Back to Journals » Nature and Science of Sleep » Volume 17
The Mediating Role of Negative Mood Affect in the Relationship Between Perceived Stress and Vulnerability to Insomnia Among Student Pharmacist Shift Workers
Authors Salahuddin MF , I BS, Bugingo R , Spencer D , Manzar MD , BaHammam AS
Received 6 January 2025
Accepted for publication 26 March 2025
Published 23 April 2025 Volume 2025:17 Pages 649—662
DOI https://doi.org/10.2147/NSS.S515923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Mohammed F Salahuddin,1 Blesson I Samuel,2 Richard Bugingo,1 Delilah Spencer,1 Md Dilshad Manzar,3 Ahmed S BaHammam4,5
1Department of Pharmaceutical Sciences, School of Pharmacy & Health Professions, Notre Dame of Maryland University, MD, 21210, USA; 2Independent Researcher, Chennai, India; 3Department of Nursing, College of Applied Medical Sciences, Majmaah University, Majmaah, 11952, Saudi Arabia; 4University Sleep Disorders Center, Department of Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 5National Plan for Science and Technology, College of Medicine, King Saud University, Riyadh, Saudi Arabia
Correspondence: Mohammed F Salahuddin, Department of Pharmaceutical Sciences, School of Pharmacy, Notre Dame of Maryland University, MD, 21210, USA, Email [email protected]
Background: Perceived stress and vulnerability to develop insomnia are closely linked, with negative mood affect playing a key role. Shift workers, particularly student pharmacists juggling academic demands and irregular work schedules, are at heightened risk for stress-related sleep disturbances. While previous studies have explored their direct relationships, limited evidence exists on the dual role of negative mood affect as both a mediator and a non-mediator in these pathways. This study investigates the mediating role of negative mood affect in the relationship between perceived stress and vulnerability to develop insomnia and assesses whether negative mood affect mediates the reverse relationship.
Methods: A cross-sectional study was conducted on 86 student pharmacist shift workers at Notre Dame of Maryland University. Participants completed validated self-report measures, including the Perceived Stress Scale (PSS), the Ford Insomnia Response to Stress Test (FIRST), and the Positive and Negative Affect Schedule (PANAS). Mediation analysis using Hayes’ PROCESS macro (Model 4) examined the mediating role of negative mood affect in both pathways. Bias-corrected bootstrapping with 5000 iterations calculated confidence intervals for indirect effects, with significance set at p < 0.05.
Results: Negative mood affect partially mediated the relationship between perceived stress and vulnerability to develop insomnia. Higher stress levels were associated with increased negative mood affect (b = 0.49, SE = 0.05, p < 0.01), which, in turn, was linked to greater insomnia vulnerability (b = 0.39, SE = 0.04, p < 0.01). The indirect effect was significant (b = 0.19, 95% CI [0.06, 0.33]). In contrast, negative mood affect did not mediate the reverse pathway (p = 0.15).
Conclusion: Negative mood affect significantly mediates the relationship between perceived stress and insomnia vulnerability but not the reverse pathway. Interventions targeting emotional regulation may help reduce stress-related sleep disturbances. Longitudinal studies are needed to confirm these findings and refine interventions.
Plain Language Summary: Student pharmacists who work irregular shifts often experience high levels of stress and disrupted sleep, which can negatively impact their health, academic performance, and overall well-being. This study explored how negative mood, including feelings of anxiety, irritability, and sadness, might explain the connection between perceived stress and vulnerability to insomnia.
The findings revealed that higher stress levels were linked to increased negative mood, which in turn heightened vulnerability to insomnia. However, when examining the reverse pathway, whether insomnia influences stress through negative mood, the results showed no significant effect. This suggests that while negative mood plays a key role in how stress contributes to sleep problems, it does not explain how poor sleep might increase stress.
These results highlight the importance of emotional regulation strategies, such as mindfulness or counseling, in managing stress and improving sleep health among student pharmacists. Addressing negative mood could break the cycle between stress and sleep disturbances. Future research should investigate these relationships over time and consider other factors, like coping mechanisms or cognitive arousal, to provide a more comprehensive understanding. Meanwhile, academic institutions and healthcare organizations can use these insights to create wellness programs that support both mental health and sleep quality for student pharmacists working demanding shifts.
Keywords: sleep disturbances, emotional regulation, psychological stress, mood-sleep connection
Graphical Abstract:

Introduction
Perceived stress and vulnerability to develop insomnia are prevalent challenges among student pharmacist shift workers, who often juggle demanding academic schedules with irregular work hours.1–3 Shift workers face heightened vulnerability to insomnia through multiple pathophysiological mechanisms.4 The misalignment of circadian rhythms from irregular work schedules disrupts the body’s natural sleep-wake cycle, leading to melatonin suppression and sustained physiological hyperarousal.1,4 This biological disruption is particularly severe in healthcare settings, where shift work disorder affects up to 41% of professionals, manifesting as chronic insomnia symptoms and excessive daytime sleepiness.4 The resulting sleep fragmentation triggers a cascade of neurobehavioral consequences, including impaired executive function, emotional dysregulation, and increased stress reactivity.5 Student pharmacists represent an especially vulnerable population, as their developing professional skills and academic demands compound these physiological challenges, creating a perfect storm for sleep disruption.2,3 These issues are interconnected, with evidence suggesting that negative mood affect may play a pivotal role in their dynamic relationship.6,7 While the direct associations between stress, negative mood affect, and vulnerability to develop insomnia have been extensively studied, the nuanced role of negative mood affect as a mediator remains less understood.8 Specifically, it is unclear whether negative mood affect consistently acts as an intermediary factor in the vulnerability to develop insomnia pathway or whether it also mediates the reverse relationship between vulnerability to develop insomnia and perceived stress.
Previous research highlights the importance of negative mood affect regulation in mitigating stress9 and promoting sleep health.5 Negative mood affect states, such as anxiety and irritability, can amplify stress perceptions and increase the vulnerability to developing insomnia, creating a vicious cycle.10 Student pharmacist shift workers represent a distinct population experiencing multiple stressors from both academic and professional domains. Working 12-hour shift rotations while maintaining full-time academic schedules, these individuals must balance the rigorous demands of pharmacy education with the challenges of irregular work hours. Many work as pharmacy technicians to gain practical experience and financial support, creating a unique environment for studying the impact of combined occupational and academic stress on sleep health. This population’s susceptibility to circadian rhythm disruption and high stress levels makes them particularly relevant for understanding the mechanisms linking perceived stress to insomnia vulnerability.2,3 Emerging evidence suggests a bidirectional relationship between stress and vulnerability to developing insomnia, particularly in healthcare workers exposed to irregular shifts and heightened job demands.5,9 Stress predicts vulnerability to developing insomnia and poor sleep quality also exacerbates stress responses, creating a self-reinforcing cycle. Understanding this dynamic is critical, especially in populations like student pharmacists who experience chronic stress and disrupted sleep patterns. Moreover, the role of negative mood affect in these pathways requires further investigation, particularly in determining whether it acts as a mediator in one direction but not the other (bidirectional relationship). This is especially relevant among populations prone to high levels of stress and vulnerability to developing insomnia, such as student pharmacists.2,3
The relationship between stress, negative mood affect, and insomnia can be understood through several theoretical frameworks, including the Transactional Model of Stress and Coping,11 Emotion Regulation Theory,10 and the Cognitive Hyperarousal Model.12 These frameworks collectively emphasize how emotional dysregulation, cognitive rumination, and physiological arousal mediate stress-induced vulnerability to developing insomnia, providing a foundation for examining these pathways bidirectionally.10–12 These frameworks provide a rationale for examining mood as both a mediator and a moderator in stress-sleep pathways.
This study aims to address this gap by examining the mediating role of negative mood affect in the relationship between perceived stress and vulnerability to develop insomnia and assessing whether negative mood affect similarly mediates the reverse pathway between vulnerability to develop insomnia and perceived stress. Using validated self-report measures and mediation analysis, we explore these pathways among a cohort of student pharmacist shift workers. We hypothesize that negative mood affect significantly mediates and moderates the relationship between perceived stress and vulnerability to develop insomnia, with higher stress levels exacerbating vulnerability to develop insomnia through its impact on negative mood affect. Findings from this study could inform targeted interventions aimed at improving emotional regulation and sleep health in this vulnerable population.
Methodology
Participants and Procedure
The study was conducted among student pharmacist shift workers at Notre Dame of Maryland University between June 2023 and May 2024. Full-time student pharmacists who had worked at least one shift (≥8 nighttime hours or rotating schedule) in the past 30 days were eligible. This 30-day criterion is based on evidence that shift work effects can persist for 4–6 weeks post-exposure.4 Participants were categorized into two groups: active shift workers (currently working shifts at the time of data collection, n=45) and recent shift workers (not currently working shifts but with shift work exposure within the past 30 days, n=40). All participants worked as pharmacy technicians during rotations, managing both academic and professional responsibilities. Participants were required to be 18 years or older, fluent in English, and to have at least one month of shift work history before participation. Individuals with visual or hearing impairments or self-reported psychiatric illnesses were excluded to ensure the reliability of the data.
Eighty-five participants (mean age: 31.9 ± 8.1 years; Table 1) completed the study. Informed consent was obtained, and Institutional Review Board (IRB) approval was secured. Participation was voluntary, with no incentives provided.
 |
Table 1 Participants’ Characteristics |
Measures
Stress was measured using the Perceived Stress Scale (PSS), a 10-item Likert scale with scores ranging from 0 to 40, where higher scores indicate greater perceived stress.13 The Ford Insomnia Response to Stress Test (FIRST), a 9-item scale, assessed vulnerability to stress-induced insomnia, with scores ranging from 9 to 36.14 Negative mood affect was evaluated using the Positive and Negative Affect Schedule (PANAS), which provides separate scores for positive and negative affect.15 The PANAS scale was selected for its well-established psychometric properties and ability to capture both positive and negative affect states. Negative affect, characterized by anxiety, irritability, and sadness, is particularly relevant in stress-sleep interactions as it reflects heightened emotional reactivity to stressors, which can disrupt sleep continuity.10,15 All instruments demonstrated strong psychometric properties, with reliability coefficients ranging from 0.80 to 0.90 in prior studies.
While our study did not directly assess clinical insomnia diagnoses, we collected information on participants’ existing health conditions, including psychiatric illnesses (PTSD, anxiety, depression, schizophrenia), hypertension, diabetes, ischemic heart disease, chronic kidney disease, bronchial asthma, and others. Because psychiatric disorders are well-documented risk factors for sleep disturbances,16 future studies should integrate validated insomnia symptom measures—such as the Insomnia Severity Index (ISI)—to better assess how these conditions influence insomnia vulnerability.
Statistical Analysis
Mediation analysis was conducted using Hayes’ PROCESS macro (Model 4) in SPSS 28.0 to examine whether negative mood affect mediated the relationship between perceived stress (PSS) and vulnerability to insomnia (FIRST). The reverse mediation pathway (insomnia vulnerability To stress) was also assessed. Bootstrapping with 5000 iterations was used to compute confidence intervals for indirect effects (p < 0.05). To ensure reproducibility, a random seed number (10000) was applied during the bootstrapping procedure in the mediation analysis. To improve interpretability, continuous variables (PSS, PANAS-Negative, and FIRST) were standardized using z-scores. Prior to analysis, normality (Shapiro–Wilk test), homoscedasticity (Breusch-Pagan test), and multicollinearity (VIF < 5) were assessed to ensure statistical validity. Prior to mediation analysis, key statistical assumptions were tested.
Referring to previous population studies exploring sleep-related outcomes,17,18 we standardized continuous variables (PSS, PANAS-Negative, and FIRST) using z-scores to enhance interpretability. Following established methodological frameworks in shift work research,4 we adjusted for key confounders known to influence sleep vulnerability: age (given its association with circadian adaptation), gender (due to documented differences in stress perception and sleep patterns), and shift duration (to account for varying degrees of circadian disruption). These adjustments were consistently applied across all analytical models to ensure robust control of potential confounding effects. Interaction terms in regression models were used to examine whether negative mood affect moderated the relationship between stress and vulnerability to develop insomnia. Results were visualized using interaction plots to illustrate the moderating role of negative mood affect on the stress-insomnia relationship, while model fit and effect sizes were also reported.
Results
Assumption Testing
Assumption testing confirmed that the residuals were normally distributed (Shapiro–Wilk, p = 0.052), homoscedasticity was satisfied (Breusch-Pagan, p = 0.433), and no multicollinearity issues were detected (VIF: Stress = 1.001, Mood = 1.001), ensuring the validity of the mediation analysis.
Associations Between Negative Mood Affect, Stress, and Insomnia: Key Findings From Correlation and Effect Size Analyses
The zero-order correlation matrix revealed significant associations between PANAS Negative Affect and both Perceived Stress (PSS) (r = −0.162, p < 0.05; Table 2) and vulnerability to Insomnia (FIRST) (r = −0.174, p < 0.05; Table 2), suggesting that higher negative mood was linked to increased stress and insomnia vulnerability. Partial correlations, controlling for gender, largely confirmed these findings, with PANAS Negative Affect maintaining its association with FIRST (r = −0.200, p < 0.05; Table 3). Gender was chosen as a control variable based on both theoretical and empirical justification, as prior research highlights significant gender-based differences in perceived stress, emotional affect, and vulnerability to developing insomnia. Preliminary analyses indicated that gender had a notable influence on these relationships, while other covariates (eg, age, shift duration) showed minimal effects. This approach ensures model parsimony while accounting for the most relevant confounding variable. Distribution characteristics showed that while most variables followed a near-normal distribution, PANAS Negative mood affect demonstrated moderate positive skewness (skewness = 0.62) and kurtosis (kurtosis = 1.11), indicating slight asymmetry and peakedness (Table 4). Finally, effect sizes highlighted a weak negative relationship between PANAS Negative Affect and vulnerability to Insomnia (FIRST) (r = −0.17, 95% CI [−0.39, 0.04]) (Table 5). Collectively, these findings underscore the modest but notable role of negative mood affect in the relationships between stress and insomnia vulnerability.
 |
Table 2 Zero-Order Correlation Matrix |
 |
Table 3 Partial Correlations (Controlling for Gender) |
 |
Table 4 Distribution Characteristics (Skewness, Kurtosis) |
 |
Table 5 Effect Sizes With Confidence Intervals |
Negative Mood Affect as a Mediator in the Effect of Perceived Stress on Vulnerability to Develop Insomnia (Forward Mediation Pathway: Stress → Negative Mood Affect → Insomnia)
To further understand these associations, mediation analysis was conducted to determine whether negative mood affect acts as a bridge between perceived stress and insomnia vulnerability. The results of the mediation analysis to assess the effect of stress on vulnerability to develop insomnia through negative mood affect (PANAS) are shown in Figure 1. The overall model explained 33.36% of the variance in vulnerability to develop insomnia (R² = 0.3336, F[2,97] = 24.28, p < 0.001).
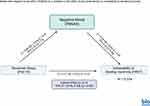 |
Figure 1 FIRST values are the unstandardized coefficients, and second values within brackets are the standard errors; p<0.01. The indirect effect of perceived stress on vulnerability to develop insomnia through negative mood affect was significant (95% CI=[0.06,0.33]). Solid line: significant path. Created in BioRender. Salahuddin, (M) (2025) https://BioRender.com/c97o425. |
Stress (PSS Total) was a significant positive predictor of negative mood affect (b = 0.49, SE = 0.05, p < 0.001, 95% CI [0.25, 0.73], indicating that individuals with higher stress levels experienced elevated negative mood affect. Negative mood affect was also a significant positive predictor of vulnerability to develop insomnia (b = 0.39, SE = 0.04, p < 0.001, 95% CI [0.22, 0.56]), suggesting that individuals with higher negative mood affect levels were more likely to suffer from increased vulnerability to develop insomnia.
The direct effect of perceived stress on vulnerability to develop insomnia remained significant after controlling for negative mood affect (b = 0.21, SE = 0.03, p = 0.039, 95% CI [0.01, 0.42]). The indirect effect of perceived stress on vulnerability to develop insomnia through negative mood affect was statistically significant (b=0.19), with a 95% confidence interval of [0.06, 0.33]. These findings suggest that negative mood affect partially mediates the effect of perceived stress on vulnerability to develop insomnia.
The total, direct, and indirect effects for both mediation models are summarized in Supplementary Table 1. The table provides detailed statistical coefficients, standard errors, and confidence intervals for each effect, offering clarity on the strength and significance of each pathway.
Negative Mood Affect Was Not a Mediator in the Effect of Vulnerability to Develop Insomnia on Perceived Stress (Reverse Mediation Pathway: Insomnia → Negative Mood Affect → Stress)
Next, a reverse mediation analysis was performed to examine whether negative mood affect mediates the pathway from insomnia vulnerability to perceived stress. The results of the mediation analysis to assess the effect of vulnerability to develop insomnia on perceived stress through negative mood affect (PANAS) are shown in Figure 2. The overall model explained 38.46% of the variance in stress (R2=0.3846, F[2,97]=30.45, p<0.001).
 |
Figure 2 First values are the unstandardized coefficients, and second values within brackets are the standard errors; p<0.01. The indirect effect of vulnerability to develop insomnia on stress through negative mood affect was non-significant (95% CI=[−0.01,0.32]). Additionally, negative mood affect was not a significant predictor of stress (p=0.15). Solid line: significant path, Dashed line: non-significant path. Created in BioRender. Salahuddin, (M) (2025) https://BioRender.com/i91x853. |
Vulnerability to develop insomnia (FIRST Total) was a significant positive predictor of negative mood affect (b=0.64, SE=0.06, p<0.01, 95% CI [0.43, 0.85]), indicating that individuals with higher vulnerability to develop insomnia levels experienced elevated negative mood affect. However, negative mood affect was not a significant predictor of stress (b=0.23, SE=0.05, p=0.15). The direct effect of vulnerability to develop insomnia on perceived stress was significant (b=0.24, SE=0.04, p<0.05).
The indirect effect of vulnerability to develop insomnia on perceived stress through negative mood affect was not statistically significant (b=0.14), with a 95% confidence interval of [−0.01,0.32]. These findings suggest that negative mood affect does not mediate the relationship between vulnerability to develop insomnia and perceived stress.
Discussion
This study explored the intricate relationships among perceived stress, negative mood affect, and vulnerability to develop insomnia in student pharmacist shift workers, providing novel insights into the psychosocial challenges faced by this population. The findings revealed that negative mood affect partially mediates the effect of perceived stress on vulnerability to develop insomnia, emphasizing its role as a psychological mechanism in this pathway. Specifically, higher stress levels predicted elevated negative mood affect, which, in turn, was associated with greater vulnerability to develop insomnia severity. These results align with existing research highlighting the critical role of emotional regulation in the interplay between stress and vulnerability to developing insomnia.5,10,19
While our mediation analysis revealed a statistically significant effect (b = 0.19), the modest effect size warrants careful interpretation. This finding suggests that negative mood affect, though important, explains only a small portion of the variance in the stress-insomnia vulnerability pathway. Rather than diminishing its clinical relevance, this modest effect size underscores the complex, multifactorial nature of stress-related sleep disturbances. Specifically, emotional dysregulation likely represents just one of several concurrent mechanisms linking stress to insomnia vulnerability. Our findings suggest that interventions targeting emotional regulation alone may yield limited benefits. Instead, comprehensive interventions integrating multiple evidence-based strategies—such as CBT-I, mindfulness-based stress reduction, and sleep hygiene education—may yield greater benefits than emotional regulation alone. This multi-modal approach acknowledges both the statistical significance and practical limitations of our findings.
Our findings provide novel insights into the mechanisms linking perceived stress to insomnia vulnerability among student pharmacist shift workers. The partial mediation by negative mood affect aligns with the Transactional Model of Stress and Coping,11 suggesting that emotional responses play a crucial but not exclusive role in stress-related sleep disturbances. The significant direct effect indicates additional pathways, possibly involving cognitive hyperarousal12 or inflammatory processes,20 that warrant further investigation. The absence of reverse mediation supports the Cognitive Hyperarousal Model’s emphasis on persistent thought patterns rather than emotional states in maintaining stress-insomnia relationships.12 This finding suggests that interventions targeting cognitive processes may be particularly important for breaking the cycle between sleep disruption and stress. These results have important implications for developing targeted interventions. While emotional regulation strategies may help mitigate the impact of stress on sleep vulnerability, comprehensive approaches addressing both emotional and cognitive factors are likely needed. Future research should explore the role of inflammatory markers as potential biological mediators and examine how individual differences in stress reactivity influence these relationships. Negative affect, characterized by emotions such as fear, anxiety, and distress, exacerbated the impact of perceived stress on vulnerability to develop insomnia. This is consistent with theories suggesting that stress-induced emotional arousal disrupts sleep continuity by increasing physiological and psychological reactivity.21,22 In contrast, interventions targeting emotional regulation, such as mindfulness-based stress reduction (MBSR) or cognitive-behavioral therapy (CBT), could help address these emotional disturbances and reduce the vulnerability to developing insomnia among shift workers.23
Interestingly, the results also showed that negative mood affect did not mediate the reverse pathway, where vulnerability to develop insomnia influences perceived stress. Although vulnerability to develop insomnia significantly predicted negative mood affect, negative mood affect was not a significant predictor of stress, and the indirect effect of vulnerability to develop insomnia on stress through negative mood affect was non-significant. An intriguing finding of this study is that negative mood affect did not mediate the reverse pathway. While previous research suggests a bidirectional relationship between stress and sleep disturbances,24 the mechanisms underlying this pathway remain unclear. One possible explanation for this finding is that cognitive hyperarousal and rumination, rather than negative mood affect, serve as stronger mediators in this reverse pathway.12,25,26 This aligns with the Cognitive Hyperarousal Model,27 which emphasizes how persistent cognitive arousal contributes to sleep disturbances. Individuals prone to rumination may experience prolonged stress-related thought patterns that sustain physiological hyperarousal, reinforcing the stress-insomnia cycle.25,26 The lack of mediation by negative mood affect in the reverse pathway suggests that cognitive factors (eg, rumination, worry) may be more dominant than emotional dysregulation in this direction of influence. Moreover, individuals experiencing poor sleep may engage in maladaptive thought patterns, such as worry and preoccupation with sleep loss, which could contribute to stress independently of mood disturbances.28 Additionally, the effects of insomnia vulnerability on stress may manifest over a longer timeframe, suggesting that longitudinal research is needed to capture potential delayed effects. These findings also align with the Transactional Model of Stress and Coping,11 which suggests that individuals experience stress appraisals based on their perceived coping resources. In this context, insomnia vulnerability may increase perceived stress through cognitive rather than emotional pathways. Future studies should explore alternative mediators, such as cognitive arousal, physiological stress responses, and resilience factors, to further elucidate the complex bidirectional relationship between stress and insomnia.
The absence of a significant mediation effect in the reverse pathway suggests that additional mechanisms beyond emotional dysregulation warrant further exploration. Cognitive hyperarousal, characterized by racing thoughts and inability to disengage from stress-related cognitions, may serve as a more potent mediator.12,27 Sleep reactivity—an individual’s predisposition to stress-induced sleep disturbances—has emerged as a stronger predictor of insomnia vulnerability compared to mood-related measures alone.21 Individual differences in stress resilience and emotion regulation capacity likely moderate these relationships. For instance, trait resilience buffers against stress-induced sleep disruption,29 while neuroticism amplifies vulnerability to both stress and sleep disturbances. These findings point to a more nuanced model in which personality traits and cognitive processes interact with emotional states to shape sleep outcomes.
An additional biological mechanism linking stress to insomnia vulnerability involves inflammatory processes. Chronic psychological stress activates the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system, triggering an inflammatory cascade that elevates key markers including C-reactive protein, interleukin-6, and tumor necrosis factor-alpha.20 These inflammatory mediators disrupt sleep architecture through multiple pathways: directly impairing sleep-wake regulation in the hypothalamus, altering circadian gene expression, and disrupting neurotransmitter systems critical for sleep initiation and maintenance.30 Recent evidence suggests a complex ‘stress-inflammation-sleep’ pathway where inflammatory markers serve as biological mediators between perceived stress and sleep disruption.30 Specifically, stress-induced inflammation may dysregulate serotonin and dopamine metabolism, exacerbating negative mood; impair hypothalamic sleep centers, increasing vulnerability to insomnia; and amplify sympathetic activation, maintaining a state of physiological hyperarousal.30 This inflammatory mechanism may be particularly relevant in shift workers, where circadian disruption can enhance inflammatory responses to psychological stress.2
From an occupational health perspective, the findings of this study emphasize the importance of addressing negative mood affect dysregulation in efforts to manage stress and improve sleep outcomes in shift workers. The differential mediation patterns observed align with theoretical models suggesting distinct forward and reverse pathway mechanisms. The observed mediation patterns align with theoretical frameworks, such as the Stress-Arousal Model26 and the Cognitive Model of Insomnia,27 which highlight emotional arousal and cognitive hyperarousal as key mechanisms linking stress and sleep disturbances. These findings support the role of emotional regulation in addressing stress-induced vulnerability to insomnia among healthcare workers. Similar to our results, these studies emphasize the exacerbating role of emotional dysregulation in stress-induced vulnerability to developing insomnia. Programs that foster emotional resilience, such as promoting positive affect through gratitude journaling, physical exercise, or workplace wellness initiatives, may mitigate the adverse effects of stress on vulnerability to develop insomnia.29,31,32 Additionally, recognizing the role of negative affect in exacerbating vulnerability to insomnia underscores the value of integrating psychological interventions into occupational health frameworks.33–35
The partial mediation observed in the forward pathway underscores the substantial but not exhaustive role of negative mood affect in translating stress into insomnia vulnerability. While significant, the remaining direct effect (b = 0.21, p < 0.01) suggests additional unexplored pathways, possibly involving cognitive factors such as hyperarousal or maladaptive coping strategies. The non-significant indirect effect in the reverse pathway suggests that negative mood affect may not act as a primary mechanism linking to stress-induced vulnerability to developing insomnia. Instead, alternative mechanisms, such as cognitive hyperarousal or persistent rumination, may play a larger role.25,26 Our findings emphasize the importance of targeting emotional regulation in interventions designed to reduce stress-related insomnia vulnerability, while also highlighting the need for further investigation into alternative reverse pathways.
Although our study measured insomnia vulnerability using FIRST, we did not directly assess clinical insomnia symptoms or formal diagnoses. However, many participants had psychiatric conditions such as PTSD, anxiety, and depression, well-established contributors to sleep disturbances.16 Future research should incorporate standardized insomnia symptom measures and examine the role of psychiatric comorbidities in mediating the relationship between stress, mood, and insomnia vulnerability.
Additionally, while our study focused on psychosocial pathways, future research should explore biological mechanisms, particularly the role of inflammatory processes in linking stress and sleep disturbances. Given that chronic stress triggers neuroimmune dysregulation, investigating inflammatory markers such as CRP, IL-6, and TNF-α could provide valuable insights into the underlying physiological mechanisms contributing to stress-related insomnia.20 Incorporating biomarker assessments into mediation models could help clarify whether inflammatory responses mediate or moderate the impact of perceived stress and negative mood on sleep vulnerability. These findings would be particularly relevant for developing targeted interventions that address both psychological and physiological contributors to sleep disturbances in shift workers.
Limitations and Future Directions
Although all participants had recent shift exposure, 47.1% (n=40) were not actively working shifts at the time of data collection. While we adjusted for days since their last shift, unmeasured factors (eg, stress recovery time, sleep timing) may still contribute to residual confounding. Future research should stratify analyses by active vs recent shift status and incorporate real-time work schedule tracking to better differentiate acute from chronic effects of shift work. Several methodological limitations warrant consideration when interpreting our findings. First, the cross-sectional design precludes establishing causal relationships between perceived stress, negative mood affect, and insomnia vulnerability. While our mediation analyses suggest potential pathways, longitudinal studies are needed to confirm temporal ordering and directional effects. Second, our reliance on self-reported measures introduces potential common method bias and recall inaccuracies. The absence of objective sleep measurements and clinical insomnia assessments limits our ability to validate subjective reports. Future studies should incorporate actigraphy, polysomnography, and standardized diagnostic interviews using validated insomnia symptom scale (insomnia severity index). This would allow for a clearer distinction between stress-induced insomnia vulnerability and pre-existing insomnia symptoms, providing deeper insight into the stress-mood-sleep relationship. Third, our sample characteristics constrain generalizability. The relatively small sample size (n=86) from a single institution may not adequately represent the broader population of healthcare shift workers. The specific focus on student pharmacists, while valuable for understanding this population, limits extrapolation to other shift work contexts. Future research should include multi-institutional studies with larger and more diverse healthcare worker samples to improve external validity and generalizability. Additionally, varying shift work status among participants at the time of data collection introduces potential confounding factors that could influence the observed relationships. The mediation analysis was conducted without additional covariates to focus on the primary relationships among perceived stress, negative mood affect, and vulnerability to developing insomnia. Another limitation is that while gender was included as a covariate in our analysis, other potential confounders, such as age and shift duration, were not accounted for. Age-related differences in stress resilience and variations in shift duration may influence sleep vulnerability and should be considered in future models. Adjusting for these additional covariates in future studies will improve the accuracy and generalizability of findings.
The findings highlight the importance of integrating targeted emotional regulation interventions into student wellness programs, occupational health initiatives, and academic support services. Interventions such as Mindfulness-Based Stress Reduction (MBSR), Cognitive-Behavioral Therapy for Insomnia (CBT-I), and structured resilience workshops can help mitigate the impact of stress and negative mood affect on sleep disturbances. Universities and healthcare institutions should prioritize implementing these strategies to support shift workers in managing stress and improving sleep health.
Conclusion
In conclusion, this study highlights negative mood affect as a significant mediator in the perceived stress-vulnerability to develop insomnia pathway but not in the vulnerability to develop insomnia-perceived stress pathway. These findings suggest that emotional regulation may play a role in moderating stress-related insomnia vulnerability in student pharmacist shift workers. While this study does not test the effectiveness of specific interventions, our findings suggest that emotional regulation may influence stress-related insomnia vulnerability. However, given the study’s cross-sectional design and sample limitations, future research is needed to establish causal relationships and intervention effectiveness.
Strategies aimed at improving emotional regulation—such as mindfulness or cognitive-behavioral approaches—may warrant further investigation for their potential role in managing stress-related sleep disturbances in this population. Understanding the role of negative mood affect in stress-induced insomnia vulnerability can help inform future research and intervention development. Given the complex interplay of stress, mood, and sleep vulnerability, future research should examine other potential mediating factors—such as cognitive hyperarousal and coping strategies—to better understand effective approaches for improving sleep health among shift workers.
Data Transparency
The data can be shared by the corresponding author upon reasonable request.
Ethical and Institutional Review Board
The present study was approved by the Notre Dame of Maryland University IRB board vide # PH053023MSDS. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Acknowledgments
We are grateful to the participants of the study.
While preparing this work, the author(s) used ChatGPT to polish the text. After using this tool/service, the author(s) reviewed and edited the content as needed and took (s) full responsibility for the publication’s content.
The graphical abstract was created in BioRender. Salahuddin, M. (2025) https://BioRender.com/b42t284. The scales used in this study, including the Perceived Stress Scale (PSS) and the Positive and Negative Affect Schedule (PANAS), are in the public domain. The respective links for access are:
- PANAS: https://eprovide.mapi-trust.org/instruments/positive-and-negative-affect-schedule
- PSS: https://eprovide.mapi-trust.org/instruments/perceived-stress-scale-10-items
Permission to use the FIRST tool has been obtained from the proprietor.
All other content, including original research data, text, and analysis, is the intellectual property of the authors and is intended solely for academic and research purposes under the applicable copyright laws.
Author Contributions
Mohammed F Salahuddin: Conceptualization, Methodology, Software, Writing – Original Draft Preparation, Writing – Reviewing and Editing. Blesson Samuel: Statistical Analysis, Writing – Reviewing and Editing. Richard Bugingo: Data Curation, Writing – Original Draft Preparation. Delilah Spencer: Data Curation, Writing – Original Draft Preparation. Md Dilshad Manzar: Visualization, Validation, Writing – Reviewing and Editing.
Ahmed S. BaHammam: Supervision, Writing – Reviewing and Editing.
All authors have drafted or written, or substantially revised or critically reviewed the article; agreed on the journal to which the article will be submitted; have reviewed and approved all versions of the article before submission, and during revision; agreed to take responsibility and be accountable for the contents of the article. All authors approve the final version of the manuscript.
Funding
The study has been supported by the Council for Faculty Research and Development of Notre Dame of Maryland University.
Disclosure
All authors declare no conflict of interest.
References
1. Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. J Psychosom Res. 2002;53(1):577–583. doi:10.1016/s0022-3999(02)00438-5
2. Soleimani E, Tahmasebi R, Daneshmandi H, Salimi SH, Aliasghari F. Work-life balance and health among pharmacists: physical activity, sleep quality, and general health. BMC Health Serv Res. 2024;24(1):1217. doi:10.1186/s12913-024-11701-w
3. Zeek ML, Savoie MJ, Song M, et al. Sleep duration and academic performance among student pharmacists. Am J Pharm Educ. 2015;79(5):63. doi:10.5688/ajpe79563
4. Wickwire EM, Geiger-Brown J, Scharf SM, Drake CL, Wickwire EM. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest. 2017;151(5):1156–1172. doi:10.1016/j.chest.2016.12.007
5. Tomaso CC, Johnson AB, Nelson TD. The effect of sleep deprivation and restriction on mood, emotion, and emotion regulation: three meta-analyses in one. Sleep. 2021;44(6):zsaa289. doi:10.1093/sleep/zsaa289
6. Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10(5):323–337. doi:10.1016/j.smrv.2005.11.001
7. Kamdar BB, Kaplan KA, Kezirian EJ, Dement WC. The impact of extended sleep on daytime alertness, vigilance, and mood. Sleep Med. 2004;5(5):441–448. doi:10.1016/j.sleep.2004.05.003
8. Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: stressor chronicity, cognitive intrusion, and coping. Sleep. 2014;37(7):1199–1208. doi:10.5665/sleep.3838
9. Kadović M, Mikšić Š, Lovrić R. Ability of emotional regulation and control as a stress predictor in healthcare professionals. Int J Environ Res Public Health. 2022;20(1):541. doi:10.3390/ijerph20010541
10. Vandekerckhove M, Wang YL. Emotion, emotion regulation and sleep: an intimate relationship. AIMS Neurosci. 2017;5(1):1–17. doi:10.3934/Neuroscience.2018.1.1
11. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company; 1984.
12. Tang NKY, Saconi B, Jansson-Fröjmark M, Ong JC, Carney CE. Cognitive factors and processes in models of insomnia: a systematic review. J Sleep Res. 2023;32(6):e13923. doi:10.1111/jsr.13923
13. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi:10.2307/2136404
14. Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. doi:10.1093/sleep/27.2.285
15. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063. doi:10.1037/0022-3514.54.6.1063
16. Krystal AD, Krystal AD. Psychiatric disorders and sleep. Neurol Clin. 2012;30(4):1389–1413. doi:10.1016/j.ncl.2012.08.018
17. You Y, Ablitip A, Chen Y, et al. Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: a cross-sectional study. BMC Public Health. 2024a;24(1):1920. doi:10.1186/s12889-024-19432-7
18. You Y. Accelerometer-measured physical activity and sedentary behaviour are associated with C-reactive protein in US adults who get insufficient sleep: a threshold and isotemporal substitution effect analysis. J Sports Sci. 2024b;42(6):527–536. doi:10.1080/02640414.2024.2348906
19. Demichelis OP, Grainger SA, Burr L, Henry JD. Emotion regulation mediates the effects of sleep on stress and aggression. J Sleep Res. 2023;32(3):e13787. doi:10.1111/jsr.13787
20. Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi:10.3389/fnhum.2017.00316
21. Kalmbach DA, Anderson JR, Drake CL. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;27(6):e12710. doi:10.1111/jsr.12710
22. Pesonen AK, Makkonen T, Elovainio M, Halonen R, Räikkönen K, Kuula L. Presleep physiological stress is associated with a higher cortical arousal in sleep and more consolidated REM sleep. Stress. 2021;24(6):667–675. doi:10.1080/10253890.2020.1869936
23. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739–749. doi:10.1016/j.psc.2017.08.008
24. Yap Y, Slavish DC, Taylor DJ, Bei B, Wiley JF. Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study. Sleep. 2020;43(3):
25. Carney CE, Harris AL, Falco A, Edinger JD. The relation between insomnia symptoms, mood, and rumination about insomnia symptoms. J Clin Sleep Med. 2013;9(6):567–575. doi:10.5664/jcsm.2752
26. Kalmbach DA, Cheng P, Drake CL. A pathogenic cycle between insomnia and cognitive arousal fuels perinatal depression: exploring the roles of nocturnal cognitive arousal and perinatal-focused rumination. Sleep. 2021;44(6):zsab028. doi:10.1093/sleep/zsab028
27. Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. doi:10.1016/S0005-7967(01)00061
28. Carney CE, Edinger JD, Morin CM, et al. Examining maladaptive beliefs about sleep across insomnia patient groups. J Psychosom Res. 2010;68(1):57–65. doi:10.1016/j.jpsychores.2009.08.007
29. Allan AC, Gamaldo AA, Gamaldo CE, et al. The promotion of sleep wellness: resilience as a protective factor. Front Sleep. 2023;2:1133347. doi:10.3389/frsle.2023.1133347
30. You Y, Li J, Zhang Y, Li X, Li X, Ma X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: insights from population-based and mice studies. CNS Neurosci Ther. 2024c;30(5):e14783. doi:10.1111/cns.14783
31. Lancaster MR, Callaghan P. The effect of exercise on resilience, its mediators and moderators, in a general population during the UK COVID-19 pandemic in 2020: a cross-sectional online study. BMC Public Health. 2022;22(1):827. doi:10.1186/s12889-022-13070-7
32. Scott AJ, Webb TL, Martyn-St James M, Rowse G, Weich S. Improving sleep quality leads to better mental health: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2021;60:101556. doi:10.1016/j.smrv.2021.101556
33. Choi DS, Kim SH. Factors affecting occupational health of shift nurses: focusing on job stress, health promotion behavior, resilience, and sleep disturbance. Saf Health Work. 2022;13(1):3–8. doi:10.1016/j.shaw.2021.09.001
34. Darvishi E, Osmani H, Aghaei A, Moloud EA. Hidden risk factors and the mediating role of sleep in work-related musculoskeletal discomforts. BMC Musculoskelet Disord. 2024;25(1):256. doi:10.1186/s12891-024-07387-0
35. Shaik L, Cheema MS, Subramanian S, Kashyap R, Surani SR. Sleep and safety among healthcare workers: the effect of obstructive sleep apnea and sleep deprivation on safety. Medicina. 2022;58(12):1723. doi:10.3390/medicina58121723
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

