Back to Journals » Journal of Pain Research » Volume 18
The Placebo Hypoalgesic Response Is Reduced in Healthy Older Adults Showing a Decline in Executive Functioning
Authors Rischer KM, Dierolf AM, Anton F , Montoya P , González-Roldán AM, van der Meulen M
Received 9 September 2024
Accepted for publication 11 February 2025
Published 1 April 2025 Volume 2025:18 Pages 1747—1763
DOI https://doi.org/10.2147/JPR.S488198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kushang V Patel
Katharina M Rischer,1 Angelika M Dierolf,1 Fernand Anton,1 Pedro Montoya,2 Ana M González-Roldán,2 Marian van der Meulen1
1Department of Behavioural and Cognitive Sciences, Institute for Health and Behaviour, University of Luxembourg, Esch-sur-Alzette, Luxembourg; 2Cognitive and Affective Neuroscience and Clinical Psychology, Research Institute of Health Sciences, Balearic Islands Health Research Institute, University of the Balearic Islands, Palma, Spain
Correspondence: Marian van der Meulen, University of Luxembourg, Maison des Sciences Humaines, 11 Porte des Sciences, Esch-sur-Alzette, L-4366, Luxembourg, Email [email protected]
Purpose: Aging is accompanied by various changes in pain perception and modulation. However, the influence of older age – and associated neurocognitive changes – on placebo hypoalgesia has not been systematically investigated. Findings to date are inconclusive, ranging from a reduced, to a preserved or even an amplified response in older adults. The aim of this study was to examine age-related changes in the placebo hypoalgesic response magnitude, and the potential modulating effect of executive functions, namely working memory, cognitive flexibility and inhibitory control.
Methods: Thirty-nine younger (18– 36 years) and 42 healthy older adults (60– 82 years) completed a series of executive functioning tests. Placebo hypoalgesic responding was assessed via a sham transcutaneous electrical nerve stimulation (TENS) intervention while participants received moderately painful electrical stimuli to their arm. An electroencephalogram (EEG) was recorded during the placebo paradigm and pain ratings were collected.
Results: Overall, both age groups showed similar robust placebo hypoalgesic effects: pain ratings and pain-related brain potentials were significantly reduced in response to the sham treatment. Interestingly, worse executive functions in older adults (in particular, working memory and cognitive flexibility) were associated with reduced placebo responses. Moreover, executive functions also moderated the overall age group difference in placebo hypoalgesia: when cognitive flexibility and inhibitory control scores were low, older adults showed a smaller placebo response than younger adults.
Conclusion: We demonstrated an age-related reduction in placebo hypoalgesia in older adults showing a decline in executive functioning. This is an important finding, considering the fact that placebo effects contribute to positive treatment outcomes. Our results advocate the assessment of executive functions when investigating the influence of aging on placebo effects, as variable aging trajectories of decline may influence overall group comparisons.
Plain Language Summary: As people age, their pain experience and pain coping skills change. This study explores how aging, and related changes in brain function, affect the ability to experience pain relief from a placebo treatment (placebo hypoalgesia). Previous research has been unclear, showing reduced, unchanged, or even increased placebo responses in older adults. We investigated if aging affects the strength of the placebo effect and whether executive functions (like working memory, flexible thinking, ignoring distractions and controlling impulsive reactions) play a role.
We studied 39 younger adults (18-36 years) and 42 older adults (60-82 years). They took tests measuring their executive functions and then received a fake electrical nerve stimulation treatment while experiencing moderately painful electrical stimuli on their arm. We recorded their brain activity and pain ratings.
Both younger and older adults reported less pain and showed reduced brain responses to pain after the fake treatment, indicating a clear placebo effect in both groups. Older adults with better executive functions, particularly in working memory and cognitive flexibility, had stronger placebo responses. Moreover, when older adults had good cognitive flexibility and inhibitory control, their placebo responses were as large as those of younger adults.
In conclusion, when executive functions are intact, normal placebo pain relief can be experienced in older age. This finding is relevant because placebo effects contribute considerably to treatment outcomes. Our results suggest that assessing a potential decline in executive functions is important when studying the impact of aging on pain (coping).
Keywords: cognitive pain modulation, aging, executive functions, event-related potentials, prefrontal cortex
Introduction
In recent years, the mechanisms underlying placebo hypoalgesia have received increasing attention, leading to the identification of numerous psychological, neurobiological and contextual variables that influence the magnitude of the placebo effect at the behavioral and neural level (see1,2 for reviews). One variable of interest is age, since aging has been associated with various changes in pain perception and modulation. Older adults, for example, often show increased acute pain thresholds and impaired top-down pain regulation, due to altered structure and function of peripheral and central nociceptive pathways.3–6 However, to date, the influence of older age on placebo effects has not been systematically investigated, and the few studies addressing the issue have ranged from observing a reduced,7 to a preserved,8,9 or even to an amplified response in older adults,10 as compared to young adults.
A possible explanation for these divergent findings could be variable aging trajectories of decline in brain areas and cognitive skills that mediate the placebo effect. The prefrontal cortex (PFC) is a prime candidate here, given its importance in placebo hypoalgesia,11–15 as well as its vulnerability to age-related atrophy and functional changes.16–18 Inhibiting the dorsolateral PFC with repetitive transcranial magnetic stimulation has, for example, been shown to disrupt placebo hypoalgesia19 whereas active transcranial direct current stimulation of the dorsolateral PFC boosts placebo hypoalgesia.20,21 Others have reported a correlation between the placebo hypoalgesic response and PFC functional connectivity,22 as well as medial PFC activity,23 in patients with (early) Alzheimer’s disease.
The PFC is also the central brain region mediating executive functioning,24,25 and executive functions may be involved in the placebo hypoalgesic response in several ways. Two core executive functions, namely working memory and cognitive flexibility, allow us to maintain and manipulate beliefs and expectations,26 which in turn drive the placebo effect.27,28 A third core executive function, inhibitory control, may promote placebo responding through its link to emotion regulation.29 Emotion and pain share a common regulation system30,31 and efficient emotion regulation may enhance placebo hypoalgesia through the reduction of pain-related anxiety and fear.32,33 In addition, both emotion regulation and placebo hypoalgesia involve cognitive (re)appraisal processes.34 Previous research has also demonstrated that inhibitory control is directly related to the efficiency of other forms of endogenous pain inhibition.35–38
Effective executive functioning should thus facilitate placebo hypoalgesia through several mechanisms, and some studies have indeed shown that individuals with impaired executive functioning (due to Alzheimer’s Disease) show weaker placebo hypoalgesic effects.22,23 More generally, poorer executive functioning has also been demonstrated in patients with chronic pain conditions and is associated with higher experimental pain sensitivity and impaired top-down pain modulation.35–42 Consistent with the vulnerability of the PFC to age-related structural and functional changes, executive functions have also been shown to decline with age, including working memory, cognitive flexibility, and inhibitory control.43,44 However, the extent and rate of decline in executive functions can vary widely among older individuals, influenced by various biological, health and lifestyle factors.45
The objective of the present study was to assess the role of age-related changes in executive functions in modulating placebo hypoalgesia in healthy aging adults, as a potential explanation for the conflicting findings regarding these effects in older adults. This question holds significant clinical relevance, considering the well-established contribution of placebo effects to analgesic treatment outcomes.46 We administered a battery of executive functioning tests to a group of healthy young and older adults, followed by a placebo hypoalgesia paradigm involving a sham treatment with transcutaneous electrical nerve stimulation (TENS). We measured the placebo effect both through pain ratings and electroencephalography (EEG), by assessing the N2/P2 complex peak-to-peak and the P3 peak amplitudes.47,48 We hypothesized that better executive functioning would be associated with a greater (behavioral and electrophysiological) placebo hypoalgesic response. We also expected that an age-related decrease in placebo hypoalgesia would be apparent only in those older adults showing a stronger decline in executive functioning (ie, we expected the relationship between age and placebo hypoalgesia to be moderated by executive functioning).
Materials and Methods
Participants
Ninety-five participants, including 48 younger adults and 47 older adults, were recruited for the study. Participants were partly recruited through re-invitations from a previous study on age-related changes in distraction from pain38 (9 younger and 15 older adults). All participants were recruited through paper and online advertisement at the University of Luxembourg, targeting regular as well as senior students. Older participants were further recruited through public presentations at institutions for senior citizens in Luxembourg and interviews in local media (radio and newspapers).
Exclusion criteria were assessed in both young and older participants, and included a current diagnosis of chronic pain or a psychiatric or neurological condition (eg, neuropathy) as well as substantial cognitive impairment as established with the Mini Mental State Examination (with a cut-off score ≤ 2449). Participants were requested not to take any pain medication or other drugs known to have an impact on sensory perception or cognition on the day of the experimental sessions. Eight young adults dropped out after inclusion and one young and two older participants were excluded due to a diagnosis of chronic pain. Finally, the data of three older participants could not be analyzed due to technical issues with the pain stimulation or excessive noise in the EEG data.
Thus, the data of 39 younger adults (22 female; mean age = 23.87, SD = 4.26; age range: 18–36 years) and 42 older adults (22 female; mean age = 67.80, SD = 5.70; age range: 60–82 years) were included in the final analyses. Participants received a compensation of between €35 or €55 for their time and effort (participants who were re-recruited from the previous study already completed the executive tests and thus received a lower compensation). In addition, all participants had the chance to win a gift voucher worth €80 in a prize draw and could request a report on their performance in the executive tests. Ethical approval was obtained from the Ethics Review Panel of the University of Luxembourg, and the study complies with the Declaration of Helsinki. All participants gave their informed consent at the start of the study. Participants were only debriefed about the real aim of the study after data collection was finished.
Procedure
Participants were invited to two test sessions; during the first session they filled out several questionnaires and we assessed executive functions, and during the second session we administered the placebo paradigm while recording an EEG. The first session lasted on average between 1.5–2 hours, and the second session between 3.5–4.5 hours. Participants could complete the experiment either in German (71.6%), English (19.8%) or French (8.6%).
Questionnaires
Participants completed a demographic questionnaire about their health status, education level, pain history and previous experience with TENS. We also obtained information about their handedness (Edinburgh Handedness Inventory; EHI50), their level of emotional distress (DASS-4251), and pain-related cognitions, ie, fear of pain (FPQ-III52), pain catastrophizing (PCS53), pain vigilance and awareness (PVAQ54) and attitudes to pain (PAQ-r55). Finally, we administered the Mini Mental State Examination (MMSE56) to assess general cognitive status.
Validated versions for all three official languages spoken at the University of Luxembourg (English, German, French) were available for the EHI,57 DASS-42,58,59 PCS,60,61 PVAQ62 and MMSE.63,64 The French version of the FPQ-III and PAQ-r were translated by a native French speaker and partly adapted from a validated short version.65 The French version of the PVAQ66 was based on a 5-point scale and scores were later transformed to a 6-point scale, to be compatible with the other language versions. The German version of the PAQ-r was translated by German native speakers.
Executive Functioning Tasks
Participants completed five standard executive tests, namely the Digit Span Test from the WAIS-IV,67 the Trail Making Test (TMT68), the Stroop Color-Word Test-Victoria version,69 the Attentional Network Test (ANT) and a speeded Go/NoGo test. These tests were specifically selected to comprise the assessment of relevant core components of executive functioning, namely working memory (Digit Span), cognitive flexibility (TMT), and, given our previous results,37,38 a special focus on inhibitory control (ANT, Stroop and Go/NoGo). The Digit Span, TMT and Stroop test were paper-and-pen versions, while the ANT and Go/NoGo were programmed in E-Prime 2.0. Validated versions of all tasks in all three languages (German, English, and French) were available.
Digit Span Test
Subtests of the Digit Span Test are the Digit Span Forward, Backward and Sequencing. Here, we only report the Sequencing subtest, as this test places the highest demand on working memory.67
Trail Making Test
For the TMT, we computed the TMT difference score by subtracting the time needed to complete part A from the time needed to complete part B. Part A of the TMT presumably reflects psychomotor speed and attention, while part B has been established as a measure of central executive processes, including cognitive flexibility, task-set inhibition, as well as the ability to maintain a response set.70,71 The difference score (B-A) is commonly used as indicator of cognitive flexibility controlled for psychomotor speed and attention.72 A smaller difference score reflects better cognitive flexibility.
Stroop Color-Word Test – Victoria Version
To assess the Stroop interference effect, we subtracted the time needed to name colors in the interference condition (color words) from the time needed to name colors in the control condition (colors).69 A smaller Stroop interference effect reflects better response inhibition abilities.26,73
Attentional Network Test
The ANT combines the Posner spatial cuing task with the Eriksen flanker task. The present version of the ANT was adapted from Fan et al74 (A full description of the ANT can be found in the Supplementary Materials). The ANT assesses three attentional networks (alerting, executive control and orienting) of which we report only the executive control network. This network is involved in the resolution of conflicting responses.74 The processing efficiency of the executive control network was assessed by computing difference scores in the reaction time for incongruent minus congruent flankers.74,75 Difference scores were based on the median reaction time for correct trials with a minimum response time of 100 ms. In order to account for age-related slowing, difference scores were based on adjusted RTs (by computing z-score transformations for each participant as suggested by Jennings et al75). A lower score indicates better inhibitory control abilities.
Go/NoGo Task
The Go/NoGo task assesses prepotent response inhibition ability. In the present version of the task, participants were presented with a series of numbers (odd and even) and required to respond with a button press to “go” stimuli (either odd or even numbers) and to withhold their response to the “nogo” stimuli (either even or odd numbers). Because the ratio of “go” and “nogo” stimuli was skewed (30% “nogo” and 70% “go” stimuli), participants had to inhibit the tendency to respond to “nogo” stimuli. (A full description of the Go/NoGo task can be found in the Supplementary Materials). We calculated the percentage of responses to “nogo” stimuli (commission errors) as an index for response inhibition. To account for speed-accuracy trade-offs and age-related slowing, we corrected this for the average reaction time for “go” stimuli, by computing unstandardized residuals from commission errors regressed on reaction times. Lower scores indicate better response inhibition (free of reaction time effects).
Placebo Paradigm
Placebo Induction
Placebo hypoalgesia was induced using sham transcutaneous electrical nerve stimulation (TENS). Participants were told that they were taking part in a study about the effects of aging on brain responses to a treatment with TENS. They were furthermore told that, to establish a baseline, they would receive painful stimuli without TENS treatment. In reality, the device had been disabled so that, at no point during the experiment, TENS stimulation could be received. To increase credibility of the manipulation, participants were told that the TENS device would deliver a low-level current at a very high frequency that is imperceptible.
To induce pain, a concentric surface electrode was attached to the inner side of the participants’ arm (see description below). For the sham TENS stimulation, two adhesive patches were placed adjacent to the pain stimulation electrode and connected to the TENS device (KRES100B, HCS Electronics, China). Participants were told that they would receive moderately painful pulses through the pain stimulation electrode, but that the TENS device would reduce their level of pain when turned on.
Calibration Phase
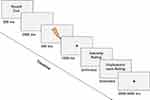
The placebo paradigm was divided into two runs with a short break in between. Each run consisted of a calibration phase followed by a conditioning and a test phase (see Figure 1). An automated calibration procedure was conducted to derive individually adapted electrical stimulus intensities that reliably evoked mild, moderate and strong pain. To this end, participants were asked to rate the intensity of a series of stimuli on a 100-point visual analogue scale, divided into the following sections (0–25: no pain; 25–50: mild pain; 50–75: moderate pain; 75–100: strong pain). The scale was a continuous scale but to facilitate its interpretation it was divided into four equally long and distinctly labeled and colored sections (green: no pain, yellow: mild, orange: moderate, red: strong) (see Supplementary Materials and Figure S1). Participants were told that the border between the green and yellow section marked the pain threshold. Target intensity ratings were VAS = 37.5 for mild pain; VAS = 62.5 for moderate pain and VAS = 87.5 for strong pain. (A detailed description of the calibration procedure can be found in the Supplementary Materials and Figure S2 schematically represents the calibration algorithm).
Conditioning and Test Phase
The placebo paradigm was divided into two runs, each containing 10 blocks. In each block, five electrical pulses (trials) were administered. The first two blocks of each run consisted of a conditioning procedure that has been shown to induce robust placebo hypoalgesic effects.34,76 The placebo effect was tested in the remaining eight test blocks in each run. At the start of each block, participants were informed whether the TENS device was turned on or off for this block by a visual cue on screen (“TENS ON” or “TENS OFF”). In addition, each electrical stimulus was preceded by a sound cue specific to each condition (ie, two beep tones with different pitches, and the association between sound and condition was counterbalanced between participants).
Participants were informed that they would receive moderately painful stimuli throughout the procedure. However, unbeknownst to them, in the conditioning procedure, mildly painful stimuli were administered in the “TENS ON” block, and strongly painful stimuli in the “TENS OFF” block, to help raise expectations of pain relief in the subsequent test blocks. In the eight test phase blocks, moderately painful stimuli were administered while participants were again told that the TENS device was alternatingly turned on (placebo condition) and off (control condition). Any difference in response to the painful stimuli between these two conditions can be attributed to the expectation of pain relief.
Presenting the placebo and control conditions in brief, alternating blocks ensures that any potential habituation or sensitization effects to the electric stimulation are unlikely to influence the placebo effect as they would either lead to a general reduction or increase in pain ratings, or the corresponding electrophysiological response, in both conditions. The order of the blocks (control condition first or placebo condition first) was counterbalanced between participants. Expectations of pain relief in the test phase were reinforced by replacing the last stimulus in the first and third placebo and control blocks with mildly and strongly painful stimuli, respectively. Responses to these reinforcement stimuli were not analyzed.
In total, 80 trials were presented in the test phase of the placebo paradigm (2 runs with 8 test blocks and 5 trials each), of which 36 trials per condition (40 minus 4 reinforcement trials) were analyzed. After each trial, participants were asked to rate the intensity and unpleasantness of the stimulus on two 100-point visual analogue scales (identical to the scale used during the calibration). Labels used for the intensity and unpleasantness scale were the same; only the headers presented above the scale (“Pain intensity” and “Unpleasantness”) differed. In each of the two runs, participants could take a short break in the middle (4th block) and in the end.
Trial Timeline
Each block started with displaying the information whether the TENS device was turned on (“TENS ON”) or off (“TENS OFF”) on the screen. In addition, each trial started with an auditory anticipation cue (500 ms), indicating whether the TENS device was turned on or off. During the anticipation phase (3500 ms), participants viewed a black screen. This was followed by the electrical stimulation (500 ms) and after an interval of 2000 ms (black screen with fixation cross), participants rated the intensity and unpleasantness of the stimulus without time limit. If participants needed less than 2500 ms for their rating, a blank interval (default: 500 ms) between the scales was increased by the remaining time. After an inter-trial interval between 2000–3000 ms, the next trial started, resulting in a minimum trial duration of 13000 ms (see Figure 2).
Pain Stimulation
Electrical stimuli were 500 ms long pulse trains that were administered using a concentric surface electrode with a platinum pin (Brainbox Ltd., Cardiff, UK) using a custom-made stimulus generator (Fa. Curio Medizinelektronik, Bonn, Germany). Each pulse train consisted of 50 10-ms biphasic square-wave pulses (positive voltage: 2 ms, negative voltage: 2ms, interval between pulse onsets: 6 ms), corresponding to a frequency of 100 Hz. The current range was set to 10 mA. Depending on skin impedance, the compliance voltage could be increased to a maximum of 70V to achieve the necessary level of pain. The electrode was attached to the volar surface of the left arm (at a distance of 60% from the wrist to the elbow) using medical tape. Before application of the electrode, the experimenter abraded the cleaned skin with a peeling and subsequently applied a moisturizing gel; any remains of the gel were removed immediately before the sensory calibration.
Electroencephalography Recording and Data Reduction
Participants were seated in a reclined chair (ca. 140 cm from the screen) in a light-attenuated and electrically shielded EEG chamber. Data were acquired with BrainAmp amplifiers (BrainVision Recorder; Brain Products GmbH, Gilching, Germany) at a sampling rate of 1000 Hz. EEG signals were recorded using a 64-channel standard BrainCap-MR with Ag/AgCl electrodes placed according to the international 10/20 system and an impedance below 10 kΩ (EASYCAP GmbH, Herrsching, Germany). AFz served as ground electrode and FCz as online reference. Additional electrodes were placed approximately 1 cm above and below the right eye and 1 cm left and right of each eye to record vertical and horizontal electrooculograms. We also recorded an electrocardiogram and electromyogram to assess potential startle and nociceptive flexion reflexes (data not reported here).
Off-line analysis was performed in BrainVision Analyzer 2 (Brain Products GmbH, Gilching, Germany). Continuous EEG data were re-referenced to the linked mastoids (TP9, TP10) and a 50 Hz notch filter was applied, as well as zero phase shift Butterworth low-pass filter with a high cutoff (half-power point) of 27 Hz (order 8). In a first step, we screened the data for major (non-ocular) artifacts, ie, voltage steps above 100 µV/s and voltage differences above 400 µV over 200 ms intervals, and activity below 0.5 µV over 100 ms intervals. In a second step, ocular artifacts were detected using semi-automatic ocular correction independent component analysis (ICA), with a meaned slope restricted infomax algorithm (ICA training continued until the modifications made to the matrices were below 1e−07, or until the maximum of 512 steps was reached). ICA components with a relative VEOG/HEOG variance over 30% were automatically marked and reviewed before removal.
In four participants, one channel (AF3, AF7, FP1 and PO8, respectively) was removed prior to running ICA due to excessive noise and interpolated after ICA using spherical splines (order of splines = 4; maximal degree of Legendre polynomials = 10; λ = 1e−5). The EEG data were segmented into epochs baseline-corrected around the pain stimulus, beginning 200 ms prior to and until 800 ms after the onset of the electrical pulse, and an additional semiautomatic artifact rejection was applied to the segmented data. The algorithm scanned for voltage steps above 50 µV/ms, voltage differences above 150 µV over 200 ms intervals, and activity below 0.5 µV over 100 ms intervals. Results were manually verified and corrected, if necessary. Subsequently, epochs were averaged for each participant and each condition. For younger adults, we included an average of 32.90 epochs in the control condition and 33.05 in the placebo condition, and for the older adults we included on average 32.43 epochs in the control condition and 32.67 in the placebo condition.
We extracted peak amplitudes for three pain-evoked event-related potentials (ERPs), ie, the N2, P2 and P3, using semi-automatic peak detection. While the N2 and P2 are considered to reflect changes in perceived pain intensity,47,76–78 the P3 (which may partly overlap with the P248) has been related to cognitive and affective processes, eg, stimulus evaluation.79 Search intervals for the respective ERP amplitudes were determined based on previous studies and confirmed by visual inspection of the grand average; all automatically detected peaks were reviewed and if necessary, manually adjusted to account for heterogeneity in peak latencies. In line with previous reports on nociceptive stimulation,80 inspection of the grand average showed two distinct peaks for the P2 in older adults; therefore, search intervals for the P2 were split in an early interval (P2a) and a late interval (P2b) for all participants, but for all subsequent analyses, we focused only on the P2a peak. Search intervals were defined at Cz (N2: 150 to 200 ms; P2a: 250 to 270 ms; P2b: 270 to 300 ms) and CPz (P3: 300 to 400 ms).76,77,81–83 The P3 was evaluated at three electrode sites (Cz, CPz, Pz) which was added as within-subject factor as the topography indicated a broad distribution.
Statistical Analyses
Statistical analyses were performed using SPSS 26 (IBM SPSS Statistics). Group differences in pain-related cognitions were assessed with 2-tailed independent sample t-tests, and group differences in executive functions were assessed with 1-tailed independent sample t-tests (as we either expected no difference or reduced performance in older adults).
The behavioral and electrophysiological placebo effects were assessed with separate repeated measures ANOVAs with the within-subject factors condition (placebo vs control) and run (run 1 vs run 2), and the between-subject factor age group (young adults vs older adults). Runs were added as within-subject factor to account for potential differences in pain perception due to the re-calibration in-between run 1 and 2. For the behavioral placebo effect, average intensity and unpleasantness ratings were evaluated. For the electrophysiological placebo effect, the difference in peak-to-peak amplitude between the N2 and P2a at electrode site Cz, and the P3 amplitude were evaluated.47,48 The P3 was evaluated at three electrode sites (Cz, CPz, Pz) as the topography indicated a broad distribution (added as an additional within-subject factor). All assumptions for a repeated measures ANOVA were checked. (Tests of normality can be found in the Supplementary Materials, Table S1 and S2).
We furthermore created a behavioral placebo effect score for intensity (PE-I) and unpleasantness ratings (PE-U) by computing the difference in VAS scores for test stimuli in the control and placebo condition (control – placebo) for each participant. Electrophysiological placebo effect scores were computed for the N2/P2a peak-to-peak amplitude by subtracting the amplitude values for the placebo condition from the control condition for each participant. A composite electrophysiological placebo effect measure for the P3 peak amplitude was computed by averaging the activity at all three electrode sites (Cz, CPz, Pz) and subtracting the peak amplitude for the placebo condition from the control condition. These scores were used for all subsequent analyses.
To investigate the relationship between executive functions and the placebo response, we ran a linear regression model with all five executive functioning test scores as standardized independent variables. We did this for the behavioral placebo effect on the intensity scale (PE-I) and for the electrophysiological placebo effect (N2/P2a complex and P3). PE-U was not assessed as this score correlated strongly with PE-I (r(81) = 0.666, p < 0.001). Bias-corrected and accelerated 95% confidence intervals were computed using bootstrapping (1000 samples).
Finally, to test whether executive functions moderated the influence of age on placebo hypoalgesia, we ran separate moderation analyses in PROCESS v.3.584 one for each executive function score as moderator, and with age group as independent variable, using bootstrapping (5000 samples). We did this for the PE-I, the N2/P2a complex and the P3 placebo effect measures as dependent variables. (We did not run separate moderation analyses with PE-U, as this score was strongly correlated with PE-I). For significant moderation models, we probed the interaction by calculating conditional effects at moderator values of −1SD, Mean and +1SD. The significance of the conditional effects was assessed using confidence intervals.
We used a significance level of α = 0.05 for all analyses. In case of multiple comparisons, we used Bonferroni-corrected post hoc tests. Partial eta squared (np2) effect size measures are reported for significant effects in the ANOVA models, where 0.01 represents a small effect, 0.06 represents a medium effect and 0.14 represents a large effect. Outlier analysis was performed, and no outliers were identified.
Power analyses (using G*Power 3.1.9) showed that a sample size of 82 participants is sufficient to obtain a power of 0.80 for an assumed medium effect size of f = 0.25 for a repeated measures ANOVA with a between-group factor and four numbers of measurements, ie, run (run 1 vs run 2) x condition (placebo vs control). In addition, a sample size between 32 and 45 is sufficient to obtain a power of 0.80 for an assumed large effect size of the population squared correlation coefficient between ρ2 = .3 and .4 for H1 and ρ2 = 0 for H0 for a 2-tailed linear multiple regression with five predictors. Finally, a sample size of 48 is sufficient to obtain a power of 0.80 for an assumed medium effect size of f = 0.25 for a moderation model, ie, a multiple regression with three predictors (age group, executive test score and their interaction).
Results
Psychological Characteristics
Mean scores and group statistics for psychological characteristics can be found in Table 1. All older adults had an MMSE score well above the cut-off, indicating that they showed no general cognitive impairment.49 There was no difference between older and young adults with respect to emotional distress (total DASS-42) and pain attitude (total PAQ-r score), but older participants reported significantly lower negative pain-related cognition scores (FPQ-III and PCS) (see Table 1). However, two-tailed Pearson correlations showed no significant relationship between these pain-related cognitions and the placebo effect size for either age group (see Supplementary Materials, Table S3).
 |
Table 1 Psychological Characteristics |
Executive Functions
Mean scores and group statistics for the executive functioning tests can be found in Table 2. Overall, older adults performed significantly worse than younger adults on the Digit Span, Stroop, and TMT. There was no group difference for the ANT and Go/NoGo. We found no correlation between executive test scores and age or education (see Supplementary Materials, Table S4).
 |
Table 2 Executive Functioning Test Scores |
Behavioral Placebo Effect
A manipulation check confirmed that all participants rated highly painful stimuli as significantly more intense and unpleasant than the mildly painful stimuli that were administered during the conditioning phase (intensity ratings: F(1,79) = 615.53, p < 0.001, np2 = 0.886; unpleasantness ratings: F(1,79) = 522.71, p < 0.001, np2 = 0.869; see Supplementary Materials for the full statistical results from the conditioning phase). For the test phase, we found a significant main effect for condition for intensity ratings (F(1,79) = 39.63, p < 0.001, np2 = 0.334, mean difference = 3.73 (95% CI: [2.55, 4.91])) and unpleasantness ratings (F(1,79) = 47.92, p < 0.001, np2 =.378, mean difference = 4.37 (95% CI: [3.11, 5.62])), with higher ratings in the control than in the placebo condition in both age groups (see Figure 3). There were no other main effects (all Fs < 3.20, all ps > 0.077, all np2s < 0.039) or interactions between any of the variables (all Fs < 0.57, all ps >.452, all np2s < 0.007) for the intensity scale. For the unpleasantness scale, we also found a main effect of age group, with older adults rating all stimuli as significantly less unpleasant than young adults, F(1,79) = 5.40, p = 0.023, np2 = 0.064. No other main effects or interactions became significant (all Fs < 1.47, all ps > 0.227, all np2s < 0.018).
Association Between Executive Functions and Behavioral Placebo Effect
The regression model for older adults with the five executive test scores as independent variables and PE-I as dependent variable was significant (F(5,35) = 3.31, p = 0.015, R2 = 0.321). In particular, higher Digit Span and lower TMT scores (ie, better performance) were associated with a greater placebo effect (see Table 3). (Collinearity statistics for this model are reported in the Supplementary Materials, Table S5). The same regression model for younger adults was not significant (F(5,32) = 1.58, p = 0.195, R2 = 0.197).
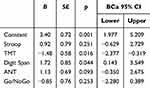 |
Table 3 Regression Model for Older Adults, with the Placebo Effect Calculated from Intensity Ratings (PE-I) as Outcome Variable and Executive Functions as Predictors |
Moderation by Executive Functions of Age Difference in Placebo Hypoalgesia
Next, we tested whether executive functioning moderated the effect of age on placebo hypoalgesia (PE-I). This revealed significant moderation by the TMT score (F(3,76) = 3.01, p = 0.035, R2 = 0.106) and the Go/NoGo score (F(3,77) = 2.75, p = 0.048, R2 = 0.097) (see Table 4). The model with the Digit Span score as moderator was marginally significant (F(3,77) = 2.40, p = 0.074, R2 = 0.086) and the ANT and Stroop scores did not significantly moderate the relationship between age group and placebo hypoalgesia (both F < 1.45, p > 0.236).
 |
Table 4 Moderation Models with the TMT and Go/NoGo Score as Moderator, Age Group as Independent Variable and the Behavioral Placebo Effect on the Intensity Scale (PE-I) as Dependent Variable |
For the model with the TMT score as moderator, conditional effects analyses revealed that older adults had a smaller placebo effect than younger adults, but only when performance on the TMT task was low (ie, when scores were 1SD above the mean). On the other hand, when performance was average or high, there was no group difference in placebo hypoalgesia (see Table 5). Similarly, conditional effects analyses for the model with the Go/NoGo as moderator revealed that older adults showed a smaller placebo effect than younger adults when performance on the Go/NoGo task was low (scores of 1SD above the mean), but not when performance was average-to-high (see Table 5). A similar trend could be observed for the Digit Span score, with older adults showing a tendency for a smaller placebo effect when performance was low (b = 3.31, SE = 1.66, t = 1.99, p = 0.051, CI[−0.007 6.620]), but not when performance was average-to-high (p = 0.740 and p = 0.146, respectively).
 |
Table 5 Conditional Effects Analyses for the Moderation by Executive Functioning of the Relationship Between Age Group and Placebo Hypoalgesia |
Electrophysiological Placebo Effect
Figure 4 shows the average ERP waveforms at the vertex (Cz) in response to the pain stimuli, separate for the two age groups and experimental conditions. For the peak-to-peak amplitude of the N2/P2a complex, there was a significant main effect for condition (F(1,79) = 6.17, p = 0.015, np2 = 0.072). Post-hoc comparisons revealed a lower mean in the placebo condition (M=16.94; SD=0.95) than in the control condition (M=17.91; SD=0.96), with a mean difference of 0.97 (95% CI: [0.19, 1.74]). Likewise, for the P3 peak amplitude, we found a significant main effect for condition (F(1,79) = 4.35, p = 0.040, np2 = 0.052). Again, the mean was lower in the placebo condition (M=13.06; SD=0.49) than in the control condition (M=13.73; SD=0.51), with a mean difference of 0.67 (95% CI: [0.03, 1.30]). There were no interactions between age group and condition for these ERP components (for the N2/P2a complex: F(1,79) = 0.24, p = 0.624, np2 = 0.003, and for the P3 peak amplitude: F(1,79) = 0.49, p = 0.488, np2 = 0.006).
 |
Figure 4 Average waveforms at the vertex (Cz) in response to pain stimuli, for each condition and age group. Notes: 0 ms indicates the start of the pain stimulation. |
Older adults showed significantly later onsets of the N2 peak (F(1,79) = 46.68, p < 0.001, np2 = 0.371) and P2a peak (F(1,79) = 21.09, p < 0.001, np2 = 0.211), but not of the P3 peak (p = 0.280) when compared to younger adults. They also showed significantly smaller peak-to-peak amplitudes for the N2/P2a complex (F(1,79) = 65.61, p < 0.001, np2 = 0.454) and smaller peak amplitudes for the P3 (F(1,79) = 74.99, p < 0.001, np2 = 0.487) compared to young adults. For the P3 we also observed a significant main effect for electrode site (F(2158) = 18.16, p < 0.001, np2 = 0.187), but no interactions between any of the factors (all Fs < 3.35, ps >.055). Bonferroni-corrected pairwise comparisons for each channel showed that the P3 activity at electrode site CPz differed significantly from the activity at site Cz (p = 0.001) and Pz (p < 0.001), whereas we found no difference between Cz and Pz (p = 0.105), with CPz showing the largest activity (M = 14.09, SE = 0.49), followed by Cz (M = 13.37, SE = 0.50) and Pz (M = 12.73, SE = 0.50).
Across participants, the electrophysiological placebo effect in the peak-to-peak amplitudes of the N2/P2a complex (ie, the difference in µV between the N2/P2a complex in the control and placebo condition) correlated positively with the behavioral placebo effect on the intensity scale (PE-I), r(81) = 0.244, p = 0.028, CI[0.018 0.474]. Correlations for the unpleasantness scale or for the P3 placebo effect did not reach significance (all p > 0.322).
Executive Functions and Electrophysiological Placebo Effect
None of the executive test scores was a significant predictor of the electrophysiological placebo effect in older adults (N2/P2a complex: F(5,35) = 0.11, p = 0.990, R2 = 0.015; P3: F(5,35) = 1.47, p = 0.226, R2 = 0.173) or in younger adults (N2/P2a complex: F(5,32) = 0.94, p = 0.467, R2 = 0.128; P3: F(5,32) = 1.18, p = 0.341, R2 = 0.156).
Moderation analyses for the N2/P2a complex and the P3 placebo effect measures as dependent variables, age group as independent variable and the different executive functioning test scores as moderators revealed no significant overall models (all Fs < 1.93, all ps > 0.132, all R2s < 0.07). However, it is noteworthy that for the model with the N2/P2a complex as placebo measure, the TMT score was a significant moderator, ie, there was a significant interaction between TMT and age group (b = 0.10, SE = 0.05, t = 2.10, p = 0.039, CI[0.01 0.19]). Conditional effects analyses revealed that, when performance on the TMT was low, older participants showed a significantly smaller ERP modulation (N2/P2a peak-to-peak amplitude reduction in the placebo vs control condition) than younger participants (b = 2.45, SE = 1.19, t = 2.06, p = 0.043, CI[0.09 4.80]), but when performance was average-to-high, there was no group difference in the placebo effect (p = 0.371 and p = 0.357, respectively). However, the overall model was not significant, suggesting that the combined explanatory power of age group, TMT score and their interaction was small.
Discussion
We investigated the role of executive functions in modulating the placebo hypoalgesic response in healthy older adults. Previous studies have shown inconsistent results regarding the effect of aging on placebo hypoalgesia.7–10 Given the relevance of the prefrontal cortex (PFC) and of executive functions in mediating placebo responses, we speculated that variable aging trajectories of decline in executive functioning may explain these divergent findings. We hypothesized that an age-related decrease in placebo hypoalgesia would be apparent only in those older adults showing a stronger decline in executive functioning.
Our results were indeed in line with this hypothesis. When we compared a group of younger and older healthy adults overall, we found no difference in placebo hypoalgesia; the two groups showed equivalent pain reduction in terms of sensory ratings and pain-related brain potentials, in response to a sham treatment manipulation. However, executive functions explained up to 32.1% of the variance in the behavioral placebo effect in healthy older adults, with the TMT difference score and Digit Span Sequencing score being the strongest predictors. Importantly, executive functioning also moderated the age difference in placebo hypoalgesia. Specifically, we found decreased (behavioral and electrophysiological) placebo hypoalgesic responses in older compared to younger adults, when their performance on the TMT and Go/NoGo tests was low (with a same tendency for the Digit Span test). When performance was average-to-high, there was no age group difference. This could explain why previous studies on the effect of age on placebo hypoalgesia reported largely inconsistent results; their samples of older adults may have differed in the extent of age-related decline in executive functions, thereby leading to differences in overall group comparisons.
While better performance on the TMT and Digit Span was associated with a stronger placebo hypoalgesic effect in older adults, performance on the Stroop, Go/NoGo and ANT did not significantly predict the degree of pain relief. The significant association between the placebo effect and the TMT and Digit Span suggests that better working memory ability and cognitive flexibility may be related to more efficient maintenance and manipulation of beliefs and expectations,26 which are integral to generating placebo effects.27,28 In addition, better working memory may be related to more efficient learning of cue-stimulus associations (conditioning),85–87 thereby facilitating conditioned placebo hypoalgesia, as implemented in the current design.
The Stroop, Go/NoGo and ANT tests, on the other hand, all involve a strong inhibitory control component. It is surprising that these test scores did not predict placebo hypoalgesia, given the growing evidence for a positive relationship between inhibition abilities and the efficacy of pain modulation.35–38 One explanation could be that most of these latter studies investigated the association between cognitive inhibition and conditioned pain modulation or distraction from pain, which likely employ different neural mechanisms from placebo hypoalgesia.38,88 Inhibitory control skills may be more important for distraction from pain, for example, as they enable participants to maintain attentional focus on the distractive task and away from incoming nociceptive information.37,38
We did, however, find evidence for a moderating role of inhibitory control skills: Go/NoGo test scores interacted with age in influencing placebo hypoalgesia. When their scores were low, older adults showed a smaller placebo response than younger adults, which was not the case when performance was average-to-high. Therefore, response inhibition appears relevant for placebo hypoalgesia to a certain degree. This might be related to the ability to suppress negative emotional responses to and interpretations of the pain, or the ability to suppress doubts or negative expectations about the effectiveness of the TENS treatment.
To date, very few studies have directly examined the effect of individual differences in executive functioning on placebo hypoalgesia. One study demonstrated that individuals with Alzheimer’s disease displaying reduced Frontal Assessment Battery (FAB) scores showed reduced placebo hypoalgesia.22 The FAB is a brief neuropsychological bedside test consisting of six subtests that evaluate different aspects of frontal executive functioning, including cognitive flexibility and response inhibition.89 Another study on individuals with early Alzheimer’s disease reported that placebo hypoalgesia was related to cognitive flexibility (as measured with the Wisconsin Card Sorting Test) and response inhibition/shifting, self-monitoring and set-shifting (as measured with the Behavioural Assessment of the Dysexecutive Syndrome; BADS), but not to a range of other executive functions tested.23 This latter study also demonstrated a positive correlation between activation in the medial prefrontal cortex during response inhibition (in particular, during NoGo trials in a Go/NoGo task) and placebo hypoalgesia. And finally, one study on adolescents demonstrated that better inhibitory control and cognitive flexibility (as measured with the Reversed Flanker task) were associated with greater conditioned nocebo-like effects on heat perception.90
All these studies thus converge in demonstrating a specific role of cognitive flexibility and inhibition skills in mediating placebo hypoalgesia. Our results extend these findings by showing the same relationship in healthy older adults, and point to an additional role for working memory skills. Our results are also in line with previous findings on the role of executive functioning in other forms of endogenous pain modulation.35–42
One limitation of our study is that the older adults were specifically recruited to be in good health, rendering it difficult to generalize our findings to more representative samples of the older population, including those with cognitive complaints or chronic pain conditions. These latter groups may have more negative treatment expectations,91 as well as a more pronounced decline in executive functioning,39 and it would be important to assess the relationship between placebo hypoalgesic effects and executive functions in these groups. Another limitation is the cross-sectional design of the study, meaning that age-related decline in cognitive functions and placebo hypoalgesia were inferred from group comparisons. Longitudinal studies would provide additional insights in dynamic changes and causal relationships between placebo effects and executive functioning across the age span. And finally, apart from trends, we did not find clear associations or moderation of the electrophysiological placebo effect by executive functions, despite a correlation between the behavioral and electrophysiological placebo effect. This is likely due to the relatively smaller effect size of the electrophysiological, compared to the behavioral, placebo effect.
Conclusions
Our results show that good executive functions, in particular, cognitive flexibility, working memory and inhibitory control, are necessary to maintain normal placebo hypoalgesic responding in healthy older adults. Given that the placebo effect is an integral part of treatment outcomes,46 an age-related decline in executive functioning may contribute to worse treatment outcomes in older adults and drive their increased risk of developing chronic pain conditions.92 Preserving executive functions, eg, with cognitive training, may have beneficial effects on the efficacy of endogenous pain modulation. Our results also highlight the importance of assessing executive functioning skills when examining the influence of aging on placebo effects, and suggest that the current discrepancy in findings may be due to variable aging trajectories of decline in brain areas and cognitive skills that mediate the placebo effect.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, MvdM, upon reasonable request.
Acknowledgments
We would like to thank the “GERO – Kompetenzzenter fir den Alter” for their help with participant recruitment as well as Immo Curio for technical support. Moreover, we would like to thank all student assistants and interns who assisted us with the data collection and all participants who took part in this study.
Funding
This work was supported by the Luxembourg National Research Fund (FNR) (grant number: C16/BM/11266318/ACHE).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kern A, Kramm C, Witt CM, Barth J. The influence of personality traits on the placebo/nocebo response: a systematic review. J Psychosomatic Res. 2020;128:109866. doi:10.1016/j.jpsychores.2019.109866
2. Koban L, Ruzic L, Wager TD. Brain Predictors of Individual Differences in Placebo Responding. In: Placebo and Pain. Elsevier; 2013:89–102.
3. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi:10.1097/00002508-200407000-00004
4. Hackett J, Naugle KE, Naugle KM. The decline of endogenous pain modulation with aging: a meta-analysis of temporal summation and conditioned pain modulation. J Pain. 2020;21(5–6):514–528. doi:10.1016/j.jpain.2019.09.005
5. Helme RD, Gibson SJ. Pain in older people. Epidemiol Pain. 1999;1999:103–112.
6. Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–113.
7. Ho T, Fan X, Rodgers A, Lines C, Winner P, Shapiro R. Age effects on placebo response rates in clinical trials of acute agents for migraine: pooled analysis of rizatriptan trials in adults. Cephalalgia. 2009;29:711–718. doi:10.1111/j.1468-2982.2008.01788.x
8. Weimer K, Colloca L, Enck P. Age and sex as moderators of the placebo response-an evaluation of systematic reviews and meta-analyses across medicine. Gerontology. 2015;61(2):97–108. doi:10.1159/000365248
9. Wrobel N, Fadai T, Brassen S, Bingel U. Preserved capacity for placebo hypoalgesia in the elderly. J Pain. 2016;17:1318–1324. doi:10.1016/j.jpain.2016.08.012
10. Daguet I, Bergeron-Vézina K, Harvey MP, Martel M, Léonard G. Transcutaneous electrical nerve stimulation and placebo hypoalgesia: is the effect the same for young and older individuals? Clin Interventions Aging. 2018;13:335–342. doi:10.2147/CIA.S152906
11. Crawford LS, Mills EP, Peek A, Macefield VG, Keay KA, Henderson LA. Function and biochemistry of the dorsolateral prefrontal cortex during placebo hypoalgesia: how the certainty of prior experiences shapes endogenous pain relief. Cereb Cortex. 2023;33:9822–9834. doi:10.1093/cercor/bhad247
12. Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian VA. Brain networks predicting placebo hypoalgesia in a clinical trial for chronic back pain. Pain. 2012;153:2393–2402. doi:10.1016/j.pain.2012.08.008
13. Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo hypoalgesic response. J Neurosci. 2009;29:4882–4887. doi:10.1523/JNEUROSCI.5634-08.2009
14. Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo hypoalgesia. PAIN. 2012;153:2210–2217. doi:10.1016/j.pain.2012.07.010
15. Zubieta JK, Bueller JA, Jackson LR. Placebo effects mediated by endogenous opioid activity on -opioid receptors. J Neurosci. 2005;25:7754–7762. doi:10.1523/JNEUROSCI.0439-05.2005
16. Crivello F, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Longitudinal assessment of global and regional rate of grey matter atrophy in 1172 healthy older adults: modulation by sex and age. PLoS One. 2014;9:e114478. doi:10.1371/journal.pone.0114478
17. Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain J Neurol. 2005;128:1964–1983.
18. Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–344. doi:10.1001/archneur.56.3.338
19. Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo hypoalgesia. Pain. 2010;148:368–374. doi:10.1016/j.pain.2009.09.033
20. Egorova N, Yu R, Kaur N, et al. Neuromodulation of conditioned placebo/nocebo in heat pain: anodal vs. cathodal transcranial direct current stimulation to the right dorsolateral prefrontal cortex. Pain. 2015;156:1342. doi:10.1097/j.pain.0000000000000163
21. Tu Y, Wilson G, Camprodon J, et al. Manipulating placebo hypoalgesia and nocebo hyperalgesia by changing brain excitability. Proc Natl Acad Sci. 2021;118:e2101273118.
22. Benedetti F, Arduino C, Costa S, et al. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain. 2006;121:133–144. doi:10.1016/j.pain.2005.12.016
23. Palermo S, Rainero I, Stanziano M, et al. A novel neurocognitive approach for placebo hypoalgesia in neurocognitive disorders. Exp Gerontol. 2019;118:106–116. doi:10.1016/j.exger.2019.01.011
24. Daigle KM, Pietrzykowski MO, Waters AB, Swenson LP, Gansler DA. Central executive network and executive function in patients with Alzheimer’s disease and healthy individuals: meta-analysis of structural and functional MRI. J Neuropsychiatry Clin Neurosci. 2022;34:204–213. doi:10.1176/appi.neuropsych.20110279
25. Zink N, Lenartowicz A, Markett S. A new era for executive function research: on the transition from centralized to distributed executive functioning. Neurosci Biobehav Rev. 2021;124:235–244. doi:10.1016/j.neubiorev.2021.02.011
26. Diamond A. Executive Functions. Annu Rev Psychol. 2013;64:135–168. doi:10.1146/annurev-psych-113011-143750
27. Kirsch I. How Expectancies Shape Experience. Washington DC: American Psychological Association; 1999.
28. Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nature Med. 2010;16(11):1277–1283. doi:10.1038/nm.2229
29. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cognitive Sci. 2005;9:242–249. doi:10.1016/j.tics.2005.03.010
30. Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–10402. doi:10.1523/JNEUROSCI.3458-05.2005
31. Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar function reflects common individual differences in emotion and pain regulation success. J Cognitive Neurosci. 2012;24(1):148–158. doi:10.1162/jocn_a_00125
32. Flaten MA, Aslaksen PM, Lyby PS, Bjorkedal E. The relation of emotions to placebo responses. Philos Trans R Soc Lond B Biol Sci. 2011;366:1818–1827. doi:10.1098/rstb.2010.0407
33. Lyby PS, Aslaksen PM, Flaten MA. Is fear of pain related to placebo analgesia? J Psychosomatic Res. 2010;68:369–377. doi:10.1016/j.jpsychores.2009.10.009
34. Van der Meulen M, Kamping S, Anton F. The role of cognitive reappraisal in placebo hypoalgesia: an fMRI study. Soc Cognit Affective Neurosci. 2017;12:1128–1137. doi:10.1093/scan/nsx033
35. Marouf R, Caron S, Lussier M, Bherer L, Piché M, Rainville P. Reduced pain inhibition is associated with reduced cognitive inhibition in healthy aging. PAIN. 2014;155(3):494–502. doi:10.1016/j.pain.2013.11.011
36. Oosterman JM, Dijkerman HC, Kessels RP, Scherder EJ. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur J Pain. 2010;14(10):1046–1050. doi:10.1016/j.ejpain.2010.04.004
37. Rischer KM, González‐Roldán AM, Montoya P, Gigl S, Anton F, Van der Meulen M. Distraction from pain: the role of selective attention and pain catastrophizing. Eur J Pain. 2020;24:1634.
38. Rischer KM, Anton F, González-Roldán AM, Montoya P, Van der Meulen M. Better executive functions are associated with more efficient cognitive pain modulation in older adults: an fMRI study. Front Aging Neurosci. 2022;14:828742.
39. Bunk S, Preis L, Zuidema S, Lautenbacher S, Kunz M. Executive functions and pain. J Neuropsychol. 2019;30(3):169–196.
40. Lithfous S, Després O, Pebayle T, Dufour A. Modification of descending analgesia in aging: critical role of the prefrontal cortex. Clin J Pain. 2019;35(1):23–30. doi:10.1097/AJP.0000000000000655
41. Serrano PV, Zortea M, Alves RL, et al. Association between descending pain modulatory system and cognitive impairment in fibromyalgia: a cross-sectional exploratory study. Front Behav Neurosci. 2022;16:917554. doi:10.3389/fnbeh.2022.917554
42. Zhou S, Després O, Pebayle T, Dufour A. Age-related decline in cognitive pain modulation induced by distraction: evidence from event-related potentials. J Pain. 2015;16(9):862–872. doi:10.1016/j.jpain.2015.05.012
43. Salthouse TA. Theoretical Perspectives on Cognitive Aging. Hillsdale, NJ: Erlbaum; 1991.
44. Kang W, Wang J, Malvaso A. Inhibitory control in aging: the compensation-related utilization of neural circuits hypothesis. Front Aging Neurosci. 2022;13:771885. doi:10.3389/fnagi.2021.771885
45. Caballero HS, McFall GP, Wiebe SA, Dixon RA. Integrating three characteristics of executive function in non-demented aging: trajectories, classification, and biomarker predictors. J Int Neuropsychol Soc. 2021;27(2):158–171. doi:10.1017/S1355617720000703
46. Atlas LY, Wielgosz J, Whittington RA, Wager TD. Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology. 2014;231:813–823. doi:10.1007/s00213-013-3296-1
47. Colloca L, Tinazzi M, Recchia S, et al. Learning potentiates neurophysiological and behavioral placebo hypoalgesic responses. Pain. 2008;139:306–314. doi:10.1016/j.pain.2008.04.021
48. Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Clin Neurophysiol. 2003;33:293–301. doi:10.1016/j.neucli.2003.10.004
49. Pezzotti P, Scalmana S, Mastromattei A, Di Lallo D. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: a prospective observational study. BMC Family Pract. 2008;9:1–11. doi:10.1186/1471-2296-9-1
50. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi:10.1016/0028-3932(71)90067-4
51. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33:335–343. doi:10.1016/0005-7967(94)00075-u
52. McNeil DW, Rainwater AJ. Development of the fear of pain questionnaire-III. J Behav Med. 1998;21:389–410. doi:10.1023/A:1018782831217
53. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assessment. 1995;7:524. doi:10.1037/1040-3590.7.4.524
54. McCracken LM. “Attention” to pain in persons with chronic pain: a behavioral approach. Behav Ther. 1997;28:271–284. doi:10.1016/S0005-7894(97)80047-0
55. Mah K, Tran KT, Gauthier LR, et al. Psychometric evaluation of the pain attitudes questionnaire-revised for people with advanced cancer. J Pain. 2017;18:811–824. doi:10.1016/j.jpain.2017.02.432
56. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6
57. Büsch D, Hagemann N, Bender N. The dimensionality of the Edinburgh Handedness Inventory: An analysis with models of the item response theory. Laterality. 2010;15(6):610–628. doi:10.1080/13576500903081806
58. Ciobanu T, Brodard F, Antonietti JP, Genoud PA, Brandner C. Screening negative affectivity in young adults: validation and psychometric evaluation of the French version of the depression anxiety stress scales. Canadian J Behav Sci/Revue Canadienne Des Sciences du Comportement. 2018;50(4):238. doi:10.1037/cbs0000110
59. Nilges P, Essau C. DASS. depressions-angst-stress-skalen-deutschsprachige Kurzfassung. 2021.
60. French DJ, Noël M, Vigneau F, French JA, Cyr CP, Evans RT. L’Échelle de dramatisation face à la douleur PCS-CF: adaptation canadienne en langue française de l’échelle «pain catastrophizing scale». Canadian J Behav Sci/Revue Canadienne Des Sciences du Comportement. 2005;37(3):181. doi:10.1037/h0087255
61. Meyer K, Sprott H, Mannion AF. Cross-cultural adaptation, reliability, and validity of the German version of the pain catastrophizing scale. J Psychosomatic Res. 2008;64(5):469–478. doi:10.1016/j.jpsychores.2007.12.004
62. Kunz M, Capito ES, Horn-Hofmann C, et al. Psychometric properties of the German version of the pain vigilance and awareness questionnaire (PVAQ) in pain-free samples and samples with acute and chronic pain. Int J Behav Med. 2017;24:260–271. doi:10.1007/s12529-016-9585-4
63. Derouesne C, Poitreneau J, Hugonot L, Kalafat M, Dubois B, Laurent B. Mini-mental state examination: a useful method for the evaluation of the cognitive status of patients by the clinician. Consensual French version. Presse Med. 1999;28(21):1141–1148.
64. Kessler J, Markowitsch HJ, Denzler P. Mini-mental-status-test(MMST) [German Version]. Beltz Test GmbH. 1990.
65. Albaret MC, Sastre MTM, Cottencin A, Mullet E. The fear of pain questionnaire: factor structure in samples of young, middle-aged and elderly European people. Eur J Pain. 2004;8:273–281. doi:10.1016/j.ejpain.2003.09.005
66. Desrochers G, Bergeron S, Khalifé S, Dupuis MJ, Jodoin M. Fear avoidance and self-efficacy in relation to pain and sexual impairment in women with provoked vestibulodynia. Clin J Pain. 2009;25:520–527. doi:10.1097/AJP.0b013e31819976e3
67. Wechsler D. Wechsler Adult Intelligence Scale “(WAIS–IV)”. Vol. 22. San Antonio, TX: NCS Pearson; 2008:1.
68. Reitan RM. Trail making test results for normal and brain-damaged children. Perceptual Motor Skills. 1971;33:575–581. doi:10.2466/pms.1971.33.2.575
69. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary.
70. Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1:2277–2281. doi:10.1038/nprot.2006.390
71. McMorris T. History of research into the acute exercise–cognition interaction: a cognitive psychology approach. In: Morris T, editor. Exercise-Cognition Interaction: Neuroscience Perspectives. Vols. 1-28. Elsevier Academic Press; 2016:1–28.
72. Kowalczyk A, McDonald S, Cranney J, McMahon M. Cognitive flexibility in the normal elderly and in persons with dementia as measured by the written and oral trail making tests. Brain Impairment. 2001;2(1):11–21. doi:10.1375/brim.2.1.11
73. Banich MT, Burgess GC, Depue BE, et al. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47:3095–3104. doi:10.1016/j.neuropsychologia.2009.07.005
74. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and Independence of attentional networks. J Cognitive Neurosci. 2002;14:340–347. doi:10.1162/089892902317361886
75. Jennings JM, Dagenbach D, Engle CM, Funke LJ. Age-related changes and the attention network task: an examination of alerting, orienting, and executive function. Aging Neuropsychol Cognit. 2007;14:353–369. doi:10.1080/13825580600788837
76. Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain Behav Immun. 2006;20:219–230. doi:10.1016/j.bbi.2006.01.007
77. Tiemann L, May ES, Postorino M, et al. Differential neurophysiological correlates of bottom-up and top-down modulations of pain. Pain. 2015;156:289–296. doi:10.1097/01.j.pain.0000460309.94442.44
78. Zhang W, Luo J. The transferable placebo effect from pain to emotion: changes in behavior and EEG activity. Psychophysiology. 2009;46:626–634. doi:10.1111/j.1469-8986.2009.00786.x
79. Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. 2010;35:129–155. doi:10.1080/87565640903526504
80. Legrain V, Perchet C, Garcia-Larrea L. Involuntary orienting of attention to nociceptive events: neural and behavioral signatures. J Neurophysiol. 2009;102:2423–2434. doi:10.1152/jn.00372.2009
81. Aslaksen PM, Flaten MA. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosomatic Med. 2008;70:811–818. doi:10.1097/PSY.0b013e31818105ed
82. Hird EJ, Jones AKP, Talmi D, El-Deredy W. A comparison between the neural correlates of laser and electric pain stimulation and their modulation by expectation. J Neurosci Meth. 2018;293:117–127. doi:10.1016/j.jneumeth.2017.09.011
83. Lyby PS, Aslaksen PM, Flaten MA. Variability in placebo hypoalgesia and the role of fear of pain—an ERP study. Pain. 2011;152:2405–2412. doi:10.1016/j.pain.2011.07.010
84. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford publications; 2017.
85. Alderman N. Central executive deficit and response to operant conditioning methods. Neuropsychol Rehabilitat. 1996;6:161–186. doi:10.1080/713755505
86. Hoefer M, Allison S, Schauer G, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain. 2008;131:1646–1657. doi:10.1093/brain/awn082
87. Weiler JA, Bellebaum C, Daum I. Aging affects acquisition and reversal of reward-based associative learning. Learn Memory. 2008;15:190–197. doi:10.1101/lm.890408
88. Buhle JT, Stevens BL, Friedman JJ, Wager TD. Distraction and placebo: two separate routes to pain control. Psychol Sci. 2012;23:246–253. doi:10.1177/0956797611427919
89. Dubois B, Slachevsky A, Litvan I, Bfab P. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. doi:10.1212/WNL.55.11.1621
90. Neuenschwander R, Weik E, Tipper CM, Jensen K, Oberlander TF. Conditioned placebo-and nocebo-like effects in adolescents: the role of conscious awareness, sensory discrimination, and executive function. Front Psych. 2020;11:586455. doi:10.3389/fpsyt.2020.586455
91. Bingel U, Colloca L, Vase L. Mechanisms and clinical implications of the placebo effect: is there a potential for the elderly? A mini-review. Gerontology. 2011;57:354–363. doi:10.1159/000322090
92. Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuro Psychopharmacol Biol Psych. 2019;93:284–290. doi:10.1016/j.pnpbp.2019.04.006
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.