Back to Journals » International Journal of Nanomedicine » Volume 19
The Effects of Mesenchymal Stem Cells-Derived Exosomes on Metabolic Reprogramming in Scar Formation and Wound Healing
Authors Gong X , Zhao Q, Zhang H, Liu R, Wu J, Zhang N, Zou Y, Zhao W, Huo R , Cui R
Received 1 June 2024
Accepted for publication 17 September 2024
Published 24 September 2024 Volume 2024:19 Pages 9871—9887
DOI https://doi.org/10.2147/IJN.S480901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Xiangan Gong,1,2 Qian Zhao,1,2 Huimin Zhang,1,2 Rui Liu,1,2 Jie Wu,1,2 Nanxin Zhang,3 Yuanxian Zou,1,2 Wen Zhao,1,2 Ran Huo,1,2,4 Rongtao Cui1– 4
1Department of Burn and Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China; 2Medical Science and Technology Innovation Center, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, People’s Republic of China; 3School of Clinical Medicine, Shandong Second Medical University, Weifang, People’s Republic of China; 4Department of Burn and Plastic Surgery, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
Correspondence: Rongtao Cui, Department of Burn and Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China, Tel +86 18653170822, Email [email protected]
Abstract: Pathological scarring results from aberrant cutaneous wound healing due to the overactivation of biological behaviors of human skin fibroblasts, characterized by local inordinate inflammation, excessive extracellular matrix and collagen deposition. Yet, its underlying pathogenesis opinions vary, which could be caused by increased local mechanical tension, enhanced and continuous inflammation, gene mutation, as well as cellular metabolic disorder, etc. Metabolic reprogramming is the process by which the metabolic pattern of cells undergoes a systematic adjustment and transformation to adapt to the changes of the external environment and meet the needs of their growth and differentiation. Therefore, the abnormality of metabolic reprogramming in cells within wounds and scars attaches great importance to scar formation. Mesenchymal stem cells-derived exosomes (MSC-Exo) are the extracellular vesicles that play an important role in tissue repair, cancer treatment as well as immune and metabolic regulation. However, there is not a systematic work to detail the relevant studies. Herein, we gave a comprehensive summary of the existing research on three main metabolisms, including glycometabolism, lipid metabolism and amino acid metabolism, and MSC-Exo regulating metabolic reprogramming in wound healing and scar formation for further research reference.
Keywords: mesenchymal stem cells-derived exosomes, metabolic reprogramming, scar, wound healing, fibroblast
Background
Hypertrophic scar (HS) and keloids are the two most common pathological scars, causing both psychological burdens and physiological deformations for patients.1,2 However, the pathogenesis and shortage of effective therapies hinder clinical treatment. Thus, it is urgent to explore the etiology and develop potential therapeutics.
The process of wound healing experiences hemostasis, inflammation, proliferation and remodeling stages.3 Although the exact pathogenesis of pathological scars remains unclear, the excessive activities of local fibroblasts were the foundation during wound healing and scar formation.4,5 However, the biological functions of fibroblasts are greatly decided by the metabolic status of fibroblasts.6–8 Thus, further studying the mechanism of metabolic reprogramming of fibroblasts during wound healing and scar formation would provide theoretical references for other researchers and develop more effective therapeutics for pathological scars.
Exosomes are nano-sized extracellular vesicles (EVs) that serve as mediators between cell communication.9 The origination of exosomes varies. However, mesenchymal stem cells-derived exosomes (MSC-Exo) have especially attracted much attention for their cell-free feature, immunomodulatory and regenerative functions in the treatment of HS and keloids by promoting wound healing and tissue repair through their cargo, including proteins, lipids and nucleic acids.9–11 Our previous study demonstrated that the miRNA-138-5p loaded in MSC-Exo could attenuate pathological scars by targeting silent information regulator 1 (SIRT1) and further inhibit the biological behaviors of human scar fibroblasts.12 Recent studies suggest that exosomes play an important role in metabolic reprogramming.13–15 The research in MSC-Exo-mediated metabolic reprogramming in wound healing and scar formation achieved great progress due to the importance of metabolic reprogramming of fibroblasts in wound healing and scar formation.16–19 Yet, the clinical application of MSC-Exo still faces major challenges such as production and isolation methods, and ideal cell sources.20,21
Presently, the principal clinical strategies for preventing and treating pathological scars involve steroid injections and the external application of silicone gel adjuncts, other treatments like the localized injection of botulinum toxin and radiation therapy are utilized. However, these treatments lack adequate effectiveness and broad applicability. Considering that inflammation is a proven initiating factor in the formation of scars, and given the distinctive metabolic and immunoregulatory mechanisms of MSC-Exo, MSC-Exo is anticipated to be an effective therapeutic agent in the treatment of pathological scars.
However, the role of MSC-Exo regulates metabolic reprogramming in wound healing and scar formation lacks a systematic review. Therefore, the present study aims to provide an overview of how MSC-Exo promotes wound healing and tissue repair processes to attenuate the formation of pathological scars by regulating metabolic reprogramming and discuss current assessment challenges and fundamental insights leading to future clinically relevant exosome therapy directions.
The Effects of MSC-Exo on Scar Formation
Overview of MSC-Exo
Exosomes are one kind of extracellular vesicle (EV) with diameters ranging from 30 to 150 nm, secreted by nearly all cells and found in all body fluids including blood, urine, saliva, cerebrospinal fluid, and semen.22 The lipid bilayer of the exosomes safeguards internal constituents like proteins, messenger RNA (mRNA), transfer RNA (tRNA), long non-coding RNA (lncRNA), mitochondrial DNA (mtDNA), microRNA (miRNA), and lipids, thus facilitating their biological roles.23–25 Exosomes identify and attach to specific receptors on the cell surface and deliver their cargo into target cells either by fusing directly with the cell membrane or by endocytosis, thus orchestrating the regulation of intercellular signaling.26–28 In addition to conveying specific signaling molecules and cytokines that impact the transcription and translation in target cells, exosomes also serve as biological vehicles for transporting a multitude of bioactive elements, contributing to signal transduction.29
Mesenchymal stem cells (MSCs), known for their strong exosome production and features such as widespread tissue presence, self-renewal, and multi-lineage differentiation potential, are applied in clinical studies across multiple areas including cancer, immune responses, neurological and cardiovascular diseases.30 It is currently understood that MSCs mainly positively influence skin wound healing via paracrine mechanisms.31 However, the use of MSCs in therapy poses issues such as tumorigenicity, immune rejection, and ethical concerns. More and more studies consider exosomes derived from MSCs (MSC-Exo) as potential substitutes for MSC-based therapies. MSC-EXO regulates local inflammation, oxidative stress, and metabolism by transferring cargoes such as proteins, lipids, and nucleic acids between cells. Compared to MSC therapy, MSC-Exo offers a cell-free treatment option with lower immunogenicity and a decreased risk of tumorigenesis.32
Function and Mechanism of MSC-Exo
MSC-Exo are primarily derived from adipose-derived stem cells (ADSC), bone marrow-derived stem cells (BMSC), human umbilical cord mesenchymal stem cells (hUC-MSC), and other stem cell types. Excessive inflammatory responses facilitate scar formation and fibrosis rather than tissue regeneration. Effectively managing the transition from the inflammatory phase to the proliferative phase may be key to minimizing scar formation and preventing pathological scars.33,34 The inflammatory stage is characterized by dysregulation of immune cells, Research indicates that macrophages, T lymphocytes, mast cells, and neutrophils each play a role in scar formation at varying degrees.35 Studies have found that various MSC-Exo influence the activity of fibroblasts, immune cells, and endothelial cells in repairing inflammatory wounds and forming pathological scars. They work by modulating immune and inflammatory responses, promoting angiogenesis, inhibiting the proliferation and differentiation of fibroblasts, and reducing the secretion and deposition of extracellular matrix (ECM) to facilitate wound repair and inhibit the formation of pathological scars.17,36,37 The study by Blazquez et al demonstrated that ADSC-Exo significantly inhibited the proliferation and differentiation of CD 4 and CD 8 T cells and markedly reduced the production of γ interferon, affecting the γ interferon-mediated inflammatory response through immunoregulation, reducing the proportion of M1 macrophage polarization mediated by γ interferon, thus diminishing the release of inflammatory factors, thereby alleviating inflammation-induced impaired wound healing and scar fibrosis.38 Li et al conducted immunohistochemical observations and qRT-PCR analyses to evaluate collagen I and III in wound areas in their research, discovering that ADSC-Exo promotes the expression of collagen I and III in the early stages of wound healing, enhancing the healing process, and suppresses collagen expression in the later stages, thereby lowering the levels of scarring, revealing that ADSC-Exo plays differing positive roles in the various stages of wound healing.39
In our previous research, we first demonstrated that exosomes derived from MSCs, serving as delivery vehicles for miR-138-5p, can downregulate SIRT1 to inhibit the growth and protein expression of hypertrophic scar fibroblasts (HSF) and mitigate pathological scarring.12 Subsequently, we showed that MSC-exo alleviates HS by inhibiting fibroblasts via the TNFSF-13/HSPG 2 signaling pathway, which confirmed that TNFSF 13 is upregulated in HS tissues and HSF, TNFSF 13 activates the NF-κB pathway by interacting with HSPG 2, regulating the proliferation, migration, fibrosis, and inflammatory response of HSF, while MSC-Exo can reduce the expression of α-SMA and COL1A1, ultimately inhibiting the proliferation, migration, fibrosis, and inflammatory response of HSF.40
The transdifferentiation of fibroblasts into myofibroblasts is a crucial step in the pathogenesis of scar formation. In keloids, macrophages are primarily anti-inflammatory phenotype M2 macrophages responsible for tissue repair and remodeling, rather than the pro-inflammatory phenotype M1 macrophages that dominate the early stages of wound healing.41 M2 macrophages can facilitate the transformation of fibroblasts into myofibroblasts by secreting transforming growth factor-beta (TGF-β). Li et al applied ADSC-Exo with high expression of miR-192-5p to HSFs, which significantly downregulating the level of the fibrogenic marker IL-17 RA, confirming that miR-192-5p in ADSC-Exo can mitigate fibrosis in HSFs and directly target IL-17 RA to regulate the role of the Smad pathway in the formation of hypertrophic scars, reducing collagen deposition and the transdifferentiation of myofibroblasts, ultimately inhibiting the formation of pathological scars.42 Research by Jiang et al has shown that BMSC-Exo can suppress the TGF-β/Smad pathway, thereby inducing proliferation in HaCaT cells and human dermal fibroblasts, which in turn aids the process of wound healing.43 Zhu et al demonstrated that hUC-MSC-Exo can promote wound healing by stimulating fibroblasts to secrete nerve growth factor (NGF), thus supporting the regeneration of skin nerves.44
Increasing evidence indicates that MSC-Exo have therapeutic effects on various types of ischemic diseases. Studies indicate that the deposition of ECM in pathological scars results from hypoxia, and hypoxia-inducible factor 1α (HIF-1α) is highly expressed in keloid tissues. HIF-1α influences the metabolism of nucleosides, amino acids, and sugars under hypoxic conditions, and also regulates the expression of immune cells and pro-inflammatory factors.45,46 Bai and others injected ADSC-Exo pretreated with low doses of hydrogen peroxide into the abdominal transplant flaps of rats with ischemia/reperfusion injury, demonstrating that ADSC-Exo can improve the survival rate of the flaps, reduce ischemia/reperfusion injury, promote neovascularization, and alleviate inflammation and apoptosis in the flaps, unveiling the crucial role of ADSC-Exo in facilitating angiogenesis.47
Application of MSC-Exo
As a promising cell-free therapeutic strategy, MSC-Exo has garnered broad interest in recent clinical studies across multiple disciplines, showing significant therapeutic potential in various diseases. Previous studies have found that sepsis increases oxidative stress in the liver, thus promoting hepatocyte death and liver damage. Research by Cai et al has proven that MSC- Exos deliver miR-26a-5p which mediates the degradation of MALAT1.48 The deficiency of MALAT1 significantly inhibits oxidative stress, and miR-26a-5p from MSC-Exo reduces liver damage in sepsis by silencing MALAT1. Cartilage damage resulting in osteochondral defects affects joint cartilage and subchondral bone tissue. The study by Zhang et al shows that MSC-Exo significantly increases the number of chondrocytes by enhancing proliferation, reducing apoptosis, and boosting recruitment, which simultaneously promotes matrix synthesis, thereby repairing and regenerating critical-sized osteochondral defects.49 This study also observed an increase in exosome-mediated gene expression related to proliferation (PCNA and FGF-2) and anti-apoptosis (Survivin and Bcl-2). In studies on muscle atrophy caused by diabetes, Song et al intravenously injected MSC-Exo into diabetic db/db mice models and found that MSC-Exo increased muscle strength and mass in db/db mice, activating autophagy mediated by AMPK/ULK1, thereby improving muscle atrophy in diabetes.50 In studies on neurological diseases, Venkat et al found that exosomes from bone marrow mesenchymal stem cells of type 2 diabetic rats promote neural repair after stroke in type 2 diabetic rats, with the miR-9/ABCA1 pathway playing a significant role.51
With the progress in the study of MSC-Exo’s mechanisms, there have been progress in the application of MSC-Exo in reducing scar formation and promoting wound healing in recent years. MSC-Exo preconditioned under specific conditions are often endowed with enhanced cytokine secretion and cell modulation capabilities. Yu et al found that atorvastatin-preconditioned BMSC-Exo promote angiogenesis and wound healing in a diabetic rat model.52 Yang and others observed that exposure to 455 nm blue light significantly enhances the angiogenic capabilities of hUC-MSC-Exo.53 Exosome-biomaterials demonstrate unique benefits in preserving exosome activity and improving delivery efficiency. Hu et al developed a GelMA hydrogel embedded with ADSC-Exo using non-covalent forces and physical encapsulation, and discovered that by modulating the mechanism of circ-Snhg 11 delivery of exosomes, diabetic wound healing is accelerated. Additionally, circ-Snhg 11-modified ADSC-Exos enhance the migration, proliferation, and angiogenic potential of endothelial cells.54 This research investigates the function and downstream targets of hypoxia-engineered exosome hydrogels in diabetic wound management, presenting a new potential approach for clinical treatment.
Numerous studies have demonstrated the targeted therapeutic actions of MSC-Exo and its function as a therapeutic carrier, and have to some extent elucidated the mechanisms by which MSC-Exo inhibits pathological scarring. However, there is still a considerable gap in translating these experimental studies into applications, and many issues remain to be resolved, such as the need to improve the extraction and purification efficiency of MSC-Exo, as well as the need for extensive clinical studies to validate the safety and therapeutic efficacy of MSC-Exo targeted treatment and carrier functions. The clinical deployment of MSC-Exo encounters numerous challenges. Therefore, future research will focus on reducing the in vivo clearance rate of MSC-Exo, enhancing its targeting capabilities, and improving the safety and efficacy of MSC-Exo treatment.
Metabolic Reprogramming in Scar Formation
Glycometabolism
Overview of Glycometabolism
Glycometabolism, also known as carbohydrate metabolism, refers to the biochemical process responsible for the synthesis, breakdown, and conversion of carbohydrates within living organisms.55,56 Carbohydrates are essential biomolecules that serve as a major energy source for the body, are involved in cellular structure, and play critical roles in various biological processes.57,58 Glycometabolism primarily focuses on the transformation of sugars and starches into energy and other necessary metabolites.58 This metabolic pathway is crucial for energy production, especially in organisms that rely heavily on glucose as their primary energy source.59,60 The process includes several key phases, such as glycolysis, the Citric Acid Cycle, oxidative phosphorylation gluconeogenesis, glycogenesis and glycogenolysis, each integral to how cells and tissues manage energy resources.61,62
Glycolysis is the initial step in the breakdown of glucose and occurs in the cytoplasm of cells.63,64 It converts glucose into pyruvate, producing a small amount of adenosine triphosphate (ATP, the energy currency of the cell) and nicotinamide adenine dinucleotide (NADH).65,66 What should be noticed is that this process is anaerobic, meaning it does not require oxygen. Following glycolysis, pyruvate is transported into mitochondria and converted into acetyl-CoA, which enters the Citric Acid Cycle.67,68 This cycle is a series of chemical reactions that generates ATP through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins.69,70 The electrons removed during glycolysis and the Citric Acid Cycle are transferred to oxygen in a series of steps in the mitochondria, a process known as oxidative phosphorylation, which produces a significant amount of ATP and is dependent on the presence of oxygen (aerobic).71 Gluconeogenesis is essentially the reverse of glycolysis and involves the synthesis of glucose from non-carbohydrate sources, such as lactate, glycerol, and amino acids.64,72 This process primarily occurs in the liver and to a lesser extent in the kidneys, ensuring that glucose levels in the blood remain stable, especially during fasting or heavy exercise.73 Glycogenesis is the process of glycogen synthesis, where excess glucose is stored as glycogen in liver and muscle tissues, which serves as a readily available storage form of glucose.74,75 Glycogenolysis refers to the breakdown of glycogen back into glucose when energy is needed, ensuring a steady supply of glucose to body tissues, particularly the brain and muscles during fasting or vigorous activity.76,77
Glycometabolism is the fundamental process for providing the energy for all cellular functions, which is intricately linked to other metabolic pathways, including lipid and protein metabolism, and is critical for brain function, muscle activity, and overall energy management.78,79 Disruptions in carbohydrate metabolism can lead to various metabolic disorders, such as diabetes mellitus, hypoglycemia, and metabolic syndrome.57,80 Therefore, Glycometabolism is a complex and essential set of processes that manage how the body utilizes, stores, and generates energy from carbohydrates. Proper regulation of these metabolic pathways is vital for maintaining health and supporting the body’s diverse physiological functions.
Glycometabolism in Scar Formation
Glycometabolism plays a critical role in wound healing and the formation of scars by providing the necessary energy and biosynthetic precursors required for cell proliferation, migration, and extracellular matrix synthesis.81 Carbohydrates, being a primary energy source, are crucial during the increased metabolic demands of tissue repair.
Rapidly dividing cells, such as fibroblasts and keratinocytes in the wound bed, require significant amounts of ATP, which is generated through glycolysis and the Citric Acid Cycle.82 Efficient energy production is essential for all phases of wound healing and scar formation, including inflammation, proliferation, and remodeling.83,84 In addition, The pentose phosphate pathway, which branches from glycolysis, generates ribose-5-phosphate for nucleotide synthesis and NADPH for fatty acid synthesis and maintaining cellular redox status.85 Both are crucial for cell division and for managing oxidative stress during wound healing. Moreover, Glucose, as a precursor, can be converted into several intermediates that serve as precursors for collagen synthesis, which is the essential component of the extracellular matrix in scar formation.86
The previous studies investigated how the upregulation of glycolytic enzymes affects the rate of wound healing, and found that enhancing glycolysis can speed up the healing process by providing more energy and biosynthetic substrates to wound sites, but excessive glycolysis might lead to fibrosis or excessive scarring.87 Numerous studies have explored how impaired glycometabolism in diabetic patients affects wound healing. These studies generally show that hyperglycemia impairs various aspects of cellular function and immune response, leading to slower wound healing rates and increased risk of infection.88 Additionally, research into how dietary modifications, such as carbohydrate intake, influence wound healing outcomes demonstrated that a balanced diet with adequate carbohydrates can improve wound healing, whereas others highlight the benefits of specific diets, like ketogenic diets, in reducing inflammation and oxidative stress.89,90
However, to thoroughly understand the impact of glycometabolism on scar formation, a multidisciplinary approach involving biochemistry, cellular biology, and clinical research is necessary. Future studies might focus on more nuanced aspects of carbohydrate metabolism to scar formation processes would provide more definitive insights, such as the role of specific glycolytic intermediates in cell signaling during wound repair and scar formation, genetic variations in glycometabolism enzymes and their impact on wound healing and scar formation, the development of therapeutic strategies that target glycometabolic pathways to enhance wound healing and reduce scarring.
Lipid Metabolism
Overview of Lipid Metabolism
Lipid metabolism is the process involved in the synthesis, breakdown, and utilization of lipids in the body, which plays a critical role in energy storage, cellular signaling, and the formation of cell membranes.91 Lipid metabolism can be broadly divided into two main categories: catabolism (breakdown of lipids) and anabolism (synthesis of lipids).92 Each of these processes involves a series of biochemical reactions that are essential for maintaining overall energy balance and health.
Lipid catabolism is subdivided into lipolysis and fatty acid oxidation. Lipolysis is the process where triglycerides (the main form of stored fat in the body) are broken down into glycerol and free fatty acids, which primarily occurs in adipose tissue and is regulated by hormones such as adrenaline, noradrenaline, glucagon, and insulin.93,94 After being released from adipose tissue, free fatty acids travel through the bloodstream to other tissues where they can be oxidized to produce energy.93 In the mitochondria of cells, fatty acids undergo fatty acid oxidation (beta-oxidation), a cycle of reactions that systematically breaks down the carbon chains of fatty acids into acetyl-CoA units, which then enter the Citric Acid Cycle to produce ATP.95,96
Whereas, lipid anabolism mainly includes fatty acid synthesis, triglyceride synthesis. Fatty acid synthesis primarily occurs in the liver and to a lesser extent in adipose tissue, involving the conversion of acetyl-CoA and malonyl-CoA (derived from carbohydrates and proteins) into fatty acids, which requires acetyl-CoA carboxylase and fatty acid synthase.97,98 Once fatty acids are synthesized, they are often esterified with glycerol to form triglycerides, which can be stored in adipose tissue or secreted into the blood as very low-density lipoproteins (VLDL).98
Additionally, cholesterol is an essential lipid that serves as a structural component of cell membranes and a precursor for the synthesis of steroid hormones, bile acids, and vitamin D.99 Cholesterol can be ingested from dietary sources or synthesized de novo in the liver.100 The regulation of cholesterol levels involves complex feedback mechanisms and is tightly regulated by the enzyme HMG-CoA reductase.99
Furthermore, lipoproteins are complexes of lipids and proteins that transport lipids through the bloodstream.101 There are several classes of lipoproteins, including chylomicrons, VLDL, low-density lipoproteins (LDL), and high-density lipoproteins (HDL). Each of them plays a specific role in lipid transport and metabolism. For example, LDL is often referred to as “bad cholesterol“ because high levels can lead to plaque formation in arteries, while HDL is known as “good cholesterol” because it helps transport cholesterol away from artery walls.102
However, lipid metabolism is crucial for providing energy, especially during prolonged exercise and periods of fasting. The dysregulation of lipid metabolism is associated with various health disorders, including obesity, type 2 diabetes, cardiovascular diseases, and metabolic syndrome.103–105 Understanding and managing lipid metabolism through diet, exercise, and medication can significantly impact an individual’s health and quality of life. Therefore, lipid metabolism encompasses a complex set of processes that are vital for energy production, cellular structure, and numerous biological functions, which are essential for maintaining health and preventing disease.
Lipid Metabolism in Scar Formation
Lipid metabolism plays a crucial role in various physiological processes, including inflammation, cell signaling, and tissue repair, which are all integral to wound healing and scar formation.106–108 In the context of wound healing and scar formation, lipid metabolism impacts several key processes.
First, fatty acids are fundamental components of cellular membranes. Rapidly proliferating cells in a healing wound require new membranes, which necessitates an increase in lipid synthesis.109 Second, carbohydrates are the primary energy source, while lipids provide a substantial secondary energy reserve, which can be particularly important in prolonged phases of wound healing.110 Third, many lipids, such as phospholipids, steroids, and eicosanoids, function as signaling molecules that regulate inflammation, cell proliferation, and other critical aspects of the healing process.108,111,112 Fourth, Lipid mediators like prostaglandins and leukotrienes, derived from arachidonic acid, are crucial in the inflammatory phase of wound healing, influencing vasodilation, vascular permeability, and leukocyte recruitment.113,114
A previous study from various clinical and experimental studies focusing on the dietary intake of omega-3 fatty acids and their metabolic effects related to wound healing showed that omega-3 fatty acids can enhance wound healing by reducing inflammation and potentially improving collagen deposition, leading to less scarring.115,116 There are studies focused on enzymes involved in lipid metabolism, such as fatty acid synthase (FAS) or lipoprotein lipase (LPL), and their roles in the wound healing process, their results illustrated that upregulation of these enzymes in wound sites correlates with improved healing rates, possibly through increased availability of lipid-based energy and structural components.117 In addition, some other studies explored the lipidomic profiles associated with different types of scarring, such as hypertrophic scars or keloids, they identified specific lipid signatures that predict scar formation or severity, offering targets for therapeutic intervention to minimize scarring.118
Therefore, future investigation into the specific pathways by which lipid metabolites affect fibroblast and keratinocyte function in wound healing as well as advanced lipidomic techniques could develop lipid-based therapies or dietary interventions that modulate the wound healing process or improve skin scarring.
Amino Acid Metabolism
Overview of Amino Acid Metabolism
Amino acid metabolism is a set of biochemical processes that govern the synthesis, breakdown, and conversion of amino acids, which are the building blocks of proteins.119 It is crucial for protein synthesis, energy production, and the generation of other important molecules such as neurotransmitters, hormones, and nucleotides.120,121 Amino acids are central to many metabolic processes and can be categorized as essential and non-essential.79
Non-essential amino acids are synthesized from intermediates of major metabolic pathways. For example, glutamate can be synthesized from α-ketoglutarate, a component of the citric acid cycle.122 While other amino acids, like serine, glycine and cysteine, are derived from compounds like 3-phosphoglycerate (a glycolytic intermediate) and methionine.123 Essential acids are primarily obtained through dietary proteins, which are broken down into their constituent amino acids during digestion and absorbed into the bloodstream.124 Interestingly, essential amino acids cannot be synthesized by the body and must be obtained from the diet, while non-essential amino acids can be synthesized internally from other metabolic intermediates.125,126
Once amino acids are available in the cell, they can be used to synthesize new proteins according to the genetic instructions encoded in DNA, which is known as translation, occurs in the ribosomes and involves the assembly of amino acids into specific sequences forming proteins, which are crucial for countless cellular functions.127,128 On the contrary, the breakdown of amino acids typically begins with the removal of the amino group through a process called deamination. The amino group is usually converted into ammonia, which is subsequently transformed into urea in the liver and excreted in the urine.129 While the remaining part of the amino acid, often referred to as the carbon skeleton, enters various metabolic pathways, primarily for energy production.130 Depending on their structure, these skeletons can be converted into pyruvate, acetyl-CoA, or one of the intermediates of the Citric Acid Cycle.130
The urea cycle is a vital process that occurs in the liver and is responsible for converting potentially toxic ammonia into urea, which is less harmful and can be excreted in the urine.131 This cycle is crucial for detoxifying ammonia produced during amino acid catabolism. Besides, amino acids are also precursors for many biologically important molecules. For instance, tryptophan is a precursor for serotonin (a neurotransmitter) and niacin (vitamin B3); tyrosine is a precursor for dopamine, norepinephrine and thyroid hormones; arginine is a precursor for nitric oxide.132–134
Anyway, amino acid metabolism is essential for maintaining protein turnover, supporting growth and repair, producing energy and synthesizing critical molecules that regulate many aspects of physiology and metabolism.135 Disorders of amino acid metabolism can lead to serious conditions, such as phenylketonuria, maple syrup urine disease, and homocystinuria, which can affect growth, development, and overall health.135,136 Thus, amino acid metabolism encompasses a complex network of processes that are fundamental to supporting life, as they contribute to the structural components of cells, the production of energy, and the synthesis of numerous vital biomolecules.
Amino Acid Metabolism in Scar Formation
Amino acid metabolism is crucial in wound healing and scar formation, primarily because amino acids are the building blocks for protein synthesis, including the production of collagen, which is essential for tissue repair and scar formation.137,138 Amino acid metabolism is also connected to various metabolic pathways that contribute to cell proliferation, immune response, and tissue remodeling.
It has been confirmed that amino acids are essential for repairing damaged tissues and regenerating new tissues. For example, Certain amino acids, like arginine and glutamine, play roles in immune function, influencing both the inflammatory response and the activity of immune cells.139 Moreover, amino acids like glycine, proline, and lysine are direct precursors to collagen, a major component of the extracellular matrix in the skin.140 Importantly, amino acids can act as signals themselves or be involved in the synthesis of signaling molecules that regulate the process of wound healing and scar formation.137
The previous study showed that the supplementation of amino acids like arginine or leucine can enhance wound healing. Thereinto, arginine supplementation enhances collagen synthesis and increases the strength of the healed wound tissue, and ornithine, derived from arginine, plays a role in the synthesis of proline, which is crucial for collagen stabilization in the extracellular matrix, indicating that dietary supplementation with arginine and ornithine might improve wound healing and reduce the risk of abnormal scar formation.141 Identically, leucine supplementation significantly accelerated wound closure rates in diabetic rats compared to controls with improved collagen deposition and reduced inflammation observed in the wounds of leucine-supplemented rats, suggesting that leucine may have therapeutic potential in enhancing wound healing and improving scar attenuation, especially in populations with impaired healing capacities such as diabetic patients.142–144 These studies highlighted potential therapeutic strategies that involve the manipulation of amino acid levels to enhance tissue repair and reduce the risk of pathological scarring.
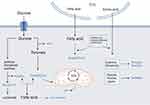
Therefore, the precise mechanisms by which specific amino acids influence the healing process at the molecular and cellular levels could provide us with target therapeutics. Additionally, genetic studies to understand variations in amino acid metabolism among individuals and how these affect wound healing and scar outcomes are promising future research directions (Figure 1).
MSC-Exo Regulates Metabolic Reprogramming in Scar Formation
MSC-Exo Regulates Glycometabolism in Scar Formation
The content and function of MSC-Exo can be influenced by the metabolic state of their cells of origin, which in turn can affect the metabolic status of recipient cells.13 This makes them significant in the context of metabolic diseases such as diabetes, obesity, and metabolic syndrome. In the context of glycometabolism, MSC-Exo can modulate insulin signaling, affect the expression of glucose transporters, and interact with metabolic enzymes and pathways, thereby impacting overall metabolic homeostasis.145–147
Importance of MSC-Exo in Glycometabolism
First of all, MSC-Exo can carry insulin receptors and other key molecules that influence insulin signaling pathways, potentially affecting insulin sensitivity in peripheral tissues.146,148 Furthermore, MSC-Exo can directly influence glucose metabolism pathways in recipient cells by transferring enzymes and regulatory proteins.149 In addition, since metabolic diseases are often associated with chronic inflammation, exosomes can modulate inflammatory responses, which are crucial in the development and progression of metabolic disorders.29,150
MSC-Exo Regulates Glycometabolism in Scar Formation
MSCs are multipotent stromal cells that can differentiate into a variety of cell types. MSC-Exo have been shown to have regenerative properties, including promoting wound healing and alleviating scar formation.150 Their role in glycometabolism during these processes is increasingly recognized as significant.
The improved wound healing and scarring mediated by MSC-Exo is mainly due to its effects on inhibiting the biological behaviors of human skin fibroblasts and promoting new blood vessel formation, which is essential for delivering nutrients and oxygen necessary for tissue repair.12,151 MSC-Exo could also modulate the inflammatory milieu of a wound site, promoting a switch from a pro-inflammatory environment to an anti-inflammatory or pro-healing environment.16,29 Specifically in the context of glycometabolism, MSC-Exo can enhance glucose utilization in wound tissues, potentially accelerating the healing process and improving the quality of the healed tissue.152
While the exact mechanisms through which MSC-Exo regulates glycometabolism in wound healing and scar formation are still under investigation, their ability to modulate glucose and energy metabolism at wound sites presents a promising therapeutic avenue. Future research should focus on elucidating these mechanisms in detail, which could lead to innovative treatments for wound management, particularly in metabolic disorders where wound healing is compromised.
MSC-Exo Regulates Lipid Metabolism in Scar Formation
As cellular communication vehicles, MSC-Exos carry a variety of molecular cargoes including lipids, proteins, and nucleic acids.24,28 They are instrumental in regulating lipid metabolism, which involves the synthesis, transport, storage, and breakdown of lipids.153 MSC-Exo influences these processes by transferring enzymes involved in lipid metabolism, signaling molecules that regulate these enzymes, and RNAs that modulate gene expression related to lipid pathways.145,153
Importance of MSC-Exo in Lipid Metabolism
MSC-Exo directly transports cholesterol, fatty acids, and other lipids between cells, influencing lipid levels and metabolism in recipient cells.154 MSC-Exo can also impact lipid profiles by transferring cholesterol, fatty acids, and enzymes involved in lipid metabolism, thus changing the storage and breakdown of lipids.155,156 Moreover, MSC-Exo carries proteins and miRNAs that can modulate the activity of enzymes involved in lipid synthesis and breakdown, such as lipases and fatty acid synthase.153 Additionally, by affecting signaling pathways, MSC-Exo attaches great importance to the formation and turnover of lipid droplets, which are key cell storage structures.157 Most importantly, MSC-Exo could communicate metabolic states between different tissues, such as adipose tissue, liver, and muscle, thus coordinating systemic lipid metabolism.158,159
MSC-Exo Regulates Lipid Metabolism in Scar Formation
Mesenchymal stem cells (MSCs) are known for their ability to differentiate into various cell types and MSC-Exo plays a pivotal role in promoting tissue repair and modulating immune responses.29,160 MSC-Exo also plays an important role in regulating lipid metabolism in wound healing and scar formation, which is crucial for energy supply and cellular functions during the repair process.
MSC-Exo enhances the utilization of lipids as an energy source in wound healing.161 This is crucial in energy-demanding processes such as cell proliferation and migration. The delivery of specific miRNAs or proteins by MSC-Exo that upregulate or downregulate enzymes involved in lipid metabolism, affecting the local availability of lipids necessary for membrane biosynthesis and signaling during tissue repair.36,162 Furthermore, MSC-Exo modulates the production of lipid mediators such as prostaglandins and leukotrienes that are involved in the inflammatory response during wound healing, regulating the subsequent healing and scarring process.163 In addition, the impact of MSC-Exo on lipid metabolic pathways related to fibroblast proliferation and synthesis of the extracellular matrix could potentially reduce abnormal scar formation.164
Therefore, MSC-Exo plays a multifaceted role in regulating lipid metabolism during wound healing and scar formation. Their ability to transport lipids and lipid-regulating molecules makes them a promising tool in regenerative medicine. Understanding and harnessing these functions could lead to novel therapeutic strategies for improving wound healing and scar attenuation, particularly in metabolic conditions characterized by impaired lipid metabolism. However, further research is still needed to fully elucidate the mechanisms through which MSC-Exo influences lipid metabolism in tissue repair processes.
MSC-Exo Regulates Amino Acid Metabolism in Scar Formation
MSC-Exo is involved in the regulation, distribution, and utilization of amino acids within and between tissues by transporting various biomolecules during the process of amino acid metabolism, including the transport of enzymes, regulatory proteins, and RNAs that are involved in the synthesis, breakdown, and conversion of protein.165
Importance of Exosomes in Amino Acid Metabolism
MSC-Exo helps coordinate metabolic activities between different cells, tissues and organs through the transfer of metabolic information, ensuring a systemic metabolic balance. By delivering microRNAs and other non-coding RNAs, MSC-Exo can influence the expression of genes involved in amino acid metabolism, thereby altering the metabolic profile of target cells.166 Additionally, the enzymes that directly participate in amino acid metabolism or proteins could be carried by MSC-Exo, allowing for the fine-tuning of metabolic pathways in recipient cells.13 Furthermore, MSC-Exo is also involved in nutrient signaling mechanisms, relaying information about nutrient availability and requirements between cells and tissues, which is crucial during physiological processes like growth, immune responses, and tissue repair.167,168
MSC-Exo Regulates Amino Acid Metabolism in Scar Formation
Protein synthesis is necessary for the repair and regeneration of damaged tissues. And the role of MSC-Exo in modulating amino acid metabolism is crucial for protein synthesis, cellular function, and energy production during wound healing and scar formation.169 Whereas the tRNAs, aminoacyl-tRNA synthetases, and mRNAs delivered by MSC-Exo play a key role in it.170,171
Amino acid metabolism is critical for the function of fibroblasts, which play a key role in wound healing and scar formation. The previous study showed that the proliferation, differentiation, and collagen synthesis of fibroblasts is greatly inhibited by MSC-Exo through the modulation of amino acid metabolism.172 As is known that autophagy is a process that recycles cellular components, including proteins, during stress and starvation.173,174 Interestingly, MSC-derived exosomes could modulate such pathways, thereby affecting amino acid recycling and availability during the healing process.175
The role of MSC-Exo in regulating amino acid metabolism during wound healing and scar formation is a promising area of research, which not only supports the structural rebuilding of tissues but also ensures the proper metabolic environment for healing processes. Therefore, it is necessary to fully understand the mechanisms by which MSC-Exo influences amino acid metabolism and how this can be harnessed in therapeutic contexts, particularly in chronic wounds or in conditions with impaired healing and scar formation.
Discussion
Pathological scars are the excessive repair of local tissues when the injury is up to the reticular dermis.2 In particular, the overactivation of local human skin fibroblasts, resulting in excessive extracellular matrix and collagen deposition, is deemed to be the main pathogenesis of aberrant scar proliferation.176,177 However, the molecular mechanisms of functional change of fibroblasts remain unclear.
Metabolic reprogramming refers to a process that the cells undergo a systematic adjustment and transformation to adapt to the changes of the external environment and meet the needs of their growth and differentiation.178,179 Thus, the metabolic reprogramming of fibroblasts, including three main metabolic processes such as glycometabolism, lipid metabolism and amino acid metabolism, could greatly affect the function status of fibroblasts.7,180 Previous studies demonstrated that metabolic reprogramming is a driver of fibroblast activation, resulting in pulmonary, renal, and liver fibrosis.7,181,182 However, few studies were reported to elucidate the metabolic reprogramming of fibroblasts in cutaneous scarring. In 2018, Qi Li et al confirmed that keloids underwent a reprogrammed metabolic phenotype of aerobic glycolysis, which was essential for keloid hyperplasia, and glycolytic inhibitors might provide a potential treatment for keloids.183 Later, Rong Huang et al showed that polypyrimidine tract binding regulates aerobic glycolysis and the cell functions of keloid fibroblasts via alternative splicing of pyruvate kinase muscle.184 Therefore, further exploration of metabolic mechanisms within wound healing and scarring is extremely needed to understand etiology and develop effective therapeutics.
MSC-Exo was proven to improve wound healing and attenuate scar formation, resting with its modulatory functions in local inflammation, oxidative stress, and metabolism by transferring cargo like protein, lipid and nucleic acid between cells.161,168,185 Our previous study proved that MSC-Exo-loaded miR-138-5p may significantly alleviate pathological scarring by inhibiting biological behaviors of human skin fibroblasts via targeting silent information regulator 1 (SIRT1).12 Additionally, recent studies illustrated that MSC-Exo plays a pivotal role in tumor progression and tumor microenvironment by rewriting the metabolic processes in tumor cells and environmental stromal cells.13,186 Moreover, the previous study by Shihao Xu et al demonstrated that MSC-Exo significantly ameliorated unilateral ureter obstruction-induced renal fibrosis by inhibiting glycolysis in tubular epithelial cells via delivery miR-21a-5p.187 Therefore, MSC-Exo may improve wound healing and scarring by mediating metabolic reprogramming of microenvironmental fibroblasts, either key enzymes or pathways, which require further study.
In conclusion, MSC-Exo regulates the metabolic status of fibroblasts is crucial to accelerated wound healing and scar alleviation. We are determined to explore the molecular mechanisms of metabolic reprogramming in wound and scar microenvironments, and then further develop MSC-Exo-related treatments, aiming to reveal the pathogenesis and therapeutics of pathological scars.
Funding
This study was financially supported by the National Natural Science Foundation of China (81901968), the Provincial Natural Science Foundation of Shandong province (ZR2019BH051) and the Postdoctoral Science Foundation of China (2018M642667), Shandong Traditional Chinese Medicine Science and Technology Project (M-2023037), Technology Project of National Administration of Traditional Chinese Medicine(GZY-KJS-SD-2023-071).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jeschke MG, Wood FM, Middelkoop E, et al. Scars. Nat Rev Dis Primers. 2023;9(1):64. doi:10.1038/s41572-023-00474-x
2. Knowles A, Glass DA. Keloids and hypertrophic scars. Dermatol Clin. 2023;41(3):509–517. doi:10.1016/j.det.2023.02.010
3. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e–S–32e–S. doi:10.1097/01.prs.0000222562.60260.f9
4. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
5. Martin RF. Wound Healing. Surg Clin North Am. 2020;100(4):ix–xi. doi:10.1016/j.suc.2020.05.012
6. Hwang S, Chung KW. Targeting fatty acid metabolism for fibrotic disorders. Arch Pharm Res. 2021;44(9–10):839–856. doi:10.1007/s12272-021-01352-4
7. Para R, Romero F, George G, Summer R. Metabolic reprogramming as a driver of fibroblast activation in pulmonary fibrosis. Am J Med Sci. 2019;357(5):394–398. doi:10.1016/j.amjms.2019.02.003
8. Gong J, Lin Y, Zhang H, et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11(4):267. doi:10.1038/s41419-020-2434-z
9. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/ijn.S264498
10. Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8):1959. doi:10.3390/cells10081959
11. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi:10.1111/cas.14563
12. Zhao W, Zhang R, Zang C, et al. Exosome derived from mesenchymal stem cells alleviates pathological scars by inhibiting the proliferation, migration and protein expression of fibroblasts via delivering miR-138-5p to target SIRT1. Int J Nanomed. 2022;17:4023–4038. doi:10.2147/ijn.S377317
13. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5(1):242. doi:10.1038/s41392-020-00359-5
14. Xi Y, Shen Y, Chen L, Tan L, Shen W, Niu X. Exosome-mediated metabolic reprogramming: implications in esophageal carcinoma progression and tumor microenvironment remodeling. Cytokine Growth Factor Rev. 2023;73:78–92. doi:10.1016/j.cytogfr.2023.08.010
15. Ye L, Li Y, Zhang S, Wang J, Lei B. Exosomes-regulated lipid metabolism in tumorigenesis and cancer progression. Cytokine Growth Factor Rev. 2023;73:27–39. doi:10.1016/j.cytogfr.2023.05.002
16. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13(1):24. doi:10.1186/s13287-021-02697-9
17. An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi:10.1111/cpr.12993
18. Narayanan S, Eliasson Angelstig S, Xu C, et al. HypoxamiR-210 accelerates wound healing in diabetic mice by improving cellular metabolism. Commun Biol. 2020;3(1):768. doi:10.1038/s42003-020-01495-y
19. Weinstein AL, Lalezarzadeh FD, Soares MA, Saadeh PB, Ceradini DJ. Normalizing dysfunctional purine metabolism accelerates diabetic wound healing. Wound Repair Regen. 2015;23(1):14–21. doi:10.1111/wrr.12249
20. Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. doi:10.7150/thno.41580
21. Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 2019;20(19):4684. doi:10.3390/ijms20194684
22. Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281–294. doi:10.1016/j.bioactmat.2021.08.029
23. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445.e418. doi:10.1016/j.cell.2019.02.029
24. Rao D, Huang D, Sang C, Zhong T, Zhang Z, Tang Z. Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front Bioeng Biotechnol. 2021;9:797359. doi:10.3389/fbioe.2021.797359
25. Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12(1):561. doi:10.1186/s13287-021-02629-7
26. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. doi:10.1186/s12964-021-00730-1
27. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585–606. doi:10.1038/s41580-020-0251-y
28. Sohrabi B, Dayeri B, Zahedi E, et al. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022;29(8–9):1105–1116. doi:10.1038/s41417-022-00427-8
29. Zhao W, Zhang H, Liu R, Cui R. Advances in immunomodulatory mechanisms of mesenchymal stem cells-derived exosome on immune cells in scar formation. Int J Nanomed. 2023;18:3643–3662. doi:10.2147/ijn.S412717
30. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
31. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi:10.3727/096368913x667709
32. Moghadasi S, Elveny M, Rahman HS, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302. doi:10.1186/s12967-021-02980-6
33. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi:10.3390/ijms18030606
34. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. doi:10.1007/s00018-016-2268-0
35. Wang ZC, Zhao WY, Cao Y, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. doi:10.3389/fimmu.2020.603187
36. Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101(9):1254–1266. doi:10.1038/s41374-021-00611-8
37. Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8(1):125. doi:10.1186/s13287-017-0578-2
38. Blazquez R, Sanchez-Margallo FM, de la Rosa O, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. doi:10.3389/fimmu.2014.00556
39. Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. doi:10.1038/srep32993
40. Zhang H, Zang C, Zhao W, et al. Exosome derived from mesenchymal stem cells alleviates hypertrophic scar by inhibiting the fibroblasts via TNFSF-13/HSPG2 signaling pathway. Int J Nanomed. 2023;18:7047–7063. doi:10.2147/ijn.S433510
41. Xu X, Gu S, Huang X, et al. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8:tkaa006. doi:10.1093/burnst/tkaa006
42. Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221. doi:10.1186/s13287-021-02290-0
43. Jiang T, Wang Z, Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther. 2020;11(1):198. doi:10.1186/s13287-020-01723-6
44. Zhu Z, Zhang X, Hao H, et al. Exosomes derived from umbilical cord mesenchymal stem cells treat cutaneous nerve damage and promote wound healing. Front Cell Neurosci. 2022;16:913009. doi:10.3389/fncel.2022.913009
45. Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int J Mol Sci. 2021;22(11):5703. doi:10.3390/ijms22115703
46. Kang Y, Roh MR, Rajadurai S, et al. Hypoxia and HIF-1α regulate collagen production in keloids. J Invest Dermatol. 2020;140(11):2157–2165. doi:10.1016/j.jid.2020.01.036
47. Bai Y, Han YD, Yan XL, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500(2):310–317. doi:10.1016/j.bbrc.2018.04.065
48. Cai J, Tang D, Hao X, Liu E, Li W, Shi J. Mesenchymal stem cell-derived exosome alleviates sepsis- associated acute liver injury by suppressing MALAT1 through microRNA-26a-5p: an innovative immunopharmacological intervention and therapeutic approach for sepsis. Front Immunol. 2023;14:1157793. doi:10.3389/fimmu.2023.1157793
49. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi:10.1016/j.biomaterials.2017.11.028
50. Song J, Liu J, Cui C, et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia, Sarcopenia Muscle. 2023;14(2):915–929. doi:10.1002/jcsm.13177
51. Venkat P, Zacharek A, Landschoot-Ward J, et al. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp Neurol. 2020;334:113456. doi:10.1016/j.expneurol.2020.113456
52. Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. doi:10.1186/s13287-020-01824-2
53. Yang K, Li D, Wang M, et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10(1):358. doi:10.1186/s13287-019-1472-x
54. Hu N, Cai Z, Jiang X, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175–186. doi:10.1016/j.actbio.2022.11.057
55. Peng G, Yan J, Chen L, Li L. Glycometabolism reprogramming: implications for cardiovascular diseases. Prog Biophys Mol Biol. 2023;179:26–37. doi:10.1016/j.pbiomolbio.2023.03.003
56. Mulukutla BC, Yongky A, Le T, Mashek DG, Hu WS. Regulation of glucose metabolism - a perspective from cell bioprocessing. Trends Biotechnol. 2016;34(8):638–651. doi:10.1016/j.tibtech.2016.04.012
57. Chandel NS. Carbohydrate Metabolism. Cold Spring Harb Perspect Biol. 2021;13(1):a040568. doi:10.1101/cshperspect.a040568
58. Zhao L, Hutchison AT, Heilbronn LK. Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2021;24(4):342–348. doi:10.1097/mco.0000000000000756
59. Sano H, Nakamura A, Yamane M, et al. The polyol pathway is an evolutionarily conserved system for sensing glucose uptake. PLoS Biol. 2022;20(6):e3001678. doi:10.1371/journal.pbio.3001678
60. Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia. 2007;55(12):1238–1250. doi:10.1002/glia.20376
61. Wei Y, Miao Q, Zhang Q, et al. Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage. Nat Neurosci. 2023;26(12):2081–2089. doi:10.1038/s41593-023-01476-4
62. Ye L, Jiang Y, Zhang M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. 2022;68:81–92. doi:10.1016/j.cytogfr.2022.11.001
63. Tang BL. Glucose, glycolysis, and neurodegenerative diseases. J Cell Physiol. 2020;235(11):7653–7662. doi:10.1002/jcp.29682
64. Hernández F. Glycolysis and gluconeogenesis: a teaching view. J Biol Chem. 2021;296:100016. doi:10.1016/j.jbc.2020.100016
65. Chi F, Sharpley MS, Nagaraj R, Roy SS, Banerjee U. Glycolysis-independent glucose metabolism distinguishes te from ICM fate during mammalian embryogenesis. Dev Cell. 2020;53(1):9–26.e24. doi:10.1016/j.devcel.2020.02.015
66. Luengo A, Li Z, Gui DY, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81(4):691–707.e696. doi:10.1016/j.molcel.2020.12.012
67. Chandel NS. Glycolysis. Cold Spring Harb Perspect Biol. 2021;13(5):a040535. doi:10.1101/cshperspect.a040535
68. Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71(14):2577–2604. doi:10.1007/s00018-013-1539-2
69. Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys. 2014;68(3):475–478. doi:10.1007/s12013-013-9750-1
70. Arnold PK, Finley LWS. Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem. 2023;299(2):102838. doi:10.1016/j.jbc.2022.102838
71. Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi:10.1016/j.redox.2020.101674
72. Wu X, Chen S. Advances in natural small molecules on pretranslational regulation of gluconeogenesis. Drug Dev Res. 2023;84(4):613–628. doi:10.1002/ddr.22053
73. Shah A, Wondisford FE. Gluconeogenesis flux in metabolic disease. Annu Rev Nutr. 2023;43:153–177. doi:10.1146/annurev-nutr-061121-091507
74. Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–678. doi:10.1152/ajpendo.00492.2002
75. Patino SC, Orrick JA. Biochemistry, Glycogenesis. In: StatPearls. StatPearls Publishing; 2024.
76. Chauhan SS, England EM. Postmortem glycolysis and glycogenolysis: insights from species comparisons. Meat Sci. 2018;144:118–126. doi:10.1016/j.meatsci.2018.06.021
77. Hertz L, Chen Y. Glycogenolysis, an astrocyte-specific reaction, is essential for both astrocytic and neuronal activities involved in learning. Neuroscience. 2018;370:27–36. doi:10.1016/j.neuroscience.2017.06.025
78. Zhang S, Lachance BB, Mattson MP, Jia X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog Neurobiol. 2021;204:102089. doi:10.1016/j.pneurobio.2021.102089
79. Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73(2):377–392. doi:10.1007/s00018-015-2070-4
80. Miller BS, Freeze HH. New disorders in carbohydrate metabolism: congenital disorders of glycosylation and their impact on the endocrine system. Rev Endocr Metab Disord. 2003;4(1):103–113. doi:10.1023/a:1021883605280
81. Clayton RP, Herndon DN, Abate N, Porter C. The effect of burn trauma on lipid and glucose metabolism: implications for insulin sensitivity. J Burn Care Res. 2018;39(5):713–723. doi:10.1093/jbcr/irx047
82. Liu Y, Liu Y, He W, et al. Fibroblasts: immunomodulatory factors in refractory diabetic wound healing. Front Immunol. 2022;13:918223. doi:10.3389/fimmu.2022.918223
83. Grada A, Phillips TJ. Nutrition and cutaneous wound healing. Clin Dermatol. 2022;40(2):103–113. doi:10.1016/j.clindermatol.2021.10.002
84. He Y, Yip SL, Cheung KK, Huang L, Wang S, Cheing GL. The effect of monochromatic infrared energy on diabetic wound healing. Int Wound J. 2013;10(6):645–652. doi:10.1111/j.1742-481X.2012.01039.x
85. TeSlaa T, Ralser M, Fan J, Rabinowitz JD. The pentose phosphate pathway in health and disease. Nat Metab. 2023;5(8):1275–1289. doi:10.1038/s42255-023-00863-2
86. Cechowska-Pasko M, Surazyński A, Bańkowski E. The effect of glucose deprivation on collagen synthesis in fibroblast cultures. Mol Cell Biochem. 2009;327(1–2):211–218. doi:10.1007/s11010-009-0059-8
87. Akkus A, Aydinuraz K, Daphan C, et al. Effect of carnitine on cutaneous wound healing in immunosuppressed rats. J Surg Res. 2009;155(2):301–305. doi:10.1016/j.jss.2008.06.010
88. Wan G, Chen Y, Chen J, et al. Regulation of endothelial progenitor cell functions during hyperglycemia: new therapeutic targets in diabetic wound healing. J Mol Med (Berl). 2022;100(4):485–498. doi:10.1007/s00109-021-02172-1
89. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25(1):61–68. doi:10.1177/0884533609358997
90. Shields BE. Diet in wound care: can nutrition impact healing? Cutis. 2021;108(6):325–328. doi:10.12788/cutis.0407
91. Petrenko V, Sinturel F, Riezman H, Dibner C. Lipid metabolism around the body clocks. Prog Lipid Res. 2023;91:101235. doi:10.1016/j.plipres.2023.101235
92. DeBose-Boyd RA. Significance and regulation of lipid metabolism. Semin Cell Dev Biol. 2018;81:97. doi:10.1016/j.semcdb.2017.12.003
93. Cho CH, Patel S, Rajbhandari P. Adipose tissue lipid metabolism: lipolysis. Curr Opin Genet Dev. 2023;83:102114. doi:10.1016/j.gde.2023.102114
94. Grabner GF, Xie H, Schweiger M, Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab. 2021;3(11):1445–1465. doi:10.1038/s42255-021-00493-6
95. Srivastava A, Srivastava P, Mathur S, et al. Lipid metabolism and mitochondria: cross talk in cancer. Curr Drug Targets. 2022;23(6):606–627. doi:10.2174/1389450122666210824144907
96. Benador IY, Veliova M, Liesa M, Shirihai OS. Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab. 2019;29(4):827–835. doi:10.1016/j.cmet.2019.02.011
97. Wedan RJ, Longenecker JZ, Nowinski SM. Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism. Cell Metab. 2024;36(1):36–47. doi:10.1016/j.cmet.2023.11.017
98. Poitelon Y, Kopec AM, Belin S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells. 2020;9(4):812. doi:10.3390/cells9040812
99. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225–245. doi:10.1038/s41580-019-0190-7
100. Schade DS, Shey L, Eaton RP. Cholesterol review: a metabolically important molecule. Endocr Pract. 2020;26(12):1514–1523. doi:10.4158/ep-2020-0347
101. Mahley RW, Innerarity TL, Rall SC, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984;25(12):1277–1294.
102. Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB j. 2001;15(12):2073–2084. doi:10.1096/fj.01-0273rev
103. Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18(6):e13048. doi:10.1111/acel.13048
104. Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell. 2021;81(18):3708–3730. doi:10.1016/j.molcel.2021.08.027
105. Zadoorian A, Du X, Yang H. Lipid droplet biogenesis and functions in health and disease. Nat Rev Endocrinol. 2023;19(8):443–459. doi:10.1038/s41574-023-00845-0
106. Andersen CJ. Lipid metabolism in inflammation and immune function. Nutrients. 2022;14(7):1414. doi:10.3390/nu14071414
107. Du W, Wang Z, Dong Y, et al. Electroacupuncture promotes skin wound repair by improving lipid metabolism and inhibiting ferroptosis. J Cell Mol Med. 2023;27(16):2308–2320. doi:10.1111/jcmm.17811
108. Sunshine H, Iruela-Arispe ML. Membrane lipids and cell signaling. Curr Opin Lipidol. 2017;28(5):408–413. doi:10.1097/mol.0000000000000443
109. Hegde RS, Keenan RJ. The mechanisms of integral membrane protein biogenesis. Nat Rev Mol Cell Biol. 2022;23(2):107–124. doi:10.1038/s41580-021-00413-2
110. Kuhla B, Metges CC, Hammon HM. Endogenous and dietary lipids influencing feed intake and energy metabolism of periparturient dairy cows. Domest Anim Endocrinol. 2016;56:S2–s10. doi:10.1016/j.domaniend.2015.12.002
111. Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34(11):1675–1699. doi:10.1016/j.cmet.2022.09.023
112. Mutlu AS, Duffy J, Wang MC. Lipid metabolism and lipid signals in aging and longevity. Dev Cell. 2021;56(10):1394–1407. doi:10.1016/j.devcel.2021.03.034
113. Das UN. Essential fatty acids and their metabolites in the pathobiology of inflammation and its resolution. Biomolecules. 2021;11(12):1873. doi:10.3390/biom11121873
114. Yoshikai Y. Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr Opin Infect Dis. 2001;14(3):257–263. doi:10.1097/00001432-200106000-00003
115. McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16(3):337–345. doi:10.1111/j.1524-475X.2008.00388.x
116. Alexander JW, Supp DM. Role of arginine and omega-3 fatty acids in wound healing and infection. Adv Wound Care (New Rochelle). 2014;3(11):682–690. doi:10.1089/wound.2013.0469
117. Pils V, Terlecki-Zaniewicz L, Schosserer M, Grillari J, Lämmermann I. The role of lipid-based signalling in wound healing and senescence. Mech Ageing Dev. 2021;198:111527. doi:10.1016/j.mad.2021.111527
118. Huang C, Ogawa R. Roles of lipid metabolism in keloid development. Lipids Health Dis. 2013;12:60. doi:10.1186/1476-511x-12-60
119. Krick T, Verstraete N, Alonso LG, et al. Amino Acid metabolism conflicts with protein diversity. Mol Biol Evol. 2014;31(11):2905–2912. doi:10.1093/molbev/msu228
120. Paulusma CC, Lamers WH, Broer S, van de Graaf SFJ. Amino acid metabolism, transport and signalling in the liver revisited. Biochem Pharmacol. 2022;201:115074. doi:10.1016/j.bcp.2022.115074
121. Yang L, Chu Z, Liu M, et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J Hematol Oncol. 2023;16(1):59. doi:10.1186/s13045-023-01453-1
122. Wiese EK, Hitosugi S, Buhrow SA, et al. Reductive amination of α-Ketoglutarate in metabolite extracts results in glutamate overestimation. J Chromatogr A. 2020;1623:461169. doi:10.1016/j.chroma.2020.461169
123. Pasini E, Corsetti G, Dioguardi FS. Behind protein synthesis: amino acids-metabokine regulators of both systemic and cellular metabolism. Nutrients. 2023;15(13):2892. doi:10.3390/nu15132892
124. Wu G. Dietary protein intake and human health. Food Funct. 2016;7(3):1251–1265. doi:10.1039/c5fo01530h
125. Church DD, Hirsch KR, Park S, et al. Essential amino acids and protein synthesis: insights into maximizing the muscle and whole-body response to feeding. Nutrients. 2020;12(12):3717. doi:10.3390/nu12123717
126. Phang JM, Liu W, Hancock C. Bridging epigenetics and metabolism: role of non-essential amino acids. Epigenetics. 2013;8(3):231–236. doi:10.4161/epi.24042
127. Fan Y, Evans CR, Ling J. Rewiring protein synthesis: from natural to synthetic amino acids. Biochim Biophys Acta Gen Subj. 2017;1861(11 Pt B):3024–3029. doi:10.1016/j.bbagen.2017.01.014
128. Tharp JM, Ad O, Amikura K, et al. Initiation of protein synthesis with non-canonical amino acids in vivo. Angew Chem Int Ed Engl. 2020;59(8):3122–3126. doi:10.1002/anie.201914671
129. Zang X, Ueno Y, Kitadai N. Photochemical synthesis of ammonia and amino acids from nitrous oxide. Astrobiology. 2022;22(4):387–398. doi:10.1089/ast.2021.0064
130. Stepien M, Azzout-Marniche D, Even PC, et al. Adaptation to a high-protein diet progressively increases the postprandial accumulation of carbon skeletons from dietary amino acids in rats. Am J Physiol Regul Integr Comp Physiol. 2016;311(4):R771–r778. doi:10.1152/ajpregu.00040.2016
131. Häberle J, Burlina A, Chakrapani A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: first revision. J Inherit Metab Dis. 2019;42(6):1192–1230. doi:10.1002/jimd.12100
132. Correia AS, Vale N. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci. 2022;23(15):8493. doi:10.3390/ijms23158493
133. Ramdani C, Vidal F, Dagher A, Carbonnell L, Hasbroucq T. Dopamine and response selection: an Acute phenylalanine/tyrosine depletion study. Psychopharmacology (Berl). 2018;235(4):1307–1316. doi:10.1007/s00213-018-4846-3
134. He HY, Henderson AC, Du YL, Ryan KS. Two-enzyme pathway links L-arginine to nitric oxide in N-nitroso biosynthesis. J Am Chem Soc. 2019;141(9):4026–4033. doi:10.1021/jacs.8b13049
135. Ziegler SG, Kim J, Ehmsen JT, Vernon HJ. Inborn errors of amino acid metabolism - from underlying pathophysiology to therapeutic advances. Dis Model Mech. 2023;16(11):dmm050233. doi:10.1242/dmm.050233
136. Kazmierczak SC. Diseases of metabolism (disorders of amino acid metabolism). Anal Chem. 1993;65(12):401r–404r. doi:10.1021/ac00060a606
137. Arribas-López E, Zand N, Ojo O, Snowden MJ, Kochhar T. The effect of amino acids on wound healing: a systematic review and meta-analysis on arginine and glutamine. Nutrients. 2021;13(8):2498. doi:10.3390/nu13082498
138. Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: a literature review. Life Sci. 2022;290:120096. doi:10.1016/j.lfs.2021.120096
139. Wellington MO, Hulshof TG, Ernst K, Balemans A, Page GI, Van Hees HMJ. Impact of L-Arginine and L-Glutamine supplementation on growth performance and immune status in weanling pigs challenged with Escherichia coli F4. J Anim Sci. 2023;101:skad138. doi:10.1093/jas/skad138
140. de Paz-Lugo P, Lupiáñez JA, Sicilia J, Meléndez-Hevia E. Control analysis of collagen synthesis by glycine, proline and lysine in bovine chondrocytes in vitro - Its relevance for medicine and nutrition. Biosystems. 2023;232:105004. doi:10.1016/j.biosystems.2023.105004
141. Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20(1):52–61. doi:10.1177/011542650502000152
142. Pereira MG, Baptista IL, Carlassara EO, Moriscot AS, Aoki MS, Miyabara EH. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS One. 2014;9(1):e85283. doi:10.1371/journal.pone.0085283
143. Pereira MG, Silva MT, Carlassara EO, et al. Leucine supplementation accelerates connective tissue repair of injured tibialis anterior muscle. Nutrients. 2014;6(10):3981–4001. doi:10.3390/nu6103981
144. Zhang XJ, Chinkes DL, Wolfe RR. Leucine supplementation has an anabolic effect on proteins in rabbit skin wound and muscle. J Nutr. 2004;134(12):3313–3318. doi:10.1093/jn/134.12.3313
145. He Q, Wang L, Zhao R, et al. Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res Ther. 2020;11(1):223. doi:10.1186/s13287-020-01731-6
146. Xiong J, Hu H, Guo R, Wang H, Jiang H. Mesenchymal stem cell exosomes as a new strategy for the treatment of diabetes complications. Front Endocrinol. 2021;12:646233. doi:10.3389/fendo.2021.646233
147. Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115(48):12158–12163. doi:10.1073/pnas.1808855115
148. Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. doi:10.1021/acsnano.7b07643
149. Shi H, Hao X, Sun Y, et al. Bone marrow mesenchymal stem cell-derived exosomes reduce insulin resistance and obesity in mice via the PI3K/AKT signaling pathway. FEBS Open Bio. 2023;13(6):1015–1026. doi:10.1002/2211-5463.13615
150. Ha DH, Kim HK, Lee J, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9(5):1157. doi:10.3390/cells9051157
151. Zhou C, Zhang B, Yang Y, et al. Stem cell-derived exosomes: emerging therapeutic opportunities for wound healing. Stem Cell Res Ther. 2023;14(1):107. doi:10.1186/s13287-023-03345-0
152. Cai F, Chen W, Zhao R, Liu Y. The capacity of exosomes derived from adipose-derived stem cells to enhance wound healing in diabetes. Front Pharmacol. 2023;14:1063458. doi:10.3389/fphar.2023.1063458
153. Wang W, Zhu N, Yan T, et al. The crosstalk: exosomes and lipid metabolism. Cell Commun Signal. 2020;18(1):119. doi:10.1186/s12964-020-00581-2
154. Baruah H, Sarma A, Basak D, Das M. Exosome: from biology to drug delivery. Drug Deliv Transl Res. 2024;14(6):1480–1516. doi:10.1007/s13346-024-01515-y
155. Reiss AB, Vernice NA, Siegart NM, De Leon J, Kasselman LJ. Exosomes in cholesterol metabolism and atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 2017;17(3):185–194. doi:10.2174/1871529x18666180103124443
156. Zein Abdin Z, Geng AZ, Chandy M. Exosomes and lipid metabolism in metabolic and cardiovascular disorders. Curr Opin Lipidol. 2023;34(2):82–91. doi:10.1097/mol.0000000000000873
157. Fujita K, Somiya M, Kuroda S, Hinuma S. Induction of lipid droplets in non-macrophage cells as well as macrophages by liposomes and exosomes. Biochem Biophys Res Commun. 2019;510(1):184–190. doi:10.1016/j.bbrc.2019.01.078
158. Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016;118(2):330–343. doi:10.1161/circresaha.115.307654
159. Deng H, Sun C, Sun Y, et al. Lipid, Protein, and MicroRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram. 2018;20(3):178–186. doi:10.1089/cell.2017.0047
160. Huang J, Xiong J, Yang L, Zhang J, Sun S, Liang Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale. 2021;13(19):8740–8750. doi:10.1039/d1nr01314a
161. Lv H, Liu H, Sun T, Wang H, Zhang X, Xu W. Exosome derived from stem cell: a promising therapeutics for wound healing. Front Pharmacol. 2022;13:957771. doi:10.3389/fphar.2022.957771
162. Ren S, Chen J, Guo J, et al. Exosomes from adipose stem cells promote diabetic wound healing through the eHSP90/LRP1/AKT axis. Cells. 2022;11(20):3229. doi:10.3390/cells11203229
163. Lin F, Chen W, Zhou J, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13(3):271. doi:10.1038/s41419-022-04708-w
164. Cui HS, Kim DH, Joo SY, Cho YS, Kim JB, Seo CH. Exosomes derived from human hypertrophic scar fibroblasts induces smad and TAK1 signaling in normal dermal fibroblasts. Arch Biochem Biophys. 2022;722:109215. doi:10.1016/j.abb.2022.109215
165. Wu B, Feng J, Guo J, et al. ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis. Stem Cell Res Ther. 2022;13(1):494. doi:10.1186/s13287-022-03049-x
166. Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843–853. doi:10.1042/bst20180079
167. Zheng X, Zhu Y, Zhao Z, Chu Y, Yang W. The role of amino acid metabolism in inflammatory bowel disease and other inflammatory diseases. Front Immunol. 2023;14:1284133. doi:10.3389/fimmu.2023.1284133
168. Zhong Y, Zhang Y, Yu A, et al. Therapeutic role of exosomes and conditioned medium in keloid and hypertrophic scar and possible mechanisms. Front Physiol. 2023;14:1247734. doi:10.3389/fphys.2023.1247734
169. Li B, Chen Y, Zhou Y, et al. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer’s disease. Neural Regen Res. 2024;19(7):1593–1601. doi:10.4103/1673-5374.385839
170. Fraga de Andrade I, Mehta C, Bresnick EH. Post-transcriptional control of cellular differentiation by the RNA exosome complex. Nucleic Acids Res. 2020;48(21):11913–11928. doi:10.1093/nar/gkaa883
171. Zheng J, Yang B, Liu S, Xu Z, Ding Z, Mo M. Applications of exosomal miRNAs from mesenchymal stem cells as skin boosters. Biomolecules. 2024;14(4):459. doi:10.3390/biom14040459
172. Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119. doi:10.3390/ijms19103119
173. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO j. 2021;40(19):e108863. doi:10.15252/embj.2021108863
174. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi:10.1002/path.2697
175. Chen G, Wang M, Ruan Z, Zhu L, Tang C. Mesenchymal stem cell-derived exosomal miR-143-3p suppresses myocardial ischemia-reperfusion injury by regulating autophagy. Life Sci. 2021;280:119742. doi:10.1016/j.lfs.2021.119742
176. Griffin MF, desJardins-Park HE, Mascharak S, Borrelli MR, Longaker MT. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech. 2020;13(6):dmm044164. doi:10.1242/dmm.044164
177. Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol. 2018;7(2):10. doi:10.1002/wdev.307
178. Han J, Li Q, Chen Y, Yang Y. Recent metabolomics analysis in tumor metabolism reprogramming. Front Mol Biosci. 2021;8:763902. doi:10.3389/fmolb.2021.763902
179. Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi:10.1186/s13046-015-0221-y
180. Eming SA, Murray PJ, Pearce EJ. Metabolic orchestration of the wound healing response. Cell Metab. 2021;33(9):1726–1743. doi:10.1016/j.cmet.2021.07.017
181. Zhu X, Jiang L, Long M, Wei X, Hou Y, Du Y. Metabolic Reprogramming and Renal Fibrosis. Front Med Lausanne. 2021;8:746920. doi:10.3389/fmed.2021.746920
182. Delgado ME, Cárdenas BI, Farran N, Fernandez M. Metabolic reprogramming of liver fibrosis. Cells. 2021;10(12):3604. doi:10.3390/cells10123604
183. Li Q, Qin Z, Nie F, et al. Metabolic reprogramming in keloid fibroblasts: aerobic glycolysis and a novel therapeutic strategy. Biochem Biophys Res Commun. 2018;496(2):641–647. doi:10.1016/j.bbrc.2018.01.068
184. Huang R, Han R, Yan Y, et al. PTB regulates the metabolic pathways and cell function of keloid fibroblasts through alternative splicing of PKM. Int J Mol Sci. 2023;24(6):5162. doi:10.3390/ijms24065162
185. Li J, Li Z, Wang S, Bi J, Huo R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered. 2022;13(4):8515–8525. doi:10.1080/21655979.2022.2051838
186. Malla RR, Shailender G, Kamal MA. Exosomes: critical mediators of tumour microenvironment reprogramming. Curr Med Chem. 2021;28(39):8182–8202. doi:10.2174/0929867328666201217105529
187. Xu S, Cheuk YC, Jia Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death Dis. 2022;13(10):876. doi:10.1038/s41419-022-05305-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.