Back to Journals » Research and Reports in Tropical Medicine » Volume 16
The Perception and Practices of Black African Subjects Toward Hemorrhoidal Disease: The Relevant Effects of Beliefs and Misconceptions in Côte d’Ivoire, West Africa
Authors Mahassadi AK , Motcheyo HC, Kouame DH, Yao-Bathaix FM
Received 28 October 2024
Accepted for publication 6 February 2025
Published 17 March 2025 Volume 2025:16 Pages 11—23
DOI https://doi.org/10.2147/RRTM.S498009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario A. Rodríguez-Pérez
Alassan Kouame Mahassadi,1– 3 Hyacinthe Chepig Motcheyo,2 Dimitri Hatrydt Kouame,1– 3 Fulgence Mamert Yao-Bathaix1– 3
1Gastroenterology and Medicine Unit, Yopougon Teaching Hospital, Abidjan, Côte d’Ivoire; 2Gastroenterology and Endoscopy Unit, Abobo General Hospital, Abidjan, Côte d’Ivoire; 3Departement des maladies de l’appareil digestif, Faculty of Medicine, Felix Houphouët Boigny University, Abidjan, Côte d’Ivoire
Correspondence: Alassan Kouame Mahassadi, Email [email protected]
Background: The perception of black African subjects toward hemorrhoidal disease is surrounded by myths and misconceptions in sub-Saharan Africa. This study aimed to determine the magnitude of knowledge, attitudes, and practices (KAPs) of black African subjects toward hemorrhoidal disease and the impact of knowledge on their attitudes and practices.
Methods: A cross-sectional KAP survey was conducted through convenience sampling of 735 participants (mean age, 38.8 years; men, 59.2%) from urban and rural areas in Côte d’Ivoire. They received an auto questionnaire of 25 items on Likert scales depicting their KAP toward hemorrhoidal disease. A mean score of KAP < 50 points was considered low. Logistic and linear regression models were used to determine the factors associated with self-reported hemorrhoidal disease and the impact of knowledge on attitudes and practices.
Results: The overall Cronbach score was 0.75, and the sample proportions of self-reported or symptom-based hemorrhoidal disease were 44.4% (9% CI: 41– 48) and 21.2% [95% CI: 18.4– 24.4], respectively. The overall mean (SD) scores of KAP were low: 49 (34.4), 43.4 (18.7), and 33.6 (21.7), respectively. The attitudes and practices of the participants remained unchanged regardless of their knowledge. Hemorrhoidal disease was negatively associated with attitudes (beta = − 3.1, p = 0.02) or practices (beta = − 3.4, p < 0.05). Overall, the participants agreed that hemorrhoidal disease led to sexual dysfunction (85.2%) and infertility (67.1%). They preferred indigenous (52.4%) over modern treatments (30.2%) and perceived surgery for hemorrhoidal disease to be dangerous (24.4%) and not recommended (56.6%).
Conclusion: Knowledge did not change the attitudes and practices of black African subjects toward hemorrhoidal disease.
Keywords: hemorrhoidal disease, myths, misconception, sub-Saharan Africa
Background
Hemorrhoidal disease is the major anal diseases affecting 4.4% of individuals in the USA and 11% of the population in self-reported studies.1–3 Hemorrhoidal disease is a debilitating disease that can be a source of absenteeism, high medication consumption, and impaired quality of life.4,5
Modern treatments for hemorrhoidal disease are well-known and include medical, instrumental, and surgical treatments.1,6 Surgical treatment is well tolerated even though postsurgical pain, fecal incontinence, and anal stenosis or stricture may occur in 2 to 15%, 5 to 7%, and 0% to 5% of cases, respectively, with conventional surgery.1 Therefore, the side effects of surgical treatment of hemorrhoidal disease may have an impact on the healthcare-seeking behaviour of patients with hemorrhoidal disease, as reported in patients who underwent colorectal surgery.7
Disease perception among the population varies according to geographical region.8 In the Western world, diseases are considered health disorders that may occur when risk factors are present, and modern treatments are the best way to achieve a curative outcome.9 However, in sub-Saharan Africa, diseases are thought to be related to mystical attacks, witchcraft, or evil powers.8–10 Moreover, healthcare-seeking behaviour varies between urban and rural communities in Africa. Besides the beliefs and misconceptions, the lack of knowledge about diseases and, an inaccessible and unaffordable healthcare service are the most important barriers to healthcare-seeking behaviour both for rural and urban communities.11
For these reasons, traditional treatments are considered to be the most valuable recovery options.9,10 Therefore, herbal medicine treatments and incantations provided by traditional healers or wizards are the first-line treatment options considered in the general population both in urban and rural areas.10
Hemorrhoidal disease is prevalent in sub-Saharan Africa, ranging from 0.8 to 31% in hospital-based studies, and data is scarce in population-based studies.12–15 The perceptions of subjects with hemorrhoidal disease may be influenced by their beliefs, as is the case for subjects who have breast cancer, which could impact their healthcare-seeking behaviour.15–17 Consequently, in Nigeria, Ray-Offor et al, reported that among 171 patients admitted to the hospital for hemorrhoidal disease, only one-third underwent medical or instrumental treatment, and 3 patients among 44 with hemorrhoidal disease classified as Goligher grade 4 received surgical treatment.18 This discrepancy in the healthcare-seeking behaviour of patients with hemorrhoidal disease is probably due to their beliefs and misconceptions related to insufficient knowledge about hemorrhoidal disease.16
In most West African countries, hemorrhoidal disease is named “Kooko” in local languages and is thought to be responsible for infertility, sexual dysfunction, eye irritation, or blindness.16,19 Despite the presence of a large proportion of educated subjects, populations are reluctant to receive modern treatment because of their beliefs and fear of general anesthesia and death.10,20
The knowledge, attitudes, and practices (KAP) survey has three domains that allow stakeholders to view the knowledge of subjects upon a disease or phenomenon that modulates their attitudes in terms of beliefs or misconceptions and practices in terms of behaviour.21,22 It is a practical and manageable survey that can be conducted in urban and rural areas via a simple and meaningful questionnaire.21
In Africa, the KAP of the population toward hemorrhoidal disease has scarcely been reported.16 Moreover, the impact of knowledge on the attitudes and practices of the sub-Saharan population toward hemorrhoidal disease is not well known.
We hypothesize that in West Africa, black African subjects express negative attitudes and practices regardless of the magnitude of their knowledge of hemorrhoidal disease. This finding will help healthcare workers to take action to communicate with and sensitize the population toward hemorrhoidal disease in West Africa.
This study aimed to assess the impact of knowledge on the attitudes and practices of a large sample of black African individuals toward hemorrhoidal disease living in urban or rural areas in Côte d’Ivoire, West Africa.
Materials and Methods
A cross-sectional study using the convenience sampling method23 was conducted in Côte d’Ivoire (West Africa) in 2023, from February 1st to March 31st. The study used a self-report questionnaire to include participants living in urban (major cities) and rural areas (villages) belonging to South (the cities of Abidjan, Adzopé, Azaguié, Bingerville, and the village of Bieby), East (Agnibilékro and the village of Kouassikro), West (Gagnoa and the village of Gnahio), Center (Toumodi and the village of Labokro), and North (Korhogo and the villages of Koni and Bodonon). For convenience, one-third and two-thirds of the participants were recruited from rural and urban areas, respectively.
Selection of Participants
Participants were included in the study if they gave their verbal consent to participate, were over 18 years old, and could read and write in French or understand the meaning of the questionnaire items translated into local languages, if necessary, by the surveyor during the survey. Only verbal consent was obtained and witnessed by the family chief if the questionnaire was distributed in the family compound or the villages by the chief or any influential community member. Most participants were recruited by approaching them in the streets, at the market at their shops, or in their compound. They were informed about the study’s purpose and then received a copy of the questionnaire if they explicitly gave their verbal consent to participate. Verbal consent was required instead of writing because of political misperception, embarrassment, and local language barriers related to the topic under study.22 They were excluded if they refused to participate, had difficulties reading French or understanding the meaning of the items translated into broken French or in their local language, or had partially filled out the questionnaire during the survey. To avoid misinterpretation when translating the questionnaire’s items into local languages, the surveyors belong to the main ethnic group of the surveyed communities. For the South (KC, KRE), the South-East and Central regions (LNP, SKD), and the North (KZ). The acronyms are related to the full names of the surveyors provided in the acknowledgment subsection.
The Questionnaire
The questionnaire has three parts: the first part included social and demographic variables; the second part included clinical symptoms of hemorrhoidal disease; and the third part included items related to knowledge, attitudes, and practices (Supplementary Table 1). The questionnaire was self-administered to the participants and included simple and meaningful words with binary or 4-point Likert scale responses and included 26 items related to their knowledge (Q5, Q6, Q12, Q13, Q14, and Q15), attitudes (Q16, Q17, Q18, Q19, Q20, Q21, Q22, Q23, Q24, Q31, Q32, and Q33), and practices (Q3, Q4, Q25, Q26, Q27, Q28, Q29, and Q30). The questionnaire was tested in ten subjects before submission for the survey. The items for knowledge of hemorrhoidal disease were formulated in binary scale responses (“Yes”, “No”, “I do not know” or “I have never seen”). For attitudes, the items were formulated on a 4-point Likert scale (“Yes, I strongly agree!”, “Yes, I agree!”, “No, I do not entirely agree!”, “No, I do not agree!”). For the practices, the questionnaire items were also formulated in binary scale responses (“Yes”, “No”, “I do not think so”, or “I do not know”) and 4 Likert scale responses (“Of course, I would accept”, “Yes, I would accept”, “Not sure, I would accept”, “No, I would not accept”) or (“Of course, I prefer”, “Yes, I prefer”, “Not sure, I would prefer”, “No, I would not prefer”). The participants filled out the questionnaire during the survey, which took almost 5 min without any help provided by the surveyor. If necessary, the items of the questionnaire were translated into local languages or in the most understandable and meaningful way, especially to those living in rural areas or not educated, and the chosen response expressed by the participants was ticked by the surveyor.
Operational Definitions
The presence of the hemorrhoidal disease was based on self-declaration or symptoms expressed by the participants. Based on self-declaration, the participants were considered to have hemorrhoidal disease if they answered “Yes” to item 1 of the questionnaire (Q1: “Do you have hemorrhoidal disease?”). Those who said “I do not know” but expressed frequent or intermittent anal prolapse during defecation with intermittent anal bleeding were added to that category. They were considered self-reported hemorrhoidal disease participants.
The participants with symptom-based hemorrhoidal disease were those with frequent or intermittent hemorrhoidal crisis or anal prolapse during defecation associated with frequent or intermittent anal bleeding.1
Those who expressed symptoms of hemorrhoidal disease during the survey received an appointment at the proctology unit for consultation if necessary. Any immediate consultation or clinical examination to confirm the presence of hemorrhoidal disease was performed during the survey.
Scores Calculation Method
Overall, binary questions were scored 1 point if correctly answered (otherwise, 0 points), and questions formulated on 4 Likert scales were scored 3, 2, 1, or 0 points according to the appropriateness of the responses. The most appropriate response was given 3 points, and the least appropriate response was given 0 points. For each participant, the score of KAP expressed as the number of points was calculated as follows: number of scores = (total score obtained in the domain of interest: knowledge, attitudes, or practices divided by the maximum score for that domain) × 100. Thus, the score ranged from 0 to 100 points, corresponding to the highest level of knowledge, attitudes, or practices toward hemorrhoidal disease and the lowest level if the score was less than 50. The questions depicting the choice of the participants between modern or indigenous treatment for their own (questions Q25 and Q26) or for their relatives (Q29 and Q30) were formulated differently, and the means of the scores obtained from the belonging questions were used in the formula (formula in Supplementary Table 1).
Study’s Objectives
For further analysis, the participants were categorized into two groups: those with hemorrhoidal disease and those without. The primary objective was to determine the overall magnitude of KAP toward hemorrhoidal disease. The secondary objective was to assess the factors associated with self-reported hemorrhoidal disease. The third objective was to determine the effect of knowledge on attitudes and practices toward hemorrhoidal disease among participants.
Study Sample Calculation
Using a prevalence (P) of 13.1% of hemorrhoidal disease published by Kibret et al,13 with a margin of error ∆ of 5%, the sample size is given by the Wald formula: N=[4*Z21-α/2*P*(1-P)]/∆2.24 The estimated sample size was 700 participants.
Ethical Considerations
This study was conducted under the Declaration of Helsinki. The protocol, and the verbal consent method used to submit the questionnaire, were approved by the Ethic Committee of the Yopougon Teaching Hospital (Comité d’éthique de la Direction Médicale et Scientifique (DMS) du Centre Hospitalier et Universitaire de Yopougon. Registration number 001–23/MSHPCMU/CHUY/DMS/THO-K/DA). All the collected data were confidential and accessible through the participants’ requests.
Statistical Analysis
Categorical variables were described as numbers and percentages, whereas continuous variables were described as the means and standard deviations. The overall distributions of the magnitudes of the KAP scores were reported and compared between the baseline characteristic groups. The chi-square test or Fisher’s exact test was used to compare categorical variables, and the Student’s T test was used for continuous variables. The Cronbach coefficient was determined to assess the reliability and consistency of the questionnaire among surveyed participants and according to their belonging areas (urban and rural).25 The correlation between KAP scores was determined with the Spearman rho rank test. The sample proportions of self-reported hemorrhoidal disease or symptom-based hemorrhoidal disease with 95% confidence intervals were determined among participants via the binomial method.26 All independent variables that reached a p-value under 20% in the univariate analysis were included in the multivariate regression analysis. A step-by-step backward procedure was used.26
The multivariate logistic regression method was used to assess factors (age, sex, residency, BMI, remunerated activity, and education level) associated with self-reported or symptom-based hemorrhoidal disease.26 The influence of knowledge on attitudes or practices toward hemorrhoidal disease was determined via a multivariate linear regression model.26 The overall mean scores of attitudes or practices were the dependent variables in the linear regression model.26 Age, sex, residency, BMI, remunerated activity, education level, the presence of hemorrhoidal disease, and knowledge score were the independent variables forced in the linear multivariate regression models. All the statistical analyses were performed via IBM SPSS Statistics for Windows, version 27.0. (Armonk, NY: IBM Corp). and R statistical software (v4.1.2; R Core Team 2021), with a two-tailed significance level of less than 0.05.
Results
Description of Baseline Characteristics
A total of 761 participants completed the questionnaire. Among them, 26 (3.4%) answered incompletely. The remaining 735 (96.6%) participants (mean ±SD: age, 38.8 ± 13.6 years; men, 59.2%) were selected for further analysis. Among them, 530 (72.7%) and 199 (27.3%) were recruited from urban and rural areas, respectively. The selected participants reached the secondary and university levels, 33.1% and 31.7%, respectively, and 22.1% of them were not educated. The overall internal consistency of the questionnaire was high (Cronbach score: 0.75), which remained stable and acceptable when it was administered to participants living in urban (Cronbach score: 0.77) or rural areas (Cronbach score: 0.66). Table 1 presents the baseline characteristics of the participants included in the analysis. The primary symptoms of hemorrhoidal disease reported during the survey were anal pain (24.6%), anal bleeding (12.7%), anal prolapse (13.9%), and hemorrhoidal crisis (19.1%), which occurred frequently or often.
 |
Table 1 Baseline Characteristics of the Participants |
Factors Associated with Self-Reported Hemorrhoidal Disease Among the Participants
Based on self-declaration, 326 (44.4%) participants were considered having hemorrhoidal disease; among them, 305 (41.5%) spontaneously claimed that they suffered from hemorrhoidal disease, and 21 (2.9%) were selected among 163 who said “I do not know” but reported symptoms of hemorrhoidal disease consisting of anal prolapse during defecation with frequent or intermittent anal bleeding. They were older (mean age = 49.3 vs 37.1 years, p < 0.0001) than those without, and 52.5% of them were between 30 and 50 years old. Overall, participants with self-reported hemorrhoidal disease complained frequently or often with anal pain (46.6% vs 7.1%, p < 0.0001), anal bleeding (34.1 vs 3.7%, P < 0.0001), anal prolapse (28.5 vs 2.2%, p < 0.0001), and hemorrhoidal crisis (39.9 vs 2.7%, p < 0.0001) compared to those without hemorrhoidal disease (Table 1). The sample proportion of self-reported hemorrhoidal disease among the participants was 44.4% [95% CI: 41–48]. This sample proportion was similar between those living in urban (43% [95% CI: 38–47]) and rural areas (47% [95% CI: 40–54]) but slightly higher in men (47.3% [95% CI: 42.6–52.1]) than in women (39.8% [95% CI: 34.2–45.6], p = 0.051). In terms of self-declaration (Table 1), participants who were highly educated (university level) reported a lower frequency of hemorrhoidal disease than those who were not educated (p = 0.001). Based on a combination of reported acute signs (hemorrhoidal crisis) and chronic signs (anal prolapse) occurring frequently with or without anal bleeding, only 156 (21.2%) participants were considered to have hemorrhoidal disease, and 51.3% of them were aged between 30 and 50 years. The estimated sample proportion of symptom-based hemorrhoidal disease was 21.2% [95% CI: 18.4–24.4]. In the logistic multivariate analysis in which self-reported hemorrhoidal disease was used as an outcome, those aged between 30 and 50 years (p = 0.01) and male sex (p = 0.04) were mostly affected. However, self-reported or symptom-based hemorrhoidal disease was uncommon in highly educated participants (Table 2).
 |
Table 2 Logistic Regression of the Baseline Characteristics Associated with the Presence of Hemorrhoids Upon Self-Declaration or Symptom Basis Among Participants |
The Knowledge of the Participants About Hemorrhoidal Disease
The overall mean (SD) score of knowledge was 49.9 (34.4). The knowledge score remained nonsignificant regardless of gender or education level (Supplementary Table 2). There was no correlation between knowledge and attitudes or practices. However, participants with self-reported hemorrhoidal disease (p<0.001), aged over 30 years (p=0.01), having a remunerated activity (p<0.001) or living in an urban area (p<0.001) express higher scores of knowledge than those who have not (Table 1, Figure 1A, Supplementary Table 2). Overall, more than 50% of the participants acknowledged the location and symptoms of hemorrhoidal disease, and nearly 45% provided inaccurate responses or did not (Table 3).
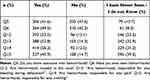 |
Table 3 Frequencies of the Responses to the Items Related to the Knowledge of the Participants Toward Hemorrhoids |
 |
Figure 1 Distribution of the KAP scores according to the residency of the participants (A) and the correlation between attitudes and practices (B). |
The Attitudes of the Participants Toward Hemorrhoidal Disease and Influencing Factors
The overall mean score (SD) of the attitudes of the participants toward hemorrhoidal disease was low: 43.4 (18.7). Attitudes were positively correlated with practices (rho = 0.66, p < 0.001; Figure 1B). Neither age nor gender was significantly associated with the attitudes of the participants toward hemorrhoidal disease (Supplementary Table 2). However, the participants living in rural areas (38.2 vs 45.4, p < 0.001, Figure 1A) or having a remunerated activity (41.8 vs 47.0, p < 0.0001) have presented low scores of attitudes compared with their counterparts (Supplementary Table 2). The mean score of attitudes was correlated with the education level, and the participants who had reached the secondary or university level expressed the highest scores of attitudes compared with those at the primary school level or who were not educated (Supplementary Table 2).
In the multivariate linear regression analysis (Table 4), the factors negatively associated with attitudes were self-reported hemorrhoidal disease (p = 0.02) and the remunerated activity (p < 0.001), and those positively associated with attitudes were age (p = 0.03), secondary (p < 0.001) or university level (p < 0.001).
 |
Table 4 The Effect of Knowledge on the Attitudes and Practices of the Participants Toward Hemorrhoids on the Basis of Self-Declaration: Results From Multivariate Linear Regression Analysis |
Table 5 shows the participant’s beliefs toward hemorrhoidal disease. Overall, 86.6% strongly agree or agree that hemorrhoidal disease interferes with eye diseases or is responsible for sexual infertility (67.1%) or sexual dysfunction (85.2%). They also strongly agree or agree that modern treatment of hemorrhoidal disease is dangerous (24.4%), surgical treatment of hemorrhoidal disease leads to infertility (51.1%), or sexual dysfunction (56.2%) and is not recommended (56.6%). They also think that traditional healers treat hemorrhoidal disease better than medical doctors (48.6%).
 |
Table 5 Frequencies of the Responses to the Items Related to the Attitudes of the Participants Toward Hemorrhoids |
Moreover, 56.5% of them strongly agree or agree that hemorrhoidal disease can be treated in hospitals. These beliefs involved participants with self-reported hemorrhoidal disease than those without, as hemorrhoidal disease interferes with eye diseases (90.8, 83.1, p = 0.002), is responsible for sexual dysfunction (89.3 vs 81.9%, p = 0.01), and traditional healers treat hemorrhoidal disease better than medical doctors do (53.1 vs 45, p = 0.03) (Figure 2A).
Practices of the Participants Toward Hemorrhoidal Disease and Influencing Factors
The overall mean score (SD) of the participant’s practices toward hemorrhoidal disease was low: 33.6 (21.7). The score of practices was similar among participants with or without self-reported hemorrhoidal disease (Table 1). The same trend of observations was found with the predictors such as gender and remunerated activity (Supplementary Table 2). In contrast, participants aged <30 years (p<0.03), living in urban areas (p < 0.001, Figure 1A), or highly educated (university level: p = 0.03) have presented high scores of practices compared with their counterparts (Supplementary Table 2). In the multivariate linear regression analysis (Table 4), living in an urban area was positively associated with practices (p < 0.001), whereas the participants with self-reported hemorrhoidal disease (p =0.05) or having a remunerated activity (p=0.01) exhibited negative practices toward hemorrhoidal disease. Overall, 29.1% of the participants claimed that they have consulted a traditional healer for hemorrhoidal disease, and 68.3% have not (Table 6). Furthermore, 52.4% of the participants preferred to be treated by a traditional healer when this option was highlighted (Q26), whereas 30.2% reported positive responses for modern treatment as the first option (Q25) to treat hemorrhoidal disease (Table 6).
 |
Table 6 Frequencies of the Responses to the Items Related to the Practices of the Participants Toward Hemorrhoids |
More precisely, when the participants claimed that they had hemorrhoidal disease, 40.4% of them had consulted a traditional healer for hemorrhoidal disease (Figure 1B), compared to those without. Or, they would prefer to be treated by a traditional healer (60.1 vs 46.2%, p < 0.001), contrasting with no preference for modern (31 vs 29.6, p = ns) or surgical treatment (15 vs 17.8%, p = ns). We found the same trend of responses for their relatives, with a significant preference for traditional (indigenous) treatment compared with modern treatment (83.2 vs 75.8, p = 0.04) (Figure 2B).
Discussion
Our study demonstrated that hemorrhoidal disease is frequently reported in Côte d’Ivoire both in rural and urban areas, affecting mainly men and those aged between 30 and 50 years. Overall, the level of knowledge, attitudes, or practices toward hemorrhoidal disease was low among participants and lower in rural compared to urban areas. Moreover, hemorrhoidal disease led to negative attitudes and practices. The effect was tempered by: the age, residency, and the participant’s education level. Finally, knowledge of hemorrhoidal disease did not affect the attitudes or practices of the participants in the multivariate analysis.
The prevalence of hemorrhoidal disease in the Western world ranges between 4.4 and 86% with specific patterns.2,3,27–29 Our findings from a population-based study found the same similarities with previous reports in sub-Saharan Africa contrasting with findings from the Western world.2,12–15
Hemorrhoidal disease is a challenging disease worldwide and is surrounded by taboos related to their anatomic location, beliefs, or misconceptions.16,30,31 In the Western world, the impact of hemorrhoidal disease on health-related quality of life has been intensively reported, revealing its negative effects on daily life and sexual activity.5,32,33 It is also correlated with psychological distress among this population.32 However, in sub-Saharan Africa, hemorrhoidal disease is implicitly related to sexual dysfunction and infertility embedded within local beliefs, with the perception of the ineffectiveness of modern treatment to cure hemorrhoidal disease mainly linked to the fear of surgery as a dangerous method in our study as reported elsewhere.16,34 Indeed, half of the participants in our study were worried about the surgery for hemorrhoidal disease probably related to the potential post-operative complications as perceived by more than 40% of the Taiwanese subjects.1,35 Our study also showed, in a formal way, the impact of these beliefs and perceptions on the willingness of the participants to seek medical care when they have suffered from hemorrhoidal disease and the reasons for the rejection of the modern treatment of hemorrhoidal disease. The rejection was not influenced by their knowledge, attitudes, or practices toward hemorrhoidal disease. However, our study revealed that the higher the score of attitudes the participants display, the higher the score of practices, suggesting that the participants who expressed fewer beliefs were mostly in favour of the modern treatment of hemorrhoidal disease. Indeed, the participants were mainly in favour of traditional treatment, as reported elsewhere in sub-Saharan Africa.34 The main explanation is the global perception of black Africans about the onset of a disease based on myths, beliefs, misconception, and ignorance as stated by the high frequency of erroneous or inaccurate responses quantifying the level of KAP toward hemorrhoidal disease in our study and previously reported in sub-Saharan Africa for other diseases.10,16,17 The belief most strongly expressed in our study was the fact that hemorrhoidal disease is responsible for eye diseases, sexual dysfunction, and infertility. This assertion is false. These beliefs were also found in Ghana, and Nigeria.16,19,34,36 Moreover, in Nigeria, authors report that in people’s minds regardless of their education levels, candies, sugar intake, or the consumption of red meat are the main causes of hemorrhoidal disease.16 These beliefs are perpetuated by traditional healers, probably for commercial purposes.36 However, it is well known that hemorrhoidal disease during the acute phase of a hemorrhoidal crisis may provoke sexual asthenia or sexual dissatisfaction that subjects may have considered as being a sexual dysfunction.32,33 These beliefs or perceptions are probably the main barriers to healthcare-seeking behaviour toward using modern treatment but favouring traditional medicine as reported by previous authors in sub-Saharan Africa and elsewhere.16,32–34 However, in our study, the participant’s opinion toward modern treatment was substantially high not for their purpose but for their family members, suggesting a shift toward Western medicine (61.2% of them favoured modern treatment for their family members). Most of the participants were reluctant to have surgery, probably related to myths accusing surgery as the main cause of sexual dysfunction or infertility, which was expressed by 50% of them in our study, regardless of their socioeconomic background. This fact was suggested by the negative correlation of a remunerated activity with the attitudes and practices of the participants in our study. However, sexual activity impairment has been reported in women, whereas improvements have been reported in men after haemorrhoidectomy, clearly demonstrating that the question of whether surgical treatment of hemorrhoidal disease may have an impact on sexual activity has not been fully answered.37,38
This study has several limitations. First were the caveats related to the translation of the questionnaire of a KAP survey into local languages in the African community. Since the questionnaire items were translated into approximately seven local languages during the survey, the meaning of these items may have been jeopardized.22 Second, the presence of hemorrhoidal disease was suggested based on self-reported data without clinical examination. Furthermore, we did not use patient-reported outcome measure instruments to ascertain the severity of the hemorrhoidal disease and its impact on the participant’s quality of life which could have probably influenced their perception.32,39 Moreover, we used a convenience sample from which the external validity is limited.23 However, the questionnaire was sufficiently reliable, suggesting that our findings could reflect how people think and behave toward hemorrhoidal disease in urban and rural areas in West Africa.
Furthermore, our study suggests that, regardless of their knowledge, the participant willingness to modern treatment of hemorrhoidal disease was strongly influenced by their beliefs and misconceptions related to hemorrhoidal disease. Our study emphasizes the need for intensive communication favouring modern treatment at the onset of symptoms suggesting hemorrhoidal disease. Moreover, intensive collaboration with traditional healers is needed to ensure good practice and patient referral with a suspected hemorrhoidal disease when the alleged symptoms persist under traditional treatment.
Conclusion
Our study, which is based on self-reported data, suggests that hemorrhoidal disease is frequently encountered in Côte d’Ivoire and is surrounded by myths and misconceptions in the general population. Traditional medicine remains the main therapy option suggesting massive communication and sensitization of subjects to modern medicine.
Further studies are needed to determine the impact of hemorrhoidal disease progression on the perception and behaviour of subjects in sub-Saharan Africa.
Acknowledgments
We want to thank all the participants who gave their consent for the study and the surveyors who surveyed participants in cities and villages cited in the manuscript, particularly Mrs. Koba Charlaine (KC), Konan Ruth Esther (KRE), Souwene Kpaquis Danielle (SKD), Loukou Nguessan Patricia (LNP), and Mr Koulibaly Zoumana (KZ). We would also like to thank Halle Ekane Mesode Arlene Nancy to have corrected the English transcription of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jacobs D. Hemorrhoids. N Engl J Med. 2014;371(10):944–951. doi:10.1056/NEJMcp1204188
2. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 1990;98(2):380–386. doi:10.1016/0016-5085(90)90828-O
3. Sheikh P, Régnier C, Goron F, Salmat G. The prevalence, characteristics and treatment of hemorrhoidal disease: results of an international web-based survey. J Comp Eff Res. 2020;9(17):1219–1232. doi:10.2217/cer-2020-0159
4. Yang JY, Peery AF, Lund JL, Pate V, Sandler RS. Burden and cost of outpatient hemorrhoids in the United States employer-insured population, 2014. Am J Gastroenterol. 2019;114(5):798–803. doi:10.14309/ajg.0000000000000143
5. Rørvik HD, Davidsen M, Gierløff MC, Brandstrup B, Olaison G. Quality of life in patients with hemorrhoidal disease. Surg Open Sci. 2023;12:22–28. doi:10.1016/j.sopen.2023.02.004
6. Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum. 2018;61(3):284–292. doi:10.1097/DCR.0000000000001030
7. Gurland BH, Merlino J, Sobol T, et al. Surgical complications impact patient perception of hospital care. J Am Coll Surg. 2013;217(5):843–849. doi:10.1016/j.jamcollsurg.2013.06.015
8. Lisa MV, Farrah J, Raymond CB. Cultural health attributions, beliefs, and practices: effects on healthcare and medical education. Open Med Educ J. 2009;2(1):64–74. doi:10.2174/1876519X00902010064
9. Kreps GL, Ruben BD, Baker MW, Rosenthal SR. Survey of public knowledge about digestive health and diseases: implications for health education. Public Health Rep. 1987;102(3):270–277.
10. Kahissay MH, Fenta TG, Boon H. Beliefs and perception of ill-health causation: a sociocultural qualitative study in rural North-Eastern Ethiopia. BMC Public Health. 2017;17(1):124. doi:10.1186/s12889-017-4052-y
11. Idriss A, Diaconu K, Zou G, Senesi R, Wurie H, Witter S. Rural–urban health-seeking behaviours for non-communicable diseases in Sierra Leone. BMJ Global Health. 2020;5:e002024. doi:10.1136/bmjgh-2019-002024
12. Iwatt AR. Epidemiology of haemorrhoids in the adult Nigerian and English: a comparative study. Centr Afr J Med. 1987;33(3):61–66.
13. Kibret AA, Oumer M, Moges AM. Prevalence and associated factors of hemorrhoids among adult patients visiting the surgical outpatient department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS One. 2021;16(4):e0249736. doi:10.1371/journal.pone.0249736
14. Sehonou J, Wanvoegbe AF, Kpossou RA, et al. Hemorrhoidal disease in Cotonou: epidemiological, clinical and anuscopic aspects. Open J Gastroenterol. 2015;5(7):77–82. doi:10.4236/ojgas.2015.57013
15. Okafor CH, Okafor NJ, Okafor IR. Hemorrhoids among people of Sifawa community in Sokoto state. J Fam Med Health Care. 2023;9(2):28–33.
16. Azeez A, Isiugo-Abanihe UC. Sociocultural context and determinants of treatments for hemorrhoids among the Nigeria police force, Oyo state command. J Soc Behav Health Sci. 2017;11(1):31–46. doi:10.5590/JSBHS.2017.11.1.02
17. Sarmah N, Sibiya MN, Khoza TE. The sociocultural influences on breast cancer screening among rural African women in South Africa. Int J Environ Res Public Health. 2023;20(21):7005. doi:10.3390/ijerph20217005
18. Ray-Offor E, Amadi S. Hemorrhoidal disease: predilection sites, pattern of presentation, and treatment. Ann Afr Med. 2019;18(1):12–16. doi:10.4103/aam.aam_4_18
19. Ntim-Ampomsah CT, Amoaku Winfried MK, Ofosu-Amaah S. Awareness and knowledge of glaucoma and other diseases associated with blindness in a Ghanaian community. Nigerian J Ophtalmol. 2004;12(4):50–54.
20. Osinaike BB, Dairo MD, Oyebamiji EO, Odesanya JO, Tanimowo A. Attitude of general public to risks associated with anaesthesia. East Afr J Public Health. 2007;4(1):40–42.
21. Andrade C, Menon V, Ameen S, Kumar Praharaj SK. Designing and conducting knowledge, attitude, and practice surveys in psychiatry: practical guidance. Indian J Psychol Med. 2020;42(5):478–481. doi:10.1177/0253717620946111
22. Launiala A. How much can a KAP survey tell us about people’s knowledge, attitudes and practices? Some observations from medical anthropology research on malaria in pregnancy in Malawi. Antropol, Matters J. 2009;11(1):1–13.
23. Andrade C. The inconvenient truth about convenience and purposive sample. Indian J Psychol Med. 2021;43(1):86–88. doi:10.1177/0253717620977000
24. Gonçalves L, de Oliveira MR, Pascoal C, Pires A. Sample size for estimating a binomial proportion: comparison of different methods. J Appl Stat. 2012;39(11):2453–2473. doi:10.1080/02664763.2012.713919
25. Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi:10.5116/ijme.4dfb.8dfd
26. Newman SC. Biostatistical Methods in Epidemiology. John Wiley & Sons; 2003.
27. Riss S, Weiser FA, Schwameis K, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27(2):215–220. doi:10.1007/s00384-011-1316-3
28. Carter D, Gabel MB, Zbar A, Segev S, Kopylov U. Prevalence and clinical associations of hemorrhoids at screening colonoscopy. World J Colorect Surg. 2013;3(2):1–13.
29. Haas P, Haas G, Schmaltz S, Fox TA. Prevalence of hemorrhoids. Dis Col Rec. 1985;26:435–439. doi:10.1007/BF02556521
30. Tse GN. Practical management of hemorrhoids: myths, pitfalls and plain sailing. Can Fam Physician. 1988;34:655–659.
31. Altomare DF, Rinaldi M, La Torre F, et al. Red hot chili pepper and hemorrhoids: the explosion of a myth: results of a prospective, randomized, placebo-controlled, crossover trial. Dis Colon Rectum. 2006;49(7):1018–1023. doi:10.1007/s10350-006-0532-3
32. Abramowitz L, Bouchard D, Siproudhis L, et al. Psychometric properties of a questionnaire (HEMO-FISS-QoL) to evaluate the burden associated with haemorrhoidal disease and anal fissures. Colorectal Dis. 2019;21(1):48–58. doi:10.1111/codi.14393
33. Keller JJ, Lin HC. Haemorrhoids are associated with erectile dysfunction: a population-based study. Int J Androl. 2012;35(6):867–872. doi:10.1111/j.1365-2605.2012.01292.x
34. Ayuk CO, Mgbenkemdi EH. Traditional medicine and its psychological effectiveness in the management of terminal diseases in Igbo land Nigeria. Int J Med Sci Appl Biosc. 2020;5(1):8–22.
35. Pin-Chun C, Chih-I C. Exploring factors impacting patient decisions in hemorrhoids surgery: a questionnaire survey in Taiwan. Surg Open Sci. 2024;20:214–221. doi:10.1016/j.sopen.2024.07.009
36. Azeez A, Isiugo-Abanihe U. Disease diagnosis and management: the experiences of hemorrhoid herbal vendors and customers in Oyo state, Nigeria. J Soc Behav Health Sci. 2019;13(1):82–97. doi:10.5590/JSBHS.2019.13.1.05
37. Lin YH, Stocker J, Liu KW, Chen HP. The impact of hemorrhoidectomy on sexual function in women: a preliminary study. Int J Impot Res. 2009;21(6):343–347. doi:10.1038/ijir.2009.37
38. Abdelaziz AS, Ghoneem AM, Elewesy EA. The impact of surgical hemorrhoidectomy on male sexual function: a preliminary study. Urol Ann. 2019;11(3):235–240. doi:10.4103/UA.UA_138_18
39. Xia W, Connoly A, Hill AG. Symptom-based scoring for haemorrhoidal disease: a systematic review. Colorectal Dis. 2020;22(11):1518–1527. doi:10.1111/codi.15253
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


