Back to Journals » Cancer Management and Research » Volume 17
The Impact of Nutritional Status and Tumor-Infiltrating Lymphocytes (CD4+ and CD8+) on Chemotherapy Response in Colorectal Cancer Patients
Authors Lukman K , Septianto R, Rudiman R , Ruchimat T, Sribudiani Y , Nugraha P , Primastari E , Budiman D
Received 31 October 2024
Accepted for publication 23 January 2025
Published 31 January 2025 Volume 2025:17 Pages 197—209
DOI https://doi.org/10.2147/CMAR.S503985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Kiki Lukman,1 Rhandy Septianto,1 Reno Rudiman,1 Tommy Ruchimat,1 Yunia Sribudiani,2 Prapanca Nugraha,1 Etis Primastari,3 Deny Budiman1
1Surgery Department, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 3The Hasan Sadikin Hospital’s Anatomical Pathology Department in Bandung, Bandung, Indonesia
Correspondence: Kiki Lukman; Rhandy Septianto, Email [email protected]; [email protected]
Introduction: The World Health Organization reports that colorectal cancer (CRC) is the second leading cause of cancer-related mortality globally, with an estimated 1.9 million new cases annually. Tumor-infiltrating lymphocytes (TILs) are frequently associated with colorectal cancer and are believed to play a significant role in the immune response to cancer cells. Regarding chemotherapy responses in patients with colorectal cancer, this study aims to investigate the association between nutritional status and infiltrating lymphocyte counts, specifically CD4+ and CD8+.
Materials and Methods: This prospective observational study analyzed the impact of nutritional status using body mass index (BMI) and TILs levels (CD4+/CD8+) on chemotherapy outcomes in CRC patients treated at a tertiary hospital in West Java, Indonesia, from July 2023 to June 2024.
Results: Thirty-six research subjects were included. Eighteen participants had high levels of TILs CD4+ and CD8+ expression. Nutritional status, age, histological type, tumor site, stage, and metastasis showed no significant correlation with the expression of either CD4+ or CD8+. Nutritional status, levels of CD4+ and CD8+ were significantly associated with chemotherapy responses in CRC patients (p< 0.001).
Conclusion: Nutritional status and elevated TIL levels (CD4+/CD8+) positively correlate with better chemotherapy response in CRC patients.
Plain Language Summary: Colorectal cancer is the second leading cause of cancer-related deaths worldwide, with 1.9 million new cases reported annually, according to the World Health Organization. This study focuses on understanding how nutrition and immune system activity affect chemotherapy responses in patients with colorectal cancer in Indonesia. The immune system plays a vital role in fighting cancer, and certain immune cells, called tumor-infiltrating lymphocytes (TILs), may help the body combat colorectal cancer. Specifically, we looked at two types of these immune cells, CD4+ and CD8+, and their connection to patients’ nutritional health during chemotherapy. We investigated 36 colorectal cancer patients receiving chemotherapy at a tertiary hospital in West Java, Indonesia, between July 2023 to June 2024. The results showed a link between better nutritional status and higher levels of CD4+ and CD8+ immune cells with improved responses to chemotherapy. These findings highlight the importance of supporting patients’ nutritional health during cancer treatment and suggest that monitoring immune cell activity could help predict treatment success. By tailoring care to these factors, healthcare providers may be able to improve outcomes for people with colorectal cancer.
Keywords: chemotherapy response, colorectal cancer, prognostic, tumor infiltrating lymphocytes
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related mortality worldwide, with 1.9 million cases reported to the World Health Organization. 45,230 new cases of rectal cancer and almost 104,270 new cases of colon cancer were estimated by the American Cancer Society, with deaths reaching 52,980. Indonesia, the fourth most populous country in the world and the largest in ASEAN, has a CRC incidence rate of 17.2 per 100,000 people, which is expected to rise annually. In 2020, the GloboCAN Indonesia database reported a total of 21,764 CRC cases, making it the second most prevalent cancer in the country, with a prevalence rate of 11.9%.1
Epidemiological studies indicate that obesity accounts for 14–35% of cancer cases and is linked to a 1.2–2 fold increased risk of colorectal cancer. Non-communicable diseases made worse by fat accounted for 73% of all deaths in 2018, which raised the prevalence of diseases like diabetes, hypertension, and cancer.2–4 The increase in Indonesia’s obesity prevalence reached an 80% increase over the last three decades, from 7% in 1980 to 12.5% in 2015, which further increased morbidity and mortality, making this a concerning phenomenon.5 One of the consequences of obesity in the pathogenesis and progression of CRC is metabolic dysfunction. This dysfunction promotes chronic inflammation, impairs insulin signaling, increases tumor-promoting growth factors such as IGF-1, and creates a pro-tumorigenic microenvironment by disrupting lipid metabolism and energy homeostasis. These changes collectively accelerate tumor initiation, progression, and resistance to therapy.6,7 It starts with the buildup of pro-inflammatory markers, elevated insulin levels, and resistance to hormones like insulin-like growth factor-1 (IGF-1). In people with obesity, there is a notable rise in a specific biomarker linked to metabolic syndrome, fibroblast growth factor (FGF-21), along with a 1.7 to 2.4 times higher risk of colorectal cancer. The general population (odds ratio [OR] 1.71, 95% CI 1.19–2.47). The pro-inflammatory environment created by obesity leads to regulatory imbalances. Proliferation, cell apoptosis, immune response disturbances, and oxidative stress triggered by obesity lead to the progression of carcinoma.6–8 Weight loss associated with malignancy is linked to anti-tumor response and disrupted immunity, resulting in decreased cancer-specific survival. The inability to maintain good nutritional status is a common constraint encountered in oncology patients, often leading to malnutrition.9
Research indicates that both obesity and being underweight, as measured by body mass index (BMI), are linked to increased all-cause mortality, cancer-specific mortality, and recurrence rates in colorectal cancer patients. These patients also experience poorer disease-free survival (DFS) when compared to those with a normal BMI. A study by Okada et al highlights a strong correlation between these factors, nutritional status during chemotherapy and adverse events as well as chemotherapy response. By the sixth month of chemotherapy, patients with good nutritional status are associated with better responses to anti-cancer therapy, thereby also enhancing progression-free survival and overall survival (OS) in FOLFOX/FIRI therapy.10
Detection of CRC through colonoscopy in at-risk population groups and confirmation via biopsy are crucial for screening in the early stages. In CRC, the prognosis can worsen if metastasis has occurred, potentially leading to increased mortality, with approximately 60% of individuals experiencing metastasis within five years. Therefore, surgical resection is the primary treatment modality for early-stage localized CRC.11 Unfortunately, surgical resection is found to be the most commonly performed modality compared to other modalities such as immunotherapy and chemotherapy. One of the reasons for this is the lack of widely promoted screening programmes in at-risk population groups, resulting in predominantly advanced-stage carcinoma cases being detected.11
Colorectal cancer is often associated with tumor-infiltrating lymphocytes (TILs), which are thought to be part of the immune system’s defense against cancer cells. Obesity, which is one of the risk factor for CRC, influences TILs through chronic inflammation, adipokine secretion, immune checkpoint dysregulation, and metabolic alterations in the tumor microenvironment, which collectively impair their anti-tumor activity and promote immune suppression.5,6 This immune response includes the activation and recruitment of cytotoxic CD8+ T lymphocytes and helper CD4+ T lymphocytes, triggered by cancer cell antigens. Studies indicate that the cytotoxic mechanisms employed by CD8+ T cells—such as perforin, granzyme, granulysin, Fas ligand, and tumor necrosis factor alpha (TNF-α)—contribute to inhibiting cancer cell proliferation and micrometastasis. In contrast, CD4+ T cells differentiate into various subsets with distinct functions, determined by the cytokines they secrete, which can aid in the destruction of tumor cells.12
Adjuvant, neoadjuvant, or palliative chemotherapy is recommended for patients with stage III colorectal cancer and stage II CRC who exhibit high-risk traits. These high-risk characteristics encompass having fewer than 12 lymph nodes assessed, poorly differentiated tumors, evidence of vascular or lymphatic invasion, perineural invasion, tumor obstruction or perforation, and a pT4 classification, along with those having a WHO performance status (PS) of 0 or 1.The chemotherapy regimen may include various agents such as 5-Fluorouracil (5-FU), followed by Capecitabine, Leucovorin/Ca-folinat, Oxaliplatin, and Irinotecan. During treatment, it is essential to monitor complete blood counts, liver function, kidney function (urea and creatinine), and blood electrolytes due to potential side effects. Patients may experience various side effects, including stomatitis, esophagopharyngitis, diarrhea, liver enzyme abnormalities, insomnia, allergies, anemia, leukopenia, neutropenia, thrombocytopenia, bradycardia, edema, hypotension, fever, anorexia, nausea, and vomiting, among others.13,14
A study by Cheng et al (2022) in China established a link between CD4+ and CD8+ T cells in the context of immunotherapy for colorectal cancer patients. It revealed a significant correlation between the ratio of tumor-infiltrating lymphocytes CD4+/CD8+ and the presence of CD4+ T cells (HR = 9.23, P = 0.004; HR = 4.83, P = 0.02). These findings suggest that higher levels of CD4+ T cells may correlate with specific patterns of disease progression or therapeutic outcomes, potentially reflecting their role in modulating the immune response within the tumor microenvironment.15
Recent advancements in biomarkers like microsatellite instability (MSI) and therapies such as immune checkpoint inhibitors have revolutionized CRC treatment, underscoring the need to investigate complementary factors like TILs and nutritional status to optimize therapeutic strategies. Nutritional status and TILs play crucial roles in modulating the immune system’s response to cancer, yet their combined impact on chemotherapy outcomes in CRC remains underexplored, addressing an important gap in current research. This study aims to investigate the association between nutritional status and infiltrating lymphocyte counts, specifically CD4+ and CD8+. By uncovering these relationships, the findings could guide personalized treatment plans, improve chemotherapy responses, enhance quality of life, and ultimately increase survival rates for CRC patients.
Materials and Methods
Study Design
This study is an observational study to evaluate the influence of nutritional status and levels of TILs CD4+ and CD8+ on the outcome of chemotherapy response in patients with CRC undergoing treatment from July 2023 to June 2024. This study was conducted and reported in accordance with the STROBE Guidelines.16
Study Sample and Selection Criteria
The inclusion criteria for this study encompass patients diagnosed with CRC, aged 18 years and older, who underwent chemotherapy procedures at the Department of Surgery. Excluded from the study are patients unwilling to participate, those lacking complete pre-chemotherapy clinical data and post-chemotherapy follow-up information, as well as deceased individuals. Additionally, patients with a prior history of malignancy or immunocompromising conditions, such as HIV or long-term steroid usage, are also excluded.
Data Collection
Body mass index (BMI) data, calculated as the ratio of weight (kilograms) to height (meters) squared, were collected from medical records prior to chemotherapy.17 The levels of tumor-infiltrating lymphocytes (TILs) CD4+ and CD8+ were measured using immunohistochemistry (IHC) from paraffin-embedded blocks of colorectal cancer patients. TIL levels were categorized as either elevated or normal, with staining expression greater than 5% classified as elevated.18 The chemotherapy response of solid tumors is typically assessed using The Response Evaluation Criteria in Solid Tumours (RECIST), which focuses on unidimensional diameter measurements, unlike the WHO criteria that consider perpendicular and bidimensional diameters. Imaging techniques such as CT scans and MRIs are commonly used to facilitate these measurements. RECIST provides a standardized method for evaluating tumor response based on progression-free survival (PFS) or overall survival (OS), categorizing outcomes into complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD). A complete response occurred when all target lesions had completely disappeared for at least one month. A partial response was defined as at least a 30% decrease in the longest diameter of a target lesion compared to baseline. Stable disease was identified when there was insufficient shrinkage to be categorized as a partial response, and insufficient growth to be labeled as progressive disease. Progressive disease was recognized by a 20% or greater increase in the longest diameter of a target lesion or the appearance of new lesions, using the smallest diameter recorded since the start of treatment as the baseline.19
Statistical Analysis
The study’s sample size was determined using the minimum sample size formula for observational quantitative studies, specifically calculated as 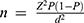 , where Z represents the Z-score for the desired confidence level, P is the estimated prevalence or proportion, and d is the acceptable margin of error. The initial calculation yielded n = 30.4, which was adjusted by adding a 10% buffer to account for potential attrition or missing data, resulting in a final sample size of 34 participants. Missing data were handled through case-wise exclusion to maintain the integrity of the statistical analyses. Data were processed using IBM® SPSS® version 26.0. For the bivariate analysis, categorical variables were compared using the chi-square test. When the assumptions for the chi-square test were not met, Fisher’s exact test was applied. Binary logistic regression was employed to calculate the odds ratio (OR). A p-value of less than 0.05 was considered statistically significant.
, where Z represents the Z-score for the desired confidence level, P is the estimated prevalence or proportion, and d is the acceptable margin of error. The initial calculation yielded n = 30.4, which was adjusted by adding a 10% buffer to account for potential attrition or missing data, resulting in a final sample size of 34 participants. Missing data were handled through case-wise exclusion to maintain the integrity of the statistical analyses. Data were processed using IBM® SPSS® version 26.0. For the bivariate analysis, categorical variables were compared using the chi-square test. When the assumptions for the chi-square test were not met, Fisher’s exact test was applied. Binary logistic regression was employed to calculate the odds ratio (OR). A p-value of less than 0.05 was considered statistically significant.
Results
Study Demography
There were 36 research subjects included in the study; data from the 36 subjects can be seen in Table 1. Out of 36 research subjects, 12 individuals (33.3%) are under 50 years old, while the other 24 individuals (66.7%) are aged 50 years or Out of the total group, 19 individuals (52.8%) were female, while 17 individuals (47.2%) were male. Histopathological findings revealed that most cases were Adenocarcinoma was the most common type, affecting 33 individuals (91.7%), while signet ring cell carcinoma was present in 2 individuals (5.6%) and mucinous adenocarcinoma in 1 individual (2.8%). Most of the subjects were classified as well-differentiated in grade (28 subjects, 77.8%). In terms of tumor location, 10 individuals (27.8%) had tumors in the colon, involving areas like the cecum, ascending colon, transverse colon, descending colon, and sigmoid colon. The other 26 individuals (72.2%) had tumors in the rectum, spanning both proximal and distal regions.
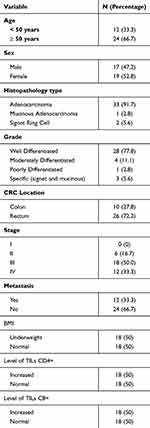 |
Table 1 Characteristics of Colorectal Cancer Patients |
BMI and Clinical Features
The majority of research subjects suffer from stage III and IV CRC, with 18 subjects (50.0%) and 12 subjects (33.3%) respectively, while 6 subjects (16.7%) have stage II CRC. Metastasis was found in 12 subjects (33.3%), with the most common metastasis occurring in the liver, found in 11 out of 12 cases (91.7%). The research results of BMI in this study show that 18 subjects (50.0%) are categorized as underweight, and 18 subjects (50.0%) are categorized as normal weight. Based on statistical analysis, BMI in relation to age, histopathological type, location of CRC, stage, and metastasis showed no significant association, while BMI has a significant association with gender as shown in Table 2.
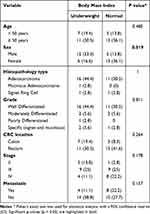 |
Table 2 Analysis of Clinical Characteristics on BMI of Colorectal Cancer Patients |
Expression of CD4+ and CD8+ TILs and Clinical Features
Table 3 reveals no notable correlation between CD4+ and CD8+ expression and factors such as age, gender, histopathological type, grade, CRC location, stage, or metastasis (p>0.05). For age, 72.2% of patients with increased CD4+ expression were ≥50 years, compared to 61.1% in the normal group (p = 0.480), while 66.7% of patients with increased CD8+ expression were ≥50 years, identical to the normal group (p = 1). Similarly, there was no significant association between BMI and T-cell expression, with 66.7% of patients having normal BMI in the increased CD4+ group compared to 33.3% in the normal group (p = 1.0).
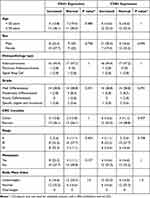 |
Table 3 Analysis of Clinical Characteristics on CD4+ and CD8+ Expression in Colorectal Cancer Patients |
BMI, Expression of TILs, and Chemotherapy Response
The findings in Table 4 demonstrate a significant association between BMI, CD4+ expression, CD8+ expression, and the chemotherapy response in CRC patients (p < 0.05). Among patients with a normal BMI, 6 patients (16.6%) achieved a complete response, whereas those with an underweight BMI exhibited higher rates of progressive disease (6 patients, 16.6%) and stable disease (12 patients, 33.3%).
 |
Table 4 Analysis of Independent Variables on Chemotherapy Response in Colorectal Cancer Patients |
Elevated CD4+ expression was associated with either a complete response in 6 patients (16.6%) or progressive disease in 12 patients (33.3%), while normal CD4+ expression was exclusively linked to stable disease in 18 patients (50%). Similarly, elevated CD8+ expression was linked to a complete response in 6 patients (16.6%), progressive disease in 6 patients (16.6%), and stable disease in 6 patients (16.6%). In contrast, normal CD8+ expression was associated with progressive disease in 6 patients (16.6%) and stable disease in 12 patients (66.7%). The odds ratio for patients with a normal BMI achieving a better chemotherapy response was 7.336 compared to those with an underweight BMI. The odds ratio for patients with a normal level of TILs CD4+ achieving a better chemotherapy response was 0.136, while for a normal level of TILs CD8+, it was 0.193. However, the p-values indicated that these odds ratios were not statistically significant as shown in Table 5.
 |
Table 5 Analysis of BMI and TILs on Chemotherapy Response in Colorectal Cancer Patients |
Discussion
Characteristics of Research Subjects
In this study, most patients diagnosed with CRC are aged 50 or older, aligning with epidemiological research indicating that the majority of CRC cases occur in this age group.20–22 While recent studies have noted a rise in CRC cases among individuals under 50, the bulk of cases still involves those 50 and older.23,24 The risk of CRC escalates significantly with age, reaching 20–30% in people aged 55–59 and above.
The number of research subjects is slightly higher for females than males, but not significantly different. When related to CRC epidemiological data, previous research data showed a higher incidence of CRC in males compared to females. Recent research findings indicate that the incidence rate is comparable between genders. In 2016, among individuals aged 45 to 49, the incidence per 100,000 people was 33.52 for men and 28.21 for women. For those aged 70 to 74, the rates were 154.50 for men and 111.41 for women.24–26 The narrowing gender gap in CRC incidence is influenced by lifestyle changes, including increased obesity, sedentary behavior, and smoking among women, which have raised their CRC risk. Improved access to screening procedures, such as colonoscopy and fecal testing, has also contributed to earlier detection and prevention in both genders, reducing disparities.11
Histopathology findings in this research is also in accordance with the current evidence, where the majority of CRC cases histopathologically manifest as adenocarcinoma, found in 90% of all cases.26,27 Moreover, the number of tumor located in the rectum is consistent with current research data of an increased incidence of CRC in the rectum, so in most cases of CRC, the tumor is found in the rectum.25,26
Nutritional Status in CRC Patients and Its Relations With Chemotherapy Responses
Based on statistical analysis, BMI in relation to age, histopathological type, location of CRC, stage, and metastasis showed no significant association, while BMI has a significant association with gender. Based on the literature, both obesity and underweight BMI in CRC patients are associated with higher all-cause mortality, cancer-specific mortality, and recurrence rates, as well as worse DFS compared to CRC patients with normal BMI.26 Moreover, both obesity and underweight BMI in CRC patients are associated with higher all-cause mortality, cancer-specific mortality, and recurrence rates, as well as worse DFS compared to CRC patients with normal BMI.28,29
Obesity leads to inflammatory conditions and alters the microenvironment, such as in liver steatosis, which can increase the risk of distant metastasis. Conversely, in underweight patients, the early infiltration of blood and lymphatic systems may heighten the risk of distant metastasis as well. These observations are consistent with research by Simkens et al and Campbell et al, which identified a significant link between BMI and gender.30,31 Additionally, this study reinforces Campbell et al findings that indicated no significant correlation between BMI and the location or stage of colorectal cancer.31 Similarly, Kalb et al 2019 study found no statistically significant differences in distant metastasis among various BMI groups.32 Molecular mechanisms, including inflammatory cytokines such as insulin-like growth factor-receptor (IGF-R) and leptin, could play a role in influencing distant metastasis in CRC patients.20
Pre-operative malnutrition is linked to complications after surgery, tumor advancement, and unfavorable clinical results. In individuals with gastrointestinal cancers, there is often a decline in nutritional status during the disease’s progression, which greatly affects patients’ quality of life, illness severity, and survival rates. The influence of BMI on the survival of colorectal cancer patients is still debated. Nonetheless, studies utilizing various nutritional measures have shown differing outcomes., scores indicate more conclusive findings that nutritional status before and after the diagnosis of CRC affects prognosis.32
The current findings suggest that immune markers like CD4+ and CD8+ expression, along with nutritional status, are associated with chemotherapy response in colorectal cancer patients. This highlights the potential for nutritional interventions as a complementary approach to enhance treatment outcomes. Clinically, tailored nutritional strategies before and after chemotherapy could help optimize immune function, reduce treatment-related toxicity, and improve overall response. For example, patients with suboptimal nutritional status might benefit from nutritional supplementation or modifications to support immune function, potentially improving their response to chemotherapy.32 Integrating such interventions into clinical practice could help personalize treatment plans and enhance patient outcomes in colorectal cancer care.
CD4+ and CD8+ Expression in CRC Patients and Its Relations With Chemotherapy Responses
This study did not find any significant correlation between CD4+ and CD8+ T cell expression and factors such as age, gender, histopathology type, location, stage, or metastasis of CRC. These results are in line with the findings of Aggeletopoulou et. Al and Kuwahara et al, who also reported that increased CD8+ expression was not associated with age, gender, tumor type, or location. In addition, Trianto et al (2023) showed no relationship between the number of tumor-infiltrating lymphocytes and tumor size, metastasis status, or location. The lack of correlation between CD4+ and CD8+ TILs and clinicopathological features can be attributed to several factors, such as the complex immune response, tumor heterogeneity, and potential variations in individual patient conditions that may obscure clear associations.21,33
Many studies have highlighted the important role of TILs, which include T cells, B cells, natural killer (NK) cells, macrophages, dendritic cells, and neutrophils. Microsatellite instability is recognized as a strong prognostic indicator in colorectal cancer patients and is closely associated with the presence of TILs, especially T cells. CD4+ helper T lymphocytes are essential for cellular immunity, assisting the activation of other immune cells such as B cells and cytotoxic T cells, thus playing a critical role in immune regulation. CD4+ T cells are further categorized into subtypes based on their functions, such as cytokine secretion and effector roles, including T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17), follicular T helper cells, and regulatory T cells (Treg).13
Th1 cells play a key role in inflammation and cell-mediated immunity by activating macrophages, B cells, and CD8+ T cells to help destroy tumor cells. The presence of Th1 cells and their cytokines, such as IFN-ɣ and TNF-α, is closely associated with positive clinical outcomes across nearly all types of cancer. CD8+ cytotoxic T cells are vital for the adaptive immune system’s defense against internal threats, including viruses, bacteria, and cancerous cells. Their ability to kill targets depends on molecules like perforin, granzyme, granulysin, Fas ligand, and tumor necrosis factor α (TNF-α). Therefore, the presence of both CD4+ and CD8+ T cells is essential for an effective anti-tumor immune response.14
Association of CD4+ And CD8+ Expression and Prognosis in CRC Patients
Yasuda et al (2011) found a strong correlation between the density of CD4+ and CD8+ T cells in rectal cancer biopsy samples and the tumors’ response to chemoradiotherapy. Furthermore, several prior studies have indicated a significant relationship between tumor-infiltrating lymphocytes in colorectal cancer and favorable treatment results.34 Research by Nakagami et al shows a significant correlation between high CD4+ density and relapse-free survival (RFS).35 In contrast, patients with low combined densities of CD4+ and FOXP3+ tumor-infiltrating lymphocytes tend to have poor 5-year RFS outcomes. Additionally, a study by Canna et al in 2005 found that low levels of CD4+ T lymphocyte infiltration are predictive of reduced cancer-specific survival.36 Furthermore, Betts et al (2012) demonstrated that the suppression of CD4+ T cells is linked to the progression of colorectal cancer.37
Cytotoxic CD8+ T lymphocytes are essential in attacking cancer cells and are crucial for anti-cancer immunity. Previous studies have shown that increased CD8+ expression is associated with better outcomes for patients with colorectal cancer. A systematic review and meta-analysis conducted by Orhan et al in 2022 highlighted that the presence of CD8+ tumor-infiltrating lymphocytes (TILs) serves as a favorable prognostic indicator for both disease-free survival (DFS) and overall survival (OS) in rectal cancer patients.38
Association of CD4+ and CD8+ Expression and Chemotherapy Response in CRC Patients
Malnutrition has a negative impact on immune response in cancer cases, thus improving nutrition is crucial for enhancing immune response. Previously, nutritional supplementation aimed solely at preventing muscle damage and weakness through protein and essential micronutrient intake. However, nowadays adequate nutritional status plays a vital role in immune function. Colorectal cancer patients are more likely to experience immunosuppression due to several factors, including advanced age, nutritional status, and direct effects of CRC.39
L-arginine is a crucial micronutrient that plays a significant role in activating T lymphocytes and enhancing the secretion of insulin and growth hormone (GH). Additionally, L-arginine serves as a nitrogen donor for the synthesis of nitric oxide (NO) in various cell types, including macrophages, polymorphonuclear leukocytes, lymphocytes, hepatocytes, vascular endothelial cells, and neurons through the action of NO synthase.34,40
The activation of T cells, particularly T lymphocytes, is essential for the body’s defense against tumors, as they target and eliminate cancer cells. Tumor-infiltrating lymphocytes (TILs) are closely associated with the prognosis of colorectal cancer (CRC), affecting factors such as tumor growth patterns, the invasion of blood and lymphatic vessels, and metastasis. A lower expression of CD8+ and a reduced CD4+/CD8+ ratio are linked to a more favorable CRC prognosis.41
Caglayan et al conducted the first study examining the effects of immunonutrition on TILs in patients with Colorectal Cancer. The results showed that nutritional interventions increased TILs (both CD4+ and CD8+), which is significantly correlated with prognosis, particularly with a notable rise in CD8+ lymphocytes related to CRC outcomes.42
Limitations and Future Improvements
Variability in research findings, including differences in the results of this study, can be attributed to limitations in the number of research subjects and differences in the characteristics of research subjects. In this study, there were no research subjects with overweight or obesity. While investigating the expression of TILs CD8+, CD4 with PD-L1 and FOXP3+ in patients with Colorectal Cancer holds promise for understanding immune responses in this population, several limitations must be acknowledged. Firstly, the potential constraint of a small sample size could hinder the generalizability of findings and limit statistical power, potentially affecting the robustness of conclusions drawn. Additionally, inherent biases, such as selection bias or measurement bias, may impact the validity of results, potentially skewing interpretations. Another limitation of this study is the reliance on BMI as the sole measure of nutritional status, which fails to account for factors like muscle mass, fat distribution, and metabolic health. The absence of extended follow-up to assess long-term outcomes, such as OS and DFS, reduces the ability to evaluate the true prognostic value of CD4+/CD8+ expressions and nutritional interventions. Future studies should incorporate more comprehensive nutritional markers and long-term follow-up to provide a deeper understanding of these relationships in colorectal cancer outcomes. The study is also limited by its six-month timeframe, which restricts the assessment of long-term chemotherapy outcomes, such as survival rates and recurrence, focusing solely on short-term responses. Furthermore, the small sample size reduces the statistical power and generalizability of the findings. Lastly, the lack of overweight and obese participants limits the comprehensiveness of the conclusions regarding the relationship between BMI, nutritional status, and chemotherapy response.
Despite these limitations, conducting research in this area offers valuable insights into the immune landscape of Colorectal Cancer patients and provides a foundation for future studies aimed at elucidating mechanisms underlying immune dysregulation in this population. Future improvements may involve addressing these limitations through larger, more diverse sample sizes and implementing rigorous study designs to mitigate biases, thereby enhancing the reliability and applicability of findings in clinical practice. Future research should explore the molecular pathways that mediate the relationship between immune markers like CD4+ and CD8+ and chemotherapy response, with a focus on cytokine profiles. Investigating cytokines such as interleukins, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) could provide valuable insights into their role in shaping the tumor microenvironment and influencing treatment outcomes. Integrating cytokine profiling could enhance the understanding of the immunological and molecular mechanisms underlying the interplay between immune markers, nutritional status, and colorectal cancer prognosis.
Conclusion
The conclusion drawn from this research is that there is a correlation between chemotherapy response and the expression of TILs CD8+ and CD4, as well as nutritional status in CRC patients. However, the study does not assess long-term outcomes such as survival rates or recurrence, reducing its clinical relevance. The study’s limitations, including the small sample size and lack of population diversity, highlight the need for future research with larger and more diverse cohorts to improve the generalizability and applicability of the findings.
Data Sharing Statement
The article contains all of the data and tables that support the study’s conclusions, and the accompanying author can provide them upon request.
Ethical Declaration
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hasan Sadikin General Hospital under Ethical Approval No. DP.04.03/D.XIV.6.5/181/2024. Prior to participation, each participant was provided with detailed information about the research and gave their informed consent to participate as a research subject.
Acknowledgments
The authors thank the resident and trainee who helped carry out this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received funding from the Universitas Padjadjaran Research Grant No. 1999/UN6.3.1/PT/00/2024.
Disclosure
The authors state that they have no disclosed financial or personal conflicts that could be perceived as influencing the study presented.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Allen J, Sears CL. Impact of the gut microbiome on the genome and epigenome of colon epithelial cells: contributions to colorectal cancer development. Genome Med. 2019;11(1):11. doi:10.1186/s13073-019-0621-2
3. Smith RA. American Cancer Society Position Statement on the Elimination of Patient Cost-Sharing Associated with Cancer Screening and Follow-up Testing. American Cancer Society. 2023. Available from: https://www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/overview/acs-position-on-cost-sharing-for-screening-and-follow-up.html.
4. Greathouse KL, White JR, Padgett RN, et al. Gut microbiome meta-analysis reveals dysbiosis is independent of body mass index in predicting risk of obesity-associated CRC. BMJ Open Gastroenterol. 2019;6(1):e000247. doi:10.1136/bmjgast-2018-000247
5. Vos T, Barber RM, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
6. Célind J, Ohlsson C, Bygdell M, Nethander M, Kindblom JM. Childhood body mass index is associated with risk of adult colon cancer in men: an association modulated by pubertal change in body mass index. Cancer Epidemiol Biomarkers Prev. 2019;28(5):974–979. doi:10.1158/1055-9965.EPI-18-1077
7. Johnson IT, Lund EK. Review article: nutrition, obesity and colorectal cancer. Aliment Pharmacol Ther. 2007;26(2):161–181. doi:10.1111/j.1365-2036.2007.03371.x
8. Harlid S, Myte R, Van Guelpen B. The metabolic syndrome, inflammation, and colorectal cancer risk: an evaluation of large panels of plasma protein markers using repeated, prediagnostic samples. Mediators Inflamm. 2017;2017:4803156. doi:10.1155/2017/4803156
9. Jaitner S, Pretzsch E, Neumann J, et al. Olfactomedin 4 associates with expression of differentiation markers but not with properties of cancer stemness, EMT nor metastatic spread in colorectal cancer. J Pathol Clin Res. 2023;9(1):73–85. doi:10.1002/cjp2.300
10. Okada S, Yamazaki S. Impact of nutritional status in the era of FOLFOX/FIRI-based chemotherapy. World J Surg Oncol. 2017;15:17. doi:10.1186/s12957-017-1226-0
11. Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134(5):1296–1310. doi:10.1053/j.gastro.2008.02.098
12. Yamagishi H, Kuroda H, Imai Y, Hiraishi H. Molecular pathogenesis of sporadic colorectal cancers. Chin J Cancer. 2016;35:4. doi:10.1186/s40880-015-0066-y
13. Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Tumor-infiltrating lymphocytes in colorectal cancer: the fundamental indication and application on immunotherapy. Front Immunol. 2021;12:808964. doi:10.3389/fimmu.2021.808964
14. Strickler JH, Hurwitz HI. Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer. Oncologist. 2012;17(4):513–524. doi:10.1634/theoncologist.2012-0003
15. Cheng YK, Chen DW, Chen P, et al. Association of peripheral blood biomarkers with response to Anti-PD-1 immunotherapy for patients with deficient mismatch repair metastatic colorectal cancer: a Multicenter Cohort Study. Front Immunol. 2022;13:809971. doi:10.3389/fimmu.2022.809971
16. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(5):31. doi:10.4103/sja.SJA_543_18
17. Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2023. PMID: 31082114.
18. Budiman D, Lukman K, Rudiman R, et al. Intra‐tumoral tumor infiltrating Lymphocyte‐T CD8+ and chemotherapy response in colorectal cancer: a prospective observational study. Trends in Immunotherapy. 2024;8(1):2815. doi:10.24294/ti.v8.i1.2815
19. Fournier L, de Geus-Oei LF, Regge D, et al. Twenty years on: RECIST as a biomarker of response in solid tumours an EORTC Imaging Group - ESOI Joint Paper. Front Oncol. 2022;11:800547. doi:10.3389/fonc.2021.800547
20. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi:10.3322/caac.21772
21. Kuwahara T, Hazama S, Suzuki N, et al. Intratumoural-infiltrating CD4+ and FOXP3+ T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br J Cancer. 2019;121(8):659–665. doi:10.1038/s41416-019-0559-6
22. Fadel MG, Malietzis G, Constantinides V, Pellino G, Tekkis P, Kontovounisios C. Clinicopathological factors and survival outcomes of signet-ring cell and mucinous carcinoma versus adenocarcinoma of the colon and rectum: a systematic review and meta-analysis. Discover Oncology. 2021;12(1):5. doi:10.1007/s12672-021-00398-6
23. Foppa C, Maroli A, Lauricella S, et al. Different Oncologic Outcomes in Early-Onset and Late-Onset Sporadic Colorectal Cancer: a Regression Analysis on 2073 Patients. Cancers. 2022;14(24):6239. doi:10.3390/cancers14246239
24. Chang SH, Patel N, Du M, Liang PS. Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database. Clin Gastroenterol Hepatol. 2022;20(6):e1365–77. doi:10.1016/j.cgh.2021.07.035
25. Raguso C, Maisonneuve N, Pichard C. Subjective Global Assessment (SGA): evaluation and followup of nutritional state. Rev Med Suisse Romande. 2004;124. PMID: 15573503.
26. Ziętarska M, Krawczyk-Lipiec J, Kraj L, Zaucha R, Małgorzewicz S. Nutritional status assessment in colorectal cancer patients qualified to systemic treatment. Contemp Oncol. 2017;21(2):157–161. doi:10.5114/wo.2017.68625
27. REACCT Collaborative. Impact of microsatellite status in early-onset colonic cancer. Br J Surg. 2022;109(7):632–636. doi:10.1093/bjs/znac108
28. Tu MY, Chien TW, Chou MT. Using a nutritional screening tool to evaluate the nutritional status of patients with colorectal cancer. Nutr Cancer. 2012;64(2):323–330. doi:10.1080/01635581.2012.650778
29. Gupta D, Lammersfeld CA, Vashi PG, Burrows J, Lis CG, Grutsch JF. Prognostic significance of Subjective Global Assessment (SGA) in advanced colorectal cancer. Eur J Clin Nutr. 2005;59(1):35–40. doi:10.1038/sj.ejcn.1602029
30. Simkens LH, Koopman M, Mol L, et al. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011;47(17):2560–2567. doi:10.1016/j.ejca.2011.06.038
31. Campbell PT, newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J clin oncol. 2012;30(1):42–52. doi:10.1200/JCO.2011.38.0287
32. Kalb L, Merkel K, Brunner B, et al. Influence of Body Mass Index on Long-Term Outcome in Patients with Rectal Cancer—A Single Centre Experience. Cancers. 2019;11(5):609. doi:10.3390/cancers11050609
33. Trianto C, Aggeletopoulou I, Kalafateli M. Chimeric Antigen Receptor T Cell Therapy for Hepatocellular Carcinoma: where Do We Stand? Int J mol Sci. 2024;25(5):2631. doi:10.3390/ijms25052631
34. Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6(1):49. doi:10.1186/1748-717X-6-49
35. Nakagami Y, Hazama S, Suzuki N, et al. CD4 and FOXP3 as predictive markers for the recurrence of T3/T4a stage II colorectal cancer: applying a novel discrete Bayes decision rule. BMC Cancer. 2022;22(1):1071. doi:10.1186/s12885-022-10181-7
36. Canna K, McArdle PA, McMillan DC, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92(4):651–654. doi:10.1038/sj.bjc.6602419
37. Betts G, Jones E, Junaid S, et al. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61(8):1163–1171. doi:10.1136/gutjnl-2011-300970
38. Orhan A, Khesrawi F, Tvilling Madsen M, et al. Tumor-infiltrating lymphocytes as biomarkers of treatment response and long-term survival in patients with rectal cancer: a systematic review and meta-analysis. Cancers. 2022;14(3):636. doi:10.3390/cancers14030636
39. Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-Based Prognostic Factors of Colorectal Cancers Are Associated With the State of the Local Immune Reaction. J clin oncol. 2011;29(6):610–618. doi:10.1200/JCO.2010.30.5425
40. Heredia M, Canales S, Sáez C, Testillano M. The nutritional status of patients with colorectal cancer undergoing chemotherapy. Farm Hosp. 2008;32(1):35–37. doi:10.1016/S1130-6343(08)72807-1
41. Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24(5):671–682. doi:10.1038/modpathol.2010.234
42. Caglayan K, Oner I, Gunerhan Y, Ata P, Koksal N, Ozkara S. The impact of preoperative immunonutrition and other nutrition models on tumor infiltrative lymphocytes in colorectal cancer patients. Am J Surg. 2012;204(4):416–421. doi:10.1016/j.amjsurg.2011.12.018
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

