Back to Journals » International Journal of Nanomedicine » Volume 19
The New Era of Neural Modulation Led by Smart Nanomaterials
Authors Hou Z
Received 3 October 2024
Accepted for publication 13 November 2024
Published 20 November 2024 Volume 2024:19 Pages 12287—12295
DOI https://doi.org/10.2147/IJN.S491440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Zhitao Hou1– 5
1College of Basic Medical and Sciences, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, 150040, People’s Republic of China; 2Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA; 3Department of Neurology, the Second Hospital Affiliated with Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, 150010, People’s Republic of China; 4Department of Neurology, the First Hospital Affiliated with Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, 150010, People’s Republic of China; 5Institute of Shaanxi Key Laboratory of Ultrasonics, Shaanxi Normal University, Xi’an, 710119, People’s Republic of China
Correspondence: Zhitao Hou, Email [email protected].
Abstract: Understanding the physiology and pathology of neural circuits is crucial in neuroscience research. A variety of techniques have been utilized in medical research, with several established methods applied in clinical therapy to enhance patient’ neurological functions. Traditional methods include generating electric fields near neural tissue using electrodes, or non-contact modulation using light, chemicals, magnetic fields, and ultrasound. The advent of nanotechnology represents a new advancement in neural modulation techniques, offering high precision and the ability to target specific cell types. Smart nanomaterials enable the conversion of remote signals (such as light, magnetic, or ultrasound) into local stimuli (eg, electric fields or heat) for neurons. Surface treatment technologies of nanomaterials have enhanced biocompatibility, making targeted delivery to specific cell types possible and paving the way for precise neural modulation. This perspective will explore neural modulation techniques supported by nanomedical materials, focusing on photoelectric, photothermal, magnetoelectric, magnetothermal, and acoustoelectric conversion mechanisms, and looking forward to their medical applications.
Keywords: neurodegenerative diseases, neural modulation, nanomaterials
Introduction
Neural modulation technology has experienced significant advancements, especially in the treatment of neurological disorders and the correction of nervous system dysfunctions.1 These technologies are widely used to restore hearing and vision, and treat diseases such as Parkinson’s,2 essential tremor,3 epilepsy,4 and obsessive-compulsive disorder.5 According to the World Health Organization, Parkinson’s disease affects approximately 8.5 million people globally, with the prevalence having doubled over the past 25 years. Essential tremor impacts around 7 million individuals in the United States alone.6,7 Additionally, epilepsy remains one of the most common neurological conditions, impacting around 50 million people worldwide. Obsessive-compulsive disorder has a global prevalence rate of 1% to 2%.8 Moreover, the peripheral nervous system’s regular function, which is essential for the operation of various organs, underscores the vast clinical potential of neural modulation technologies targeting peripheral nerves. However, traditional methods, such as vagus nerve stimulation, often necessitate surgical electrode implantation, raising concerns about biosafety, surgical trauma, and the limitations posed by electrical field attenuation.9,10
In recent years, to tackle these challenges, researchers have devised optical, thermal, and chemogenetic tools, allowing for more minimally invasive and cell-specific neural modulation.2,11–13 These methods allow researchers to control the activation or inhibition of neurons through external stimuli, such as light or heat. While these technologies have demonstrated potential in animal experiments, the reliance on genetic engineering and translating from animal models to clinical use presents challenges. Furthermore, the timeliness and conversion efficacy of these methods still need further enhancement.
The utilization of smart nanomaterials has opened new opportunities in nervous system research (Figure 1). Unlike traditional methods, smart nanomaterials not only serve as drug delivery vehicles but also act as energy converters in neural stimulation.14 These materials, such as metallic or inorganic compound nanomaterials, respond to external stimuli—including light, heat, or electromagnetic fields—facilitating neural activation.15,16 Spatial selectivity optimization is key, achievable through precise placement of stimulation signals or nanomaterials. In many experiments, smart nanomaterials have played a key role in neural activation through mechanical, thermal, and electromagnetic effects, which are crucial for understanding how smart nanomaterials function in the nervous system.
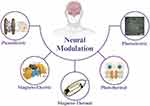 |
Figure 1 Application of Smart Nanomaterials in Neural Modulation. |
Distinct from existing reviews, this perspective uniquely synthesizes the latest advancements in smart nanomaterials for neural modulation, offering a comprehensive overview of their multifunctional roles beyond conventional applications. We aim to provide a critical analysis of how these materials transcend traditional paradigms, particularly focusing on their potential to address the limitations of current neural modulation techniques. This perspective is timely, considering the rapid evolution of nanotechnology in neuroscience, and serves as an essential resource for researchers aiming to explore the frontier of neural modulation with smart nanomaterials.
Key Mechanisms of Nanotechnology in Neural Modulation
Application of the Photoelectric Effect
Quantum dots (QD), as semiconductor nanoparticles ranging from 2 to 6 nanometers in diameter, exhibit photoconductive properties, demonstrating potential as optically-controlled neural stimulation mediators.17 Under light stimulation, QDs generate dipole moments and electric fields, potentially sufficient to activate neurons’ voltage-gated ion channels. Currently, research mainly employs two methods to direct QDs to the surface of neurons:18–20 the first is by modifying QDs with antibodies or peptides to bind them to the neuron membrane; the second is fixing QDs on a specific material substrate, then attaching them to the neuron surface.
However, several limitations arise when QDs are directly bound to neuron cell membranes. One major issue is the potential internalization of QDs by neuronal cells, which compromises the stability and efficacy of the photoelectric interface. Additionally, the binding specificity of antibodies can be insufficient, making it difficult to achieve consistent and stable neural regulation. To address these challenges, alternative techniques have been developed. One such method involves coupling QDs to carriers to form thin films, reducing the likelihood of internalization. While this approach improves stability, it often only achieves short-term efficacy. For instance, research has demonstrated that multilayered Hg-Te-QD films can generate photocurrents capable of triggering action potentials in neuroblastoma NG108 cells, thereby activating neuronal responses.21–23
Further advancements have been made using materials like Cd-Te-QD and Cd-Se-QD films, which have been utilized to create photoelectric interfaces with different cell types, including LnCap prostate cancer cells and cortical neurons. When illuminated, these cells exhibit various responses, from depolarization to hyperpolarization, and in some cases, action potentials are induced in cortical neurons. Nevertheless, QD-mediated photoelectric stimulation remains limited, with studies showing that only around 11% of neurons are effectively activated.23 Additionally, cellular responses can vary significantly, with some neurons depolarizing and others hyperpolarizing under identical stimuli.
To improve performance and biocompatibility, researchers have engineered composite materials by chemically coupling Cd-Se/Cd-S core-shell nanorods with carbon nanotubes. These composites have demonstrated the ability to activate under pulsed light at a wavelength of 405 nm,18 successfully stimulating chick retinas lacking developed photoreceptors. However, their clinical application is still limited to superficial neural stimulation due to the poor tissue penetration of visible light. Notably, Maya-Vetencourt et al24 demonstrated that subretinally injected semiconducting polymer nanoparticles can rescue vision in a rat model of retinal dystrophy by mediating light-evoked stimulation of retinal neurons, highlighting the potential of semiconducting polymer nanoparticles in vision restoration.
The Role of Photothermal Effects in Neural Stimulation
The temperature sensitivity of neuronal activity makes it possible to utilize photothermal effects for neural stimulation. Gold nanomaterials, due to their localized surface plasmon resonance properties, have become ideal photoabsorbers. Under illumination at resonant frequencies, gold nanomaterials generate electronic oscillations and collisions, inducing a photothermal effect and successfully activating neurons. However, the internalization of gold nanorods and the inconsistency in neuronal responses remain key issues to be addressed. Research indicates that spatial local temperature gradients on neuronal tissues are the cause of increased membrane capacitance and action potentials.25,26 Conversely, due to the regulatory role of ion channels, slow and prolonged heating can inhibit neuronal activity. Additionally, the specific temperature sensitivity of transient receptor potential vanilloid (TRPV)27 is another widely studied trigger for neuronal modulation. To generate localized heat for stimulating neurons, nanoparticles are used as photoabsorbers to convert light into heat.28 Due to localized surface plasmon resonance, gold nanomaterials are particularly suitable as photoabsorbers for neural stimulation.29,30 Under illumination from light sources at resonance frequencies, electrons in gold nanomaterials oscillate and collide, producing photothermal effects.
Gold nanorods coated with silica have been observed to successfully activate rat auditory neurons in vitro.31,32 Under pulsed laser illumination at a resonant wavelength of 780 nm, these gold nanorods activate nearby neurons, with a linear relationship to the duration of the laser pulses. Researchers also found that internalized gold nanorods, under continuous and pulsed irradiation of near-infrared at a resonant wavelength of 780 nm, promoted synaptic outgrowth in NG108-15 cells and induced Ca2+ influx.33,34 Under pulsed laser irradiation at the near-infrared resonance wavelength of 980 nm, the response of the sciatic nerve injected with gold nanorods to compound action potentials increased by nearly 6 times, and the action potential threshold decreased by 3 times. Therefore, this method can significantly reduce the power and duration of laser stimulation, thereby significantly reducing the risk of tissue damage. In an in vivo animal study, researchers injected polyethylene glycol-modified PEG-Au NRs into the left stellate ganglion of dogs and irradiated them with 810 nm near-infrared light. They found that the photothermal effect reversibly suppressed the function and activity of the stellate ganglion, significantly reducing ventricular arrhythmias after myocardial ischemia. Furthermore, a decrease in c-fos protein expression was observed in the stellate ganglion, providing a solution for future non-invasive optical control of the autonomic nervous system.35
However, the internalization of gold nanorods remains a significant issue for these photothermal neuronal stimulations, often leading to variable outcomes, inconsistent phenomena, and tissue damage.32,33 Studies indicate that the internalization of gold nanorods may lead to a decrease in Ca2+ influx when pulsed laser irradiance is enhanced.33 Studies have also observed that gold nanorods, electrostatically attached to neuronal membranes, exert inhibitory effects on hippocampal, cortical, and olfactory bulb neurons.36 These phenomena are hypothesized to be caused by the temperature-sensitive inhibitory TREK-1 channels. Consequently, another challenge is to precisely determine the different pathways of photothermal regulation by nanomaterials, enabling accurate control of neuronal responses. A recent study by Hescham et al37 presents a wireless magnetothermal approach to deep brain stimulation (DBS) that alleviates parkinsonian-like symptoms in mice using synthetic magnetic nanoparticles and alternating magnetic fields to activate heat-sensitive TRPV1-expressing neurons, enhancing the discussion on wireless magnetothermal neuromodulation for Parkinson’s disease treatment (Figure 2).
 |
Figure 2 Photothermal Effects in Neural Stimulation – Mechanisms, Applications, Challenges, and Advancements. |
Application of Magneto-Electric Effect in Neural Stimulation
Researchers have proposed a new method of controlling neural stimulation using magnetic fields, based on the magneto-electric effect of magneto-electric nanoparticles.38 These nanoparticles are capable of converting magnetic fields into electric fields, thereby activating neurons. Building on this effect, researchers have validated the use of core-shell structured magneto-electric nanoparticles (10 µg CoFe2O4-BaTiO3 30 nm) in mice, demonstrating these materials’ capability to modulate deep brain circuits under low-intensity magnetic fields.39 However, more research is needed in this area to assess the stability of their physicochemical properties and the feasibility of long-term biocompatibility and safety in the body. Gregurec et al40 explored the use of magnetic vortex nanodiscs for remote magnetomechanical neural stimulation. The transition from vortex to in-plane magnetization in iron oxide nanodiscs allows for the modulation of mechanosensory cells, triggering Ca2+ influx in low magnetic fields. Additionally, Kozielski et al41 introduced injectable, magnetoelectric nanoelectrodes that wirelessly transmit electrical signals to the brain in response to an external magnetic field, enabling less invasive deep brain stimulation in freely moving mice. Lee et al42 introduced m-Torquer, a magnetic toolkit that delivers piconewton-scale forces to cells, enabling non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. The application of biological magnetic nanomaterials, such as magnetotactic bacteria and synthetic magnetic nanoparticles, holds promise for developing biocompatible, neuron-targeted magneto-electric stimulation strategies.43
Neural Stimulation Strategies Using the Magneto-Thermal Effect
Superparamagnetic nanoparticles achieve magneto-thermal neural stimulation by converting alternating magnetic fields into localized heat through magneto-thermal conversion.44 Studies have utilized streptavidin-modified superparamagnetic Mn Fe2O4 nanoparticles, and with genetic engineering, induced neurons to express temperature-gated TRPV1 ion channels and anchored biotinylated peptides, allowing nanoparticles to specifically bind to target neuronal membranes. Upon application of a radio frequency magnetic field, the localized thermal effect of magneto-thermal conduction induces Ca2+ influx through the TRPV1 ion channels, causing neuronal depolarization and the generation of action potentials.45
Given that TRPV1 ion channels can be uniformly expressed in target cells across a tissue through genetic engineering, the magneto-thermal neural stimulation enabled by superparamagnetic nanoparticles can uniformly stimulate targeted cell populations.45–47Although both photothermal and magneto-thermal stimulation use thermal effects as local stimulation signals, the latter involves genetic modification of target neurons for specific cellular and tissue targeting.46,47 However, due to the use of genetic engineering and the high permeability of TRPV1 ion channels to Ca2+, other temperature-gated ion channels are gradually being explored for application.48 Thermally induced nanomaterials, such as those covered in recent studies, offer innovative ways to achieve spatial and temporal control over neuronal activity, which can be especially useful in developing minimally invasive neuromodulation techniques.49
The study “Subsecond multichannel magnetic control of select neural circuits in freely moving flies”50 achieves subsecond, multichannel stimulation of different groups of neurons in Drosophila melanogaster using magnetic nanoparticles, enabling precise temporal modulation of neural activity. This reference can be integrated to discuss advancements in multichannel magnetic control for neural circuits.
Application of the Piezoelectric Effect in Neural Regulation
Piezoelectric materials can convert mechanical stimuli into electrical signals, generating a localized electromagnetic field (EMF). There is evidence that EMF can affect various voltage-gated ion channels on the cell surface, influencing the influx and efflux of electrolytic ions inside and outside the cell, thus regulating cell function and activity, impacting ion channels on the cell surface.51,52 Piezoelectric nanomaterials have been successfully used in experiments to activate specific neuronal cells, demonstrating their potential in non-invasive neural regulation.53
After treating SH-SY5Y neuron-like cells with piezoelectric nanomaterial barium titanate nanoparticles (BTNPs) and applying ultrasonic treatment, particle dynamic fluorescence imaging indicated significant cellular responses in terms of calcium and sodium flux. The process was validated using appropriate blockers to be caused by the activation of voltage-gated membrane channels. When cells were treated with cubic non-piezoelectric barium titanate nanoparticles, this phenomenon disappeared, confirming the hypothesis of piezoelectric stimulation in neuron-like cells.54 Following this, research by Rojas et al55 demonstrated that adsorbing piezoelectric barium titanate nanoparticles onto neuronal membranes enabled the activation of specific neuronal cells with piezoelectric material using ultrasound. This activation ceased with the discontinuation of ultrasound, indicating high specificity and selectivity in neural regulation by piezoelectric materials, as neurons without piezoelectric nanoparticles or with non-piezoelectric nanoparticles were not activated by ultrasound. Zhao et al56 study applied this technology to regulate specific neurological disease models. Carbon-coated piezoelectric Ba Ti O3 nanoparticles entering TH neurons in a Parkinson’s disease model zebrafish could affect voltage-sensitive L-type calcium channels under ultrasound induction, influencing intracellular calcium ion flow in neurons, modulating the plasticity of impaired substantia nigra neurons, and improving symptoms in the experimental animals. In a later animal study, researchers injected chitosan-coated Ba Ti O3 nanoparticles into the right inferior cardiac ganglia of beagles. Using ultrasound-mediated activation, they stimulated ganglion activity, effectively lowering the ventricular rate in a canine model of atrial fibrillation.57 This series of experiments fully foreshadows the prospects of piezoelectric nanomaterials in neural modulation. In the near future, piezoelectric materials are expected to serve as an effective means of autonomic nervous regulation of organs, used in more clinical disease diagnostics, research, and translation (Table 1).
 |
Table 1 Key Mechanisms of Nanotechnology in Neural Modulation |
Prospects and Challenges of Smart Nanomaterials in Neural Modulation
This perspective provides an overview of the mechanisms by which smart nanomaterials function in neural stimulation and their potential clinical uses. Most nanomaterials achieve neural regulation by directly acting on ion channels in neuronal cell membranes, while a minority influence by modulating intracellular pH and reactive oxygen species.58 Despite being at an early stage, the field is growing with the advancement of nanomaterials and neurobiology, leading to a rise in research on stimulus-responsive nanoparticles that specifically target ion channels. These novel nanomaterials not only propel research in non-genetic neural regulation across multidisciplinary fields but also open new possibilities for effective treatment of nervous system diseases and understanding the deep mechanisms of the human nervous system.
Current research in nanomaterial design and signal selection broadly categorizes nanoparticles into two types: those with single signal conversion, like photothermally regulated gold nanorods, and those with multiple signal conversion capabilities, such as core-shell structured piezoelectric nanoparticles capable of ultrasound/electrical conversion. These designs are often influenced by the researchers’ expertise, typically favoring the development of multifunctional rather than specialized nanoparticles for specific clinical challenges. Future research should prioritize designing multifunctional nanoparticles tailored to address particular clinical needs, such as creating targeted delivery systems for neurodegenerative disorders. Additionally, the trend in nanomaterial development is shifting from inorganic nanoparticles to hybrid organic-inorganic materials and even purely organic systems. While inorganic nanomaterials excel in signal conversion and transmission, their limitations in biocompatibility and specificity hinder widespread clinical adoption. Thus, developing organic-inorganic hybrid nanoparticles that incorporate organic groups, antibodies, or other organic nanostructures is critical for enhancing clinical relevance.
However, it is worth noting the emergence of additional neuromodulation nanotechnologies, such as the magnetomechanical effect and NIR-based optogenetics.59,60 The magnetomechanical effect leverages magnetic nanomaterials to induce mechanical forces capable of modulating neuronal activity, offering an innovative alternative to conventional electrical stimulation methods. In contrast, NIR-based optogenetics utilizes near-infrared light to activate optogenetic constructs, providing advantages such as deeper tissue penetration and minimized thermal damage. This non-invasive technique holds significant promise for achieving precise and controlled neural modulation. Collectively, these advanced approaches complement traditional methods and broaden the repertoire of neuromodulation strategies, paving the way for more effective and versatile applications in neuroscience and clinical practice.
Key challenges in translating smart nanomaterials to clinical applications include precise targeting, minimally invasive delivery, and the accurate control of stimulation signals to modulate neuronal excitation or inhibition. Currently, there are few effective methods or materials to overcome these challenges. Some studies have leveraged gene editing to express specific molecules on target cells, enabling the attachment of complementary antibodies to nanoparticles for precise binding. With advancements in gene editing technology, future strategies are expected to become safer and more reliable, offering refined approaches for targeted and efficient neuromodulation. Furthermore, addressing these application issues requires interdisciplinary collaboration between materials science, genetics, and clinical research.
Potential future action lines include developing highly specific, non-invasive delivery techniques, improving the biocompatibility and targeting efficiency of nanomaterials, and exploring alternative non-genetic strategies for neural modulation. In addition, research should aim to integrate real-time monitoring and feedback mechanisms into smart nanomaterials to achieve dynamic and adaptive control of neuronal activity. In the future, non-genetic neural regulation based on smart nanomaterials is anticipated to emerge as a groundbreaking interdisciplinary field with tremendous potential in treating neurological diseases, including currently untreatable conditions like Parkinson’s and Alzheimer’s. Beyond therapy, these materials could become indispensable tools in neuroscience research, facilitating exploration into the mechanisms of the human nervous system and the regulation of autonomic functions in organs and systems, such as those involved in cardiovascular and neuroendocrine diseases.
While smart nanomaterials are still in the early stages of clinical translation, there is a pressing need for continued innovation. Developing specific intervention methods, optimizing existing strategies, and enhancing the application and translational potential of these advanced materials will be crucial as technology continues to advance.
Data Sharing Statement
This manuscript is a review article and does not involve content related to raw data.
Acknowledgments
Dr. Zhitao Hou would like to thank Prof. Chenghui Wang and Prof. Shuyu Lin for providing access to interdisciplinary platforms and skills in the field of biomedical engineering in China. Additionally, appreciate Dr. Jacob S. Brenner for his assistance in facilitating an overseas learning opportunity, which greatly contributed to the early stages of this work. Zhitao Hou wrote this review article, with Prof. Chenghui Wang and Prof. Shuyu Lin providing guidance on the content structure and professional knowledge.
Funding
This research received funding from various sources, including the Foundation of China Scholarship Council (CSC No. 202208230108), the National Natural Sciences Foundation of China (grant Nos. 81904307 and 82274395), the Natural Science Foundation of Heilongjiang Province for Outstanding Young Scholars (grant No. YQ2022H019), the Ministry of Education of China’s Young Backbone Scholar Program for the Central and Western Regions (No. 202412), Heilongjiang University of Chinese Medicine Youth Science and Technology Innovation Capacity Building Program (No. 202405), and Heilongjiang Provincial Undergraduate University Basic Scientific Research Fund Project (No. 202405).
Disclosure
All authors of this research group have no conflicts of interest related to the publication of this article to declare.
References
1. Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies. Nat Rev Mater. 2017;2:1–6.
2. Chen Y, Hong Z, Wang J, et al. Circuit-specific gene therapy reverses core symptoms in a primate Parkinson’s disease model. Cell. 2023;186(24):5394–5410e5318. doi:10.1016/j.cell.2023.10.004
3. Chandrabhatla AS, Pomeraniec IJ, Horgan TM, Wat EK, Ksendzovsky A. Landscape and future directions of machine learning applications in closed-loop brain stimulation. NPJ Digit Med. 2023;6(1):79. doi:10.1038/s41746-023-00779-x
4. Zhang Y, Wu X, Ding J, et al. Wireless-powering deep brain stimulation platform based on 1d-structured magnetoelectric nanochains applied in antiepilepsy treatment. ACS Nano. 2023;17(16):15796–15809. doi:10.1021/acsnano.3c03661
5. Grover S, Nguyen JA, Viswanathan V, Reinhart RMG. High-frequency neuromodulation improves obsessive-compulsive behavior. Nat Med. 2021;27(2):232–238. doi:10.1038/s41591-020-01173-w
6. Ding C, Wu Y, Chen X, et al. Global, regional, and national burden and attributable risk factors of neurological disorders: the global burden of disease study 1990-2019. Front Public Health. 2022;10:952161. doi:10.3389/fpubh.2022.952161
7. Huang Y, Li Y, Pan H, Han L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J Glob Health. 2023;13:04160. doi:10.7189/jogh.13.04160
8. Collaborators GBDNSD. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 2024;23(4):344–381. doi:10.1016/S1474-4422(24)00038-3
9. van der Meij A, Wermer MJH. Vagus nerve stimulation: a potential new treatment for ischaemic stroke. Lancet. 2021;397(10284):1520–1521. doi:10.1016/S0140-6736(21)00667-X
10. Dawson J, Liu CY, Francisco GE, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–1553. doi:10.1016/S0140-6736(21)00475-X
11. Ahnood A, Chambers A, Gelmi A, Yong KT, Kavehei O. Semiconducting electrodes for neural interfacing: a review. Chem Soc Rev. 2023;52(4):1491–1518. doi:10.1039/D2CS00830K
12. Sahasrabudhe A, Rupprecht LE, Orguc S, et al. Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits. Nat Biotechnol. 2024;42(6):892–904.
13. Rao S, Chen R, LaRocca AA, et al. Remotely controlled chemomagnetic modulation of targeted neural circuits. Nat Nanotechnol. 2019;14(10):967–973. doi:10.1038/s41565-019-0521-z
14. Li X, Xiong H, Rommelfanger N, et al. Nanotransducers for wireless neuromodulation. Matter. 2021;4(5):1484–1510. doi:10.1016/j.matt.2021.02.012
15. Karatum O, Gwak MJ, Hyun J, et al. Optical neuromodulation at all scales: from nanomaterials to wireless optoelectronics and integrated systems. Chem Soc Rev. 2023;52:3326–3352.
16. Gholami Derami H, Gupta P, Weng K-C, et al. Reversible photothermal modulation of electrical activity of excitable cells using polydopamine nanoparticles. Adv Mater. 2021;33(32):e2008809. doi:10.1002/adma.202008809
17. Algar WR, Massey M, Rees K, et al. Photoluminescent nanoparticles for chemical and biological analysis and imaging. Chem Rev. 2021;121(15):9243–9358. doi:10.1021/acs.chemrev.0c01176
18. Bareket L, Waiskopf N, Rand D, et al. Semiconductor nanorod-carbon nanotube biomimetic films for wire-free photostimulation of blind retinas. Nano Lett. 2014;14(11):6685–6692. doi:10.1021/nl5034304
19. Rand D, Jakešová M, Lubin G, et al. Direct electrical neurostimulation with organic pigment photocapacitors. Adv Mater. 2018;30(25):e1707292. doi:10.1002/adma.201707292
20. Abbott J, Ye T, Ham D, Park H. Optimizing nanoelectrode arrays for scalable intracellular electrophysiology. Acc Chem Res. 2018;51(3):600–608. doi:10.1021/acs.accounts.7b00519
21. Si MJ, Jee S, Yang M, et al. Colloidal InAs quantum dot-based infrared optoelectronics enabled by universal dual-ligand passivation. Adv Sci. 2024;11(13):e2306798. doi:10.1002/advs.202306798
22. Meng L, Xu Q, Zhang J, Wang X. Colloidal quantum dot materials for next-generation near-infrared optoelectronics. Chem Commun. 2024;60(9):1072–1088. doi:10.1039/D3CC04315K
23. Pappas TC, Wickramanyake WM, Jan E, Motamedi M, Brodwick M, Kotov NA. Nanoscale engineering of a cellular interface with semiconductor nanoparticle films for photoelectric stimulation of neurons. Nano Lett. 2007;7(2):513–519. doi:10.1021/nl062513v
24. Maya-Vetencourt JF, Manfredi G, Mete M, et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat Nanotechnol. 2020;15(8):698–708. doi:10.1038/s41565-020-0696-3
25. Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3(1):736. doi:10.1038/ncomms1742
26. Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F. Corrigendum: infrared light excites cells by changing their electrical capacitance. Nat Commun. 2017;8(1):16148. doi:10.1038/ncomms16148
27. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi:10.1038/39807
28. Macpherson S, Doherty TAS, Winchester AJ, et al. Local nanoscale phase impurities are degradation sites in halide perovskites. Nature. 2022;607(7918):294–300. doi:10.1038/s41586-022-04872-1
29. Wang H, Meyer SM, Murphy CJ, Chen YS, Zhao Y. Visualizing ultrafast photothermal dynamics with decoupled optical force nanoscopy. Nat Commun. 2023;14(1):7267. doi:10.1038/s41467-023-42666-9
30. Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86(1):207–217. doi:10.1016/j.neuron.2015.02.033
31. Mou Z, You M, Xue W. Gold nanorod-assisted near-infrared stimulation of bullfrog sciatic nerve. Lasers Med Sci. 2018;33(9):1907–1912. doi:10.1007/s10103-018-2554-1
32. Yong J, Needham K, Brown WGA, et al. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv Healthc Mater. 2014;3(11):1862–1868. doi:10.1002/adhm.201400027
33. Paviolo C, Haycock JW, Cadusch PJ, McArthur SL, Stoddart PR. Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophotonics. 2014;7(10):761–765. doi:10.1002/jbio.201300043
34. Paviolo C, McArthur SL, Stoddart PR. Gold nanorod-assisted optical stimulation of neuronal cells. J Vis Exp. 2015;(98). doi:10.3791/52566-v
35. Xu XJ, Li Y, Hui H, Liu CL, Fu Y, Yang HY. Dual-targeting multifunctional hyaluronic acid ligand-capped gold nanorods with enhanced endo-lysosomal escape ability for synergetic photodynamic-photothermal therapy of cancer. Biotechnol Bioeng. 2023;120(8):2333–2344. doi:10.1002/bit.28462
36. Yoo S, Hong S, Choi Y, Park JH, Nam Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano. 2014;8(8):8040–8049. doi:10.1021/nn5020775
37. Hescham SA, Chiang P-H, Gregurec D, et al. Magnetothermal nanoparticle technology alleviates parkinsonian-like symptoms in mice. Nat Commun. 2021;12(1):5569. doi:10.1038/s41467-021-25837-4
38. Kruk S, Poddubny A, Smirnova D, et al. Nonlinear light generation in topological nanostructures. Nat Nanotechnol. 2019;14(2):126–130. doi:10.1038/s41565-018-0324-7
39. Guduru R, Liang P, Hong J, et al. Magnetoelectric ‘spin’ on stimulating the brain. Nanomedicine. 2015;10(13):2051–2061. doi:10.2217/nnm.15.52
40. Gregurec D, Senko AW, Chuvilin A, et al. Magnetic vortex nanodiscs enable remote magnetomechanical neural stimulation. ACS Nano. 2020;14(7):8036–8045. doi:10.1021/acsnano.0c00562
41. Kozielski KL, Jahanshahi A, Gilbert HB, et al. Nonresonant powering of injectable nanoelectrodes enables wireless deep brain stimulation in freely moving mice. Sci Adv. 2021;7(3). doi:10.1126/sciadv.abc4189
42. Lee J-U, Shin W, Lim Y, et al. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat Mater. 2021;20(7):1029–1036. doi:10.1038/s41563-020-00896-y
43. Winkler R, Ciria M, Ahmad M, Plank H, Marcuello C. A review of the current state of magnetic force microscopy to unravel the magnetic properties of nanomaterials applied in biological systems and future directions for quantum technologies. Nanomaterials. 2023;13:2585.
44. Barbic M. Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife. 2019;8. doi:10.7554/eLife.45807
45. Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol. 2010;5(8):602–606. doi:10.1038/nnano.2010.125
46. Castillo-Torres SA, Paez-Maggio MJ. Magnetothermal neurostimulation: a minimally invasive and “wireless” alternative for deep brain stimulation in movement disorders? Mov Disord Clin Pract. 2022;9(4):454–455. doi:10.1002/mdc3.13420
47. Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347(6229):1477–1480. doi:10.1126/science.1261821
48. Li L, Li M. Modular engineering of aptamer-based nanobiotechnology for conditional control of ATP sensing. Adv Mater. 2023;36(22):e2302972. doi:10.1002/adma.202302972
49. Yang C, Park S. Nanomaterials-assisted thermally induced neuromodulation. Biomed Eng Lett. 2021;11(3):163–170. doi:10.1007/s13534-021-00193-w
50. Sebesta C, Torres Hinojosa D, Wang B, et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. Nat Mater. 2022;21(8):951–958. doi:10.1038/s41563-022-01281-7
51. Zandi O, Agrawal A, Shearer AB, et al. Impacts of surface depletion on the plasmonic properties of doped semiconductor nanocrystals. Nat Mater. 2018;17(8):710–717. doi:10.1038/s41563-018-0130-5
52. Zheng ZB, Li J-T, Ma T, et al. Tailoring of electromagnetic field localizations by two-dimensional graphene nanostructures. Light Sci Appl. 2017;6(10):e17057. doi:10.1038/lsa.2017.57
53. Kim D, Han SA, Kim JH, Lee JH, Kim SW, Lee SW. Biomolecular piezoelectric materials: from amino acids to living tissues. Adv Mater. 2020;32(14):e1906989. doi:10.1002/adma.201906989
54. Marino A, Arai S, Hou Y, et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano. 2015;9(7):7678–7689. doi:10.1021/acsnano.5b03162
55. Rojas C, Tedesco M, Massobrio P, et al. Acoustic stimulation can induce a selective neural network response mediated by piezoelectric nanoparticles. J Neural Eng. 2018;15(3):036016. doi:10.1088/1741-2552/aaa140
56. Zhao D, Feng PJ, Liu JH, et al. Electromagnetized-nanoparticle-modulated neural plasticity and recovery of degenerative dopaminergic neurons in the mid-brain. Adv Mater. 2020;32:e2003800.
57. Han J, Zhang Y, Wang X, et al. Ultrasound-mediated piezoelectric nanoparticle modulation of intrinsic cardiac autonomic nervous system for rate control in atrial fibrillation. Biomater Sci. 2023;11(2):655–665. doi:10.1039/D2BM01733D
58. Garcia-Etxarri A, Yuste R. Time for NanoNeuro. Nat Methods. 2021;18(11):1287–1293. doi:10.1038/s41592-021-01270-9
59. Uji M, Kondo J, Hara‐Miyauchi C, et al. In vivo optogenetics based on heavy metal-free photon upconversion nanoparticles. Adv Mater. 2024;36(46):e2405509. doi:10.1002/adma.202405509
60. Park JE, Kwon SH, Lu Q, Choi HJ, Wie JJ. Synergistic inclusion effects of hard magnetic nanorods on the magnetomechanical actuation of soft magnetic microsphere-based polymer composites. Small. 2024;20(6):e2305272. doi:10.1002/smll.202305272
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

