Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
The Higher Incidence of Liver Injury in HCC Patients Compared to Other Malignancies During Immune-Checkpoint Inhibitor Therapy is Primarily Due to Tumor Progression
Authors Wang Y , Liu L, Zhao M, Chen W, Chen Y, Zhao X
Received 30 December 2024
Accepted for publication 19 May 2025
Published 25 June 2025 Volume 2025:21 Pages 963—974
DOI https://doi.org/10.2147/TCRM.S514868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Yan Wang,1,* Liwei Liu,2,* Mengyu Zhao,1,* Wei Chen,3 Yu Chen,2 Xinyan Zhao1
1Liver Research Center, Beijing Friendship Hospital, Key Laboratory on Translational Medicine on Cirrhosis, National Clinical Research Center for Digestive Disease, Capital Medical University, Beijing, People’s Republic of China; 2Fourth Department of Liver Disease (Difficult & Complicated Liver Diseases and Artificial Liver Center), Beijing You’an Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Gastroenterology, Beijing Friendship Hospital, Beijing Key Laboratory for Precancerous Lesion of Digestive Disease, National Clinical Research Center for Digestive Disease, Beijing Digestive Disease Center, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinyan Zhao, Liver Research Center, Beijing Friendship Hospital, Capital Medical University, Beijing Key Laboratory on Translational Medicine on Cirrhosis, National Clinical Research Center for Digestive Disease, 95 Yong-an Road, Xi-Cheng District, Beijing, 100050, People’s Republic of China, Email [email protected] Yu Chen, Fourth Department of Liver Disease (Difficult & Complicated Liver Diseases and Artificial Liver Center), Beijing You’an Hospital, Capital Medical University, No. 8, Xi Tou Tiao, Youanmen Wai, Fengtai District, Beijing, 100069, People’s Republic of China, Email [email protected]
Background: The study explores the incidence and clinical features of immune-related liver injury (irLI) in hepatocellular carcinoma (HCC) patients compared to those with other malignancies receiving immune checkpoint inhibitors (ICIs).
Methods: A retrospective analysis was conducted on patients treated with ICIs at Beijing Friendship Hospital. Individuals who experienced liver injury consistent with the criteria specified in the Common Terminology Criteria for Advanced Event version 5.0 for irLI were included in the study. The cohort was divided into an HCC group and a non-HCC malignancy group. HCC patients were further classified into three subgroups based on liver injury: no injury, irLI, or non-immune-related liver injury. Data on demographics, laboratory results, and mortality rates were compared.
Results: The study included 292 hCC patients and 1248 patients with other malignancies. Both groups underwent a similar number of ICIs cycles (p=0.237). Liver injury was more common in HCC patients [98 (33.6%) vs 288 (23.1%), p< 0.001], but the irLI incidence was comparable between the groups [17 (5.8%) vs 62 (5.0%), p=0.556]. Tumor progression-related liver injury was higher in HCC patients (12.0%) compared to other malignancies (4.6%). Mortality rates showed no significant differences between groups.
Conclusion: HCC patients with underlying liver disease are more prone to liver injury during ICIs therapy, mainly due to tumor progression rather than irLI.
Keywords: immune checkpoint inhibitors, hepatocarcinoma, liver injury
Graphical Abstract:

Introduction
Immune checkpoint inhibitors (ICIs) have demonstrated significant therapeutic efficacy across multiple malignancies. Through blockade of key immune regulators including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and its ligand PD-L1, ICIs augment antitumor immune responses. Nevertheless, this enhanced immunologic activity may induce immune-related adverse events (irAEs), with the liver representing one of the most frequently affected organs.1,2
PD-1-targeted immunotherapy has shown improved overall survival in both multicenter clinical trials and real-world studies involving patients with advanced hepatocellular carcinoma (HCC). The US Food and Drug Administration (FDA) granted approval to nivolumab in September 2017 for sorafenib-refractory HCC treatment, followed by the 2020 approval of nivolumab-ipilimumab combination therapy for the same indication.3 Furthermore, the American Gastroenterological Association (AGA) clinical guidelines recommend PD-1/PD-L1 inhibitors (atezolizumab as first-line and pembrolizumab as second-line therapy) for advanced HCC management.4
Although the majority of clinical trials have demonstrated the safety and efficacy of PD-1/PD-L1 inhibitors for advanced HCC, there have been reports of irLI. IrLI ranges from asymptomatic liver enzyme elevation to severe liver injury, including fever, abdominal pain, jaundice, coagulation issues, and potentially fatal acute liver failure. It is categorized into immune-mediated hepatitis, which affects hepatocytes and raises transaminases, and immune-mediated cholangitis, which targets bile duct epithelial cells, elevating alkaline phosphatase and gamma-glutamyl transferase. Pathological manifestations include active hepatitis characterized by spotty or confluent necrosis, alongside mild to moderate periportal activity.5 In rare cases, immune-related cholangitis may manifest as diffuse bile duct dilatation and multifocal stenosis, resembling sclerosing cholangitis as observed in magnetic resonance cholangiopancreatography (MRCP).6–8 The diagnosis of irLI requires a documented history of ICIs administration and must fulfill the biochemical criteria outlined in the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, with alternative causes for liver injury excluded.2,9
HCC predominantly arises in patients with chronic liver disease, most notably in those with cirrhosis. The influence of pre-existing hepatic impairment and cirrhosis on the incidence and clinical manifestations of PD-1/PD-L1 inhibitor-induced hepatotoxicity requires further elucidation. This study systematically compares the incidence and clinical profiles of PD-1/PD-L1-associated hepatotoxicity between cirrhotic HCC patients and non-hepatic malignancy patients without chronic liver disease. Through comparative analysis, we evaluate how baseline hepatic dysfunction and cirrhotic status modulate the development and phenotypic characteristics of PD-1/PD-L1 inhibitor-induced liver injury.
Materials and Methods
This retrospective cohort study analyzed patients with malignancies who underwent PD-1/PD-L1 inhibitor monotherapy or combination therapy at Beijing Friendship Hospital from April 2016 to December 2022. Multidimensional clinical data were systematically collected, encompassing demographic profiles, biochemical parameters, diagnostic imaging results, therapeutic protocols, and longitudinal follow-up data. The study protocol received approval by the Ethic Review Board of Beijing Friendship Hospital, Capital Medical University (ethics approval number: 2022-P2-269-01). The requirement for written consent was waived because this retrospective study involved the analysis of pre-existing anonymized data, presenting minimal risk to participants. The study was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki.
HCC diagnosis was confirmed using contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI), with or without concomitant α-fetoprotein (AFP) levels ≥400 ng/mL.4,10 Tumor staging was conducted in accordance with the Barcelona Clinic Liver Cancer (BCLC) classification system.11 Non-HCC malignancies were diagnosed following disease-specific diagnostic criteria delineated in current clinical guidelines.
Exclusion Criteria
(1) Cases initiating anti-PD-1/PD-L1 therapy at external medical institutions; (2) Viral hepatotoxicity attributable to Epstein-Barr virus (EBV) or cytomegalovirus (CMV); (3) Concurrent hemophagocytic lymphohistiocytosis; (4) Cases with incomplete clinical datasets.
Diagnosis and Differential Diagnosis of Liver Injury
Patients was defined as all-cause liver injury if they met the any of the following criteria outlined in CTCAE version 5.0 if they had: 1) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels exceeding 1.0 times the upper limit of normal (ULN) if normal at baseline, or over 1.5 times the baseline if abnormal, excluding AST elevation from muscle damage; 2) alkaline phosphatase (ALP) levels over 1.0 times the ULN if normal at baseline, or over 2.0 times the baseline if abnormal, excluding ALP elevation from bone metastases; or 3) total bilirubin (TBil) levels over 1.0 times the ULN if normal at baseline, or over 1.0 times the baseline if abnormal.2
In this investigation, liver injury arising during tumor treatment and satisfying CTCAE criteria was evaluated by a trio of hepatology specialists, who comprehensively reviewed clinical manifestations, laboratory analyses, imaging results, and treatment responses to ascertain the etiology. Liver injury was attributed to tumor progression if imaging post-injury revealed hepatic metastasis or biliary obstruction in the absence of pre-treatment liver injury. Elevations in liver enzymes following surgical procedures, TACE, or analogous treatments were categorized as treatment-related. Instances of liver dysfunction concurrent with severe infection, sepsis, or complications such as hypotension, shock, or hypoxia were classified as infection- or ischemia/hypoxia-induced. The diagnosis of irLI adheres to the protocol for drug-induced liver injury, necessitating biochemical evidence of liver injury and the exclusion of alternative causes. The diagnosis of irLI was reserved for cases where all aforementioned causes were eliminated, competing etiologies like infection of hepatitis A, B, C, E, EBV, CMV, or biliary obstruction caused by biliary stones were excluded, too. The severity of irAEs were graded by National Cancer Institute Common Terminology Criteria for Adverse Events, v5.0.2
Statistical Analysis
Qualitative data were analyzed using the χ²-test or Fisher’s exact test (expected cell counts <5) and were presented as frequencies and percentages (n [%]). Continuous variables were analyzed using either the Mann–Whitney U-test (two-group) or the Kruskal–Wallis test (multi-group) and were expressed as medians with interquartile ranges (median [first quartile, third quartile]). The cumulative incidences of all-cause liver injury and irLI were estimated using Kaplan-Meier statistics, and comparisons between survival curves were performed using Kaplan-Meier survival analysis. Otherwise, we conducted stratified analyses by age and gender to compare the cumulative incidence of all-cause liver injury and irLI between HCC and other malignancies, the statistical analysis as previously described.
All statistical analyses were conducted using Statistical Package for Social Science 22.0 (SPSS 22.0, IBM Corporation, SPSS, Armonk, NY) software and all figures were conducted using GraphPad Prism software (version 8).
Results
Clinical Characteristics of the Cases with HCC and Other Malignancies
As shown in Figure 1, between April 2016 and December 2023, a total of 1859 patients received ICIs immunotherapy at Beijing Friendship Hospital. After excluding 175 patients with EBV infection or hemophagocytic syndrome and 144 with incomplete follow-up data, 1540 patients were analyzed. Among these, 292 patients had HCC with cirrhosis, and 1248 patients had other malignancies without pre-existing liver diseases.
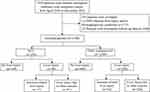 |
Figure 1 Flowchart of this study. |
The primary cause of cirrhosis was viral hepatitis (214 hepatitis B, 21 hepatitis C), 13 alcoholic cirrhosis, 2 nonalcoholic steatohepatitis, and 44 other causes (eg, cholestatic liver diseases, Budd-Chiari syndrome, etc). Liver function in the HCC patients was assessed using the Child-Turcotte-Pugh (CTP) score: 183 (62.7%) CTP A, 99 (33.9%) CTP B, and 10 (3.4%) CTP C. During follow-up, 194 hCC cases had no liver injury, while 98 developed liver injury (17 irLI, 81 other causes). Among the 1248 other malignancy cases, 960 had no liver injury, while 288 developed liver injury (62 irLI, 226 other causes).
In the HCC group, 17 (5.8%) patients developed irLI, 194 (66.4%) had no liver injury, 35(12.0%) had tumor progression, 27 (9.2%) had injury from surgery, TACE, or ablation, and 14 (4.8%) had infection, ischemia or hypoxia. In the other malignancies group, 960 (76.9%) individuals had no liver injury, 62 (5.0%) developed irLI, 57 (4.6%) had tumor progression and 49 (3.9%) had concurrent medication induced liver injury.
Patients were divided into HCC and other malignancies groups. The other malignancies group mainly included gastrointestinal cancer (502 [32.6%]), lung cancer (348 [22.6%]), and urological cancer (126 [8.2%]), which represented the top three tumor types (Figure 2). The majority of cases received PD-1 inhibitors, with fewer on PD-L1 inhibitors or combined of PD-1/PD-L1 inhibitors. HCC patients were younger than other malignancies patients (61[54, 68] vs 66[59, 71], P<0.001) (Table 1). As expected, pretreatment laboratory data revealed that the levels of ALT, AST, ALP, GGT, and TB were significantly higher in the HCC group compared to the other malignancies group.
 |
Table 1 Comparison of the Demographic and Laboratory Data of Cases with Hepatocellular Carcinoma and Other Malignancies |
 |
Figure 2 Types and proportions of tumors in our study. |
Clinical Characteristics of the Cases with HCC Who Had No Liver Injury, irLI and Liver Injury with Other Causes
Among 292 hCC cases, 21 (7.2%) received anti-PD-1 monotherapy, while others had combination therapy: 160 with TACE, 171 with TKI or EGFR inhibitors, 71 with bevacizumab, and 4 with anti-CTLA-4. HCC patients were divided into three groups based on liver injury: no liver injury, irLI, and liver injury due to other causes. No significant differences were found in age, sex distribution, total ICIs cycles, or the types of the ICIs administered among the groups. Pretreatment ALT, AST and TBIL levels were similar, but baseline ALP and GGT levels were higher in the irLI group and other-cause injury group compared to the non-injury group. Post-treatment peak levels of ALT, AST, ALP, GGT, and TBIL were significantly higher in both injury groups than in the on-injury group (Table 2). Furthermore, irLI patients had a higher rate of non-hepatic irAEs (6 [35.3%] vs 2 [1.0%] vs 2 [2.5%], p<0.001).
 |
Table 2 Comparison of the Demographic and Laboratory Data of Those Cases with HCC Who Had No Liver Injury, Immune-Related Liver Injury and Liver Injury Induced by Other Causes |
Comparison of the Cumulative Incidence of All-Cause Liver Injury and irLI
The HCC group had a significantly higher rate of all-cause liver injury than the other malignancies group (98[33.6%] vs 288[23.1%], p<0.001) (Figure 3A), but the incidence of irLI was similar between the two groups (17[5.8%] vs 62[5.0%], p=0.556) (Figure 3B). A total of 81 cases in the HCC group and 226 cases in the other malignancies group experienced liver injury due to non-immune-mediated causes. Among these, tumor progression accounted for 35 (12.0%) of HCC cases 57 (4.6%) of other malignancy cases. Surgical resection, TACE or ablation caused liver injury in 27 (9.2%) of HCC cases 30 (2.4%) of other malignancy cases. Infection, ischemia, or hypoxia led to injury in 14 (4.8%) of HCC cases and 58 (4.6%) of other malignancies cases. Other anti-tumor drugs caused liver injury in 3 (1.0%) of HCC cases and 49 (3.9%) of other malignancy cases (Supplement Table 1). The HCC group had significantly higher rates of thyroiditis and dermatitis compared to the other malignancies group. However, rates of myopericarditis, pneumonia, myositis, nephritis showed no significant differences between the two groups (Figure 3C).
Otherwise, we conducted stratified analyses by age (categorized using the approximate median age 60y) and gender to compare the cumulative incidence of all-cause liver injury and irLI between HCC and other malignancies (Figure S1). In the >60-year age subgroup, the HCC group exhibited significantly higher cumulative incidence of all-cause liver injury than the other malignancies group (p=0.007), with a similar trend observed in the <60-year subgroup(p=0.180). However, no significant differences in irLI were detected across age subgroups. These findings align with our overall comparative results. Regarding gender stratification, male HCC patients showed significantly higher cumulative incidence of all-cause liver injury compared to other malignancie group, whereas no significant differences in irLI incidence were observed. In female patients, no statistically significant differences were identified in either all-cause or irLI between the two groups.
Characteristics of HCC Cases with irLI
The demographic and laboratory data of the 17 hCC patients with irLI are summarized in Table 3. Most patients were in their 50s to 70s. Six patients were treated with Sintilimab, three with Camrelizumab, two with Tislelizumab, two with Nivolumab, and one each with Atezolizumab, Toripalimab, Envafolimab and STRIDE regimen. Six patients experienced non-hepatic irAEs. Ten patients exhibited significant elevations in ALT and AST levels, while six patients primarily showed increases in ALP, GGT and/or TBIL. Four patients received glucocorticoid therapy, and all achieved recovery. Only one patient experienced hepatotoxicity-related mortality.
 |
Table 3 Characteristics and Laboratory Data of the HCC Case with Immune-Related Liver Injury |
Follow-up time was similar between the HCC group and other malignancies group (p=0.040), with no statistical difference in survival probability (p=0.097). Among HCC patients, follow-up times did not differ significantly across the subgroups: no liver injury, irLI, and other-cause liver injury. Survival probabilities were also comparable among these subgroups (p=0.281).
Discussion
Compared to patients with other malignancies, HCC patients demonstrated a significantly higher incidence of all-cause liver injury (33.6% vs 23.1%, p<0.001), whereas no significant difference was observed in immune-related liver injury (irLI) (5.8% vs 5.0%, p=0.556). Notably, HCC cases exhibited a greater proportion of liver injury attributable to tumor progression. These findings indicate that HCC progression exerts a more pronounced influence on hepatic biochemical parameters compared to other malignancies, potentially due to the unique pathophysiology of HCC involving compromised synthetic function and architectural disruption.
In a systematic review of clinical trials by Brown et al,12 HCC patients exhibited significantly higher rates of elevated AST and ALT levels compared to non-small cell lung cancer and melanoma cohorts. This observation implies that pre-existing hepatic pathologies, particularly cirrhosis, may contribute to the increased likelihood of abnormal liver chemistries in HCC patients. Nevertheless, the precise mechanisms underlying transaminase elevation in this population remain incompletely elucidated and warrant mechanistic investigation. Our study further corroborates that HCC patients face a 1.8-fold increased risk of liver injury relative to those with other malignancies. However, this increased incidence of liver injury was primarily attributed to HCC progression and interventions such as surgical resection, TACE, or ablation, rather than irLI. Additionally, no statistically significant differences were observed in non-hepatic immune-related adverse events between HCC and non-HCC groups. These findings align with prior evidence13 suggesting that irLI is more frequently linked to hepatic metastatic burden than direct drug-induced hepatotoxicity, emphasizing the need for etiology-specific management strategies in HCC patients receiving immunotherapy.
In a landmark study by Celsa et al, HCC exhibited higher rates of any-grade irLI compared to patients with other types of cancer (11.4% versus 2.6%).14 The discrepancies between their findings and ours may be attributed to differences in patient demographics (European/American versus Asian), etiologies of HCC (hepatitis B versus hepatitis C/alcohol), and treatment regimens (atezolizumab/bevacizumab versus our regimen). Notably, their study also showed no increase in non-hepatic irAEs in HCC patients, aligning with our findings. These included gastrointestinal, endocrine, dermatological, pulmonary, and neuromuscular/rheumatologic irAEs.
Our study found slightly higher rates of irLI and all-cause liver injury in unresectable HCC patients compared to clinical trials. For instance, the KEYNOTE-224 study reported a 3% irLI rate with pembrolizumab,15,16 while a Phase I/II study on tremelimumab plus durvalumab showed a 2.7% rate of hepatitis and hepatic failure.17 The CheckMate 040 trial observed elevated ALT (15%-23.5%) and AST (21%-25.3%) levels in advanced HCC patients treated with nivolumab, camrelizumab, or pembrolizumab.16,18,19 Our findings align with real-world studies, which reported all-cause liver injury rates of 34.5% for elevated ALT, 27.5% for elevated AST, and 34.5% for elevated TBIL20 and a 5% irLI rate.21 These differences may be due to the exclusion of patients with poor liver function in trials, leading to fewer CTP B and C cases, whereas our study and real-world data included more of these cases. Furthermore, real-world cases often involved combination therapy, unlike the monotherapy typically used in trials, potentially contributing to higher liver injury rates.
PD-1/PD-L1 inhibitors can be used alone or with CTLA-4 inhibitors22,23 or other HCC treatments. Many studies show combing PD-1 antibodies with tyrosine kinase inhibitors (TKIs) improves HCC patient survival.24 The AGA guidelines recommend atezolizumab plus bevacizumab for advanced HCC.4 Current trails are evaluating the efficacy of PD-1 antibodies in combination with TACE for advanced HCC treatment.25 Previous studies have reported a higher incidence of adverse events with combination therapy than monotherapy.26,27 In our study, only 7.2% received anti-PD-1 monotherapy, while 92.8% underwent combination therapy. The high prevalence of combination therapy in our cohort may influence the incidence and clinical presentation of liver injury.
We found that HCC patients with elevated AFP levels in the other-cause liver injury group had significantly higher AFP levels compared to those in the no liver injury group and the irLI group. Given that nearly half (35/81, 43.2%) of these patients had abnormal liver chemistries due to HCC progression, elevated AFP levels may help to predict HCC progression risk. Previous studies support this, showing AFP stimulates HCC cell proliferation.28–30 Furthermore, Chen T et al found AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway.31
The precise mechanisms underlying irLI remain only partially understood. This condition occurs when ICIs activate the immune system, thereby disrupting hepatic and biliary tolerance and resulting in immune-mediated hepatic damage. IrLI may present as immune-mediated hepatitis, characterized by elevated transaminase levels, or as cholangitis, indicated by increased levels of ALP and GGT. Simultaneously, the progression of HCC causes hepatic damage by destroying tissue, impairing liver function, and potentially invading blood vessels. Furthermore, HCC can obstruct bile ducts, resulting in cholestasis, jaundice, and infections. Despite the overlap in certain biochemical markers of liver dysfunction between irLI and HCC progression, advanced imaging modalities such as MRI or CT, combined with systemic clinical presentations can assist in differentiating between tumor progression and immune-related liver injury.
This study has three principal limitations requiring consideration. Firstly, the retrospective design and moderate sample size from a single-center may limit statistical power and external validity, necessitating confirmation through multicenter prospective studies with adequate power calculation. Secondly, the treatment approach in our study diverged from that of randomized clinical trials, as the majority of HCC patients received combination therapy involving PD-1/PD-L1 antibodies alongside other medications, rather than monotherapy. However, it is important to emphasize that combination therapy has shown promising therapeutic efficacy across various tumor types and is supported by clinical guidelines. Thirdly, a subset of patients lacked available data on ALP or GGT, which may have lead to an underestimation of immune-mediated bile duct injury.
In summary, our study demonstrated that HCC patients with impaired liver function and cirrhosis experienced a higher incidence of all-cause liver injury during PD-1/PD-L1 inhibitor therapy. However, the incidence of irLI did not significantly increase compared to other malignancies. The elevated incidence of liver injury appears to be primarily driven by HCC progression rather than irLI induced by PD-1/PD-L1 inhibitors. These findings suggest that HCC progression has a more pronounced impact on liver chemistries compared to other malignancies.
Abbreviation
AFP, alpha-fetoprotein; AGA, American Gastroenterological Association; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CTP score, Child-Turcotte-Pugh score; FDA, Food and Drug Administration; GGT, gamma glutamyl transpeptidase; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; INR, international normalized ratio; irAEs, immune-related adverse events; irLI, immune-related liver injury; MELD, model for end-stage liver disease; MRCP, Magnetic resonance cholangiopancreatography; NSCLC, non-small cell lung cancer; PD-1, anti-programmed death; PD-L1, anti-programmed death ligand-1; TACE, transarterial chemoembolization; TB, total bilirubin; TKIs, tyrosine kinase inhibitors; ULN, upper limit of normal; VEGF, vascular endothelial growth factor.
Ethical Statement
The study protocol received approval by the Ethic Review Board of Beijing Friendship Hospital, Capital Medical University (ethics approval number: 2022-P2-269-01). The requirement for written consent was waived because this retrospective study involved the analysis of pre-existing anonymized data, presenting minimal risk to participants. The study was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Key Research and Development Program of China (Grant No. 2022YFC2304402), Beijing Hospitals Authority’s Ascent Plan (Grant No. DFL20221501), R&D Program of Beijing Municipal Education Commission (Grant No. KM202310025009), Beijing iGandan Foundation- Artificial Liver Special Fund (Grant No. iGandanF-1082024-RGG113), National Natural Science Foundation of China (No. 82470625), the National Key Clinical Specialty Construction Project (2022), and WBE Liver Fibrosis Foundation (Grant No. CFHPC2025036).
Disclosure
Yan Wang, Liwei Liu and Mengyu Zhao are co-first authors for this study. Yu Chen and Xinyan Zhao are co-correspondence authors for this study. The authors have no conflicts of interest in this work.
References
1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
2. Washington: US Department of Health and Human Services NIoH, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 5.0 2017.U.S. Department Of Health And Human Services. National Institutes of Health. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
3. Wright K. FDA approves nivolumab plus ipilimumab for the treatment of advanced HCC. Oncology. 2020;34(4):693606.
4. Su GL, Altayar O, O’Shea R, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 2022;162(3):920–934. doi:10.1053/j.gastro.2021.12.276
5. De Martin E, Michot J-M, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–1190. doi:10.1016/j.jhep.2018.01.033
6. Ogawa K, Kamimura K, Terai S. Antiprogrammed cell death-1 immunotherapy-related secondary sclerosing cholangitis. Hepatology. 2019;69(2):914–916. doi:10.1002/hep.30189
7. Yoshikawa Y, Imamura M, Yamaoka K, et al. A case with life-threatening secondary sclerosing cholangitis caused by nivolumab. Clin J Gastroenterol. 2021;14(1):283–287. doi:10.1007/s12328-020-01287-1
8. Pi B, Wang J, Tong Y, Yang Q, Lv F, Yu Y. Immune-related cholangitis induced by immune checkpoint inhibitors: a systematic review of clinical features and management. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e858–e867. doi:10.1097/MEG.0000000000002280
9. Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology. 2021;160(4):1384–1393. doi:10.1053/j.gastro.2020.08.063
10. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
11. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
12. Brown ZJ, Heinrich B, Steinberg SM, Yu SJ, Greten TF. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer. 2017;5(1):93. doi:10.1186/s40425-017-0298-2
13. Tsung I, Dolan R, Lao CD, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther. 2019;50(7):800–808. doi:10.1111/apt.15413
14. Celsa C, Cabibbo G, Fulgenzi CAM, et al. Characteristics and outcomes of immunotherapy-related liver injury in patients with hepatocellular carcinoma versus other advanced solid tumours. J Hepatol. 2024;80(3):431–442. doi:10.1016/j.jhep.2023.10.040
15. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
16. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
17. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/JCO.20.03555
18. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
19. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
20. Feun LG, Li YY, Wu C, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125(20):3603–3614. doi:10.1002/cncr.32339
21. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323–1333. doi:10.1111/apt.15245
22. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. doi:10.1001/jamaoncol.2020.4564
23. Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93 Suppl 1(Suppl. 1):147–159. doi:10.1159/000481245
24. Xie D, Sun Q, Wang X, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. 2021;9(8):652. doi:10.21037/atm-20-7037
25. Liu BJ, Gao S, Zhu X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13(17):1395–1405. doi:10.2217/imt-2021-0192
26. Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851–3858. doi:10.1200/JCO.2016.72.1985
27. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi:10.1093/annonc/mdx642
28. Li M, Zhou S, Liu X, Li P, McNutt MA, Li G. Alpha-Fetoprotein shields hepatocellular carcinoma cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Lett. 2007;249(2):227–234. doi:10.1016/j.canlet.2006.09.004
29. Li MS, Ma QL, Chen Q, et al. Alpha-fetoprotein triggers hepatoma cells escaping from immune surveillance through altering the expression of Fas/FasL and tumor necrosis factor related apoptosis-inducing ligand and its receptor of lymphocytes and liver cancer cells. World J Gastroenterol. 2005;11(17):2564–2569. doi:10.3748/wjg.v11.i17.2564
30. Parpart S, Roessler S, Dong F, et al. Modulation of miR-29 expression by alpha-fetoprotein is linked to the hepatocellular carcinoma epigenome. Hepatology. 2014;60(3):872–883. doi:10.1002/hep.27200
31. Chen T, Dai X, Dai J, et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11(10):822. doi:10.1038/s41419-020-03030-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


