Back to Journals » Drug Design, Development and Therapy » Volume 19
The Influence of Age on the Effective Dosage of Intravenous Remimazolam for the Relief of Preoperative Anxiety in Pediatric Patients at Median and 95% Effective Doses: A Prospective Study
Authors Chen Y, Zhang W, Ma J, Liu W, Song X, Chen X
Received 6 January 2025
Accepted for publication 22 May 2025
Published 1 June 2025 Volume 2025:19 Pages 4605—4615
DOI https://doi.org/10.2147/DDDT.S515924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Tamer Ibrahim
Yueyue Chen,1 Wenhua Zhang,2 Junyi Ma,1 Wenxing Liu,1 Xingrong Song,1 Xi Chen1
1Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou, People’s Republic of China; 2Department of Anesthesiology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China
Correspondence: Xi Chen, Department of Anesthesiology, Guangzhou Medical University Affiliated Women and Children’s Medical Center, Guangzhou Medical University, 9 Jinsui Road, Tianhe District, Guangdong, Guangzhou Province, 510623, People’s Republic of China, Tel +86 18620688567, Fax +86 020 38076243, Email [email protected]
Purpose: Preoperative anxiety is an urgent problem in pediatric patients. This trial evaluated intravenous remimazolam for preoperative sedation in pediatric patients, assessing efficacy, safety, and age-dependent dose effects.
Patients and Methods: In this two-part study, Aged 1~6 years old, 293 ASA I–II children [Parental Separation Anxiety Score (PSAS) ≥ 3 after nonpharmacological interventions] were enrolled. Part I: children were divided into 5 groups according to their age, and the trial was conducted by the Dixon-Massey sequential method. The first child in each group received a dose of 0.3 mg/kg of remimazolam, with a drug dose gradient of 0.05 mg/kg. Part II: 150 children were randomly selected and assigned to receive remimazolam 0.2– 0.3 mg/kg. The main observations of this study were sedation effect and safety.
Results: The ED50 and 95% confidence interval (CI) for children aged 1– 2 years was 0.14 (0.11– 0.16) mg/kg, for children aged 2– 3 years was 0.14 (0.11– 0.17) mg/kg, for children aged 3– 4 years was 0.16 (0.12– 0.19) mg/kg, and for children aged 4– 5 years was 0.14 (0.11– 0.16) mg/kg, 5– 6 years 0.13 (0.10– 0.16) mg/kg, with no significant difference between age groups (P=0.525). The ED95 for preoperative sedation in children aged 1– 6 years was 0.29 mg/kg (95% CI: 0.27– 0.40). The difference in MOAA/S scores between the different dose groups in Part II was statistically significant (p< 0.001) at 2 minutes after dosing. None of the adverse events that occurred after the use of remimazolam in this trial required the use of medication for intervention.
Conclusion: Remimazolam can be effectively used for preoperative sedation in children aged 1– 6 years with low circulatory and respiratory effects, and there was no difference in the effective dose of the drug by age.
Keywords: pediatric patient, sedation with wakefulness, dose-effect relationship, drug
Introduction
Preoperative anxiety is a common perioperative phenomenon, with a prevalence as high as 60% in pediatric patients.1 This anxiety often manifests as fear of surgery, emotional distress, and resistance to separation from parents, which can interfere with the surgical procedure and lead to prolonged hospitalization, heightened pain sensitivity, and long-term behavioral problems. Consequently, alleviating preoperative anxiety in pediatric patients has become a critical clinical priority.2 Anesthesiologists have increasingly recognized the need to address preoperative anxiety in children and have employed various interventions. Although nonpharmacological methods—such as playing videos, using electronic devices, engaging in playful interactions, and parental accompaniment—can be effective in some cases,3 their efficacy is limited. Pharmacological treatments, therefore, remain the primary approach for managing preoperative anxiety in children due to their clear and predictable effects.
Midazolam and dexmedetomidine are commonly used sedatives for children prior to surgery. Midazolam is a traditional benzodiazepine with significant anxiolytic, hypnotic, and parasympathetic amnesic effects, and it is administered in a variety of ways, either orally, intravenously, or by nasal drip. However, the use of midazolam may result in adverse effects such as respiratory depression, drowsiness during recovery from anesthesia, prolonged recovery time to, and agitation during awakening.4,5 In addition, midazolam use may be linked to long-term behavioral problems and cognitive impairment in children,6,7 such as nightmares, night terrors, food rejection, anxiety, negativism. Dexmedetomidine, producing sedative, analgesic, and anxiolytic effects while reducing glandular secretion, thereby decreasing the incidence of postoperative nausea and vomiting. However, dexmedetomidine has a slower onset of action, a longer recovery time, and may cause significant side effects, such as bradycardia and hypotension, especially when administered in high doses.8,9 About propofol, it is well known that compared with adults, children have poorer oxygen reserve capacity, and when a certain degree of hypoxia occurs, it can affect the respiratory system and even lead to cardiac arrest, therefore, respiratory management has always been an important part of pediatric anesthesia. In a multicenter clinical trial, the incidence of hypotension and respiratory depression with remimazolam for gastroscopy sedation was significantly lower than in the propofol group.10 Another study comparing the use of remimazolam and propofol for general anesthesia in children showed that remimazolam was as effective as propofol for induction of anesthesia with fewer adverse events.11 Additionally, approximately 85% of pediatric patients experience significant pain when injecting propofol, with a higher incidence in younger children,12 and this adverse effect is not present with remimazolam. The pain due to medication is detrimental to the child who already has preoperative anxiety. However, the biggest advantage of remimazolam, is the absence of respiratory depression, which is safe for pediatric patients and especially preferable for children with potential risks to the respiratory system.
Remimazolam, a new short-acting benzodiazepine, exerts its sedative effects by binding to GABAA receptors. Its sedative effects can be rapidly reversed by the antagonist flumazenil. Remimazolam is quickly hydrolyzed by nonspecific esterases in plasma, producing the inactive metabolite CNS7054, which has significantly reduced GABAA receptor binding capacity—showed around 300 times lower affinity than remimazolam —thereby minimizing the risk of drug accumulation and avoiding prolonged sedation.13 According to Rex DK, remimazolam produces fewer circulatory effects than midazolam during painless colonoscopy.14 Furthermore, a pharmacokinetic study in children following intravenous infusion of remimazolam reported a half-life of 67 (49, 85) minutes and a clearance rate of 15.9 (12.9, 18.2) mL kg−1 min−1, which is comparable to the data observed in adults While remimazolam is widely used in adults,15 there are fewer studies investigating its efficacy for preoperative anxiety relief in children. The aim of this study was to investigate the efficacy and safety of intravenous remimazolam for sedation in children aged 1–6 years, and to examine whether the age factor affects the ED50 and ED95 of this drug, to provide a reference for the clinically safe use of remimazolam and its dosing strategy in children in this age group.
Materials and Methods
The trial was a prospective, single-arm sequential trial registered with the China Clinical Trial Registry (ChiCTR2300074480) on August 8, 2023 and approved by the Ethical Review Committee of Guangzhou Women and Children Medical Centre affiliated to Guangzhou Medical University. Prior to participation, all subjects’ parents or legal guardians signed an informed consent form. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Consolidated Standards of Reporting of Trials (CONSORT) guidelines.
Patients
Children undergoing elective surgery at the Guangzhou Women and Children Medical Center affiliated with Guangzhou Medical University were selected for inclusion in the study. Inclusion criteria: (1) children aged 1–6 years old; (2) any gender; (3) children who underwent elective general anaesthesia and were admitted to the ward; (4) American Society of Anesthesiologists (ASA) class I or II; (5) children whose preoperative anxiety was not relieved after intervention with non-pharmacological means. Exclusion criteria: (1) American Society of Anesthesiologists (ASA) classification III or above; (2) patients allergic to benzodiazepines and/or remimazolam; (3) children with contraindications to preoperative sedation; (4) children with Liver and renal dysfunction; (5) children with severe neurological, psychiatric, respiratory, or cardiovascular disorders; (6) children who have been sedated with other sedative medications within one week; (7) children who have had previous perioperative adverse events; and (7) children who are unable to establish a peripheral vein before entering the surgical preparation room.
Research Design
All children were not on preoperative medication, fasted from solid food for 8 hours, formula and milk for 6 hours, breast milk for 4 hours, and drinks for 2 hours the day before surgery, and peripheral venous access was opened in the ward. The anaesthesia made a preoperative visit one day before the surgery, monitored the child’s vital signs in a quiet state using a Mindray BeneVision N1 monitor (Shenzhen Mindray Bio-Medical Electronics Co., Ltd)., and recorded these data as the child’s baseline vital sign values. On the day of surgery, the children were accompanied by their parents into the preoperative preparation room. The child’s vital signs were routinely and continuously monitored and recorded before drug administration. Preoperative anxiety was assessed using the Parental Separation Anxiety Score (PSAS, Table 1)16,17 and Modified Observer’s Assessment Alertness/Sedation (MOAA/S, Table 1).18,19 For children whose PSAS remained ≥3 after pharmacologic intervention, who cried when separated from their parents, preoperative sedation with remimazolam IV was performed.
 |
Table 1 Evaluation Scale |
Remimazolam Tosilate for injection (Jiangsu Hengrui Medicine Co., Ltd.) was diluted to 1 mg/mL with 0.9% sodium chloride solution, and was administered according to the test protocol based on the child’s weight (kg), and the child’s vital signs were continuously monitored after drug administration of the drug. If the child’s PSAS = 1 after administration, the child was admitted to the operating room for induction of anaesthesia; if the PSAS score was still ≥ 3 after 2 minutes of observation after administration, intravenous propofol 1–2 mg/kg was administered for rescue treatment, and the child was admitted to the operating room when the PSAS = 1.
Intravenous propofol 2–5 mg/kg, sufentanil 0.3–0.5 ug/kg, and cis-atracurium 0.2 mg/kg were administered for induction of anaesthesia to complete tracheal intubation or laryngeal mask airway (LMA) insertion. The ventilator was activated in pressure-controlled mechanical ventilation mode to maintain intraoperative EtCO2 at 35–45 mmHg, and a 50% oxygen/air mixture was inhaled at a constant flow rate of 2 L/min. Anaesthesia was maintained using intravenous pumped propofol and inhaled sevoflurane 1%–2.5%, with additional 1–2 ug/kg sufentanil and/or cis-atracurium 1 mg/kg given as needed. All anaesthetics drugs were discontinued at the end of the procedure, oxygen flow was adjusted to 5 L/min, and the ventilation device was removed when the child resumed normal spontaneous respiration and spontaneous movement, and then the child was transported to the postanesthesia monitoring and surveillance treatment room (PACU). The patients were returned to the ward when they met the discharge criteria (Aldrete score ≥9). Children were excluded from the trial if they had a perioperative adverse event (eg, hemorrhage, cardiac arrest, intraoperative knowledge, reflux aspiration, malignant hyperthermia, etc).
In order to obtain more accurate ED50 and ED95 of remimazolam for preoperative sedation in children aged 1~6 years, this study was divided into two parts. Part I: The aim was to calculate the ED50 and its 95% CI for the preoperative use of remimazolam in children of different ages from 1 to 6 years old and to verify whether the age factor could have an effect on the effective dose of the drug. The children were categorized into 1~2 years old group, 2~3 years old group, 3~4 years old group, 4~5 years old group and 5~6 years old group according to their age. According to Dixon,20 in this “one up, one down” experimental design, the first child in each group received a dose of 0.3 mg/kg of remimazolam, and the dose of the next child was determined by the sedative effect of the previous child, with a drug dose gradient of 0.05 mg/kg. If the child’s sedative effect was satisfactory, the current dose was considered to be effective and was recorded as a positive result, and the subsequent dose of remimazolam received by the child was lowered by one gradient (by 0.05 mg/kg). Conversely, if sedation was unsatisfactory, ie, the current dose was deemed ineffective and recorded as a negative result, the subsequent dose received by the child would be increased by one gradient (by 0.05 mg/kg). For example, if a child receives 0.2 mg/kg of remimazolam and fails to be sedated, then the next child will receive a dose of 0.25 mg/kg of the drug. In the first part of the trial, children received a dose of remimazolam with an upper limit of 0.3 mg/kg and a lower limit of 0.05 mg/kg. If a subject withdrew from the trial during the course of the experiment, the next child would receive the same dose as the withdrawn child. The first part of the trial was completed when there were 7 alternating “positive-negative” waveforms. The part II of the trial was a randomized controlled study designed to calculate the drug ED95 and its 95% CI. Based on the results of the first part of the trial, six dose levels of the drug that were all higher than the ED50 for preoperative sedation in children aged 1–6 years with remimazolam were determined. One hundred and fifty pediatric patients were randomly selected and assigned to six different treatment groups with the same criteria for sedation success and failure as in the first part of the trial. The specific groups were as follows: group A, group B, group C, group D, group E, and group F. They received 0.2 mg/kg, 0.22 mg/kg, 0.24 mg/kg, 0.26 mg/kg, 0.28 mg/kg, and 0.3 mg/kg of remimazolam mesylate intravenously, and the success rate of the different dosages of remimazolam used for preoperative sedation in the children was recorded, and drug ED95 and its 95% CI. In this study, satisfactory sedation was defined as a PSAS=1 score when the child was separated from the parents after administration of the drug, and vice versa was defined as unsatisfactory sedation. If the child was not satisfactorily sedated with remimazolam, PSAS ≥ 2 points, intravenous propofol 1–2 mg/kg was administered to remedy the situation, and the child was separated from the parents when PSAS = 1 and entered the operating room for induction of anesthesia.
Primary Outcome
Part I: whether the remimazolam dose provided satisfactory preoperative sedation in children (PSAS=1); Part II: number of children with satisfactory sedation.
Second Outcome
The secondary outcome measures of this trial are as follows:
(1) Vital signs of the children on the preoperative day when they were quiet, before intravenous remimazolam administration, 2 minutes after administration, and after induction of anaesthesia.
(2) Sedation assessment: using the MOAA/S scale to observe the sedation of the children before and 2 minutes after the administration of the drug.
(3) The four-point Mask Acceptance Score (MAS, Table 1)16,21 was used to assess the patient’s behavior when the mask was placed over the patient’s mouth and nose while receiving oxygen at the time of induction, and a score of 1 or 2 was considered “satisfactory”.
(4) Adverse events were recorded from the time of administration to the time of induction: nausea, vomiting, hypoxemia, hypotension, hypertension, bradycardia, tachycardia, respiratory depression, and allergy (bradycardia or tachycardia: a decrease or increase in heart rate of more caithan 20% of basal value, hypotension or hypertension: a decrease or increase in blood pressure of more than 20% of basal value, and hypoxemia: an SPO2 of <94%).
Sample Size Estimation, Randomization and Study Blindness
Part I used the up-and-down approach, and due to its design characteristics, the required sample size could not be predetermined. Part II was a dose-escalation study designed to estimate the 95% effective dose of remimazolam in preoperative sedation in children (ED95) by probit regression analysis. The sample size for Part II was determined by combining the results of Part I in order to improve the efficiency of the trial due to the lack of a direct formula for estimating the required sample size and the impossibility of predicting the success rate of each dose, and therefore the inability to determine the sample size by simulation.
Study subjects were randomly assigned to different drug dose groups through a random sequence to ensure that each participant had an equal probability of receiving either dose. The determination and administration of drug doses was performed by two independent anesthesiologists, and the administering physician had no knowledge of the dose group to which the patient belonged. At the same time, all evaluators, as well as patients and their parents, will not be given any specific information about the grouping. In addition, there will be dedicated personnel responsible for overseeing the data collection and analysis process to ensure the quality and safety of the trail. This rigorous blinding and randomization will minimize bias and improve the reliability of the study results.
Statistical Analysis
Data were analyzed using SPSS version 26.0 for Windows (SPSS Inc., Chicago, IL, USA). For count data, we used frequencies or medians and the corresponding interquartile range (IQR). To test for differences in the distribution of these data, we used the chi-square test or Fisher’s exact probability method. For approximately normally distributed measure data, we used means and standard deviations (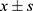 ) to describe them and one-way analysis of variance (ANOVA) to compare differences between groups. For skewed distribution of measurement data, we instead used median (M) and interquartile spacing (IQR) to describe them and rank sum test (Mann–Whitney U-test) to compare between groups. The Dixon-Massey method20 was used to calculate the ED50 and 95% confidence interval (CI); Probit regression22 was used to calculate the ED95 and 95% confidence intervals; and rank sum test was used to compare the ED50 between groups. p < 0.05 was considered statistically significant.
) to describe them and one-way analysis of variance (ANOVA) to compare differences between groups. For skewed distribution of measurement data, we instead used median (M) and interquartile spacing (IQR) to describe them and rank sum test (Mann–Whitney U-test) to compare between groups. The Dixon-Massey method20 was used to calculate the ED50 and 95% confidence interval (CI); Probit regression22 was used to calculate the ED95 and 95% confidence intervals; and rank sum test was used to compare the ED50 between groups. p < 0.05 was considered statistically significant.
Results
From August 2023 to April 2024, a total of 293 children were enrolled in this study. 8 were excluded from Part I (5 were excluded because they did not meet the inclusion criteria, and 3 were excluded because they refused to participate), and 120 were finally included. 120 were statistically analyzed, of which 26 were in the 1–2 year age group, 23 in the 2–3 year age group, 21 in the 3–4 year age group, 27 in the 4–5 year age group, and 23 in the 5–6 year age group. 15 were excluded from Part II (6 were excluded because they did not meet the inclusion criteria, and 9 were excluded because they refused to participate), resulting in the inclusion of 150 patients and statistical analysis of 146. The specific flow of the trial is shown in Figures 1 and 2, and the demographic characteristics are shown in Table 2.
 |
Table 2 Demographic Data and Patient’s Characters |
 |
Figure 1 Flow diagram of Part I. |
 |
Figure 2 Flow diagram of Part II. |
The sequential method to determine the order of median effective dose success and failure results of remimazolam for preoperative sedation in children of different age groups is shown in Figure 3. According to Part I results, the ED50 and 95% confidence interval (CI) for children aged 1–2 years was 0.14 (0.11–0.16) mg/kg, 2–3 years 0.14 (0.11–0.17) mg/kg, 3–4 years 0.16 (0.12–0.19) mg/kg, 4–5 years 0.14(0.11–0.16) mg/kg, and 5–6 years 0.13(0.10–0.16) mg/kg, with no difference between the groups (P = 0.525).In Part II, the ED50 and 95% CI of remimazolam used for preoperative sedation in children aged 1–6 years was calculated to be 0.16 (0.01–0.20) mg/kg using probit regression, with a ED95 and 95% CI was 0.29 (0.27–0.40) mg/kg (Table 3). Table 4 shows the PSAS scores, MOAA/S scores, and MAS scores of the children before and after the administration of remimazolam. There was a statistically significant difference in the MOAA/S scores after 2 minutes of administration between the different dosage groups of remimazolam in Part II (n = 146), P<0.001, and the difference between the rest of the score groups was not statistically significant.
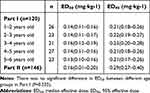 |
Table 3 ED50 and ED95 of Remimazolam for Preoperative Sedation in Pediatric Patients(mg/Kg) |
 |
Table 4 Parental Separation Anxiety Score (PSAS) and Modified Observer’s Assessment Alertness/Sedation (MOAA/S) After Drug Administration |
Adverse events at 2 minutes post-dose included bradycardia, tachycardia, hypotension, hypertension, and hypoxemia, as detailed in Table 5. None of the adverse events that occurred after the use of remimazolam in this trial required the use of medication for intervention.
 |
Table 5 Adverse Event |
Discussion
This study determined the median effective dose (ED50) and 95% confidence interval (CI) for preoperative sedation with remimazolam in children. The ED50 was found to be 0.14 mg/kg (95% CI: 0.11–0.16) for children aged 1–2 years, 0.14 mg/kg (95% CI: 0.11–0.17) for those aged 2–3 years, 0.16 mg/kg (95% CI: 0.12–0.19) for those aged 3–4 years, 0.14mg/kg (95% CI: 0.11–0.16) for those aged 4–5 years, and 0.13 mg/kg (95% CI: 0.10–0.16) for those aged 5–6 years, with no statistically significant difference in ED50 across age groups. The ED95 for preoperative sedation in children aged 1–6 years was 0.29 mg/kg (95% CI: 0.27–0.40). In this study, we investigated the use of remimazolam for preoperative sedation in children of different ages, evaluating its ED50 and ED95, and analyzing whether age influences the effective dose of the drug. The results indicated no significant difference in ED50 between the age groups when remimazolam was used for preoperative sedation in children. Studies have shown that the ED50 for remimazolam for preanesthetic induction sedation in adult patients had an ED50 of 0.11 mg/kg, with the ED50 in 18- to 40-year-old patients being higher than >80 patients.23 Although the results of this study did not show a correlation between the age factor and the preoperative sedation effect of remimazolam in children aged 1–6 years. Although the results of our study showed that the age factor did not influence the preoperative sedation effect of remimazolam in children aged 1 to 6 years, the ED50 for preoperative sedation with remimazolam in the children in this study was higher than that of the adult patients in the above study, which also suggests a difference in the effectiveness of remimazolam sedation with increasing age. Our trial included only children aged 1 to 6 years. This trial only included children aged 1–6 years, which is a small age range, and subsequent trials may expand the age range of the subjects to further explore the pharmacodynamics of preoperative sedation with remimazolam.
In a clinical trial of intravenous remimazolam in children aged 1 month-6 years with congenital heart disease in left-to-right shunts, researchers grouped children similarly according to age and measured the effective dose of the drug using the sequential method. The results showed that the ED50 for successful sedation of infants (1 month-1 year), toddlers (1–3 years), and preschoolers (3–5 years) with remimazolam was 0.209, 0.259, and 0.266 mg/kg, respectively, and the ED95 was 0.356, 0.404, and 0.408 mg/kg, respectively.24 In another study of the same single intravenous injection of remimazolam used in children, researchers found that when a single injection of remimazolam 0.45–0.60 mg/kg’ was used in children 1–6, it resulted in loss of consciousness and completion of anesthetic induction.25 In the present study, however, the results of the study showed that the ED50 and ED95 values of the drug were smaller than those of the two clinical trials mentioned above. After analyzing the reasons for this, we concluded that although both were administered via the intravenous route, the differences in the main endpoints of the trials, assessment scales, etc., led to the differences in the results of the studies. In our trial, we concluded that if the children were sedated to a level where they could be easily separated from their parents (PSAS=1), preoperative anxiety would disappear, which was the primary outcome of the present study, without the need to achieve a score of MOAA/S ≤ 3 as required by Jin et al24 or even the deep state of sedation with loss of consciousness pursued by Cai et al25 without the need to achieve deep sedation with MOAA/S≤3 or even loss of consciousness. This primary outcome is effective for preoperative sedation without the need to use high doses of sedative drugs, which increases the risk of medication. At the same time, no serious adverse effects were observed in the subjects of either clinical study, even when the drug was used in infants and young children up to 1 month of age or in doses of 0.45–0.60 mg/kg. In the present study, the minimum age of the subjects was 1 year, and the maximum dose of remimazolam was 0.3 mg/kg, and there were also no adverse reactions requiring pharmacological intervention, which proves that the selection of the subjects and the dose of the drug in the present trial were safe and ethical.
Previous studies have demonstrated that remimazolam, as a novel anesthetic, has been safely and effectively used for the induction and maintenance of general anesthesia in children.26,27 A recent clinical trial extended the application of remimazolam, confirming its efficacy in alleviating preoperative anxiety in children when administered intranasally, with minimal respiratory and hemodynamic effects.28 However, intranasal administration of remimazolam can cause a strong burning sensation, potentially limiting its use as the preferred route of administration. Therefore, in this study, we opted for intravenous administration of remimazolam to rapidly relieve preoperative anxiety while avoiding discomfort from nasal irritation.
The Part I of this trial employed the Dixon-Massey sequential method to explore the median effective dose (ED50) of remimazolam, a recognized research method known for its small sample size, convenience, accuracy, and stepwise intervention adjustment to minimize complication rates.20–29 In Part II, to calculate a more accurate 95% effective dose (ED95), we used Probit regression analysis. Based on Part I results, which showed no variability in remimazolam’s effectiveness across different age groups for preoperative sedation, Part II only included experimental groups with varying drug dosages to refine the dose-effect relationship further. The trial’s final results were obtained by combining these two Parts, referencing established methodologies.30 Borkett et al31 reported that success rates for gastroscopy in adult patients using remimazolam alone at doses of 0.10, 0.15, and 0.20 mg/kg were 32%, 56%, and 64%, respectively, with a favorable safety profile. In our preliminary tests, satisfactory sedation was achieved in most children at a dose of 0.3 mg/kg of remimazolam. Based on the principle of maximizing the child’s best interest and minimizing the need for remedial sedation, we set the starting dose at 0.3 mg/kg in Part I. In Part II, to ensure safety and efficacy, no test group was established below the ED50. The ED95 for intravenous remimazolam injection in this study was 0.29 mg/kg (95% CI: 0.27–0.40), which is lower than the dosage reported by Xiang Long et al.28 This difference may be attributed to the different routes of administration, as Xiang Long et al used transnasal administration, whereas our study used intravenous injection. Different administration routes can affect the drug’s absorption rate and bioavailability, influencing the effective dose. Additionally, the sedation depth required in this study differed from that of Xiang Long et al. In our study, a PASA score of 1 was defined as the relief of preoperative anxiety. Satisfactory sedation was achieved with a small dose of intravenous remimazolam, allowing successful separation of children from their parents. Most children experienced mild sedation, remained able to converse, and rarely fell asleep. This fine control of light sedation depth not only helps to minimize drug dosage and potential side effects but also ensures that the child remains appropriately awake preoperatively to facilitate effective communication with doctors. A study by Huichen Zhu et al32 found that in adult patients, when combined with 5 micrograms of sufentanil, the success rates of remimazolam at doses of 0.15 mg/kg and 0.2 mg/kg during gastroscopy were 88.5% and 98.7%, respectively, suggesting that sedation depth is dose-dependent. In our study, all children were awake before receiving the drug. In Part II, the children’s MOAA/S scores showed a statistically significant difference 2 minutes after drug administration, indicating that sedation depth increased with higher drug doses.
Previous studies by Antonik et al33 and Schuttler et al34 have confirmed that intravenous remimazolam can accelerate heart rate. This phenomenon was also observed in our preoperative sedation study, where the incidence of tachycardia was 9.8%, likely related to the drug’s use. Additionally, this trial recorded all adverse reactions, including in children who failed to sedate, acknowledging that hemodynamic changes might also be due to the children’s psychological distress after sedation failure. One case of hypoxemia was recorded, but the child’s respiration recovered rapidly after the mandibular support maneuver, requiring no further intervention. The child was undergoing partial adenoidectomy, and it is likely that the hypoxemia was related to sleep apnea syndrome associated with the children’s underlying condition.
Conclusion
In conclusion, remimazolam IV. can be effectively used for preoperative sedation in children aged 1–6 years with low effects on the circulatory and respiratory systems. The drug ED95 and 95% CI was 0.29 (0.27–0.40) mg/kg, and there was no difference in the half effective dose of the drug between age groups.
Data Sharing Statement
The data collected for this study can be shared with researchers in de-identified form after the publication date, and in the presence of a data transfer agreement, and if it complies with China legislation. Requests for data and study proposal should be directed to [email protected].
Disclosure
The authors report no conflicts of interest in this work.
References
1. West N, Christopher N, Stratton K, Görges M, Brown Z. Reducing preoperative anxiety with Child Life preparation prior to intravenous induction of anesthesia: a randomized controlled trial. Paediatr Anaesth. 2020;30(2):168–180. doi:10.1111/pan.13802
2. Nair T, Choo CSC, Abdullah NS, et al. Home-Initiated-Programme-to-Prepare-for-Operation: evaluating the effect of an animation video on peri-operative anxiety in children: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(8):880–887. doi:10.1097/EJA.0000000000001385
3. Manyande A, Cyna AM, Yip P, Chooi C, Middleton P. Non-pharmacological interventions for assisting the induction of anaesthesia in children. Cochrane Database Syst Rev. 2015;2015(7):CD006447. doi:10.1002/14651858.CD006447.pub3
4. Coté CJ. Sedation for the pediatric patient. A review. Pediatr Clin North Am. 1994;41(1):31–58.
5. Zhang MQ, Xu MZ, He Y, Su YW, Ma J, Zuo YX. Comparison of S-ketamine and midazolam for intravenous preoperative sedative and anxiolytic effects in preschool children: study protocol for a randomized controlled clinical trial. Trials. 2023;24(1):724. doi:10.1186/s13063-023-07767-2
6. Impellizzeri P, Vinci E, Gugliandolo MC, et al. Premedication with melatonin vs midazolam: efficacy on anxiety and compliance in paediatric surgical patients. Eur J Pediatr. 2017;176(7):947–953. doi:10.1007/s00431-017-2933-9
7. McGraw T, Kendrick A. Oral midazolam premedication and postoperative behaviour in children. Paediatr Anaesth. 1998;8(2):117–121. doi:10.1046/j.1460-9592.1998.00724.x
8. Mason KP, Zurakowski D, Zgleszewski SE, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18(5):403–411.
9. Kim HJ, Shin WJ, Park S, Ahn HS, Oh JH. The sedative effects of the intranasal administration of dexmedetomidine in children undergoing surgeries compared to other sedation methods: a systematic review and meta-analysis. J Clin Anesth. 2017;38:33–39.
10. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, Phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481.
11. Fang YB, Zhong JW, Szmuk P, et al. Safety and efficacy of remimazolam tosilate for general anaesthesia in paediatric patients undergoing elective surgery: a multicentre, randomised, single-blind, controlled trial. Anaesthesia. 2025;80(3):259–268. doi:10.1111/anae.16475
12. Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29(7):543–563.
13. Zhang F, Chang H, Qing W, Yu R, Liao Q, Tong J. Remimazolam tosylate combined with low-dose propofol improves sedation and safety in hysteroscopy. Drug Des Devel Ther. 2022;16:4101–4108. doi:10.2147/DDDT.S390403
14. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi:10.1016/j.gie.2018.04.2351
15. Gao YQ, Ihmsen H, Hu ZY, et al. Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br J Anaesth. 2023;131(5):914–920. doi:10.1016/j.bja.2023.08.019
16. Abdel-Ghaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. Br J Anaesth. 2018;121(2):445–452.
17. Liao Y, Xie S, Zhuo Y, et al. Intranasal dexmedetomidine-esketamine combination premedication versus monotherapy for reducing emergence delirium and postoperative behavioral changes in pediatric tonsillectomy and/or adenoidectomy: a randomized controlled trial. Drug Des Devel Ther. 2024;18:4693–4703.
18. Zhang W, Fan Y, Zhao T, Chen J, Zhang G, Song X. Median effective dose of intranasal dexmedetomidine for rescue sedation in pediatric patients undergoing magnetic resonance imaging. Anesthesiology. 2016;125(6):1130–1135. doi:10.1097/ALN.0000000000001353
19. Yoo YC, Park CH, Shin S, Park Y, Lee SK, Min KT. A comparison of sedation protocols for gastric endoscopic submucosal dissection: moderate sedation with analgesic supplementation vs analgesia targeted light sedation. Br J Anaesth. 2015;115(1):84–88. doi:10.1093/bja/aeu555
20. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50.
21. Cai YH, Wang CY, Fang YB, et al. Preoperative anxiolytic and sedative effects of intranasal remimazolam and dexmedetomidine: a randomized controlled clinical study in children undergoing general surgeries. Drug Des Devel Ther. 2024;18:1613–1625. doi:10.2147/DDDT.S461122
22. Yu AL, Critchley LA, Lee A, Gin T. Alfentanil dosage when inserting the classic laryngeal mask airway. Anesthesiology. 2006;105(4):684–688. doi:10.1097/00000542-200610000-00012
23. Ni MJ, Jin YT, Wu QL, et al. Effective dose of intranasal remimazolam for preoperative sedation in preschool children: a dose-finding study using Dixon’s up-and-down method. Front Pharmacol. 2024;15:1372139. doi:10.3389/fphar.2024.1372139
24. Jin M, Lin H, Qiu L, Xu H, Zhang H, Hou S. Remimazolam for successful sedation in children with left-to-right shunt congenital heart disease: an up-and-down sequential allocation trial. Eur J Anaesthesiol. 2025.
25. Cai YH, Dong LQ, Zhong JW, et al. ED50 and ED95 of remimazolam for loss of consciousness in young children. Br J Anaesth. 2025;134:S0007–0912(25)00093–5. doi:10.1016/j.bja.2025.02.004
26. Yang X, Lin C, Chen S, Huang Y, Cheng Q, Yao Y. Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des Devel Ther. 2022;16:3413–3420. doi:10.2147/DDDT.S381611
27. Chu T, Zhou S, Wan Y, et al. Comparison of remimazolam and propofol combined with low dose esketamine for pediatric same-day painless bidirectional endoscopy: a randomized, controlled clinical trial. Front Pharmacol. 2024;15:1298409.
28. Long X, Wen LX, Yang H, et al. ED95 of remimazolam in nasal administration for attenuating preoperative anxiety in children. Front Med. 2023;10:1253738.
29. Ace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
30. Frawley G, Smith KR, Ingelmo P. Relative potencies of bupivacaine, levobupivacaine, and ropivacaine for neonatal spinal anaesthesia. Br J Anaesth. 2009;103(5):731–738.
31. Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780.
32. Zhu H, Su Z, Zhou H, et al. Remimazolam dosing for gastroscopy: a randomized noninferiority trial. Anesthesiology. 2024;140(3):409–416. doi:10.1097/ALN.0000000000004851
33. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled Phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–283. doi:10.1213/ANE.0b013e31823f0c28
34. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi:10.1097/ALN.0000000000003103
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


