR.14 Goazigo et al have reported that the CXCR4 receptors are primarily located on neuronal plasma membranes, occupying both presynaptic and postsynaptic positions in the terminals of the central nervous system. This distribution suggests a significant role for CXCR4 in neuronal communication.15 When CXCL12 engages with CXCR4, it predominantly triggers G protein-mediated signal transduction. This process commences with the separation of the Gβγ and Gα subunits from the intracellular domains of CXCR4, thereby initiating a cascade of cellular responses.16,17 The interaction between CXCL12 and CXCR4 activates a series of G protein-coupled signaling kinases. Specifically, this binding event initiates the phosphorylation and subsequent activation of kinases including PI3K/mTOR and MEK/ERK, which play crucial roles in cellular signaling pathways.16 The binding of CXCL12 to the extracellular region of CXCR4 stimulates the generation of cyclic AMP (cAMP) by prompting the Gα subunit to exchange its bound GDP for GTP. This exchange leads to the separation of the Gαi and Gβγ subunits, initiating a signaling cascade that influences gene expression through the activation of various downstream effectors. These include key signaling molecules such as protein kinase B (Akt), c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase kinase (MEK), and extracellular signal-regulated kinase-1/2 (ERK1/2). Concurrently, the family of regulator of G protein signaling (RGS) proteins offers an alternative pathway to modulate the CXCR4 response to CXCL12 binding, ensuring the fine-tuning of this signaling axis18,19 (Figure 1). The RGS proteins interact with the Gα subunit of the CXCR4 receptor, enhancing its GTPase activity, which in turn accelerates the hydrolysis of GTP to GDP. This increase in GTPase activity results in the inhibition of further signaling cascades and chemotactic responses. Notably, this regulatory effect is significant regardless of the high levels of CXCR4 expression, demonstrating a critical role for RGS proteins in modulating the receptor’s activity.20 The CXCR4 protein is involved in a dynamic process of membrane trafficking and expression regulation, leading to higher concentrations of the protein inside the cell compared to the cell surface. This intracellular retention suggests a complex mechanism that affects the receptor’s availability for signaling. Moreover, accumulating research indicates that the CXCL12/CXCR4 signaling pathway is often disrupted in chronic pain states, contributing to the development and persistence of such conditions. This dysregulation underscores the potential significance of the CXCL12/CXCR4 axis in the pathophysiology of chronic pain, highlighting it as a crucial area for further investigation and therapeutic intervention.13
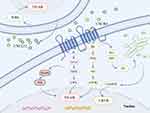 |
Figure 1 CXCL12 is produced in response to inflammation or damage, which activates CXCR4. The primary mechanism by which this process starts G-protein-mediated signal transduction is the separation of the Gβy and Gα subunits attached to the intracellular ring of CXCR4. Subsequent dissociation of the Gαi and Gβγ subunits enables them to activate different downstream signaling pathways. Pain processes can be alleviated by controlling ion channel activity and the synthesis of inflammatory mediators. Created in BioRender. kaisi, y. (2025) https://BioRender.com/bgsnsow. |
CXCL12/CXCR4 Axis in Chronic Pain
CXCL12/CXCR4 Axis in Neuropathic Pain
Neuropathic pain typically arises from damage or disorders of the somatosensory system, which can occur either within the PNS or the CNS. This type of pain is often a result of insults or dysfunctions at these levels, leading to altered sensory processing and the experience of pain.21 A systematic review indicates that the average incidence of neuropathic pain among adults is 7%. This condition notably diminishes the quality of life and disrupts daily activities and employment due to the chronic and often debilitating nature of the pain.22 Growing research suggests that the heightened responsiveness of dorsal horn neurons (DRG) is crucial in the process of neuropathic pain. Dorsal horn neurons’ heightened excitability could stem from the triggering of signaling routes following injury to peripheral nerves. To date, multiple research works have indicated the involvement of the CXCL12/CXCR4 axis in neuropathic pain, a topic we will elaborate on subsequently.
CXCL12/CXCR4 Axis in Nerve Injury-Induced Neuropathic Pain
Research utilizing the spared nerve injury (SNI) model demonstrated a sustained elevation in the levels of classical monocytes and CXCL12 in the bloodstream following SNI. Furthermore, the effects of SNI were replicated in naive mice through the intravenous administration of CXCL12 at concentrations that are considered pathological, indicating a role for this chemokine in mediating the responses associated with nerve injury.23
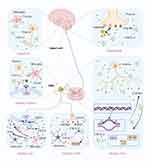 |
Figure 2 In DRG, the activation of TNF-α, ERK, NF-κB, TLR4 and Nav1.8 are mainly expressed in glia and astrocytes, which contribute to CXCL12/CXCR4 signal transduction, and the expression of TLR4 is also increased in microglia.24–28 These conditions may be influenced by neuronal expression of CXCR4, which initiates glial-neuron crosstalk. Created in BioRender. kaisi, y. (2025) https://BioRender.com/30t5cmp. |
Following SNI, there is an upregulation of both CXCL12 and its corresponding receptor, CXCR4, within the neurons and satellite glial cells of the lumbar DRG. This suggests that the CXCL12/CXCR4 axis may play a significant role in the neural and glial responses to nerve injury in the lumbar region.15 SNI has been observed to cause a prolonged increase in the levels of CXCL12 and CXCR4 within the dorsal horn of the ipsilateral L4-5 spinal cord. This upregulation is characterized by the presence of CXCL12 in both neuronal and microglial cells, while CXCR4 is found in neuronal and astrocytic populations. Moreover, SNI triggers an enduring increase in the expression of tumor necrosis factor (TNF)-α in the DRG and spinal cord, indicating a significant inflammatory response and the potential involvement of TNF-α in the pathogenesis of neuropathic pain following SNI28 (Figure 2). The administration of thalidomide via intraperitoneal injection, which inhibits the production of TNF-α, has been shown to alleviate mechanical hypersensitivity induced by spared nerve injury (SNI) and to suppress the expression of CXCL12 in DRG neurons and the spinal cord. Furthermore, the application of AMD3100, a specific antagonist of CXCR4, either intragastrically (ig) or intraperitoneally (ip) on day 8 post-SNI, has effectively reduced abnormal pain behaviors in rats. Additionally, the repeated use of AMD3100 via the intrathecal (i.t). Route has been found to prevent the activation of extracellular signal-regulated kinase (ERK) in the spinal cord. Interestingly, pre-treating naive rats with MEK inhibitor PD98059 has also been demonstrated to lessen mechanical hypersensitivity that was induced by the intrathecal administration of CXCL12, highlighting the potential of these treatments in modulating pain signaling pathways.28 Collectively, the collective research indicates that tumor necrosis factor-alpha (TNF-α) might play a role in increasing the expression of CXCL12 in the DRG and spinal cord following spared nerve injury (SNI). Furthermore, the signaling pathway involving CXCL12 and its receptor CXCR4, which is activated by extracellular signal-regulated kinase (ERK), appears to be involved in the onset and persistence of neuropathic pain. Consequently, targeting the CXCL12/CXCR4 axis could present a promising therapeutic approach for neuropathic pain management. Notably, AMD3100, a selective CXCR4 antagonist, has demonstrated the ability to block the interaction between CXCR4 and its ligand CXCL12, offering a potential avenue for treatment by inhibiting the pro-nociceptive effects of this signaling axis.29 Additional studies have elucidated the mechanisms by which AMD3100, a specific antagonist of CXCR4, can provide analgesic effects in neuropathic pain conditions. Research indicates that AMD3100 not only alleviates pain but also restores the function of inhibitory neurotransmission, such as that mediated by Glycine Receptor Alpha-3 (GlyRα3). This restoration is significant as it helps to counteract the hyperexcitability often associated with neuropathic pain, offering a more comprehensive therapeutic effect. By targeting the CXCR4 receptor and modulating neurotransmission, AMD3100 has the potential to address both the symptoms and underlying pathophysiology of neuropathic pain.24 Li et al’s research has revealed that AMD3100, a CXCR4 antagonist, can provide partial relief from neuropathic pain through a novel mechanism. They found that AMD3100 increases the secretion of endogenous opioids derived from leukocytes, which may contribute to its analgesic effects. Additionally, the early and repeated administration of AMD3100 resulted in a dose-dependent alleviation of neuropathic pain, suggesting that the chemokine CXCL12 acts through its receptor to mediate chronic neuropathic pain (Table 1). Therefore, targeting the CXCL12/CXCR4 pathway may represent an effective approach for treating this condition.25 A recent study has identified that neurons in the DRG expressing the proto-oncogene PIM1 also exhibit colocalization with markers of nociceptive neurons, including calcitonin gene-related peptide (CGRP), isolectin B4 (IB4), and substance P, with an increase in expression post-SNL surgery. PIM1, a serine/threonine protein kinase belonging to the PIM family, plays a role in various cellular processes. Notably, PIM1 has been found to modulate the signal transduction of the CXCL12/CXCR4 pathway in neuroblastoma cells (neuro-2a). This modulation is achieved by regulating the phosphorylation of CXCR4 at serine 339 (pCXCR4), which may have implications for the signaling mechanisms involved in neuropathic pain and the potential therapeutic targets within the CXCL12/CXCR4 axis.30
 |
Table 1 Application of AMD3100 for Treating Different Pain Conditions |
Within DRG tissues, the study identified a co-expression pattern of PIM1 and CXCR4. The study’s findings indicated that when PIM1 was deleted, there was a notable decrease in the expression of pCXCR4(ser 339), while the overall expression levels of CXCR4 protein remained largely unaffected following spinal nerve ligation (SNL) surgery. This suggests that PIM1 may play a specific role in the phosphorylation of CXCR4, potentially influencing the activation state of the receptor without altering the total CXCR4 protein levels, which could have implications for the understanding of pain signaling and the role of these proteins in neuropathic pain conditions.30 The study’s results imply that PIM1 plays a role in mitigating the heightened sensitivity to pain (nociceptive hypersensitivity) that can result from nerve injury. This is achieved by PIM1’s inhibitory effect on the signal transduction processes within the CXCL12/CXCR4 pathway, which is integral to pain signaling. Furthermore, the alterations in the CXCL12/CXCR4 axis are not confined to the dorsal root ganglia (DRGs) that are directly related to the damaged nerves; the influence extends to DRGs that are not in direct association with the injured nerves. This broader impact indicates that the CXCL12/CXCR4 pathway may have a more extensive role in the body’s response to nerve injury and the resulting pain, potentially affecting a wider neural network than previously considered.45 Based on these findings, it is reasonable to hypothesize that modulating the CXCL12/CXCR4 pathway could emerge as a paramount strategy for managing neuropathic pain in future therapeutic approaches. The potential of this pathway as a target for intervention is underscored by its involvement in pain signaling and the broader implications for DRGs, both affected and unaffected by direct nerve injury. This speculation suggests that future research and clinical development may focus on harnessing the CXCL12/CXCR4 axis to alleviate neuropathic pain effectively.
During the early stages of a thalamic hemorrhage state, CXCL12/CXCR4 signaling up-regulates central post-stroke pain (CPSP), a refractory central neuropathic pain. CXCL12 and its receptor CXCR4 may be a potentially attractive molecular target for therapeutic therapy of CPSP, although in the latter phase, they are more crucial in the microglia-astrocytome-neuron connection mediated by hypoxia-inducing factor 1α (HIF-1α).42 In the context of the chronic constriction injury (CCI) model of neuropathic pain, an increase in the expression levels of CXCL12, CXCR4, TXNIP, components of the NLRP3 inflammasome, IL-1β, and IL-18 was observed in neurons and glial cells of the ipsilateral spinal dorsal horn (SDH) on day 7 post-CCI. This enhancement in protein expression was mitigated following treatment with an unspecified ‘login’ therapy. Additionally, Dong et al found that in CCI rats, the microRNA miR-130a-5p was downregulated, leading to overexpression of MEG3, CXCL12, and CXCR4. The overexpression of MEG3 was associated with exacerbated neuropathic pain and increased levels of pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6. This indicates that MEG3 may play a role in intensifying neuropathic pain in CCI rats via the miR-130a-5p/CXCL12/CXCR4 pathway, suggesting a complex interplay between these molecules in the modulation of pain and inflammation in this model of neuropathy.54
In a model of neuropathic pain induced by the chronic compression of the dorsal root ganglion (CCD), the expression of CXCL12 and its receptor CXCR4 was found to be increased within the DRG. This upregulation occurred in a subset of neurons that also tested positive for nociceptive markers such as IB4, TRPV1, CGRP, and Substance P. The heightened sensitivity to mechanical and thermal stimuli, known as hypernociception, which is a characteristic of neuropathic pain following CCD, was reduced with the administration of the CXCR4 antagonist AMD3100. This suggests that the CXCL12/CXCR4 signaling axis plays a significant role in mediating hypernociception in this neuropathic pain model and that targeting CXCR4 with antagonists like AMD3100 may offer a therapeutic strategy to alleviate neuropathic pain symptoms.55 This finding’s results suggest that the CXCL12/CXCR4 pathway is crucial in hypernociception post-chronic constriction injury (CCD), and focusing on this pathway could aid in reducing neuropathic discomfort. The chronic and acute neuropathic pain linked to HIV significantly deteriorates life quality. Inhibiting the CXCR4/SDF1 pathway within the L4/5 dorsal root ganglia (DRG) and spinal dorsal horn, along with blocking the expression of the pro-inflammatory cytokine TNF-α, can mitigate HIV-associated neuropathic pain.47
After spinal nerve ligation (SNL),neurons predominantly showed elevated levels of CXCL12 expression, whereas CXCR4 was present in both spinal dorsal horn astrocytes and neurons.Moreover, the CXCL12 peptide in rats elevated the mRNA quantities of c-Fos, GFAP, and iba-1.In a rat model of SNL, either single or consecutive use of CXCL12 neutralizing antibody inhibited the occurrence and duration of neuropathic pain through the reduction of GFAP and iba-1 levels in the spinal cord’s dorsal horn.31
In a study examining the effects of partial sciatic nerve ligation (pSNL) on mice, the spinal cords were analyzed for microRNAs (miRNAs), CXCR4, and associated downstream signaling molecules. It was observed that the expression of CXCR4 in spinal cord glial cells increased in mice subjected to pSNL, which corresponded to an exacerbation of pain behavior. Intriguingly, miR-23a, a specific microRNA, was found to bind directly to the 3’ untranslated region (UTR) of the CXCR4 mRNA, suggesting a regulatory role for miR-23a in CXCR4 expression. Notably, pSNL-induced neuropathic pain was associated with a significant decrease in miR-23a mRNA levels. Conversely, the intrathecal administration of miR-23a inhibitors, which reduced miR-23a levels, induced pain-like behaviors in mice, while the inhibition of CXCR4 lessened such behaviors. These findings highlight the intricate relationship between miR-23a, CXCR4, and the manifestation of neuropathic pain, indicating that miR-23a may modulate pain sensitivity by targeting CXCR4 in the spinal cord, and thus could be a potential therapeutic target for the treatment of neuropathic pain.56 Indeed, the data suggest that miR-23a plays a regulatory role in neuropathic pain by directly targeting CXCR4 in spinal cord glial cells. This mechanism implies that miR-23a could be a key modulator of the CXCL12/CXCR4 signaling pathway, which is implicated in the pathogenesis of neuropathic pain. Furthermore, the potential to intervene epigenetically by targeting CXCR4 offers a novel strategy for developing treatments aimed at reducing nociceptive hypersensitivity that results from peripheral nerve injury. By modulating the expression or activity of CXCR4, it may be possible to alleviate the symptoms of neuropathic pain, providing a new direction for therapeutic interventions in this area.
Painful diabetic neuropathy (PDN), a complex and challenging complication associated with diabetes, is marked by neuropathic pain. In a study utilizing Wistar rats, PDN was induced through the intraperitoneal injection of streptozotocin (STZ). Notably, the administration of AMD3100, a CXCR4 antagonist, was found to alleviate hypersensitivity in these diabetic rats within two hours of treatment. Concurrently, the study observed elevated levels of interleukin-6 (IL-6) and intracellular calcium ([Ca2+]i) in the spinal synaptosomes of the diabetic rats. The therapeutic intervention with AMD3100 not only reduced hypersensitivity but also decreased the elevated IL-6 and calcium influx in the synaptosomes of diabetic rats. This suggests that the CXCR4/CXCL12 axis may modulate PDN, potentially through the involvement of voltage-dependent calcium channels, indicating a complex interplay between these signaling elements in the pathophysiology of PDN. These findings point to the possibility that targeting the CXCR4/CXCL12 interaction could offer a novel therapeutic strategy for managing PDN, highlighting the importance of further investigating the role of these molecules in the development and maintenance of neuropathic pain associated with diabetes.40 The outcomes aligned with the key discoveries made by Jayaraj et al.57 Research has pinpointed the excitatory signaling of CXCR4/CXCL12 within neurons of the dorsal root ganglion (DRG) that express the sodium channel NAV1.8 as a significant contributor to the pathophysiology of small-fiber degeneration and mechanical pain in mouse models of painful diabetic neuropathy (PDN). By curbing CXCR4 signaling or the excitability of these neurons, it is possible to prevent and ameliorate issues associated with mechanical pain. This insight underscores the potential therapeutic value of interventions that target the CXCR4 pathway to manage mechanical pain in PDN, offering a promising direction for developing treatments that could alleviate a key symptom of this prevalent complication of diabetes.
Interestingly, by blocking the CXCL12/CXCR4 signaling pathway, not only the neuralgia but also the itch can be alleviated. The research revealed that inhibiting the CXCL12/CXCR4 signaling route reduced the levels of CXCL12 and CXCR4 in trigeminal ganglion neurons, which deteriorated post-skin injury, aiding in alleviating itching and pain linked to allergic contact dermatitis.39
Therefore, these studies suggest that high expression of CXCL12/CXCR4 may potentially play a role in nerve injury-induced neuropathic pain and inflammation.
CXCL12/CXCR4 Axis in Complex Regional Pain Syndrome Type-I (CRPS-I)
Complex Regional Pain Syndrome Type I (CRPS-I) is a chronic neurological condition that predominantly impacts the limbs of individuals. Often triggered by trauma, fractures, nerve damage, or ischemia following an initial harmful event, CRPS-I is characterized by a range of symptoms including swelling, alterations in blood flow to the skin, and heightened sensitivity to both thermal and mechanical stimuli in the affected region, manifesting as hyperalgesia or allodynia. This syndrome presents a significant challenge in pain management due to its complex and varied clinical presentation.58 Therapies for CRPS-I encompass a range of strategies, such as physical therapy, interventions to block the sympathetic nervous system, the use of corticosteroid drugs, and medications classified as non-steroidal anti-inflammatory agents.59 Regrettably, the efficacy of the aforementioned treatment approaches for CRPS-I has been found to be inadequate, which complicates the clinical handling of pain-related conditions. Research utilizing the Chronic post-ischemia pain (CPIP) model in adult animals aims to replicate the early stages of hyperemia and edema, followed by ongoing manifestations of neuropathic-like pain. This includes symptoms such as continuous pain, enduring sensitivity to mechanical stimuli, and increased reactivity to temperature changes.58,60 Such manifestations are characteristic of CRPS-I as observed in human patients.
In an effort to gain further insights into the mechanisms underlying CRPS-I, our recent study focused on examining the alterations in gene expression within the ipsilateral DRG and the spinal cord dorsal horn (SCDH) in a CPIP model of rats. This analysis was conducted utilizing RNA sequencing techniques.61 Our investigation has uncovered a heightened expression of the CXCL12/CXCR4 axis within the SCDH of rats in the CPIP model. This upregulation is attributed to the modification of spinal neuronal and glial cell activation in the SCDH, coupled with the subsequent triggering of the ERK signaling pathway. The pharmacological blockade of CXCR4, facilitated by the intrathecal delivery of AMD3100—a specific antagonist for CXCR4—resulted in a reduction of c-Fos-positively stained cells, a decrease in glial cell activation in the ipsilateral SCDH, and an alleviation of mechanical allodynia in the CPIP model rats. These findings suggest that targeting the CXCL12/CXCR4 pathway could be a promising therapeutic strategy for managing CRPS-I symptoms26 (Figure 2). Consequently, our findings suggest that targeting the CXCL12/CXCR4 axis and the downstream ERK pathway within the SCDH could present a novel therapeutic approach for the management of CRPS-I. This intervention may offer a new direction for treating the symptoms associated with CRPS-I by modulating the molecular pathways that contribute to the syndrome’s pathophysiology.
CXCL12/CXCR4 Axis in Chemotherapy-Induced Peripheral Neuropathy (CIPN)
CIPN is one of the most common side effects caused by chemotherapeutic drugs.62 Primary manifestations include discomfort and numbness in the feet and hands, a result of paclitaxel build-up in the dorsal root ganglia. Beyond the pain associated with it, CIPN is marked by a reduction in the ability to sense vibration, a symptom linked to the effects on large-diameter sensory neurons. This condition is also accompanied by dysesthesia and an increased sensitivity to cold and mechanical stimuli, which can severely affect a patient’s quality of life. The use of Paclitaxel in treatment protocols has been observed to increase the expression of CXCR4 and RAGE receptors in the DRG. Interestingly, the allodynia induced by Paclitaxel can be prevented through the use of CXCR4 and RAGE antagonists, highlighting a potential therapeutic strategy for managing CIPN symptoms.22,62
The latest research has shown that the systemic delivery of paclitaxel triggers an increase in the expression of glial fibrillary acidic protein (GFAP), a marker indicative of astrocyte activation. Furthermore, paclitaxel has been found to induce astrocyte activation in vitro, leading to a significant rise in the levels of TNF-α and SDF-1 proteins. The activation response and subsequent increase in these proteins were substantially reduced by treating with neutralizing antibodies specific to TNF-α and SDF-1, demonstrating a potential method to counteract the activation of astrocytes and the associated inflammatory response triggered by paclitaxel.63 In line with these findings, pharmacological inhibition of CXCL12 signaling in vivo has been shown to reduce the onset of analgesic tolerance and hyperalgesia induced by paclitaxel in rats. This suggests that targeting the CXCL12 pathway could be a viable strategy to mitigate the pain-related side effects associated with paclitaxel treatment.22 Conversely, a study by Ransohoff RM et al demonstrated a link between paclitaxel-induced hyperalgesia and the activation of the NF-κB pathway by TNF-α, which is triggered by CXCL12. This activation can lead to the sustained production of pro-inflammatory cytokines, contributing to the development and progression of hyperalgesia. This finding highlights the complex interplay between chemokines, cytokines, and their signaling pathways in the context of chemotherapy-induced pain, and underscores the potential therapeutic relevance of modulating these interactions to manage paclitaxel-induced hyperalgesia.64
Another CIPN model, triggered by another anti-tubulin chemotherapy drugs vincristine, showed a notable rise in CXCL12 expression within the spinal cord. Additional findings from Western blotting revealed vincristine’s ability to alter the acetylation level of histones in the CXCL12 promoter area. Crucially, the impact of vincristine on the acetylation of H4 was reduced in rats with the application of AAV-Cre-GFP–injected transcription activator 3 (STAT3)flox/flox mice.22 These results indicated that anti-tubulin chemotherapeutic agents enhanced histone acetylation at the CXCL12 promoter through STAT3 signaling.
Furthermore, in an animal model of oxaliplatin-induced CIPN, there was an observed increase in the expression of CXCL12, TNF-α, and IL-1β within the dorsal root ganglia. Notably, the research also revealed that the administration of an anti-CXCL12 neutralizing antibody or CXCL12 small interfering RNA (siRNA) intrathecally led to a reduction in mechanical allodynia and thermal hyperalgesia. These findings underscore the unparalleled significance of the CXCL12/CXCR4 axis in the context of CIPN, indicating that interventions targeting this axis may hold substantial promise for alleviating the painful symptoms associated with this condition.65 Of interest, immunohistochemical results manifested that CXCL12 was only expressed in IB4- and nf-200 positive cells, and surprisingly, it was not expressed in GFAP-positive cells, respectively, in a groundbreaking study, researchers discovered that the administration of an anti-CXCL12 neutralizing antibody for 10 days effectively reduced the development of mechanical allodynia and thermal hyperalgesia caused by oxaliplatin treatment. This finding highlights the crucial role of CXCL12 in neuropathic pain associated with CIPN. By targeting CXCL12, we may have found a promising avenue for alleviating the debilitating symptoms of CIPN and improving the quality of life for cancer patients undergoing chemotherapy. Thus, targeting peripheral and central CXCL12 neuronal-glial interactions, in addition to previously recognized inflammatory cytokines and combating oxidative stress, also might be a prognostic biomarker and a promising target for chemotherapy-induced persistent pain.
CXCL12/CXCR4 Axis in Inflammatory Pain
An increasing body of research suggests that chemokines play a crucial role in the genesis of different types of pain, including inflammatory, postoperative, and chronic pancreatitis-related pain. Their involvement is linked to the ability of chemokines to trigger the production and release of a range of inflammatory factors. These factors encompass TNF-α, nuclear factor kappa B (NF-κB), IL-6, and other pro-inflammatory cytokines, along with other related molecules, which contribute to the complex pathophysiology of pain conditions.27,66,67 Additionally, these substances serve to heighten the mechanical sensitivity of peripheral nociceptors, thereby inducing and mediating inflammatory pain.
Complete Freund’s Adjuvant (CFA)-Induced Inflammatory Pain
In the realm of CFA-induced inflammatory pain, the dynamic interplay between various inflammatory elements, such as IL-2 and TNF-α, intricately governs the activation of CXCL12/CXCR4. This pivotal axis serves as a master regulator, deftly orchestrating signaling cascades like NF-κB, ERK, and PI3K to fine-tune the intricate machinery of pain perception.41 Due to the promoter characteristics of the CXCR4 gene in CFA-induced inflammatory rats, DNA demethylation is accelerated, leading to upregulation of CXCR4 expression and increased recruitment of NF-κB p65.44 This insight sheds light on the mechanism behind the increased expression of CXCR4 in inflammatory pain, indicating that targeting CXCR4 down-regulation could be a potent strategy for managing such pain. By employing methylation of the CXCR4 gene promoter region in DRGs, it may be possible to suppress the gene expression, thereby affecting the pain signals. Recently, Yang et al have provided pioneering evidence that supports the notion that the activation of ERK and PI3K-AKT signaling pathways in primary afferent neurons is facilitated through peripheral CXCR4 upon the injection of stromal cell-derived factor 1 (SDF1) into the plantar region. This research highlights the intricate relationship between chemokine signaling and pain perception, offering new avenues for therapeutic intervention in inflammatory pain conditions.68
Specifically, they demonstrated that SDF1-CXCR4 signaling plays a pivotal role in the development of primary mechanical hyperalgesia, thereby contributing to the shift from acute to chronic pain states. By modulating protein translation via the ERK and PI3K-AKT pathways, the SDF1-CXCR4 signaling pathway facilitates this transition and ultimately contributes to the establishment and persistence of chronic pain. Remarkably, the continuous knock-down of CXCR4 prior to stimulation effectively impedes the development of mechanical hyperalgesia and can even reverse the established chronic inflammatory pain (Figure 2). The studies in question have provided strong evidence that the intraplantar injection of SDF1 triggers the concurrent activation of both the ERK and PI3K-AKT signaling pathways within primary afferent neurons, mediated through peripheral CXCR4. This dual activation underscores the significant role of CXCR4 signaling in the transition from acute to chronic pain, particularly in the context of primary mechanical hyperalgesia. Consequently, these findings suggest that modulating CXCR4 signaling could be a viable approach to influence this transition and potentially offer therapeutic benefits in managing pain that becomes chronic.
Chronic Pancreatitis (CP) Pain
CP is a chronic inflammatory and fibrotic disorder of the pancreas. Pain is the most commonly symptom in CP, which involves in the metabolism of many inflammatory factors. Pancreatic stellate cell (PMC) is the target factors in chronic pancreatitis pain. The activation of PSC promotes pancreatic stellate cell promotes the expression of cytokine and production of extracellular matrix proteins, triggers a series of local pro-inflammatory cytokines be released, such as TNF-α, IL-1, and IL-6, leads to chronic inflammation, collagen deposition, and fibrosis in the progression results in chronic pancreatitis.68
There is growing evidence that pancreatitis-induced inflammation and pain response depend on TLR4 release.55 Glial TLR4 has been shown to play a vital role in the evolution of pain processing by generating sorts of inflammatory mediators, especially the chemokines CXCL12, which influent the development and maintenance of inflammatory pain.69 Blocking the TLR4 signaling pathway could attenuate the severity of pancreatitis, reduce the acinar cells necrosis and inflammatory response in pancreatitis.29 In a study on chronic pancreatitis-induced mechanical allodynia, researchers observed a significant upregulation of TLR4 in astrocytes located in the spinal dorsal horn. This finding shed light on the potential role of TLR4 in mediating pain sensitivity in this particular model. The heightened expression of TLR4 in astrocytes suggests a possible involvement of neuroinflammation in the development of chronic pain conditions. Further investigation into the mechanisms underlying this phenomenon could lead to novel therapeutic targets for managing chronic pain associated with pancreatitis.24 Research now shows that when TLR4 overlaps with CXCL12 and CXCR4, it kicks off a positive feedback loop between astrocytes and microglia.25 Interestingly, jabbing neutralizing antibodies against CXCL12 keeps IL-1β and TNF-α whispers-low. This hints that under CP conditions, targeting TLR4 might be the golden ticket to dialing down the overactive CXCL2/CXCR4 system.
On top of that, using trinitrobenzene sulfonic acid (TNBS) injections to stir up chronic pain in the pancreas lights up DRG neurons like a Christmas tree and cranks up their CXCL12 and CXCR pathways.66
ERK is a key nociceptive signal which enables and mediates neuron sensitization in different pain conditions and is prone to be activated by some chemokine receptors.67 Since in a CP rat model by intrathecal injection CXCR4 inhibitor, shows much-reversed hyperexcitability of DRG neurons and p-ERK in the DRGs as well as downregulating Nav1.8 sodium channels in DRGs.27 Hence, the initiation of CXCL12-CXCR4 signaling triggers an ERK-dependent amplification of Nav1.8 expression, thereby sustaining DRG neuronal hyperexcitability and ongoing hypersensitivity to pancreatic pain in the context of CP.41 These studies and tests that inhibition of CXCL12 pathway in the initial stage of pain can inhibit the occurrence and development of CP inflammatory pain, which provide support in facilitating this method to get to the clinical transformation stage.
At present, therapies targeting against CXCR4 expression has been extensively explored as a prospective strategy for relieving inflammation and directing CP treatment, which could inhibit inflammatory factors release to ameliorate the effects of fibrosis in pancreatic tissue, although exact immunological and neurological mechanisms of CP is not exactly known, but its effectiveness in anti-inflammatory and anti-stromal therapeutic approaches are of crucial importance.70 Therefore, targeting TLR4 and CXCR4 may offer notably therapeutic options for CP. At the same time, although these findings do not mention CXCL12, it is important to know that CXCR4 only has CXCL12 as a ligand.
CXCL12/CXCR4 Axis in Cancer Related Pain
Pain associated with cancer is a prevalent and challenging symptom, with over 70% of cancer patients experiencing it. This type of pain ranks among the most frequently reported issues, significantly impacting the well-being of those battling the disease.71 Even with the existence of potent pain management therapies, cancer-related pain remains undertreated, affecting up to 50% of patients. Particularly in pancreatic ductal adenocarcinoma (PDAC), a type of cancer known for its pronounced neuropathy, there is a high incidence of tumor cells infiltrating nerves, which can result in intense neuropathic pain. Remarkably, research utilizing genetically engineered mouse models has demonstrated that in the early stages of PDAC, the cancer can prompt the formation of nerves ready for tumor spread by secreting the chemokine CXCL12. This chemokine acts to attract glial cells, specifically Schwann cells, which are integral to nerve function. This mechanism suggests that early pancreatic cancer lesions may paradoxically suppress pain through the chemoattraction mediated by CXCL12, highlighting the complex interplay between cancer progression and pain signaling.72 Lastly, while further research is required to validate these findings in the context of advanced cancer stages, the mechanistic evidence highlighting the role of the CXCL12/CXCR4/CXCR7 axis in reactive gliosis within PDAC could offer valuable insights. Understanding this axis’s function may lead to a better comprehension of cancer-related pain and the complex interactions between nerves and cancer in a variety of malignancies. This knowledge could potentially pave the way for more targeted and effective pain management strategies in cancer care.
Studies have noted that Cancer-induced bone pain (CIBP) is associated with the induction of CXCR4, along with the persistent upregulation of phosphorylated calcium/calmodulin-dependent protein kinase II (p-CaMKII) and phosphorylated cAMP response element-binding protein (p-CREB) in spinal neurons. These molecular markers mediate the expression of p-CaMKII and p-CREB following tumor cell implantation (TCI). Therapeutic intervention with Plerixafor or the phospholipase C (PLC) inhibitor U73122, both of which are CXCR4-specific inhibitors, has been shown to significantly reduce SDF-1-induced pain behaviors and to suppress the TCI-induced upregulation of N-methyl-D-aspartate receptor 1 (NMDAR1) mRNA and protein within the CaMKII/CREB signaling pathway. This suggests a potential regulatory role of CXCR4 in the CaMKII/CREB pathway, which may be involved in the development of CIBP due to primary bone tumors or metastases to the bone. These findings underscore the importance of the CXCR4 signaling pathway in the complex mechanisms underlying bone pain in cancer and could have significant implications for the development of targeted therapies for CIBP.38 Recent discoveries have highlighted the role of CXCL12/CXCR4 signaling in the genesis of diverse pain types associated with bone cancer. This signaling pathway has been shown to activate mitogen-activated protein kinases (MAPKs) within glial cells, which are integral to the transmission and modulation of pain signals in the context of bone cancer. The activation of MAPKs suggests a significant role for this signaling cascade in the pathogenesis of cancer-related bone pain, potentially offering a target for therapeutic intervention to alleviate pain in patients with bone cancer.73 Immunofluorescence studies conducted in a tumor cell implantation (TCI) model have demonstrated that on day 14, there is activation of microglia in the spinal cord, characterized by the co-expression of CXCL12/CXCR4 along with phosphorylated forms of mitogen-activated protein kinases (p-MAPKs), including p-JNK, p-ERK, and p-p38 MAPK, as well as phosphorylated c-Jun (p-c-Jun). These markers indicate a heightened state of microglial activity associated with pain signaling. Importantly, the administration of the CXCR4 antagonist AMD3100 directly into the spinal cord (intrathecal injection) has been shown to decrease the phosphorylation of JNK and c-Jun. This reduction in phosphorylation is associated with a decrease in neuronal sensitivity and an attenuation of pain hypersensitivity in the context of cancer-induced bone pain, suggesting that targeting CXCR4 signaling may provide a therapeutic approach to manage pain in patients with bone cancer.51 Indeed, research has also established the RhoA/ROCK2 pathway as a pivotal downstream target in the context of spinal neuronal sensitization and heightened pain sensitivity in CXCR4-mediated bone cancer pain. This pathway is instrumental in the transmission and modulation of pain signals, particularly in scenarios where CXCR4 is implicated. The demonstration of the RhoA/ROCK2 pathway’s involvement underscores the complex network of molecular mechanisms that contribute to the experience of pain in the setting of bone cancer and suggests potential therapeutic avenues for targeting this pathway to alleviate cancer-related pain.52 Moreover, analyses using Western blotting and immunofluorescence have revealed that CIBP leads to a prompt and substantial increase in the levels of CXCL12 protein and the phosphorylation of NF-κB in the ventrolateral periaqueductal gray (vlPAG) from day 6 to day 12. In a group treated with bilateral electroacupuncture (EA) at 2/100 hz, the administration of anti-CXCL12 neutralizing antibodies in the vlPAG notably elevated the mechanical pain threshold in the hind limb of the CIBP model. This effect is believed to be mediated by the inhibition of the upregulation of phosphorylated NF-κB (pNF-κB) and CXCL12 in the vlPAG of the CIBP model, suggesting that EA may exert its analgesic effects, at least in part, by modulating these pain-related molecular changes.74 That is, the analgesic mechanism of EA may be to reduce the expression of CXCL12 by inhibiting the activation of NF-κB, thus inhibiting the promotion effect of CIBP. These approaches may open opportunities for acupuncture analgesia in mechanism research. Further study should focus on clinical research to confirm this hypothesis.
Pain associated with bone cancer (BCP) frequently affects individuals with advanced stages of breast, lung, and prostate cancer due to these cancers’ high propensity to spread to the bones. According to epidemiological research, a substantial majority—ranging from 75 to 90%—of patients with cancer that has metastasized or reached an advanced stage endure considerable pain due to their disease. This pain significantly impairs their overall quality of life.75 Mechanistically, the general nature of BCP shares similarities with both inflammatory and neuropathic pain under certain conditions. However, the traditional mechanisms that account for chronic pain are insufficient to fully elucidate the complex processing of BCP. This is because BCP is initiated by a confluence of factors, including inflammation, nerve damage, and tumor growth. Each of these components contributes to the pain experience, making it a multifaceted issue that cannot be attributed to a single cause. To better understand BCP, it’s important to recognize the interplay between these elements. Inflammatory pain arises from the body’s immune response to tissue damage or infection, which can be exacerbated by the presence of cancer cells. Neuropathic pain is caused by direct damage to the nervous system, which can occur as tumors infiltrate or compress nerves. Tumorigenic pain, on the other hand, is pain that is generated by the cancer itself, either through the release of pain-inducing substances or by the physical distortion of bone tissue. The complexity of BCP is further compounded by the fact that these three pain types can overlap and interact, leading to a unique and challenging pain state that is not well-addressed by classic pain management strategies. This highlights the need for a more comprehensive approach to BCP management, one that takes into account the multifactorial nature of the pain and the specific characteristics of each contributing component.75,76 In the spinal cord, CXCL12 expression is mainly concentrated in astrocytes. Studies have shown that the production of CXCL12 induced by tumor cell implantation (TCI) can be effectively reduced by intrathecal injection of specific inhibitors of astrocyte metabolism, such as fluorocitric acid, or by the use of specific inhibitors of JNK signaling pathways, such as SP600125, which is demonstrated in the BCP model. These findings shed light on the role of astrocytes in BCP and potential therapeutic strategies to control CXCL12 expression by inhibiting metabolic or signaling pathways in these cells. As a metabolic inhibitor, fluorocitric acid can block the energy metabolism of astrocytes, thus reducing the synthesis of CXCL12. SP600125 can affect the expression and regulation of CXCL12 by inhibiting JNK signaling pathway. The discovery of these mechanisms provides an important molecular target for the development of CXCL12 as a new treatment for BCP.37
Intrathecal administration of AMD3100, a CXCR4 antagonist, has been shown to significantly delay and reduce both the onset and the duration of Bone Cancer Pain (BCP) in the early stages of the disease. Recent studies suggest that downstream signaling pathways of CXCR4 are involved in the release of key regulatory proteins such as phosphorylated RhoA (p-RhoA) and phosphorylated ROCK2 (p-ROCK2) within neurons of the spinal cord. Notably, intrathecal injection of CXCL12, which exclusively binds to CXCR4, has been observed to increase p-RhoA expression in control rats. This increase was counteracted by the subsequent administration of the CXCR4 inhibitor Plerixafor (AMD3100) or the ROCK2 inhibitor Fasudil. These findings highlight the potential of targeting CXCR4 and associated pathways for the management of BCP, indicating that these interventions may modulate the expression of proteins implicated in cancer-related pain pathways.52 Indeed, these findings indicate that the spinal CXCL12/CXCR4 axis could potentially serve as a novel therapeutic target for the treatment of BCP. By modulating this axis, it may be possible to develop new strategies to alleviate pain associated with bone cancer, offering hope for more effective pain management in this patient population.
Postoperative Pain
Postoperative pain, arising from surgical procedures, is a prevalent form of nociceptive condition. It is widely believed that postoperative pain can be attributed to either inflammation or neuropathic processes.68 The elevation of cytokines and chemokines induces neuronal hyperexcitability, consequently giving rise to postoperative pain.69,77 In the research conducted by Feixing and colleagues, an overabundance of the chemokines CXCL12 and their receptors CXCR4 was observed within the dorsal horn of the spinal cord. This finding was associated with the postoperative pain that ensued following plantar incision in the hind paw, a model used to study postoperative pain.43 The dorsal horn of the spinal cord exhibited elevated levels of CXCL12 and CXCR4 following plantar incision (PI) in the hind paw of rats. Through the application of double immunofluorescence staining, it was revealed that CXCL12 was present in both neuronal and astrocytic cells. In contrast, CXCR4 was found to be specifically co-localized with neuronal cells.
PI not only increases the phosphorylation of NF-κB p65 in the spinal cord and extracellular Akt in the spinal cord, but also leads to mechanical allodynia and thermal hyperalgesia.28 However, intrathecal injection of PDTC, a specific inhibitor of NF-κB activation, relieved postoperative pain caused by plantar incisions and reduced CXCL12 expression in the spinal cord. At the same time, pre-intraperitoneal injection of AMD3100 prevented the activation of extracellular signal-regulated kinases in the spinal cord.43 Thus, activation of NF-κB signaling induced by plantar incision may mediate the upregulation of CXCL12 in the spinal cord, and CXCL12/CXCR4 axis is involved in the occurrence of postoperative pain through extracellular signaling regulation of kinase activation.
This work thus provides scientific evidence to support that intervention with CXCL12/CXCR4 axis is particularly effective for postoperative chronic pain, especially PI.
CXCL12 in Morphine-Induced Hyperalgesia and Tolerance Morphine-Induced Hyperalgesia (MIH)
\MIH represents a significant and severe complication associated with opiates and Morphine.32 MIH is often accompanied by hyperalgesia and anti-nociceptive tolerance, which frequently impede medical adherence.33 Paradoxically, long-term opioid therapy for chronic pain in patients can lead to heightened pain sensitivity, thereby limiting the clinical use of morphine.28 The signaling between astrocytes and neurons plays a critical role in pain hypersensitivity.34 Following the administration of morphine, there was a noticeable increase in the transcription of CXCL12 within sensory neurons. Concurrently, CXCR4 expression was prominently observed in satellite glial cells. This suggests that morphine influences the expression patterns of these chemokines and their receptors in distinct cellular populations within the nervous system.49
In a murine model subjected to morphine, the glial IL-33 signaling cascade through the ST2-to-CXCL12 axis in the spinal cord plays a pivotal role in intracellular communication within both neurons and astrocytes, which are essential contributors to the progression of chronic pain. Notably, an astrocyte-driven pathway that includes tumor necrosis factor receptor-associated factor 6 (TRAF6) and the kinase JNK is critical for IL-33’s role in pain modulation. This pathway facilitates the synthesis of the chemokine CXCL12 in the spinal cord, thereby influencing pain signaling.35 In addition, the manifestation of mechanical allodynia (MIH) is contingent upon the expression of CXCR4 and the secretion of the chemokine monocyte chemoattractant protein-1 (MCP1/CCL2) by satellite glial cells. This process occurs within a line of F11 neuroblastoma-sensory neuron hybrid cells derived from the dorsal root ganglia. Notably, the intraperitoneal administration of AMD3100 has been shown to fully reverse MIH in rats. Furthermore, the research identified that TNF-α is implicated in MIH through the CXCL12/CXCR4 signaling system. Inhibition of the signaling pathways of these pro-inflammatory molecules was found to alleviate MIH.36 Therefore, by targeting both peripheral neuronal-glial interactions, as well as the previously acknowledged central neuronal-glial interactions, it becomes possible to enhance opioid analgesia.
The neuroinflammatory response involving chemokines and nociceptive transmission through the N-methyl-D-aspartate receptor (NMDAR) are central to the development of opioid-induced hyperalgesia (OIH). The neurotoxic effects of CXCL12 are linked to the activation of NMDAR. Acute exposure to refentanil can trigger mechanical allodynia and thermal hyperalgesia, which are accompanied by an upregulation of CXCL12/CXCR4 expression in the spinal cord. Importantly, pretreatment with AMD3100 has been shown to mitigate hyperalgesia and reduce NMDAR phosphorylation, specifically at the NR2B subunit. Furthermore, the administration of exogenous CXCL12 leads to pain hypersensitivity and NMDAR activation in young rats, indicating that the spinal cord CXCL12/CXCR4 axis and NMDAR pathways containing NR2B are implicated in the antinociceptive resistance associated with OIH.48
Collectively, these results suggest that the CXCL12 pathway, which is involved in central neuronal-glial interactions, along with the already established peripheral neuronal-glial interactions, can contribute to the enduring analgesic effects of opioids. This dual interaction may be key in the effective management of chronic pain, highlighting the importance of the CXCL12 pathway in both central and peripheral pain modulation mechanisms.
CXCL12/CXCR4 Axis in Visceral Pain
Visceral pain is a complex phenomenon that can be initiated by a multitude of stimuli, such as mechanical stretching, inflammatory processes, ischemic conditions, disruptions in pH levels, the presence of bacteria, immune system mediators, and neurotransmitter activity. The complexity of treating visceral pain is often exacerbated by the limited knowledge of the sensory mechanisms and contributing factors that underpin its development. This gap in understanding is especially challenging in instances of “functional” visceral disorders, where no clear pathological cause can be identified, and pain is the predominant symptom reported by patients. The management of such conditions is further complicated by the absence of a concrete pathological target, necessitating a more nuanced approach to understanding and treating the underlying mechanisms of visceral pain. A study conducted by White et al,78 the roles and mechanisms of monocyte chemoattractant protein-1 (MCP1/CCL2) and CXCL12 in the pathogenesis of chronic pelvic pain (CPP) were investigated. To mimic the key pathophysiological features of CPP in human patients, a rat model of LPC (lysophosphatidylcholine-induced focal demyelination injury) was established. In the LPC model mice, there was a pronounced increase in tactile sensitivity, indicative of hyperalgesia, along with a heightened frequency of urination. Researchers also noted a substantial increase in the expression levels of chemokines CXCL12 and CCL2 within the dorsal root ganglia (DRG). Notably, localized demyelination of the sciatic nerve resulted in an elevated count of injured L4-L5 and non-injured L6-S2 sensory neurons associated with the bladder. These neurons demonstrated a responsiveness to MCP1 and CXCL12. Consequently, it is hypothesized that the upregulation of the CXCL12/CXCR4 signaling axis in visceral sensory neurons could be instrumental in the transmission of nociceptive signals that bridge somatic and visceral sensations.
Previous studies have found that the expression of CXCR4 in peripheral T-cell of patients with ulcerative colitis increases with the increase of disease activity. In addition, chemically-induced colitis in mice resulted in increased expression of CXCR4-positive leukocytes and CXCL12 in colon tissue.79 The use of CXCR4 antagonists can reduce these inflammatory effects. Therefore, in order to further investigate the role of CXCL12/CXCR4 axis pathway in normal urination and inflammatory bladder hyperreflexes, some scholars found that the expression of CXCL12 and CXCR4 in the whole bladder, especially the urinary tract epithelium, was significantly increased in female rats after cyclophosphamide (CYP) injection. Interestingly, blocking the CXCR4 receptor with AMD3100 resulted in a reduction in CYP-induced bladder hyperexcitability.50 Zhang et al 53 further found that increased CXCR4 in L6-S1 DRG and SDH contribute to colitis-induced bladder hyperactivity and hyperalgesia through phosphorylation of spinal ERK. This conclusion was reached by intravaginal injection of AMD3100 and PD98059 (an ERK inhibitor) in a colitis model established by rectal infusion of trinitrobenesulfonic acid, and evaluation of CXCR4 expression and distribution by Western blot and immunofluorescence, and pain behavior in rats demonstrated by intravesical infusion of resinotoxin. Therefore, the CXCL12/CXCR4 axis pathway may be an important signaling pathway for the pain caused by the inability to urinate normally after the treatment of bladder inflammation.
CXCL12 and CXCR4 undergo further upregulation within the dorsal root ganglion subsequent to the induction of chronic pancreatitis pain via trinitrobenzene sulfonic acid injection. Furthermore, the direct administration of AMD3100 into the spinal canal, along with the inhibition of ERK activation through the use of intrathecal U0126, has been shown to markedly reduce the persistent pain caused by TNBS and the overexpression of Nav1.8 in the dorsal root ganglia. This suggests that targeting the CXCL12/CXCR4 axis could be a pivotal strategy in managing pancreatic pain, and that the ERK-dependent upregulation of Nav1.8 might contribute to the increased excitability of primary nociceptive neurons in rats suffering from chronic pancreatitis. These findings underscore the potential therapeutic value of modulating these pathways in the treatment of chronic pain conditions,41 these findings furnish evidence supporting the targeting of the CXCL12/CXCR4 axis pathway as a potential therapeutic strategy for effectively managing pain in patients afflicted with visceral pain.
CXCL12/CXCR4 Axis in Migraine
Migraine, the predominant form of recurring and incapacitating headache, is characterized by a pulsating or throbbing sensation ranging from moderate to severe. This distressing condition significantly impacts the daily functioning of afflicted individuals.80 Its prevalence is substantial both in the United States and worldwide. Epidemiological studies have revealed a higher incidence of migraine in females compared to males.81 The precise role of human CXCL12 signaling in regulating migraines remains poorly understood. It is plausible that human astrocytes fulfill a more intricate function in chronic pain compared to their rodent counterparts. Indeed, a variety of activated microglia within the human brain may converge to drive the progression of the disease through the release of cytokines. Notably, Stromal cell-derived factor-1 alpha (SDF-1α) has emerged as the most powerful chemoattractant for endothelial progenitor cells (EPCs). Reduced levels of SDF-1α may contribute to endothelial dysfunction in migraines by hindering the mobilization of EPCs from the bone marrow.80
CXCL12 in Electro-Acupuncture Therapy
Electro-acupuncture (EA) has demonstrated its efficacy in treating both inflammatory and neuropathic pain by activating a range of bioactive chemicals through peripheral, spinal, and supra-spinal mechanisms. The effectiveness of EA is enhanced by the use of different stimulation frequencies, which can target various pain pathways more effectively. This approach has broadened the applicability of EA in pain management, offering a non-pharmacological option for patients suffering from chronic pain conditions.82 Our recent research has made significant strides in understanding the role of CXCL12 in the pain response observed in the CPIP model rats. We have found that EA can notably reduce allodynia in an animal model that mimics CRPS-I. This reduction in pain sensitivity is attributed to EA’s ability to suppress the CXCL12/CXCR4 signaling pathway, which is integral to the transmission of pain signals. Our findings indicate that EA not only lessens the activation of neurons and glial cells in the SCDH but also diminishes the activation of the ERK pathway, a key player in the processing of pain. Moreover, we have determined the optimal EA frequency and treatment parameters, which could position EA as a promising therapeutic intervention. This could potentially revolutionize the clinical management of CRPS-I by offering a targeted and effective treatment approach.83 This study has shed light on the role of glial cell activation and regulation in the context of acupuncture. Among the various mechanisms by which acupuncture exerts its effects, the modulation of central sensitization through neuroglial plasticity stands out as a key factor. This discovery offers a novel perspective on the analgesic effects of acupuncture and unveils new targets for the treatment of pain disorders. By understanding these mechanisms, we can enhance the efficacy of acupuncture in clinical practice and develop innovative pain management strategies.12
Furthermore, EA is known for its anti-inflammatory properties, which may be attributed to its capacity to reduce the expression of CXCL12 by dampening the activation of the NF-κB signaling pathway. This mechanism is particularly relevant in the context of cancer-induced bone pain, where EA could potentially counteract the descending facilitatory effects that intensify pain signals. By modulating the NF-κB pathway, EA could provide a therapeutic strategy to alleviate the painful symptoms associated with bone cancer. This approach targets the underlying inflammatory processes that contribute to the severity and persistence of pain in such conditions, offering a non-pharmacological method to manage pain and improve the quality of life for patients suffering from bone cancer.74 These approaches may open opportunities for acupuncture analgesia in mechanism research. Further study should focus on clinical research to confirm this hypothesis.
Conclusions and Future Directions
This review highlights recent progress in understanding the role of the CXCL12/CXCR4 signaling axis in various forms of pathological pain, with particular emphasis on chronic pain.
It has increasingly become evident that CXCL12/CXCR4 contributes to central sensitization through mechanisms involving not only neuronal and glial processes regulating peripheral sensitization but also neuroinflammation and neuralgia.
Further studies have shown that modulating the upstream and downstream activation of these chemokines can result in neuroinflammation or peripheral neuronal hyperactivity within the spinal cord and its surrounding regions (Figure 2) These mechanisms can trigger, sustain, and further exacerbate pain conditions.
Despite significant advances in this field, several challenges and gaps remain that warrant further investigation. First, the identification of CXCL12/CXCR4 expression has largely relied on immunostaining, which may suffer from antibody specificity issues. To achieve more precise localization of these proteins, alternative, more reliable techniques, such as in situ hybridization or RNA-based methods, should be employed. Second, although CXCL12/CXCR4 targeted therapy has attracted significant attention in the fields of chronic pain and oncology, its clinical application still faces multiple challenges. CXCR4 antagonists, such as AMD3100, may interfere with normal physiological functions (eg, hematopoietic stem cell homing), leading to side-effects like neutropenia and exerting off-target effects on immune system cells.84 Moreover, CXCL12 can also mediate downstream effects through the CXCR7 receptor. Most existing antagonists only target CXCR4, which may result in treatment resistance and signal pathway redundancy.85 Therefore, in the future, it is necessary to develop dual - receptor inhibitors or combinatorial targeting strategies (eg dual blockade of CXCR4/CXCR7) and screen sensitive patient subgroups using biomarkers.
Third, CXCR4 is expressed across multiple systems in the body, including the nervous, immune, and circulatory systems, and is involved in a broad spectrum of physiological processes.86 This widespread expression complicates the potential for systemic therapies aimed at blocking CXCR4 for analgesic purposes, as it is unlikely that such interventions would be free of unpredictable side effects. A more targeted approach, such as the intra-ganglion injection of specific CXCR4 siRNA, may offer a more precise and effective treatment strategy.
 |
Table 2 Expression of CXCL12 and CXCR4 Components Under Different Pain Conditions |
In summary, accumulating evidence demonstrates that the CXCL12/CXCR4 pathway plays a critical role in the development of chronic pain. We consolidate several promising therapeutic approaches that target this axis for pain relief, including the small molecule CXCR4 inhibitor Plerixafor (AMD3100), along with techniques such as gene knockdown/knockout and electroacupuncture. Targeting the regulation of CXCL12/CXCR4 or employing electroacupuncture may offer novel treatment options for pain relief (Table 2).
Data Sharing Statement
All data associated with this study are present in the paper.
Author Contributions
LBY defined the research topic. CZW, XYF performed document retrieval, designed the figures and wrote the first draft. FJQ, LBY and HQM made important changes and approved the final version of the manuscript. These authors contributed equally: CZW, XYF. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was supported by Supporting research funds of the Third Affiliated Hospital of Zhejiang Chinese Medicine University (KYP-2023006).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e283. doi:10.1016/j.bja.2019.03.023
2. Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of Chronic Pain: domains, Methods, and Mechanisms. J Pain. 2016;17(9 Suppl):T10–20. doi:10.1016/j.jpain.2015.08.010
3. Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38(4):237–246. doi:10.1016/j.tins.2015.02.001
4. Wyse MM, Goicoechea S, Garcia-Mata R, Nestor-Kalinoski AL, Eisenmann KM. mDia2 and CXCL12/CXCR4 chemokine signaling intersect to drive tumor cell amoeboid morphological transitions. Biochem Biophys Res Commun. 2017;484(2):255–261. doi:10.1016/j.bbrc.2017.01.087
5. Gonzalez EJ, Arms L, Vizzard MA. The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction. BioMed Res Int. 2014;2014:120525. doi:10.1155/2014/120525
6. Tripathi A, Saini V, Marchese A, Volkman BF, Tang WJ, Majetschak M. Modulation of the CXC chemokine receptor 4 agonist activity of ubiquitin through C-terminal protein modification. Biochemistry. 2013;52(24):4184–4192. doi:10.1021/bi400254f
7. Dillenburg-Pilla P, Patel V, Mikelis CM, et al. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/mTORC1 axis. FASEB J off Publ Fed Am Soc Exp Biol. 2015;29(3):1056–1068. doi:10.1096/fj.14-260083
8. Heinrich EL, Lee W, Lu J, Lowy AM, Kim J. Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways in pancreatic cancer cells. J Transl Med. 2012;10(1):68. doi:10.1186/1479-5876-10-68
9. Masci AM, Galgani M, Cassano S, et al. HIV-1 gp120 induces anergy in naive T lymphocytes through CD4-independent protein kinase-A-mediated signaling. J Leukoc Biol. 2003;74(6):1117–1124. doi:10.1189/jlb.0503239
10. Liu X, Zha J, Nishitani J, Chen H, Zack JA. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307(1):37–44. doi:10.1016/s0042-6822(02)00031-4
11. Banerjee A, Li L, Pirrone V, Krebs FC, Wigdahl B, Nonnemacher MR. cAMP Signaling Enhances HIV-1 Long Terminal Repeat (LTR)-directed Transcription and Viral Replication in Bone Marrow Progenitor Cells. Clin Med Insights Pathol. 2017;10:1179555717694535. doi:10.1177/1179555717694535
12. Lyu Z, Guo Y, Gong Y, et al. The Role of Neuroglial Crosstalk and Synaptic Plasticity-Mediated Central Sensitization in Acupuncture Analgesia. Neural Plast. 2021;2021:8881557. doi:10.1155/2021/8881557
13. Luo X, Wang X, Xia Z, Chung SK, Cheung CW. CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neurosci. 2016;27(1):83–92. doi:10.1515/revneuro-2015-0016
14. Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):5263):872–877. doi:10.1126/science.272.5263.872
15. Reaux-Le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci. 2012;36(5):2619–2631. doi:10.1111/j.1460-9568.2012.08179.x
16. Luo Y, Lathia J, Mughal M, Mattson MP. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283(36):24789–24800. doi:10.1074/jbc.M800649200
17. KI Cheng, SL Chen, JH Hsu, et al. cAMP-Response-element-binding-protein-binding protein silencing inhibits thrombin-induced endothelial progenitor cell migration via downregulation of CXCR4 expression. Biol Pharm Bull. 2010;33(5):792. doi:10.1248/bpb.33.792
18. de Lourdes Perim A, Amarante MK, Guembarovski RL, de Oliveira CEC, Watanabe MAE. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell Mol Life Sci CMLS. 2015;72(9):1715–1723. doi:10.1007/s00018-014-1830-x
19. Mousavi A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol Lett. 2020;217:91–115. doi:10.1016/j.imlet.2019.11.007
20. Dirat B, Ader I, Golzio M, et al. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells. Mol Cancer Ther. 2015;14(2):586–596. doi:10.1158/1535-7163.MCT-14-0102
21. KI Cheng, SL Chen, JH Hsu, et al. Loganin prevents CXCL12/CXCR4-regulated neuropathic pain via the NLRP3 inflammasome axis in nerve-injured rats. Phytomedicine Int J Phytother Phytopharm. 2021;92:153734. doi:10.1016/j.phymed.2021.153734
22. Xu T, Zhang XL, Ou-Yang HD, et al. Epigenetic upregulation of CXCL12 expression mediates antitubulin chemotherapeutics-induced neuropathic pain. Pain. 2017;158(4):637–648. doi:10.1097/j.pain.0000000000000805
23. Mai CL, Tan Z, Xu YN, et al. CXCL12-mediated monocyte transmigration into brain perivascular space leads to neuroinflammation and memory deficit in neuropathic pain. Theranostics. 2021;11(3):1059–1078. doi:10.7150/thno.44364
24. Wang Y-S, Li -Y-Y, Wang L-H. Tanshinone IIA Attenuates Chronic Pancreatitis-Induced Pain in Rats via Downregulation of HMGB1 and TRL4 Expression in the Spinal Cord. Pain Physician. 2015;18(4):E615–628.
25. Li XQ, Zhang ZL, Tan WF, Sun XJ, Ma H. Down-Regulation of CXCL12/CXCR4 Expression Alleviates Ischemia-Reperfusion-Induced Inflammatory Pain via Inhibiting Glial TLR4 Activation in the Spinal Cord. PLoS One. 2016;11(10):e0163807. doi:10.1371/journal.pone.0163807
26. Hu Q, Zheng X, Chen R, et al. Chronic Post-Ischemia Pain Model for Complex Regional Pain Syndrome Type-I in Rats. J Vis Exp. 2020;2020(155):e60562. doi:10.3791/60562
27. GY Xu, JH Winston, M Shenoy, et al. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G424. doi:10.1152/ajpgi.00560.2005
28. Bai L, Wang X, Li Z, et al. Upregulation of Chemokine CXCL12 in the Dorsal Root Ganglia and Spinal Cord Contributes to the Development and Maintenance of Neuropathic Pain Following Spared Nerve Injury in Rats. Neurosci Bull. 2016;32(1):27–40. doi:10.1007/s12264-015-0007-4
29. Wu J, Ma X, Chen W, et al. Protective effects of HTD4010, a Reg3α/PAP-derived peptide, in mouse model of acute pancreatitis via toll-like receptor 4 pathway. Biochem Biophys Res Commun. 2019;512(4):670–672. doi:10.1016/j.bbrc.2019.03.107
30. Zou Y, Cao Y, Liu Y, Zhang X, Li J, Xiong Y. The role of dorsal root ganglia PIM1 in peripheral nerve injury-induced neuropathic pain. Neurosci Lett. 2019;709:134375. doi:10.1016/j.neulet.2019.134375
31. Liu ZY, Song ZW, Guo SW, et al. CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model. CNS Neurosci Ther. 2019;25(9):922–936. doi:10.1111/cns.13128
32. Xie F, Wang Y, Li X, Chao YC, Yue Y. Early Repeated Administration of CXCR4 Antagonist AMD3100 Dose-Dependently Improves Neuropathic Pain in Rats After L5 Spinal Nerve Ligation. Neurochem Res. 2016;41(9):2289–2299. doi:10.1007/s11064-016-1943-8
33. Luo X, Tai WL, Sun L, et al. Central administration of C-X-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PLoS One. 2014;9(8):e104860. doi:10.1371/journal.pone.0104860
34. Yu Y, Huang X, Di Y, Qu L, Fan N. Effect of CXCL12/CXCR4 signaling on neuropathic pain after chronic compression of dorsal root ganglion. Sci Rep. 2017;7(1):5707. doi:10.1038/s41598-017-05954-1
35. Bhangoo SK, Ren D, Miller RJ, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21(5):581–591. doi:10.1016/j.bbi.2006.12.003
36. Huang W, Zheng W, Ouyang H, et al. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by herpes simplex virus vector through the SDF1α/CXCR4 system in rats. Anesth Analg. 2014;118(3):671–680. doi:10.1213/ANE.0000000000000079
37. Shen W, Hu XM, Liu YN, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation. 2014;11:75. doi:10.1186/1742-2094-11-75
38. Hu XM, Zhang H, Xu H, et al. Chemokine receptor CXCR4 regulates CaMKII/CREB pathway in spinal neurons that underlies cancer-induced bone pain. Sci Rep. 2017;7(1):1. doi:10.1038/s41598-017-04198-3
39. Su W, Yu J, Liu Q. CXCL12/CXCR4 signaling induced itch and pain sensation in a murine model of allergic contact dermatitis. Mol Pain. 2020;16:3. doi:10.1177/1744806920926426
40. da Silva Junior CA, de Castro Junior CJ, Pereira EMR, et al. The inhibitory effect of Phα1β toxin on diabetic neuropathic pain involves the CXCR4 chemokine receptor. Pharmacol Rep PR. 2020;72(1):2. doi:10.1007/s43440-019-00002-3
41. Zhu HY, Liu X, Miao X, et al. Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol Pain. 2017;13:1744806917697979. doi:10.1177/1744806917697979
42. Yang F, Luo WJ, Sun W, et al. SDF1-CXCR4 Signaling Maintains Central Post-Stroke Pain through Mediation of Glial-Neuronal Interactions. Front Mol Neurosci. 2017;10:26. doi:10.3389/fnmol.2017.00226
43. Xing F, Kong C, Bai L, et al. CXCL12/CXCR4 signaling mediated ERK1/2 activation in spinal cord contributes to the pathogenesis of postsurgical pain in rats. Mol Pain. 2017;13:1744806917718753. doi:10.1177/1744806917718753
44. F Li, ZY Xue, Y Yuan, et al. Upregulation of CXCR4 through promoter demethylation contributes to inflammatory hyperalgesia in rats. CNS Neurosci Ther. 2018;24(10). doi:10.1111/cns.12845
45. Dubový P, Klusáková I, Svízenská I, Brázda V. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol. 2010;133(3):323–337. doi:10.1007/s00418-010-0675-0
46. Yang F, Sun W, Luo WJ, et al. SDF1-CXCR4 Signaling Contributes to the Transition from Acute to Chronic Pain State. Mol Neurobiol. 2017;54(4):2763–2775. doi:10.1007/s12035-016-9875-5
47. Huang W, Zheng W, Liu S, et al. HSV-mediated p55TNFSR reduces neuropathic pain induced by HIV gp120 in rats through CXCR4 activity. Gene Ther. 2014;21(3):1. doi:10.1038/gt.2013.90
48. Li T, Wang H, Wang J. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci Res. 2019;144:48–55. doi:10.1016/j.neures.2018.07.007
49. Wilson NM, Jung H, Ripsch MS, et al. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun. 2011;25(3):565. doi:10.1016/j.bbi.2010.12.014
50. Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298(3):F589–600. doi:10.1152/ajprenal.00628.2009
51. Duan C, Zhu Y, Zhang Z, et al. Esketamine inhibits the c-Jun N-terminal kinase pathway in the spinal dorsal horn to relieve bone cancer pain in rats. Mol Pain. 2024;20:17448069241239232. doi:10.1177/17448069241239231
52. Xu H, Peng C, Chen XT, et al. Chemokine receptor CXCR4 activates the RhoA/ROCK2 pathway in spinal neurons that induces bone cancer pain. Mol Pain. 2020;16:1744806920919568. doi:10.1177/1744806920919568
53. Zhang H, Dong X, Yang Z, et al. Inhibition of CXCR4 in Spinal Cord and DRG with AMD3100 Attenuates Colon-Bladder Cross-Organ Sensitization. Drug Des Devel Ther. 2022;16:67–81. doi:10.2147/DDDT.S336242
54. Dong J, Xia R, Zhang Z, Xu C. lncRNA MEG3 aggravated neuropathic pain and astrocyte overaction through mediating miR-130a-5p/CXCL12/CXCR4 axis. Aging. 2021;13(19):23004–23019. doi:10.18632/aging.203592
55. Hong Y-P, Yu J, Su Y-R. High-Fat Diet Aggravates Acute Pancreatitis via TLR4-Mediated Necroptosis and Inflammation in Rats. Oxid Med Cell Longev. 2020;2020:8172714. doi:10.1155/2020/8172714
56. Pan Z, Shan Q, Gu P, et al. miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation. 2018;15(1):29. doi:10.1186/s12974-018-1073-0
57. Jayaraj ND, Bhattacharyya BJ, Belmadani AA, et al. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest. 2018;128(6):2205–2225. doi:10.1172/JCI92117
58. Hu Q, Wang Q, Wang C, et al. TRPV1 Channel Contributes to the Behavioral Hypersensitivity in a Rat Model of Complex Regional Pain Syndrome Type 1. Front Pharmacol. 2019;10:453. doi:10.3389/fphar.2019.00453
59. De Logu F, de Prá SDT, de David Antoniazzi CT, et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav Immun. 2020;88:535–546. doi:10.1016/j.bbi.2020.04.037
60. Tj C, Gj B. Objectifying CRPS-I. Pain. 2008;138(1):1. doi:10.1016/j.pain.2008.06.006
61. Yin C, Hu Q, Liu B, et al. Transcriptome profiling of dorsal root ganglia in a rat model of complex regional pain syndrome type-I reveals potential mechanisms involved in pain. J Pain Res. 2019;12:1201–1216. doi:10.2147/JPR.S188758
62. Klein I, Lehmann HC. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics. 2021;9(10):229. doi:10.3390/toxics9100229
63. Liu X, Tonello R, Ling Y, et al. Paclitaxel-activated astrocytes produce mechanical allodynia in mice by releasing tumor necrosis factor-α and stromal-derived cell factor 1. J Neuroinflammation. 2019;16(1). doi:10.1186/s12974-019-1619-9
64. Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108(3):425–435. doi:10.1172/JCI12629
65. Li YY, Li H, Liu ZL, et al. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol Pain. 2017;13:1744806917747425. doi:10.1177/1744806917747425
66. Zhang X, Zheng H, Zhu HY, et al. Acute Effects of Transforming Growth Factor-β1 on Neuronal Excitability and Involvement in the Pain of Rats with Chronic Pancreatitis. J Neurogastroenterol Motil. 2016;22(2). doi:10.5056/jnm15127
67. Lee SJ, Namkoong S, Kim YM, et al. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol. 2006;291(6). doi:10.1152/ajpheart.00113.2006
68. Kichler A, Jang S. Chronic Pancreatitis: epidemiology, Diagnosis, and Management Updates. Drugs. 2020;80(12):1155–1168. doi:10.1007/s40265-020-01360-6
69. XQ Li, XZ Cao, J Wang, et al. Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats. Mol Brain. 2014;7. doi:10.1186/s13041-014-0069-7
70. Neesse A, Ellenrieder V. NEMO-CXCL12/CXCR4 axis: a novel vantage point for antifibrotic therapies in chronic pancreatitis? Gut. 2017;66(2):211–212. doi:10.1136/gutjnl-2016-312874
71. Wiffen PJ, Cooper TE, Anderson AK, et al. Opioids for cancer-related pain in children and adolescents. Cochrane Database Syst Rev. 2017;7(7):CD012564. doi:10.1002/14651858.CD012564.pub2
72. Demir IE, Kujundzic K, Pfitzinger PL, et al. Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proc Natl Acad Sci U S A. 2017;114(1):E85–E94. doi:10.1073/pnas.1606909114
73. Carpenter RS, Marbourg JM, Brennan FH, et al. Spinal cord injury causes chronic bone marrow failure. Nat Commun. 2020;11(1):3702. doi:10.1038/s41467-020-17564-z
74. Xu M, Fei Y, He Q, et al. Electroacupuncture Attenuates Cancer-Induced Bone Pain via NF-κB/CXCL12 Signaling in Midbrain Periaqueductal Gray. ACS Chem Neurosci. 2021;12(18):3323–3334. doi:10.1021/acschemneuro.1c00224
75. Zajączkowska R, Kocot-Kępska M, Leppert W, Wordliczek J. Bone Pain in Cancer Patients: mechanisms and Current Treatment. Int J Mol Sci. 2019;20(23):6047. doi:10.3390/ijms20236047
76. Wan Y. New Mechanism of Bone Cancer Pain: tumor Tissue-Derived Endogenous Formaldehyde Induced Bone Cancer Pain via TRPV1 Activation. Adv Exp Med Biol. 2016;904:41–58. doi:10.1007/978-94-017-7537-3_4
77. Hong YP, Yu J, Su YR, et al. High-Fat Diet Aggravates Acute Pancreatitis via TLR4-Mediated Necroptosis and Inflammation in Rats. Oxid Med Cell Longev. 2020;2020:8172714. doi:10.1155/2020/8172714
78. Foster R, Jung J, Farooq A, et al. Sciatic nerve injury induces functional pro-nociceptive chemokine receptors in bladder-associated primary afferent neurons in the rat. Neuroscience. 2011;183:230–237. doi:10.1016/j.neuroscience.2011.03.035
79. Mikami S, Nakase H, Yamamoto S, et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327(2):383–392. doi:10.1124/jpet.108.141085
80. Liman TG, Neeb L, Rosinski J, Reuter U, Endres M. Stromal Cell-Derived Factor-1 Alpha Is Decreased in Women With Migraine With Aura. Headache. 2016;56(8):1274–1279. doi:10.1111/head.12839
81. Hassan M, Belavadi R, Gudigopuram SVR. Migraine and Stroke: in Search of Shared Pathways, Mechanisms, and Risk Factors. Cureus. 2021;13(12). doi:10.7759/cureus.20202
82. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):101. doi:10.1097/ALN.0000000000000101
83. Hu Q, Zheng X, Li X, et al. Electroacupuncture Alleviates Mechanical Allodynia in a Rat Model of Complex Regional Pain Syndrome Type-I via Suppressing Spinal CXCL12/CXCR4 Signaling. J Pain. 2020;21(9–10):1060–1074. doi:10.1016/j.jpain.2020.01.007
84. Heidegger I, Fotakis G, Offermann A, et al. Comprehensive characterization of the prostate tumor microenvironment identifies CXCR4/CXCL12 crosstalk as a novel antiangiogenic therapeutic target in prostate cancer. Mol Cancer. 2022;21(1):132. doi:10.1186/s12943-022-01597-7
85. Morandi F, Amoroso L, Dondero A, et al. Updated clinical and biological information from the two-stage Phase II study of imatinib mesylate in subjects with relapsed/refractory neuroblastoma. Oncoimmunology. 2018;7(9):e1468953. doi:10.1080/2162402X.2018.1468953
86. Chatterjee M, Rath D, Gawaz M. Role of chemokine receptors CXCR4 and CXCR7 for platelet function. Biochem Soc Trans. 2015;43(4):720–726. doi:10.1042/BST20150113
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
CXCL12 and CXCR4 as Potential Early Biomarkers for Luminal A and Luminal B Subtypes of Breast Cancer
Motyka J, Gacuta E, Kicman A, Kulesza M, Malinowski P, Ławicki S
Cancer Management and Research 2023, 15:573-589
Published Date: 4 July 2023

