Back to Journals » Drug Design, Development and Therapy » Volume 19
The Median Effective Concentration (EC50) of Alfentanil Combined with Propofol Closed-Loop Targeted Infusion in Super-Elderly Patients Undergoing Endoscopic Retrograde Cholangiopancreatography (ERCP)
Authors Sun Z, Li W, Zhong Y, Lang B, Luo Y
Received 20 February 2025
Accepted for publication 3 May 2025
Published 16 May 2025 Volume 2025:19 Pages 4011—4019
DOI https://doi.org/10.2147/DDDT.S520421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Tamer Ibrahim
Zenggang Sun,1,2 Weiqiang Li,1 Yuqi Zhong,2 Bao Lang,2,* Yanhua Luo2,*
1School of Anesthesiology, Shandong Second Medical University, Weifang, Shandong Province, 261053, People’s Republic of China; 2Department of Anesthesiology, Weifang People’s Hospital, Weifang, Shandong Province, 261041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanhua Luo, Department of Anesthesiology, Weifang People’s Hospital, No. 151 Guangwen Street, Kuiwen District, Weifang, Shandong Province, 261041, People’s Republic of China, Tel +86-15964037909, Email [email protected] Bao Lang, Department of Anesthesiology, Weifang People’s Hospital, No. 151 Guangwen Street, Kuiwen District, Weifang, Shandong Province, 261041, People’s Republic of China, Tel +86-13515400823, Email [email protected]
Purpose: To investigate the median effective concentration (EC50) of alfentanil combined with propofol closed-loop targeted infusion when used for sedation in super-elderly endoscopic retrograde cholangiopancreatography (ERCP) patients.
Patients and Methods: A total of 28 super-elderly patients (> 85 years old) undergoing elective ERCP from April to September 2024 were enrolled to receive target-controlled continuous infusion of alfentanil with an initial infusion concentration of 50ng/mL, and the continuous infusion concentration was determined according to the Dixon up and down method, and the incremental/decremental gradient of alfentanil was set at 10ng/mL. The observation was terminated after 8 reflexes. The EC50 of target-controlled infusion of alfentanil combined with propofol in the operation of ERCP in super-aged patients was calculated. HR and MAP were recorded at the time of entering the room at rest (T0), after anesthesia induction (T1), before duodenoscope placement (T2), and within 3 minutes after duodenoscope placement (T3).
Results: In this study, the EC50 of alfentanil combined with propofol target-controlled infusion during ERCP in super-elderly patients was determined to be 36.52ng/mL (95% confidence interval [CI]: 32.12– 41.71ng/mL). Among the 28 patients, 27 patients (96.43%) had a change in HR less than 20% of baseline during duodenoscopy, and 1 patient (3.57%) had a change in HR more than 20% of baseline during duodenoscopy. Of the 28 patients, 26 (92.86%) had a MAP change of less than 20% of baseline during duodenoscopy, and 2 (7.14%) had a MAP change of more than 20% of baseline.
Conclusion: The EC50 of alfentanil combined with propofol by closed-loop target-controlled infusion during ERCP in super-elderly patients is 36.52ng/mL. At this concentration level, the patients can be satisfied with surgical anesthesia and maintain relatively stable hemodynamics.
Keywords: the median effective concentration, alfentanil, super-elderly patients, endoscopic retrograde cholangiopancreatography
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is the preferred treatment for biliary diseases.1,2 By retrograde insertion of a choledochoscope for biliary examination and drainage tube placement, it can effectively relieve biliary obstruction and prevent acute obstructive suppurative cholangitis and other critical conditions.3,4 As a high-risk population for biliary diseases, super- elderly patients often present with multisystem organ function decline, resulting in significantly reduced tolerance to surgery and anesthesia.5,6 Therefore, the selection of anesthesia protocols should prioritize agents that provide adequate sedation, rapid recovery, and minimal cardiopulmonary impact. Propofol is most commonly used due to its superior pharmacokinetic properties.7 Although midazolam combined with fentanyl can achieve moderate sedation, and dexmedetomidine has minimal effects on respiratory function, these regimens may have delayed onset issues.8,9 For super-elderly patients (>85 years), short-acting drugs administered via precise target-controlled anesthesia are recommended to optimize anesthesia duration.8
Alfentanil is a novel short-acting opioid with good controllability and a low incidence of respiratory depression and delayed awakening, which makes it suitable for application in the elderly.10,11 Propofol, an alkylphenol intravenous anesthetic, is widely used in short procedures such as painless endoscopy. However, due to its lack of analgesic properties, larger doses are often required when used alone. Notably, as the propofol dosage increases, the incidence of associated adverse effects including cardiovascular depression, respiratory suppression, injection pain, and delayed recovery rises significantly.12–14 Current studies demonstrate that the combination of alfentanil and propofol exhibits a significant synergistic effect.15–17 This combination not only enhances anesthetic efficacy but also reduces the required dosage of each drug, thereby lowering anesthesia costs,16 and demonstrating distinct advantages in outpatient examinations and short surgical procedures.18 However, most existing research has focused on younger or general elderly populations, with limited studies on super-elderly patients (>85 years), dose optimization studies are still lacking.
Target-Controlled Infusion (TCI) refers to the administration of intravenous anesthetics based on pharmacokinetic and pharmacodynamic principles, regulating plasma or effect-site drug concentrations to achieve and maintain optimal anesthetic depth. This method offers superior controllability, precision, and operational convenience.19 In addition, Dixon up-and-down method was used to determine the median effective concentration (EC50) in this study, which utilizes a positive-negative feedback mechanism between adjacent dose responses to accurately identify the EC50 value.20 This method is particularly suitable for dose exploration studies in special populations such as super-elderly patients. Based on the above basis, this study for the first time determined the EC50 of alfentanil combined with propofol for closed-loop TCI in super-elderly patients undergoing ERCP, which filled the research gap in this field, evaluated the safety and effectiveness of this anesthesia regimen, aiming to provide a theoretical foundation for personalized anesthesia protocols for super-elderly patients undergoing ERCP.
Materials and Methods
Ethics Statement
This study was approved by the Medical Ethics Committee of Weifang People’s Hospital (KYLL20240318-6) and the China Clinical Trial Registry (Registration No. ChiCTR2400082734), and adhered to the Declaration of Helsinki and CONSORT standards. All patients in this study obtained informed consent and signed an informed consent form.
Participants and Inclusion Criteria
The study was conducted from April to September 2024. Participants in the study were super-elderly (>85 years old) patients undergoing elective ERCP; American Society of Anesthesiology (ASA) Medical condition Class II or III. Patients who were allergic to propofol or alfentanil; patients with blood pressure fluctuations of more than 20% after admission, or with prolonged reintubation/intubation time, or without follow-up records; patients not taking the specified drug combinations, especially those with a high impact on clinical outcomes, efficacy, and safety; and patients with severe cardiovascular and cerebrovascular diseases were excluded.
Study Protocol
On admission, intravenous access was established and sodium lactate Ringer injection was infused intravenously. A multiparameter monitor was connected to monitor electrocardiogram (ECG), heart rate (HR), and pulse oximetry (SpO2). Allen’s test was routinely performed prior to radial artery cannulation to ensure that the puncture could be performed without serious complications. Radial artery puncture under local anesthesia and catheterize for arterial blood pressure (ABP) monitoring were established. Alfentanil was applied for induction of anesthesia, and the induction dose was 5 μg/Kg pushed slowly. A closed-loop target-controlled drug infuser was connected for continuous monitoring of the electroencephalographic dual-frequency index (BIS) and Closed loop target-controlled infusion of propofol. A syringe filled with propofol (1%) was connected to an EEG-monitoring TCI syringe pump (SLGO BCP-100A model), and patient demographics were entered. Based on a study by Imagawa et al21 they recommend that when using a target-controlled Infusion (TCI) pump, the initial Target blood concentration of propofol is set at 1.2μg/mL and Cp is increased by 0.2 μg/mL every two minutes. Maintain BIS between 40 and 60. After the patient’s consciousness disappeared (OAA/S≤2), a target-controlled continuous infusion of alfentanil was given, with a starting concentration of 50 ng/mL. The continuous infusion concentration was determined in accordance with the Dixon up-and-down method,20 the patient’s plasma target-controlled concentration was determined by the response of the previous patient within 3 min after duodenoscopy placement, and if the patient showed a positive response after duodenoscopy placement (choking, body movement, swallowing), the next patient was given a higher concentration. If no such reaction occurs, the next patient is considered to have a negative reaction, and the next patient applies a lower concentration, setting the gradient of increasing and decreasing adjacent concentrations of alfentanil at 10 ng/mL. The enrolled samples were counted from the previous case in which the reaction after duodenoscopy placement occurred, and the observation was terminated after 8 reflexes.
Hypotension, defined as a 30% decrease in blood pressure from baseline, was treated with fluid therapy with accelerated infusion of Ringer’s lactate and infusion of 6–10 mg ephedrine. Bradycardia (HR < 50 bpm) was treated with infusion of 0.5 mg atropine. Continuous oxygen (3 L.min-1) Refractory respiratory depression (SpO2 < 90%) is corrected by freehand airway opening. If pulse oximetry does not improve or spontaneous respiration persists, artificial ventilation support is provided.22
Blinding
An independent anesthesiologist recorded all observational data and unidirectionally communicated positive/negative response outcomes to a senior anesthesiologist. The senior anesthesiologist calculated the appropriate alfentanil dosage based on preceding patients’ responses, preset the TCI parameters, and concealed the display interface using opaque labels to maintain blinding. Both the patients and the performing anesthesiologists responsible for induction/maintenance were only aware of case numbers. No inter-group communication occurred among other anesthesiology team members.
Observations
Primary observation index: EC50 of alfentanil combined with propofol closed-loop target-controlled infusion applied in ERCP in super-elderly patients; secondary observation index: heart rate (HR) and mean arterial pressure (MBP) at the time of admission to the room for sedentary lying (T0), after induction of anesthesia (T1), before duodenoscope placement (T2), and at the time of duodenoscope placement for 3 min (T3) were recorded.
Statistical Analysis
Data were analyzed using Excel 2007 and SPSS25.0 software. Normally distributed measurements were expressed as mean ± standard deviation ( ± s) with independent samples t test, non-normally distributed measurements were expressed as median (quartile) [M (Q1, Q3)] with Wilcoxon rank sum test, and count data were expressed as [n (%)] with χ2 test or Fisher’s exact probability test. Differences were considered statistically significant at P < 0.05. Alfentanil EC50 was calculated by the modified Dixon up-down method (MDUDM).23 Dose-response curves and 95% confidence intervals (CIs) for EC50 were determined using probabilistic regression analysis.
± s) with independent samples t test, non-normally distributed measurements were expressed as median (quartile) [M (Q1, Q3)] with Wilcoxon rank sum test, and count data were expressed as [n (%)] with χ2 test or Fisher’s exact probability test. Differences were considered statistically significant at P < 0.05. Alfentanil EC50 was calculated by the modified Dixon up-down method (MDUDM).23 Dose-response curves and 95% confidence intervals (CIs) for EC50 were determined using probabilistic regression analysis.
Results
A total of 35 patients were initially screened for this study. Following exclusion of 7 ineligible patients (4 due to withdrawal of consent, 1 on long-term anticoagulation therapy, and 2 with severe cardiovascular diseases), 28 patients ultimately completed the study protocol. The demographic characteristics of the study population are presented in Table 1.
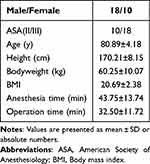 |
Table 1 Demographic Data of Study Population |
In this study, we found that the EC50 of alfentanil combined with propofol target-controlled infusion applied in ERCP in super-elderly patients was 36.52ng/mL (95% CI: 32.12–41.71ng/mL). Dose-response data for alfentanil-targeted infusion obtained by the up-and-down method (Figure 1). The response curves of target-controlled infusion concentrations of alfentanil during ERCP in ultra-elderly patients are shown in Figure 2.
 |
Figure 1 The concentration of alfentanil was determined according to the Dixon’s up-and-down method. |
 |
Figure 2 Response curve of target controlled infusion concentration of alfentanil in super-aged patients undergoing ERCP. EC50: The median effective concentration; 95% CI: 95% confidence interval. |
Twenty-seven of 28 patients (96.43%) had a change in HR of <20% of baseline during duodenoscopy placement, and 1 patients (3.57%) had a change in HR of >20% of baseline during duodenoscopy placement. Twenty-six of 28 patients (92.86%) had a change in MAP of <20% of baseline during duodenoscopy placement, and 2 patients (7.14%) had a change in MAP >20% of baseline during duodenoscopy placement. The magnitude of change in hemodynamic indices (HR and MAP) in each patient is shown in Figures 3 and 4.
 |
Figure 3 The heart rate values of 28 patients before and after duodenoscope were shown. T2: before duodenoscope placement; T3: within 3 minutes after duodenoscope placement. |
 |
Figure 4 The mean arterial pressure of 28 patients before and after duodenoscope were shown. T2: before duodenoscope placement; T3: within 3 minutes after duodenoscope placement. |
Discussion
With the aging of the global population and the high prevalence of pancreatic and biliary diseases in elderly patients,24,25 ERCP has been used more and more clinically as the gold standard for the treatment of biliary and pancreatic diseases.26 However, super- elderly patients present unique anesthetic challenges due to multiple comorbidities and declined organ function, resulting in significantly reduced metabolic capacity and drug tolerance.27 Safe sedation thus becomes a critical concern in this population, requiring careful balance between adequate sedation and avoidance of oversedation-related adverse events to optimize perioperative management and clinical outcomes.24 A network meta-analysis conducted by Li et al28 systematically evaluated various anesthetic regimens for ERCP and demonstrated that propofol-alfentanil combination offers superior safety profiles with reduced risks of respiratory depression and postoperative nausea/vomiting, a finding consistent with Mazanikov et al‘s research.29 The pharmacodynamic synergy between alfentanil and propofol enhances both analgesic and sedative effects.30 Notably, Mertens et al31 revealed that propofol significantly alters alfentanil’s pharmacokinetics, decreasing its terminal clearance by 15%, rapid distribution clearance by 68%, and slow distribution clearance by 51%. These findings underscore the necessity for infusion protocol adjustments in very elderly patients to prevent drug accumulation. Furthermore, the distinct central nervous system effects of this drug combination allow for balanced analgesia-sedation, potentially improving procedural comfort and patient experience.
Current research on optimal alfentanil dosing for very elderly patients remains limited, with existing recommended doses primarily derived from studies involving general elderly populations (65–85 years).32,33 The pharmacokinetic differences in super- elderly patients (eg, decreased hepatic/renal function, altered plasma protein binding) may lead to increased drug sensitivity, necessitating more precise dose optimization.34 EC50 refers to the drug dose that produces 50% of maximal response intensity in quantitative assays or elicits positive effects in 50% of subjects in qualitative studies. It serves as a key parameter for investigating dose-response relationships in clinical practice. This study employed the Dixon sequential method to determine EC50. Traditional dose-finding approaches involve predetermining several dose levels with corresponding patient numbers, randomly assigning patients to these doses, and finally calculating through Probit regression analysis. In contrast, the Dixon up-and-down method can achieve target results with two to three times fewer samples while maintaining accuracy,35 and has been widely adopted in clinical research. Previous studies have shown that the median minimum effective analgesic concentration (MEAC) of alfentanil ranges between 43–65 ng/mL.36 Therefore, we set the initial plasma target concentration of alfentanil at 50 ng/mL. For dose adjustment intervals, Dixon et al37 recommended setting the step size at 10% or 20% of the initial dose. Consequently, this study established a 10 ng/mL adjustment interval for alfentanil concentration. Regarding the number of crossover points, Dixon et al20 suggested terminating the trial after 4 crossovers, while Paul et al demonstrated that more crossover points could improve result accuracy. Based on extensive literature review, we determined to terminate the experiment after observing 8 positive-negative response crossovers. Our findings demonstrate that during ERCP procedures in super- elderly patients receiving propofol coadministration, the EC50 of alfentanil target-controlled infusion was 36.52 ng/mL, representing approximately 25–30% reduction compared to conventional dosing. This observation aligns with age-related pharmacokinetic changes. With advancing age, the self-regulatory capacity of the autonomic nervous system weakens, while significant alterations occur in pharmacokinetic parameters including drug absorption, distribution, metabolism and excretion, leading to enhanced opioid sensitivity in elderly patients.38 Compared to younger adults, elderly patients require significantly lower opioid doses,39 which further validates our findings. These results suggest that for super- elderly patients over 85 years old, setting the alfentanil target-controlled infusion concentration at 40 ng/mL can provide satisfactory analgesic effects for over half of the population. In clinical practice, alfentanil dosing regimens should be adjusted according to patient age to achieve personalized precision anesthesia.
Given the heightened pharmacological sensitivity in very elderly patients, conventional anesthetic administration often results in drug overdosing or underdosing. To address this challenge, our study implemented TCI to achieve more precise and safer anesthetic management. Regarding precision, the increased drug sensitivity in this population makes traditional bolus administration prone to dosage inaccuracy. TCI’s real-time monitoring-feedback-adjustment mechanism ensures adequate anesthetic depth while minimizing adverse cardiovascular effects,40 providing optimal safety for highly stimulating procedures like ERCP. Regarding safety, by simulating drug pharmacokinetics through computer modeling, TCI maintains stable plasma drug concentrations and appropriate anesthetic depth. This approach reduces surgical stress responses while ensuring rapid, stable recovery post-procedure.41 Our results demonstrated excellent hemodynamic stability: At four critical timepoints (T0-T3), only 1 patient (3.57%) exhibited >20% heart rate variation during duodenoscope insertion. Merely 2 patients (7.14%) showed >20% mean arterial pressure fluctuation during instrumentation These findings confirm that alfentanil TCI combined with propofol provides stable hemodynamics in very elderly patients, significantly enhancing procedural safety.
This study has several limitations that warrant consideration. First, the study population exclusively comprised super-elderly patients (>85 years) with a significant male predominance (64.3%). These demographic characteristics may limit the generalizability of our findings to other age groups or more gender-balanced populations. Future studies should validate the EC50 and safety profile of alfentanil target-controlled infusion combined with propofol in broader age ranges and gender-balanced cohorts. Second, the definition of successful sedation in this study considered positive responses as the occurrence of coughing, body movement, or swallowing during duodenoscope insertion, with their absence classified as negative responses. However, varying definitions of successful sedation across studies may affect outcome comparisons. Therefore, caution should be exercised when comparing results from different investigations. Third, based on clinical practicality and common dosing practices, we employed a 10 ng/mL dose increment. While this approach was clinically relevant, smaller dose steps with larger sample sizes could enhance statistical precision. Subsequent research could refine the EC50 estimation by implementing smaller dose increments while maintaining adequate power.
Conclusion
In this study, the EC50 of TAI alfentanil combined with propofol in the operation of ERCP in super- elderly patients was 36.52ng/mL, at which concentration level could satisfy the requirements of surgical anesthesia and maintain the relative stability of hemodynamics in patients. This finding fills the gap in the study of the dosage of TCI of alfentanil for the anesthesia of ERCP in super- elderly patients, and provides direct evidence for the individualized administration of the drug in this special population.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the Zenggang Sun on reasonable request.
Ethical Approval and Informed Consent
This study was approved by the Medical Ethics Committee of Weifang People’s Hospital (KYLL20240318-6) and the China Clinical Trial Registry (Registration No. ChiCTR2400082734), and adhered to the Declaration of Helsinki and CONSORT standards. All patients in this study obtained informed consent and signed an informed consent form.
Acknowledgments
The authors would like to thank the study investigators, and all individuals who took part in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project YXH2022ZX05275 was supported by Shandong Medical Association.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gallo C, Boškoski I, Matteo MV, Orlandini B, Costamagna G. Training in endoscopic retrograde cholangio-pancreatography: a critical assessment of the broad scenario of training programs and models. Expert Rev Gastroenterol Hepatol. 2021;15(6):675–688. doi:10.1080/17474124.2021.1886078
2. Cianci P, Restini E. Management of cholelithiasis with choledocholithiasis: endoscopic and surgical approaches. World J Gastroenterol. 2021;27(28):4536–4554. doi:10.3748/wjg.v27.i28.4536
3. Chandra S, Klair JS, Soota K, Livorsi DJ, Johlin FC. Endoscopic retrograde cholangio-pancreatography-obtained bile culture can guide antibiotic therapy in acute cholangitis. Dig Dis Basel Switz. 2019;37(2):155–160. doi:10.1159/000493579
4. Sbeit W, Kadah A, Shahin A, Khoury T. Same day endoscopic retrograde cholangio-pancreatography immediately after endoscopic ultrasound for choledocholithiasis is feasible, safe and cost-effective. Scand J Gastroenterol. 2021;56(10):1243–1247. doi:10.1080/00365521.2021.1955148
5. Macfarlane K, Wilson R, Fischer NJ, Wei H. Endoscopic retrograde cholangiopancreatography in the comorbid elderly: a retrospective comparative study in New Zealand. N Z Med J. 2022;135(1567):21–30. doi:10.26635/6965.5947
6. Yue-Hua L, Feng Q, Jian-Ping G, Yu-Xuan Y. Endoscopic retrograde cholangiopancreatography in the elderly: non-sedation or general endotracheal anesthesia? Asian J Surg. 2022;45(9):1762. doi:10.1016/j.asjsur.2022.03.030
7. Behrens A, Kreuzmayr A, Manner H, et al. Acute sedation-associated complications in GI endoscopy (ProSed 2 Study): results from the prospective multicentre electronic registry of sedation-associated complications. Gut. 2019;68(3):445–452. doi:10.1136/gutjnl-2015-311037
8. Godoroja-Diarto D, Constantin A, Moldovan C, Rusu E, Sorbello M. Efficacy and safety of deep sedation and anaesthesia for complex endoscopic procedures-A narrative review. Diagn Basel Switz. 2022;12(7):1523. doi:10.3390/diagnostics12071523
9. Chen M, Sun Y, Li X, et al. Effectiveness of single loading dose of dexmedetomidine combined with propofol for deep sedation of endoscopic retrograde cholangiopancreatography (ERCP) in elderly patients: a prospective randomized study. BMC Anesthesiology. 2022;22(1):85. doi:10.1186/s12871-022-01630-8
10. Zhao N, Zeng J, Fan L, et al. The effect of alfentanil on emergence delirium following general anesthesia in children: a randomized clinical trial. Paediatr Drugs. 2022;24(4):413–421. doi:10.1007/s40272-022-00510-5
11. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401. doi:10.1097/EJA.0000000000001134
12. Wong GTC, Irwin MG. Post-induction hypotension: a fluid relationship? Anaesthesia. 2021;76(1):15–18. doi:10.1111/anae.15065
13. Kamal F, Khan MA, Lee-Smith W, et al. Efficacy and safety of supplemental intravenous lidocaine for sedation in gastrointestinal endoscopic procedures: systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2021;93(6):1241–1249.e6. doi:10.1016/j.gie.2021.01.008
14. Sridharan K, Sivaramakrishnan G. Comparison of fentanyl, remifentanil, sufentanil and alfentanil in combination with propofol for general anesthesia: a systematic review and meta-analysis of randomized controlled trials. Curr Clin Pharmacol. 2019;14(2):116–124. doi:10.2174/1567201816666190313160438
15. Miner JR, Driver BE, Moore JC, et al. Randomized clinical trial of propofol versus alfentanil for moderate procedural sedation in the emergency department. Am J Emerg Med. 2017;35(10):1451–1456. doi:10.1016/j.ajem.2017.04.041
16. Jia N, Zuo X, Guo C, et al. Synergistic antinociceptive effects of alfentanil and propofol in the formalin test. Mol Med Rep. 2017;15(4):1893–1899. doi:10.3892/mmr.2017.6174
17. Yoon SW, Choi GJ, Lee OH, et al. Comparison of propofol monotherapy and propofol combination therapy for sedation during gastrointestinal endoscopy: a systematic review and meta-analysis. Dig Endosc off J Jpn Gastroenterol Endosc Soc. 2018;30(5):580–591. doi:10.1111/den.13050
18. Akhondzadeh R, Olapour A, Rashidi M, Elyasinia F. Comparison of sedation with dexmedetomidine alfentanil versus ketamine-alfentanil in patients undergoing closed reduction of nasal fractures. Anesthesiol Pain Med. 2020;10(4):e102946. doi:10.5812/aapm.102946
19. Huang SE, Zhang D. Effect of target-controlled infusion of sufentanil with different low effect-site concentrations combined with propofol on anesthesia in laparoscopic surgery. Aerospace Med J. 2022;33:967–969.
20. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50. doi:10.1016/s0149-7634(05)80090-9
21. Imagawa A, Hata H, Nakatsu M, et al. A target-controlled infusion system with bispectral index monitoring of propofol sedation during endoscopic submucosal dissection. Endosc Int Open. 2015;3(1):E2–6. doi:10.1055/s-0034-1377519
22. Li S, Yu F, Zhu H, Yang Y, Yang L, Lian J. The median effective concentration (EC50) of propofol with different doses of fentanyl during colonoscopy in elderly patients. BMC Anesthesiol. 2016;16(1):24. doi:10.1186/s12871-016-0189-y
23. Xu Q, Zhou Z, Ai L, Liu J, Tian X. Sufentanil EC50 for endotracheal intubation with aerosol inhalation of carbonated lidocaine by ultrasonic atomizer. BMC Anesthesiol. 2021;21(1):144. doi:10.1186/s12871-021-01367-w
24. Zhang Y, Zhang N, Hu J, Liu C, Li G. Safety and efficacy of a low-dose combination of midazolam, alfentanil, and propofol for deep sedation of elderly patients undergoing ERCP. BMC Gastroenterol. 2024;24(1):124. doi:10.1186/s12876-024-03197-9
25. Ukkonen M, Siiki A, Antila A, Tyrväinen T, Sand J, Laukkarinen J. Safety and efficacy of acute endoscopic retrograde cholangiopancreatography in the elderly. Dig Dis Sci. 2016;61(11):3302–3308. doi:10.1007/s10620-016-4283-2
26. Galeazzi M, Mazzola P, Valcarcel B, et al. Endoscopic retrograde cholangiopancreatography in the elderly: results of a retrospective study and a geriatricians’ point of view. BMC Gastroenterol. 2018;18(1):38. doi:10.1186/s12876-018-0764-4
27. Ogawa T, Tomoda T, Kato H, Akimoto Y, Tanaka S, Okada H. Propofol sedation with a target-controlled infusion pump in elderly patients undergoing ERCP. Gastrointest Endosc. 2020;92(2):301–307. doi:10.1016/j.gie.2020.03.002
28. Li S, Sheng G, Teng Y, Sun M. Systematic review of anaesthetic medication for ERCP based on a network meta-analysis. Int J Surg Lond Engl. 2018;51:56–62. doi:10.1016/j.ijsu.2018.01.018
29. Mazanikov M, Udd M, Kylänpää L, et al. Patient-controlled sedation for ERCP: a randomized double-blind comparison of alfentanil and remifentanil. Endoscopy. 2012;44(5):487–492. doi:10.1055/s-0031-1291655
30. Liu WC, Liang SJ, Wang XL. Effectiveness and safety of propofol combined with esketamine for sedation during endoscopic retrograde cholangiopancreatography. World Clinical Drugs. 2022;43:1124–1129. doi:10.13683/j.wph.2022.09.008
31. Mertens MJ, Vuyk J, Olofsen E, Bovill JG, Burm AG. Propofol alters the pharmacokinetics of alfentanil in healthy male volunteers. Anesthesiology. 2001;94(6):949–957. doi:10.1097/00000542-200106000-00006
32. Dong SA, Guo Y, Liu SS, et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J Clin Anesth. 2023;86:111077. doi:10.1016/j.jclinane.2023.111077
33. Hu J, Gu X, Zhu W, et al. Comparison of anesthetic effects of different doses of alfentanil combined with ciprofol in elderly patients undergoing ERCP: a randomized controlled trial. BMC Anesthesiol. 2023;23(1):353. doi:10.1186/s12871-023-02325-4
34. Andres TM, McGrane T, McEvoy MD, Allen BFS. Geriatric pharmacology: an update. Anesthesiol Clin. 2019;37(3):475–492. doi:10.1016/j.anclin.2019.04.007
35. Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
36. Li YH, Yang LJ, Leung YP, Liu CL. Effects of different plasma target-controlled concentrations of alfentanil combined with remazolam for hysteroscopic surgery on patients’ hemodynamics and respiration. J Clin Experimental Med. 2024;23:777–781.
37. Yang H, Zhao Q, Chen HY, et al. The median effective concentration of propofol with different doses of esketamine during gastrointestinal endoscopy in elderly patients: a randomized controlled trial. Br J Clin Pharmacol. 2022;88(3):1279–1287. doi:10.1111/bcp.15072
38. Naples JG, Gellad WF, Hanlon JT. The role of opioid analgesics in geriatric pain management. Clin Geriatr Med. 2016;32(4):725–735. doi:10.1016/j.cger.2016.06.006
39. Chen X, Han M, Shu A, Zhou M, Wang K, Cheng C. Effects of different doses of alfentanil on cardiovascular response to rapid sequence intubation in elderly patients: a parallel-controlled randomized trial. BMC Anesthesiol. 2024;24(1):290. doi:10.1186/s12871-024-02663-x
40. Egan TD, Minto CF, Schnider TW. Steady-state trumps accuracy: target-controlled infusion as a gain switch. Br J Anaesth. 2024;133(4):726–729. doi:10.1016/j.bja.2024.07.014
41. Bidkar PU, Dey A, Chatterjee P, Ramadurai R, Joy JJ. Target-controlled infusion – past, present, and future. J Anaesthesiol Clin Pharmacol. 2024;40(3):371–380. doi:10.4103/joacp.joacp_64_23
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

