Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
The Effects of Hyperlipidemia on Postoperative Cognitive Dysfunction in Patients Undergoing Laparoscopic Gynecological Tumor Surgery
Authors Shen H , Xiong Y, Liang Y, Liu Z, Guo L, Qin Y, He J, Lin F
Received 14 November 2024
Accepted for publication 4 February 2025
Published 13 February 2025 Volume 2025:21 Pages 259—269
DOI https://doi.org/10.2147/NDT.S506570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yu-Ping Ning
Honglei Shen,1,2,* Yao Xiong,2,* Yubing Liang,1 Zhen Liu,1 Liang Guo,1 Yi Qin,2 Jing He,1,2 Fei Lin1
1Department of Anesthesiology, Guangxi Medical University Cancer Hospital, Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China; 2Guangxi Medical University, Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Lin, Guangxi Medical University Cancer Hospital, 71 hedi Road, Qingxiu District, Nanning, Guangxi, CA 530021, People’s Republic of China, Tel +8613707886172, Email [email protected]
Background: POCD is a common postoperative complication associated with anesthesia and surgery. Its occurrence reduces the quality of life after surgery and increases the risk of death and disability. Related studies demonstrated that hyperlipidemia could affect the occurrence and development of patients’ cognitive dysfunction. However, the association between hyperlipidemia and POCD has not been well examined.
Methods: A total of 98 patients were divided into hyperlipidemia group and normal lipid group based on blood lipid level. Minimum Mental State Examination was used to evaluate the preoperative cognitive status. The patients were assessed with the Montreal Cognitive Assessment 1 day before operation and 7 days after operation. The Telephone Interview for Cognitive Status-Modified was applied to evaluate the cognitive ability 1 and 3 months after surgery. We analyzed the incidence of POCD and its potential risk factors according to the scores on each scale after operation.
Results: A total of 98 patients including 52 patients in the hyperlipidemia group and 46 patients in the normal blood lipid group completed all postoperative follow-up. POCD occurred in 27 patients 7 days after surgery, including 19 patients (36.54%) in the hyperlipidemia group and 8 patients (17.39%) in the normal group. One month after surgery, 23 patients experienced POCD, with 17 (32.69%) in the hyperlipidemia group and 6 (13.04%) in the normal group. And three months after surgery, 20 patients experienced POCD, including 14 (26.92%) in the hyperlipidemia group and 6 (13.04%) in the normal group. Through univariate and multivariate regression analysis, we found that age and total cholesterol level were significantly associated with the occurrence of POCD.
Conclusion: Preoperative hyperlipidemia was associated with the occurrence of POCD 7 days and 1 month after laparoscopic gynecologic tumor surgery. Age and preoperative hypercholesterolemia were considered independent risk factors for POCD development.
Keywords: POCD, lipid metabolism disorders, perioperative management, laparoscopic surgery
Introduction
Perioperative neurocognitive disorder (PND) is a type of cognitive dysfunction that occurs both before and after surgery. It includes conditions such as postoperative delirium (POD), postoperative cognitive dysfunction (POCD), and other related manifestations. According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the diagnostic criteria for PND include preoperative cognitive decline, postoperative delirium, delayed neurocognitive recovery, and postoperative cognitive dysfunction.1,2
Postoperative delirium (POD) is a temporary condition marked by acute cognitive impairment. It is characterized by reduced awareness of the environment, varying symptoms throughout the day, and difficulty in focusing, maintaining, or shifting attention. The symptoms of postoperative delirium can vary. Patients may exhibit hyperactive, hypoactive, or mixed cognitive and motor states. In contrast, hypoactive patients often appear calm but may show inattention, reduced mobility, and difficulty answering simple orientation questions.3
Postoperative cognitive dysfunction (POCD) is a common postoperative complication, especially in elderly patients. POCD typically manifests as a decline in cognitive functions, such as the decline of learning and memory ability, inattention, and executive dysfunction.1 Assessment of POCD typically relies on standardized neuropsychological tests that are administered before and after surgery in order to compare changes in cognitive function in patients. Preoperative tests should not be performed on the day of surgery because the results can be affected by pain, anxiety, and perioperative medications. Postoperative evaluation should also be performed only after the acute effects of surgery and anesthesia have completely dissipated. Postoperative examinations should be performed at 1 week and 3 months after surgery, depending on the clinical situation.4
Studies indicate that lipid metabolism is closely linked to several physiological processes, including hormone synthesis, vitamin absorption, and nervous system function. In the nervous system, lipids like phospholipids and cholesterol are essential for the growth, repair, and maintenance of neurons.5 However, abnormal blood lipid levels can lead to various diseases, including cardiovascular disease, metabolic syndrome, and neurodegenerative diseases. Therefore, maintaining normal lipid levels is crucial for overall health and neurocognitive function.
In recent years, an increasing number of studies have focused on the relationship between dyslipidemia and perioperative neurocognitive disorders (PND). Numerous studies considered hypertension, diabetes, hyperlipidemia, cerebrovascular disease, and microthrombosis as independent risk factors affecting cognitive function.6–11 Related studies have proved that high levels of low-density lipoprotein cholesterol (LDL-C) and triglyceride are considered to be potential factors that increase the risk of POD and POCD.12 Furthermore, studies involving elderly surgical patients with metabolic syndrome indicate that lower levels of HDL-C are significantly associated with POD. Higher preoperative BMI is also identified as a risk factor for developing POCD.13 Clinical studies have demonstrated that monitoring lipid levels before and after surgery can help identify high-risk patients and support personalized perioperative management. Additionally, more researches have shown that dyslipidemia may worsen the decline in perioperative cognitive function by promoting neuroinflammation and oxidative stress.14
In conclusion, we propose an association between hyperlipidemia and POCD. Therefore, this article mainly explores whether preoperative hyperlipidemia is related to the occurrence and development of POCD, and explores the risk factors of POCD. By analyzing the potential causes, precipitating factors, and risk factors of POCD, we aim to provide effective prevention and treatment strategies, improve prognosis, and enhance the quality of life for patients after anesthesia.
Methods
The study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (approval number: KY2021002), complied with the Declaration of Helsinki, and strictly adhered to local data confidentiality and safety regulations. The current research was registered with the China Clinical Trial Center prior to the commencement of the trial (registration number: ChiCTR2100044287 https://www.chictr.org.cn/showproj.html?proj=123276, Principal investigator: Yao Xiong, Date of registration: 2021-03-14), and a registration number was obtained to ensure the accuracy, authenticity and integrity of the trial. Informed consent was provided to the subjects by the investigators and all subjects and their authorized persons were given sufficient time to consider whether or not to participate and those who consented signed the written informed consent form.
Protocol
A total of 145 patients were included in the study, with 98 patients of them had completion of all postoperative follow-up. Patients who underwent secondary surgery, lost follow-up, and refused to participate were excluded. According to the preoperative blood test results and diagnostic criteria, all the patients were divided into hyperlipemia and normolipemia groups. And two complete cognitive ability evaluations were performed before and 7 days after surgery. According to the plasma lipoprotein profile, hyperlipidemia can be divided into four types, namely, hypercholesterolemia (TC ≥5.2 mmol/l), hypertriglyceridemia (TG ≥1.7 mmol/L), mixed hyperlipidemia (TC ≥5.2 mmol/l, TG ≥1.7 mmol/l), and low high-density lipoprotein cholesterol (HDL-C <1.0 mmol/L).15 Those who met one of the above criteria were diagnosed with hyperlipidemia and therefore included in the hyperlipidemia group.
Inclusion Criteria and Exclusion Criteria
Inclusion criteria: 1. The age of the subjects was older than or equal to 18 years old and younger than 80 years old; 2. Patients who had the ability to read and communicate effectively; 3. No history of serious heart, brain, liver, or kidney-related system diseases; 4. ASA grades I–II; 5. General anesthesia was used for elective or limited laparoscopic gynecological tumor resection; 6. Anesthesia time was 120 minutes or more.
Exclusion criteria: 1. A history of mental illness (such as dementia, depression, or delirium), cerebral infarction, or other neurological diseases; 2. Subjects who were unable to communicate effectively; 3. Subjects with significantly impaired cognitive function were excluded by MMSE score; 4. Subjects who refused to participate in this study.
Date Collection
Baseline data, including demographic data, comorbidities, and the main results of examinations, were collected preoperatively. The demographic data included age, body mass index (BMI), and education level. The main results of the examinations included TC, TG, HDL-C, LDL-C, etc. The patients’ overall status was systematically assessed using the American Society of Anesthesiologists (ASA) classification. Cognitive function was evaluated with the MMSE and the MoCA. Based on educational attainment, individuals were classified as having cognitive impairment if they scored below the following thresholds on the MMSE: illiterates scored less than 17 points, primary school graduates scored less than 20 points, junior high and technical secondary school graduates scored less than 24 points, and high school graduates and above scored less than 27 points.16 We used MoCA to score various cognitive functions in patients, including visuospatial ability, executive function, vocabulary abstraction ability, calculation and attention ability, delayed recall ability, language ability, and spatio-temporal localization ability; the maximum score is 30, with scores of 26 or higher indicating normal cognitive function, and participants with 12 years of education or less receiving an additional point.17 The self-rating depression scale (SDS) was used to determine if the subjects had depression and the severity of depression. The duration of anesthesia, type and dose of anesthetic and additional medicines, fluid balance during anesthesia, and blood pressure were all recorded intraoperatively. Following the operation, the extubation time and VAS score were recorded. MoCA and QoR-15 were used to analyze the incidence of POCD 7 days after surgery, and TICS-M was used to assess POCD incidence 1 and 3 months after surgery.
Anesthesia
A routine preoperative diet was fasted, and no anticholinergic drugs were used. Anesthesia induction protocol consisted of intravenous bolus injections of 0.04 mg/kg Midazolam, 1~1.5 mg/kg Propofol, 0.3~0.5 ug/kg Sufentanil in sequence, and a bolus injection of 0.6 mg/kg Rocuronium bromide after a patient lost consciousness. Anesthesia maintenance using 4~12mg/ (kg∙ h) Propofol + 0. 2~0. 4ug/(kg∙ h) Sufentanil + 1~2ug/ (kg∙ min) Cisatracurium besilate was pumped continuously to maintain the anesthesia, and the muscle relaxant was terminated 30 minutes before the end of surgery. Hemodynamic stability can be maintained by supplementing effective blood volume or using vasoactive drugs during anesthesia when necessary. Infusions were performed according to the clinical practice of the 4–2–1 rule to ensure hemodynamic stability and normal urine output during surgery. Specifically, the fluctuation of heart rate and mean arterial pressure did not exceed the patient’s baseline level by more than 20%, and urine output should not be fewer than 1mL/kg per hour. All the patients were resuscitated in the postanesthsia care unit (PACU) and were routinely monitored. Flurbiprofen axetil was given to assist analgesia, and Roxatidine was given to protect the gastric mucosa. When the patients were awake, the tracheal tube was removed after assessing the conditions for extubation. Then aerosol treatment was performed, and the postoperative intravenous patient-controlled analgesia pump was connected. After 30 minutes of observation, the patient was sent back to the ward if there was no obvious adverse reaction, the vital signs were stable, and the Steward score reached 6 or above. For the first 2 days after the operation, all the patients received continuous pain relief by using a patient-controlled analgesia pump.
Statistical Analysis
At present, there is no clear diagnostic criteria for POCD in the world. As this study did not set up a healthy control group, the Z-score method18 was therefore not used to determine POCD, and the standard deviation method recommended by ISPOCD that mainly uses the variability of an individual before and after in the population to reflect changes in cognitive function was applied. The specific method was as follows: The total scores of each MOCA subtest for each subject one day before surgery were calculated, and these scores were compared with the postoperative scores to obtain the difference. The occurrence of POCD was defined as a difference of at least one standard deviation. Cognitive function was assessed by the TICS-M score at 1 month and 3 months after surgery. If the TICS-M score was lower than 33, a patient was considered to have POCD.19–21
By reviewing the literature and previous studies,22–24 we hypothesized that the incidence of POCD was about 35% in patients with hyperlipidemia and about 10% in patients with normal lipids. We set α (the probability of class I error) = 0.05, 1-β (test efficacy) = 0.9 to obtain z1-0.05 = 1.64 (one-sided) and z1-β = 1.28, respectively. It was assumed that the number of cases in the two groups was the same and substituted the above values with the formula 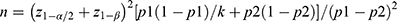 . The estimated sample size of both groups was 43 cases, that is, when the sample size of the two groups was no fewer than 43 cases, and we can detect a statistical significance level of 0.05 at 0.90 test efficacy.
. The estimated sample size of both groups was 43 cases, that is, when the sample size of the two groups was no fewer than 43 cases, and we can detect a statistical significance level of 0.05 at 0.90 test efficacy.
SPSS 25.0 software was employed for statistical analysis, with numerical variables presented as the mean (SD) or median (IQR) depending on distribution. T-test using two independent samples was used to determine the difference between groups if it conformed to the normal distribution, while if it was skewed, the nonparametric rank sum test was used. Categorical variables were shown as counts (%) and analyzed by χ2 test.
Univariate statistical analysis was performed to explore the relationship between variables and the occurrence of POCD. To identify risk factors potentially associated with POCD, multiple-regression models were generated by combining potential confounders with exposure factors. Therefore, factors with p < 0.2 were subjected to the logistic regression model for exploring the relationship between exposure factors and POCD while controlling for confounding bias. In the analytical model, odds ratios (ORs) were calculated to determine which preoperative and intraoperative factors might be risk factors for POCD. If p < 0.05, the difference was considered as statistically significant.
Results
Among the 145 patients, 111 patients met the inclusion criteria, of whom 1 underwent reoperation, 5 were lost to follow-up, and 7 withdrew from the study. Finally, a total of 98 patients were included in the study, and the patients were divided into hyperlipidemia group (n = 52) and normal lipid group (n = 46) (Figure 1). Preoperative data including age, ASA grade, years of education, preoperative hemoglobin, homocysteine, albumin, GFP, fasting blood glucose, operative history, preoperative complications, MMSE score, MOCA score, and preoperative chemotherapy were analyzed. We did not detect significant difference between the hyperlipidaemia group and the normal blood lipid group (Table 1).
 |
Table 1 Comparison of the Perioperative Data of Hyperlipidemia and Normal Lipid Group |
 |
Figure 1 Flowchart for enrolling patients in the samples. |
The results of statistical analysis of the intraoperative data on the length of surgery, duration of anesthesia, use of anesthetic drugs, amount of input and output as well as post-operative data such as the depression score, extubation time, VAS rating, QoR-15 score, and durations of hospitalization showed no statistical differences between the two groups (Table 1).
Seven days after surgery, 19 patients (36.54% of 52 cases) in the hyperlipidemia group and 8 patients (17.39% of 46 cases) in the normal blood lipid group developed POCD. 1 month after surgery, 17 patients (32.69% of 52 cases) in the hyperlipidemia group and 6 patients (13.04% of 46 cases) in the normal blood lipid group developed POCD. 3 months after surgery, 14 patients (26.92% of 52 cases) in the hyperlipidemia group and 6 patients (13.04% of 46 cases) in the normal blood lipid group developed POCD. After operation, the incidence of POCD in the hyperlipidemia group was significantly higher than that in the control group at 7 days (p = 0.034) and 1 month (p = 0.022), without significant difference between the two groups at 3 months postoperatively (Table 2).
 |
Table 2 Comparison of Incidence Rates of POCD Between the Two Groups |
We conducted binary logistic regression analysis on the results and the factors. The results showed that age (p < 0.001, OR: 1.165, 95% CI: 1.087–1.248), years of education (p < 0.001, OR: 0.548, 95% CI: 0.402–0.748), total cholesterol (p < 0.001, OR: 3.804, 95% CI: 1.973–7.334), low-density lipoprotein level (p = 0.001, OR: 2.914, 95% CI: 1.582–5.367) were significantly associated with POCD 7 days after surgery (Figure 2A). Age (p < 0.001, OR: 1.112, 95% CI: 1.050–1.178), years of education (p = 0.001, OR: 0.635, 95% CI: 0.481–0.837), total cholesterol (p < 0.001, OR: 7.539, 95% CI: 3.009–18.888), low-density lipoprotein level (p < 0.001, OR: 6.122, 95% CI: 2.614–14.336) were significantly associated with POCD 1 month after operation (Figure 2B).
In multivariate analysis, age (p = 0.001, B = 0.397, OR: 1.487, 95% CI: 1.167–1.895), total cholesterol (p = 0.003, B = 2.623, OR: 13.772, 95% CI: 2.457–77.205) were found to be the independent risk factors for POCD 7 days after surgery (Figure 3A). And age (p = 0.014, B = 0.263, OR: 1.301, 95% CI: 1.054–1.607), total cholesterol (p = 0.005, B = 2.755, OR: 15.732, 95% CI: 2.306–107.222) were found to be the independent risk factors for POCD 1 month after surgery (Figure 3B).
Discussions
The current results showed a significant difference in the incidence of POCD between the hyperlipidemia group and the control group 7 days and 1 month after the operation. Multivariate analysis demonstrated that age and preoperative serum total cholesterol were the independent risk factors for developing POCD at 7 days and 1 month postoperatively.
Abnormal lipid metabolism due to hyperlipidemia is closely related to neuroinflammation. Studies indicate that dysregulated lipid metabolism affects systemic metabolism and triggers an inflammatory response in the central nervous system. For instance, lipid metabolism disorders can activate microglia. Activated microglia release various proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which are significant in neurodegenerative diseases like Alzheimer’s disease.25 Additionally, abnormal lipid metabolism may alter the composition of cell membranes, affecting neuron function and survival, which can further exacerbate neuroinflammation. On the other hand, hyperlipidemia increases the permeability of the blood-brain barrier (BBB), allowing inflammatory factors and harmful substances to enter the central nervous system, leading to neuroinflammation and neuronal damage.26 A study on blood-brain barrier damage and hyperlipidemia found that lipid metabolism disorders, especially increased cholesterol and triglyceride levels, negatively impact the expression and function of tight junction proteins. This deterioration compromises the integrity of the BBB.27 And the inflammatory response can lead to neuronal damage and promote neurodegenerative changes, creating a vicious cycle. Therefore, modulation of lipid metabolism may be an important strategy to alleviate neuroinflammation and improve cognitive function.
Cholesterol is essential for the nervous system, and if its metabolism is disrupted, it can cause various neurological dysfunctions. Cholesterol is a vital component of cell membranes and also plays a role in releasing neurotransmitters and forming synapses.28 High cholesterol levels disrupt neuronal membrane fluidity and signaling, negatively affecting neuronal survival and function.27 Research indicates that too much cholesterol can increase neuroinflammation, which is closely linked to the development of POCD.29 Additionally, alterations in cholesterol metabolism can influence the expression of non-coding RNAs, further regulating the neuroinflammation and cell death processes related to POCD.30 The exploration of these mechanisms provides a basis for the development of intervention strategies against POCD caused by hypercholesterolemia.
Clinical studies found that elderly patients with hyperlipidemia experienced a significantly higher incidence of postoperative cognitive dysfunction compared to those without hyperlipidemia after surgery.31 In addition, POCD in patients with hyperlipidemia is positively correlated with lipid levels, especially the increase of low-density lipoprotein (LDL) and triglyceride levels, which are closely related to cognitive decline.12 This view is also confirmed in our study. This implies that hyperlipidemia may contribute to cognitive decline by promoting atherosclerosis and microangiopathy, which in turn affects cerebral blood flow and neuronal function. Therefore, interventions targeting hyperlipidemia may help to reduce the risk of postoperative cognitive dysfunction in elderly patients.
Animal experiments have demonstrated the effect of hyperlipidemia on cognitive function. Mice on a high-fat diet show significant deficits in spatial learning and memory tests. These studies revealed neuronal damage and increased inflammation in the hippocampus, further supporting the link between hyperlipidemia and cognitive dysfunction.32 Additionally, studies have shown that high cholesterol levels cause neuronal dysfunction and are linked to neuroinflammation and apoptosis.33 And dysregulated cholesterol metabolism increases neurons’ sensitivity to oxidative stress, accelerating their degeneration.34 Furthermore, cholesterol oxidation products, like 27-hydroxycholesterol, have been shown to induce neuronal damage and apoptosis, possibly serving as a significant mechanism of POCD caused by hypercholesterolemia.35 These findings underscore the essential role of cholesterol metabolism in maintaining neuronal health and cognitive function.
In recent years, research on biomarkers for hyperlipidemia and postoperative cognitive dysfunction has grown. Researchers discovered that specific serum biomarkers, including cholesterol and triglycerides, along with inflammatory markers like C-reactive protein, are strongly linked to changes in cognitive function. These biomarkers not only reflect the severity of hyperlipidemia but also act as potential indicators for predicting postoperative cognitive dysfunction.36 Studies indicate that lower levels of brain-derived neurotrophic factor (BDNF) are linked to cognitive decline in hyperlipidemic patients, suggesting BDNF’s significant role in cognitive impairment. Therefore, future studies should concentrate on the clinical application of these biomarkers to improve assessment and prediction of cognitive function changes in elderly patients’ post-surgery. Moreover, large-scale clinical trials are essential to establish the causal link between hyperlipidemia and postoperative cognitive dysfunction. Most current studies are small observational ones that do not have enough sample size to support the generalizability of their conclusions.37 The collaborative multidisciplinary research approach is crucial for investigating the link between hyperlipidemia and postoperative cognitive dysfunction in elderly patients. Since this field encompasses various disciplines, including internal medicine, surgery, neuroscience, and nutrition, interdisciplinary collaboration can enhance knowledge sharing and spur innovation.38
Our study has certain limitations. Firstly, the sample size included was small, implying a potential presence of heterogeneity. Secondly, the follow-up period was short, and there were no studies on the long-term incidence of POCD. Thirdly, MOCA and TICS-M were employed to evaluate the diagnosis of POCD in this study, and the cognitive range of the assessment was not as wide as that of using multiple tests. Moreover, there could be some learning effects that caused assessment errors. Finally, all the subjects in this study were females. Previous studies also found that the occurrence of POCD in postmenopausal women may be associated with decreased estrogen levels, which may affect the analysis of risk factors for POCD.
Conclusion
Preoperative hyperlipidemia was related to the occurrence of POCD 7 days and 1 month after surgery but was not closely related to the incidence of POCD at the third month postoperatively. Age and preoperative hypercholesterolemia were confirmed as the independent risk factors for the development of POCD.
Abbreviations
ASA, American Society of Anesthesiologists; BMI, Body Mass Index; HDL-C, High Density Lipoprotein Cholesterol; ISPOCD, International Study of Postoperative Cognitive Dysfunction; LDL-C, Low Density Lipoprotein Cholesterol; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; POD, Postoperative delirium; POCD, Postoperative cognitive dysfunction; PND, Perioperative neurocognitive disorders; QoR-15, Quality of Recovery score 15; SDS, Self Rating Depression Scale; TC, Total Cholesterol; TG, Triglyceride; TICS-M, Telephone Interview for Cognitive Status Modified; VAS, Visual Analogue Scale; TNF-α, Tumor necrosis factor-α; IL-1β, Interleukin-1β; BBB, Blood-brain barrier; BDNF, Brain-derived neurotrophic factor.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, Fei Lin, upon reasonable request.
Statement
An unauthorized version of the Chinese MMSE was used by the study team without permission, however this has now been rectified with PAR. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (https://www.parinc.com).
Acknowledgments
We wish to thank the Guangxi Clinical Research Center for Anesthesiology (GK AD22035214), the Guangxi Engineering Research Center for Tissue & Organ Injury and Repair Medicine, and the Guangxi Health Commission Key Laboratory of Basic Science and Prevention of Perioperative Organ Disfunction for their support in this research. And we thank the anesthesiology department of Guangxi Medical University Cancer Hospital for patient recruitment, data collection, and interviews.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81960022, 81560018), Guangxi Natural Science Fund General Project (2020GXNSFAA159123), Guangxi Science Research and Technology Development Program (GK AB18126061), Guangxi Thousands of Young and Middle-Aged Backbone Teacher Training Program and Guangxi Medical High-Level Talents Program (G201903011), Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University, Innovation Project of Guangxi Graduate Education (YCSW2024265).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Tasbihgou SR, Absalom AR. Postoperative neurocognitive disorders. Korean J Anesthesiol. 2021;74(1):15–22. doi:10.4097/kja.20294
2. Xu X, Hu Y, Yan E, Zhan G, Liu C, Yang C. Perioperative neurocognitive dysfunction: thinking from the gut? Aging (Albany NY). 2020;12(15):15797–15817. doi:10.18632/aging.103738
3. Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17(4):376–381. doi:10.1097/MCC.0b013e328348bece
4. SNACC Newsletter. Fall 2018 - Education Corner - Postoperative Cognitive Dysfunction: basics Revisited. Available from: https://snacc.org/wp-content/uploads/2018/fall/ed-corner5.html#top.
5. Guo Q, Cao S, Wang X. Betatrophin and Insulin Resistance. Metabolites. 2022;12(10):925. doi:10.3390/metabo12100925
6. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and Control of Hypertension: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(11):1278–1293. doi:10.1016/j.jacc.2018.07.008
7. Feinkohl I, Winterer G, Pischon T. Diabetes is associated with risk of postoperative cognitive dysfunction: a meta-analysis. Diabetes Metab Res Rev. 2017;33(5). doi:10.1002/dmrr.2884
8. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi:10.1212/01.WNL.0000149519.47454.F2
9. Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi:10.1159/000231980
10. Mielke MM, Zandi PP, Shao H, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75(21):1888–1895. doi:10.1212/WNL.0b013e3181feb2bf
11. Korf ESC, Scheltens P, Barkhof F, de Leeuw FE. Blood pressure, white matter lesions and medial temporal lobe atrophy: closing the gap between vascular pathology and Alzheimer’s disease? Dement Geriatr Cogn Disord. 2005;20(6):331–337. doi:10.1159/000088464
12. Li R, Zhang Y, Zhu Q, Wu Y, Song W. The role of anesthesia in peri‑operative neurocognitive disorders: molecular mechanisms and preventive strategies. Fundam Res. 2024;4(4):797–805. doi:10.1016/j.fmre.2023.02.007
13. Feinkohl I, Janke J, Slooter AJC, et al. Metabolic syndrome and the risk of postoperative delirium and postoperative cognitive dysfunction: a multi-centre cohort study. Br J Anaesth. 2023;131(2):338–347. doi:10.1016/j.bja.2023.04.031
14. Liu L, Shang L, Jin D, Wu X, Long B. General anesthesia bullies the gut: a toxic relationship with dysbiosis and cognitive dysfunction. Psychopharmacology (Berl). 2022;239(3):709–728. doi:10.1007/s00213-022-06096-7
15. Joint committee issued Chinese guideline for the management of dyslipidemia in adults. 2016 Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(10):833–853. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
16. Tsoi KKF, Chan JYC, Hirai HW, Wong SYS, Kwok TCY. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450–1458. doi:10.1001/jamainternmed.2015.2152
17. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J American Geriatrics Society. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
18. Moller J, Cluitmans P, Rasmussen L, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. The Lancet. 1998;351(9106):857–861. doi:10.1016/S0140-6736(97)07382-0
19. de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18(4):318–324. doi:10.1002/gps.830
20. Bentvelzen AC, Crawford JD, Theobald A, et al. Validation and normative data for the modified telephone interview for cognitive status: the Sydney memory and ageing study. J Am Geriatr Soc. 2019;67(10):2108–2115. doi:10.1111/jgs.16033
21. Cook SE, Marsiske M, McCoy KJM. The use of the modified telephone interview for cognitive status (tics-m) in the detection of amnestic mild cognitive Impairment. J Geriatr Psychiatry Neurol. 2009;22(2):103–109. doi:10.1177/0891988708328214
22. Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. JAD. 2017;56(1):215–228. doi:10.3233/JAD-160826
23. Evered LA, Silbert BS. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth Analg. 2018;127(2):496–505. doi:10.1213/ANE.0000000000003514
24. He Q, Li Q, Zhao J, et al. Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: a case-control study. Lipids Health Dis. 2016;15(1):146. doi:10.1186/s12944-016-0320-6
25. Shippy DC, Ulland TK. Lipid metabolism transcriptomics of murine microglia in Alzheimer’s disease and neuroinflammation. Sci Rep. 2023;13(1):14800. doi:10.1038/s41598-023-41897-6
26. Tóth ME, Dukay B, Hoyk Z, Sántha M. Cerebrovascular Changes and Neurodegeneration Related to Hyperlipidemia: characteristics of the Human ApoB-100 Transgenic Mice. Curr Pharm Des. 2020;26(13):1486–1494. doi:10.2174/1381612826666200218101818
27. Xie P, Kancherla K, Chandramohan S, et al. Involvement of single nucleotide polymorphisms of junction adhesion molecule with small vessel vascular dementia. Aging Med (Milton). 2023;6(4):347–352. doi:10.1002/agm2.12278
28. Shaheen A, Richter Gorey CL, Sghaier A, Dason JS. Cholesterol is required for activity-dependent synaptic growth. J Cell Sci. 2023;136(22):jcs261563. doi:10.1242/jcs.261563
29. Wilson KA, Wang L, O’Mara ML. Site of Cholesterol Oxidation Impacts Its Localization and Domain Formation in the Neuronal Plasma Membrane. ACS Chem Neurosci. 2021;12(20):3873–3884. doi:10.1021/acschemneuro.1c00395
30. Yang YS, He SL, Chen WC, et al. Recent progress on the role of non-coding RNA in postoperative cognitive dysfunction. Front Cell Neurosci. 2022;16:1024475. doi:10.3389/fncel.2022.1024475
31. You F, Ma C, Sun F, Liu L, Zhong X. The risk factors of heart failure in elderly patients with Hip fracture: what should we care. BMC Musculoskelet Disord. 2021;22(1):832. doi:10.1186/s12891-021-04686-8
32. Solomonides A. Review of Clinical Research Informatics. Yearb Med Inform. 2020;29(1):193–202. doi:10.1055/s-0040-1701988
33. Pinchaud K, Masson C, Dayre B, et al. Cell-Type Specific Regulation of Cholesterogenesis by CYP46A1 Re-Expression in zQ175 hD Mouse Striatum. Int J mol Sci. 2023;24(13):11001. doi:10.3390/ijms241311001
34. Allende LG, Natalí L, Cragnolini AB, et al. Lysosomal cholesterol accumulation in aged astrocytes impairs cholesterol delivery to neurons and can be rescued by cannabinoids. Glia. 2024;72(10):1746–1765. doi:10.1002/glia.24580
35. Loera-Valencia R, Ismail MAM, Goikolea J, et al. Hypercholesterolemia and 27-hydroxycholesterol increase s100a8 and rage expression in the brain: a link between cholesterol, alarmins, and neurodegeneration. mol Neurobiol. 2021;58(12):6063–6076. doi:10.1007/s12035-021-02521-8
36. Tinkler L, Robertson S, Tod A. Multi-professional perceptions of clinical research delivery and the Clinical Research Nurse role: a realist review. J Res Nurs. 2022;27(1–2):9–29. doi:10.1177/17449871211068017
37. Wang P, Yin X, Chen G, et al. Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: a randomised double-blind and placebo-controlled trial. Clin Nutr. 2021;40(1):64–71. doi:10.1016/j.clnu.2020.05.001
38. Ren S, Yuan F, Yuan S, Zang C, Zhang Y, Lang B. Early Cognitive Dysfunction in Elderly Patients after Total Knee Arthroplasty: an Analysis of Risk Factors and Cognitive Functional Levels. Biomed Res Int. 2022;2022(1):5372603. doi:10.1155/2022/5372603
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



