Back to Journals » Infection and Drug Resistance » Volume 18
The Effectiveness of Antiretroviral Therapy in Mitigating New HIV Infections in Southwest China: An Ecological Study
Authors Sun X, Chen H, Liang S, Xiao T, Zeng Y, Liu H, Feng L, Zhou D
Received 5 February 2025
Accepted for publication 17 May 2025
Published 9 June 2025 Volume 2025:18 Pages 2943—2950
DOI https://doi.org/10.2147/IDR.S505706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiwei Sun,1 Hang Chen,2 Shu Liang,3 Ticheng Xiao,2 Yali Zeng,3 Hong Liu,2 Liao Feng,3 Dinglun Zhou1
1West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Center for AIDS/STD Control and Prevention, Luzhou Center for Disease Control and Prevention, Luzhou, Sichuan, People’s Republic of China; 3Center for AIDS/STD Control and Prevention, Sichuan Center for Disease Control and Prevention, Chengdu, Sichuan, People’s Republic of China
Correspondence: Liao Feng, Email [email protected] Dinglun Zhou, Email [email protected]
Background: Antiretroviral therapy (ART) has been shown to reduce the number of local HIV (Human Immunodeficiency Virus) reported cases; however, there is insufficient research on the relationship between new HIV infections and ART. This study utilized real-world data to evaluate the community-level effectiveness of ART in reducing new HIV infections.
Methods: Ecological study was designed to establish the relationship between ART quality and new HIV infections. New HIV infections were identified through an expanded testing system in 2018– 2023; ART quality was evaluated based on ART-treated clients in 2016– 2023, and non-probabilistic sampling was performed. Generalized linear models was employed to assess associations between metrics of ART effectiveness and new HIV infections. Statistical significance was set at α = 0.05 with 95% confidence intervals.
Results: A total of 3836 new HIV infections were identified, yielding an overall incidence of 2.1%. Treatment coverage for the entire population was 80.8%, and the proportion of clients with an increased CD4 count was 61.0% of the entire population. In the generalized linear modeling, four key factors were associated with reduced new HIV infections: a greater number of clients undergoing treatment, a higher proportion of clients demonstrated CD4 count improvement, higher level of CD4 in baseline, and a reduction in reported cases (β = − 0.04, − 0.03, − 0.01, 0.17 respectively).
Conclusion: This ecological study verified that expanding treatment coverage, optimizing the effectiveness of antiretroviral treatment and elevating baseline CD4 counts could curb community-level new HIV infections. Early detection, prompt treatment, and effective ART are crucial for curbing HIV transmission in regions mainly driven by sexual transmission and with high cumulative incidence rates.
Keywords: HIV, ART, new infections, ecological study
Introduction
The human immunodeficiency virus (HIV) pandemic continues to expand, posing a significant burden on global health. According to the World Health Organization (WHO), approximately 39.9 million people were living with HIV, with 1.3 million people became newly infected with HIV in 2023.1 The United Nations Programme on HIV/AIDS (UNAIDS) has further outlined an overarching vision to end AIDS as a public health threat by 2030, and set ambitious 95–95-95 targets to combat HIV and AIDS globally by 2025,2 aiming for 95% of people living with HIV to know their status, 95% of those diagnosed to receive antiretroviral therapy (ART), and 95% of those on treatment to achieve viral suppression. Achieving the 95–95-95 targets by 2025 is scientifically projected to disrupt viral transmission chains, thereby creating an essential foundation for the 2030 goals. A cornerstone of this strategy is Treatment as Prevention (TasP), which aims to reduce HIV transmission by suppressing viral load through widespread and sustained ART, a global systematic review confirms that individuals on ART with suppressed viral loads (pVL < 1000 copies/mL) have near-zero sexual transmission risk,3 supporting the “Undetectable = Untransmittable” principle.4
China reported 1,355,017 currently living HIV/AIDS cases, including 749,839 hIV carriers and 605,178 AIDS patients5 in the end of 2024. In China, the HIV/AIDS epidemic exhibits regional disparities, with sexual transmission being the primary mode in many areas, followed by injection drug use and mother-to-child transmission. The Chinese government has made significant strides in HIV/AIDS prevention and control, with the implementation of free ART policies since 2003,6 and the free ART cover all people living with HIV regardless of CD4 levels in 20167, covered over 80% of diagnosed clients and achieved a viral suppression rate of 91%6 in 2017, demonstrating the country’s unwavering commitment and robust execution in public health. Despite these efforts, challenges such as drug resistance,8 adherence to ART,9 treatment failure,10 diagnostic delays11 and increased risky sexual behavior persist12 as condomless sex, multiple sexual partners, or commercial sex engagement, complicating the relationship between ART effectiveness and reduced HIV incidence.
To address these issues, we conducted an ecological study to explore the effectiveness of ART in reducing new HIV infections in a city in southwest China. Ecological studies, such as those applied to analyze HIV pre-exposure prophylaxis administration and social determinants of health in Brazil,13 which analyze data at the population level rather than the individual level, have been used to investigate the broader impacts of public health interventions. This approach allows for a more comprehensive understanding of the epidemic dynamics and helps identify gaps in current HIV/AIDS prevention and control strategies.
In this study, we focus on new HIV infections, which provide a more realistic, timely, and actionable assessment of epidemic dynamics compared to the traditional HIV/AIDS case reporting system. The latter has limitations, including an inability to distinguish incident infections from prevalent cases and substantial data delays. In 2018, China’s reported diagnoses fell far short of model-estimated infections, leaving 360,000 undiagnosed individuals.14 By examining new infections, this study aimed to explore the association between ART and reducing new HIV infections at the community level using real-world data from a city in southwest China where the primary mode of HIV transmission is sexual behavior, providing empirical evidence for HIV epidemic control in Southwest China where sexual transmission predominates. The findings offer direct guidance for optimizing local testing strategies and treatment management, with significant practical implications for achieving UNAIDS 95–95-95 targets.
Methods
Study Design and Participants Procedure
This ecological study was conducted in a city located in southwest China, with a permanent population of 4.3 million by the end of 2020 and consisting of seven regions. We used county-level data from two sources: (1) an expanded HIV testing system covering 73.7% of residents (2018–2023), with 3836 incident HIV cases observed across counties, and (2) antiretroviral therapy databases containing complete records of 12,981 treat individuals (2016–2023) managed by the city CDC (Centers for Disease Control and Prevention), non-probabilistic sampling was performed.
First, new HIV infections were identified in an expanding HIV testing system that began in 2018 in the city. The testing system was established at the medical institutions in the city. In this system, rapid HIV screening tests are offered to all inpatients and outpatients from key departments (STD clinic, obstetrics, infection department, etc). Across all age groups as well as to individuals undergoing a health examination during their medical visit or examination. Each participant was assigned a unique identifier (ID). The same unique ID may have multiple test records, as individuals accessing healthcare services repeatedly (eg, prenatal visits, STD follow-ups, or annual health checks) would be retested during subsequent encounters, allowing longitudinal tracking of seroconversion events. Data were curated to form a dynamic HIV cohort study. For this study, we used data from clients who had multiple HIV test records where the first result was negative and then identified new HIV infections where clients had a positive test result during follow-up tests between 2018 and 2023 (Figure 1). New HIV infections were defined as individuals with an initial negative HIV test followed by a confirmed positive result during follow-up. Cumulative incidence was calculated as the number of new infections divided by the total population with multiple tests (initial negative), expressed per 1000 persons.
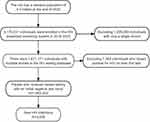 |
Figure 1 The flowchart of study populations of new HIV infected. |
The second data source was data related to HIV antiviral therapy in 2016–2023 collected by the city CDC. HIV infections in the city underwent a CD4 test during their first medical visit and received CD4 tests annually in the following years. Six metrics reflecting the characteristics and results of treatment were examined: treatment coverage, time from diagnosis to the start of treatment, CD4 level at the start of treatment, CD4 change from the start of treatment to the last follow-up CD4 test, proportion of clients with increased CD4 in all treated clients, multiplicative value of treatment coverage, and proportion of clients with increased CD4. The time from diagnosis to the start of treatment reflects treatment timeliness, while CD4 change and proportion of clients with increased CD4 levels reflect treatment effectiveness, and the multiplicative value of treatment coverage and proportion of clients with increased CD4 levels reflect real treatment effectiveness.
Statistical Analysis
Frequencies and percentages of categorical variables are reported. Generalized linear modeling was used to assess the association between antiviral treatment and number of new HIV infections. Bivariate and multivariable generalized linear modeling were performed to determine the independent correlates of HIV and the effectiveness of ART quality. Statistical significance was defined as α = 0.05 (two-tailed), with 95% confidence intervals (CIs) reported for all effect estimates. Variables showing bivariate associations at p < 0.05 advanced to multivariable modeling. All analyses were performed using R, version 4.1.3. This analysis was conducted at the county level.
Results
Number of New HIV Infections in 2018-2023
From 2018 to 2023, the city’s HIV testing system screened 3,170,431 individuals, representing 73.7% of the resident population. Among these, 1,863,802 (58.8%) underwent multiple tests with an initial negative result, from which 3836 new HIV infections were identified, yielding an overall incidence of 2.1% population. Pronounced gender disparities emerged: males exhibited a 2.5-fold higher incidence (3.3%) compared to females (1.3%), with county-level male incidence ranging from 1.5% in County 7 to 5.0% in County 5. Female incidence remained consistently lower across all counties (1.1–1.8%), and County 6 reported the highest female rate (1.8%) (Table 1).
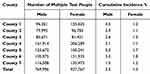 |
Table 1 Number and Incidence of New HIV/AIDS Infections in 2018–2023 |
The Characteristics and Results of Antiviral Therapy for HIV in the City
From 2016 to 2023, 12,981 clients received antiviral therapy, with a treatment coverage of 80.8% of the population. There was little difference in treatment coverage across the counties. The average time between diagnosis and treatment was 3.0 months in all cases.
The mean baseline CD4 was 303.8/μL. The proportion of clients with an increased CD4 count was 61.0% of the entire population. The mean change in CD4 was 100.0/μL. The multiplicative value of treatment coverage and proportion of clients with an increased CD4 count were 49.3% (Table 2).
 |
Table 2 Overview of Antiviral Therapy in 2016–2023 (N=12,981) |
Correlates between Antiviral Therapy Metrics and the Number of New HIV Infections
The correlation between the number of new HIV infections in year 2018–2023 and the metrics of antiviral therapy are shown in Tables 3 and 4.
 |
Table 3 Univariate Generalized Linear Modeling Between the Number of New Cases and the Metrics of Antiviral Therapy |
 |
Table 4 Multivariable Generalized Linear Modeling Between the Number of New Cases and the Metrics of Antiviral Therapy |
The generalized linear model analysis revealed significant correlations between the number of new HIV infections in 2018–2023 and the metrics of antiviral therapy as well as all HIV infections in 2016–2023. Specifically, an increase in reporting systems was positively correlated with new HIV infections (β = 0.17, p < 0.001), whereas higher baseline CD4 counts and a greater proportion of clients with CD4 counts were negatively correlated with new HIV infections (β = −0.03, p = 0.013; β = −0.01, p < 0.001).
In bivariate analysis, an increase in the number of individuals under treatment was positively correlated with the number of new HIV infections (r = 0.14, p < 0.001). Upon inclusion in multivariate analysis, the number of individuals under treatment did not significantly affect the number of new HIV infections.
Discussion
Our results showed that effectiveness of ART is associated with a decrease in new HIV infections, provides three key insights: First, achieving higher ART coverage creates a population-level prevention, consistent with WHO’s 95–95-95 target progression. Second, CD4 recovery modifies transmission risk. Third, baseline CD4 amplified treatment impact, underscoring early detection’s role in China’s “Treat All” policy.
HIV prevention aims to achieve the “three 95s” targets and eliminate AIDS by 2030. In this study, the average antiretroviral therapy coverage in the study area was 80.8%, although it did not reach the target, the coverage still represented a relatively high level, which is close to and slightly higher than the national average level.6. The antiretroviral treatment coverage varies greatly among countries. A global systematic review shows that ART coverage ranges widely, from 2.6% to 81.9%,15 indicating the challenges in reaching the target levels. It aligns with global goals and is achievable considering resource differences among countries. It can serve as a starting point for resource-limited regions to gradually improve. Moreover, with current resources and conditions, measures like improving testing accessibility, increasing drug investment, and enhancing public education can help further expand the coverage rate, which is crucial for global HIV prevention. ART reduces the likelihood of sexual transmission of HIV to uninfected partners via viral suppression.16
Viral load data were unavailable due to regional resource constraints, but CD4 levels could serve as a partial proxy for treatment efficacy, consistent with prior ecological studies in resource-limited settings.17 Our ecological models demonstrate that scaling ART coverage with CD4 monitoring can reduce transmission in resource-constrained, sexually driven epidemics. This approach does not replace VL (viral load)-driven TasP but offers interim guidance where VL testing is inaccessible. A critical limitation is the lack of direct virologic data; future implementation research should integrate CD4 and VL metrics to refine thresholds. However, many factors such as psychosocial interventions18 can affect patient adherence to treatment, and may result in treatment discontinuation or compromise the effectiveness of ART. Incomplete adherence not only diminishes individual treatment outcomes but also undermines the population-level benefits of ART by sustaining transmission risk.19 Structural barriers (eg, limited healthcare access)20 and psychosocial factors (eg, stigma, mental health21) are key drivers of poor adherence, highlighting the need to integrate adherence support into HIV prevention strategies. Treatment discontinuation results in lower CD4 count,22 increased drug resistance.23 In this study, we assessed two metrics: the proportion of clients with increased CD4 counts and the baseline CD4 counts. We found that both of these factors were associated with a decrease in HIV cases. The significance of baseline CD4 levels underscores the importance of early detection for HIV management. Notably, during the initial stages when CD4 counts remain elevated, symptoms may be scarcely noticeable, rendering it essential to promote comprehensive HIV testing. By embracing this pivotal window of opportunity, early intervention and timely treatment can be facilitated, effectively curbing the incidence of HIV.
This study revealed that from 2018 to 2023, the cumulative incidence rate of HIV infection in the target cities reached 2.3%, which was significantly higher than China’s overall HIV/AIDS incidence rate of 2.36/100,000 in 2017.24 This stark contrast not only underscores the unique and formidable challenges faced by the target cities in prevention and control but also highlights the regional disparities in HIV incidence nationwide. Notably, despite the higher HIV/AIDS incidence, the target cities have excelled in ART, achieving a treatment coverage rate of 80.8% from 2016 to 2023. This achievement underscores the immense efforts and remarkable successes made by target cities to enhance medical service quality and ensure effective treatment for clients.
China’s free ART policy represents a cost-effective public health investment, with studies showing ART reduces long-term healthcare costs by preventing opportunistic infections and treatment of adverse reactions.25 As key practitioners of this policy, the significant increase in treatment coverage rates in target cities serves as a testament to the effective implementation of national policies. However, behind these accomplishments, target cities must confront multifaceted challenges, including sustaining momentum in prevention and control efforts, addressing drug resistance, and preventing new HIV infections. Our findings align with global ART benefits, this study uniquely addresses Southwest China’s high-incidence context, where sexual transmission dominates and treatment access faces geographic barriers.
This study was based on etiological research, which cannot evaluate the effects on the transmission of HIV. Individuals with unsuppressed viral loads (due to inconsistent adherence or drug resistance) and undiagnosed PLWH with limited access to testing services remain key drivers of transmission. Future studies should consider constructing a molecular network and HIV-related social-behavior network to accurately locate communication individuals and then build the relationship between individual viral load, CD4 counts, and HIV transmission capability. In addition, the 6-year data from to 2016–2023 were packaged into a whole to evaluate the impact of antiviral quality on new HIV infections at the county level without observing the effect of the time dimension. With the extension of follow-up time, newly acquired HIV incidence can be calculated every year to verify the correlation between the time change trend of antiviral treatment quality and the time change trend of new HIV infections, and to evaluate whether the impact of antiviral quality on new HIV infections has a time-delay effect.
Conclusion
This study explored the relationship between the effectiveness of antiviral treatments and new HIV infections in the community. Our results suggest that comprehensively improving the number of clients receiving treatment and the effectiveness of antiviral treatments could help curb the HIV pandemic at the community level. Furthermore, a higher baseline CD4 count (which generally implies timely detection and treatment) at the start of treatment has led to a reduction in new infections. Early detection, prompt treatment, and effective ART are crucial for curbing HIV transmission in regions mainly driven by sexual transmission and with high cumulative incidence rates.
Ethical Approval
The use of anonymous clinical/demographic and sequence data was reviewed, and the study protocol was approved by the Ethics Committee of the West China Fourth Hospital and West China School of Public Health, Sichuan University (Gwll2022062). The study was conducted following the Helsinki Declaration of 1964.
Informed Consent for Publication
All personally identifiable information in this study had been anonymized. All participants were provided with comprehensive information about the study’s purpose, methods, potential risks, and benefits before they provided their consent.
Funding
This work was supported by the Sichuan University and Luzhou City (grant number 2022CDLZ-22). The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. UNAIDS. Global HIV & AIDS statistics - fact sheet; 2024. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. UNAIDS. 2025 AIDS targets: the next generation of goals for the global AIDS response; 2021. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2021/july/20210721_2025-aids-targets.
3. Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet. 2023;402(10400):464–471. doi:10.1016/S0140-6736(23)00877-2
4. Bekker LG, Smith P, Ntusi NAB. HIV is sexually untransmittable when viral load is undetectable. Lancet. 2023;402(10400):428–429. doi:10.1016/S0140-6736(23)01519-2
5. National Center for AIDS/STD Control and Prevention CCfDCaP. National HIV/STD epidemic status in december 2024. ChiN J AIDS STD. 2025;31(3):225.
6. Xu JJ, Han MJ, Jiang YJ, et al. Prevention and control of HIV/AIDS in China: lessons from the past three decades. Chin Med J. 2021;134(23):2799–2809. doi:10.1097/CM9.0000000000001842
7. Wu X, Wu G, Ma P, et al. Immediate and long-term outcomes after treat-all among people living with HIV in China: an interrupted time series analysis. Infect Dis Poverty. 2023;12(1):73. doi:10.1186/s40249-023-01119-7
8. Liu X, Wang D, Hu J, et al. Changes in HIV-1 subtypes/sub-subtypes, and transmitted drug resistance among ART-Naïve HIV-infected individuals - China, 2004-2022. China CDC Wkly. 2023;5(30):664–671. doi:10.46234/ccdcw2023.129
9. Msosa TC, Swai I, Aarnoutse R, et al. The effect of real-time medication monitoring-based digital adherence tools on adherence to antiretroviral therapy and viral suppression in people living with HIV: a systematic literature review and meta-analysis. J Acquir Immune Defic Syndr. 2024;96(5):411–420. doi:10.1097/QAI.0000000000003449
10. Bernabé KJ, Siedner M, Tsai AC, Marconi VC, Murphy RA. Detection of HIV virologic failure and switch to second-line therapy: a systematic review and meta-analysis of data from sub-saharan Africa. Open Forum Infect Dis. 2022;9(5):ofac121. doi:10.1093/ofid/ofac121
11. He N. Research progress in the epidemiology of HIV/AIDS in China. China CDC Wkly. 2021;3(48):1022–1030. doi:10.46234/ccdcw2021.249
12. Kitonsa J, Kansiime S, Kusemererwa S, et al. Changes in self-reported risky sexual behaviour indicators among adults receiving regular risk reduction counselling and optional initiation of pre-exposure prophylaxis in an HIV vaccine preparedness study in Masaka, Uganda. Glob Health Action. 2023;16(1):2242672. doi:10.1080/16549716.2023.2242672
13. Mendonça Gil PK, Conrado DDS, Nascimento AID, et al. HIV pre-exposure prophylaxis and incidence of sexually transmitted infections in Brazil, 2018 to 2022: an ecological study of PrEP administration, syphilis, and socioeconomic indicators. PLoS Negl Trop Dis. 2023;17(8):e0011548. doi:10.1371/journal.pntd.0011548
14. Wu Z, McGoogan JM, Detels R. The enigma of the human immunodeficiency virus (HIV) epidemic in China. Clin Infect Dis. 2021;72(5):876–881. doi:10.1093/cid/ciaa835
15. Hajarizadeh B, Kairouz A, Ottaviano S, et al. Global, regional, and country-level coverage of testing and treatment for HIV and hepatitis C infection among people who inject drugs: a systematic review. Lancet Glob Health. 2023;11(12):e1885–e98. doi:10.1016/S2214-109X(23)00461-8
16. Attia S, Egger M, Mueller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–1404. doi:10.1097/QAD.0b013e32832b7dca
17. Yapa HM, Kim HY, Petoumenos K, et al. CD4+ T-cell count at antiretroviral therapy initiation in the “treat-all” era in rural South Africa: an interrupted time series analysis. Clin Infect Dis. 2022;74(8):1350–1359. doi:10.1093/cid/ciab650
18. Ford N, Eshun-Wilson I, Ameyan W, et al. Future directions for HIV service delivery research: research gaps identified through WHO guideline development. PLoS Med. 2021;18(9):e1003812. doi:10.1371/journal.pmed.1003812
19. Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–e47. doi:10.1016/S2352-3018(18)30132-2
20. Lockman S, Holme MP, Makhema J, et al. Implementation of universal HIV testing and treatment to reduce HIV incidence in Botswana: the Ya Tsie study. Curr HIV/AIDS Rep. 2020;17(5):478–486. doi:10.1007/s11904-020-00523-0
21. Msefula MC, Umar E. Correlates of depression in ART adherence among youths in Lilongwe, Malawi. Trop Med Infect Dis. 2023;9(1). doi:10.3390/tropicalmed9010002
22. Tebas P, Henry K, Mondy K, et al. Effect of prolonged discontinuation of successful antiretroviral therapy on CD4(+) T cell decline in human immunodeficiency virus-infected patients: implications for intermittent therapeutic strategies. J Infect Dis. 2002;186(6):851–854. doi:10.1086/342603
23. Pilotto JH, Grinsztejn B, Veloso V, et al. HIV drug resistance after HAART discontinuation among treatment-naive women who initiate antiretroviral therapy for the prevention of mother-to-child transmission in Rio de Janeiro, Brazil. Antivir Ther. 2009;14(4):A167–A.
24. Liu XJ, McGoogan JM, Wu ZY. Human immunodeficiency virus/acquired immunodeficiency syndrome prevalence, incidence, and mortality in China, 1990 to 2017: a secondary analysis of the global burden of disease study 2017 data. Chin Med J. 2021;134(10):1175–1180. doi:10.1097/CM9.0000000000001447
25. Li M, Cao Y, Huang H, et al. Cost-effectiveness analysis of antiretroviral drugs for treatment-naive HIV infection in China. BMC Public Health. 2023;23(1):2228.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

