Back to Journals » Infection and Drug Resistance » Volume 18
The Predictive Value of Lactate Dehydrogenase for Viral Suppression in Newly Diagnosed People Living With HIV on Antiretroviral Therapy: A Retrospective Cohort Study
Authors Jin Y, Wang Y, Xia T, Ma Q
Received 23 July 2024
Accepted for publication 22 January 2025
Published 30 January 2025 Volume 2025:18 Pages 601—611
DOI https://doi.org/10.2147/IDR.S488220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yong Jin,1,* Yan Wang,1,* Ting Xia,1 Qichao Ma2
1Department of Internal Medicine, Ningbo Yinzhou No.2 hospital, Ningbo, Zhejiang, People’s Republic of China; 2Department of Dermatology and Venereology, Ningbo Yinzhou No.2 hospital, Ningbo, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qichao Ma, Department of Dermatology and Venereology, Ningbo Yinzhou No.2 hospital, No.998 Qianhe North Road, Ningbo, Zhejiang, 315101, People’s Republic of China, Email [email protected]
Purpose: Rapid initiation of antiretroviral therapy (ART) in people living with HIV (PLWH) is crucial for achieving viral suppression and improving clinical outcomes. Serum lactate dehydrogenase (LDH) levels serve as a readily accessible and rapid clinical biomarker, has significant predictive potential in various viral diseases. This study aims to evaluate the predictive value of LDH levels for viral suppression in the context of rapid ART initiation.
Patients and Methods: LDH levels were measured in 393 newly diagnosed PLWH who received rapid initiation of ART and were subsequently followed up. The PLWH were stratified based on tertile LDH levels and study endpoints. Kaplan-Meier analysis was conducted to generate survival curves, and Cox regression analysis was utilized to confirm the independent prognostic factors for viral suppression.
Results: The overall viral suppression rate was 94.1%. Compared to the low LDH tertile, the middle and high LDH tertiles exhibited longer times to first viral suppression (38 vs 84 vs 88 days, respectively, P < 0.001). Kaplan-Meier analysis revealed that PLWH in high LDH tertile showed the worst prognosis for viral suppression (Log rank test, P< 0.001). Multivariate Cox regression analysis identified LDH tertile as a significant predictor of viral suppression (HR = 0.714, 95% CI = 0.553– 0.922, P = 0.010 for middle vs low tertile; HR = 0.575, 95% CI = 0.443– 0.747, P < 0.001 for high vs low tertile).
Conclusion: LDH levels function as a prognostic indicator for predicting the timing of viral suppression in PLWH on ART. A comprehensive evaluation of LDH levels is beneficial in establishing risk stratification in the context of rapid ART initiation.
Keywords: predictive value, lactate dehydrogenase, viral suppression, HIV
Graphical Abstract:

Introduction
The implementation of antiretroviral therapy (ART) has effectively reversed the previously increasing trends in Human Immunodeficiency Virus (HIV) related mortality, morbidity, and disease transmission. This success underscores the importance of achieving and maintaining viral suppression in healthcare management. Achieving and maintaining viral suppression stand as a paramount objective in managing individuals with HIV, with profound implications for disease progression, treatment effectiveness, and the emergence of HIV-1-related comorbidities.1 Additionally, the benefits of viral suppression extend to both individual and public health.2 Substantial evidence supports that people living with HIV (PLWH) cannot sexually transmit the virus if their viral load is maintained at or below 200 hIV RNA copies/mL.3–6 In the global HIV pandemic context, there is growing interest in viral suppression in clinical and epidemiological studies.
The Rapid initiation of ART in PLWH is essential for improving clinical outcomes and controlling disease progression. Early initiation of ART significantly reduces viral load and prevents immune system deterioration, thereby decreasing morbidity and mortality from opportunistic infections and AIDS-defining illnesses.7 Additionally, prompt ART initiation improves time to viral suppression and retention in care, offering significant psychological benefits.8 Rapid ART aligns with the UNAIDS 95–95-95 targets by expediting viral suppression in a greater proportion of the HIV-positive population, contributing to epidemic control.9 However, not all antiretroviral drugs are appropriate for rapid ART initiation.10 The unequal distribution of medical resources and inconsistent economic conditions often delay access to optimal drugs recommended for rapid ART. Viral suppression is influenced by several factors, such as baseline viral load, adherence, ART regimens, treatment duration and the emergence of drug-resistant strains.11 In this context, early identification of viral suppression in PLWH is critically important.
Lactate dehydrogenase (LDH) is an intracellular enzyme present in various organ systems, and deviations from normal values can result from multi-organ injuries, infections, and other conditions involving the upregulation of glycolytic processes.12 Serum LDH levels serve as a readily accessible and rapid clinical biomarker, potentially benefiting viral suppression in the context of rapid ART initiation. Serum LDH levels are crucial for predicting the prognosis of various viral diseases. In chronic lymphocytic leukemia patients,13 a positive correlation existed between LDH levels and EBV-DNA copy numbers, which was linked to diminished survival time and accelerated disease progression. In pediatric Mycoplasma pneumoniae pneumonia patients,14 those with persistent Mycoplasma DNA positivity beyond 30 days from disease onset had significantly higher LDH levels than those with DNA negativity. This suggested a strong association between LDH levels and Mycoplasma DNA clearance. Nishijima et al15 compared serum LDH levels in individuals with primary measles virus infection and reinfection, finding significantly higher LDH levels and viral loads in primary infections. The area under the curve for overall LDH levels was 0.87 (95% CI 0.74–1.00), indicating LDH as a valuable marker for differentiating between primary and reinfection. In COVID-19 patients,16 serum LDH levels were found to correlate with COVID-19 Ct values, demonstrating a positive association with higher viral loads.
Despite the considerable predictive potential of serum LDH levels in various diseases, limited data exist on its use as a biomarker for predicting viral suppression in PLWH. Predicting viral suppression in PLWH remains a clinical challenge. In resource-limited regions, cost-effective, compliant, and timely diagnostic indicators are imperative. LDH, as a routine clinical parameter, offers distinct advantages. Economically, incorporating LDH may provide a cost-efficient approach to diagnosis and monitoring, enhancing medical resource utilization. For patient compliance, LDH implementation can simplify treatment, improve adherence, and support long-term management. Timely LDH detection results facilitate swift disease status evaluation and treatment adjustments. Consequently, LDH as a comprehensive indicator promises economic benefits, improves compliance, and expedites diagnosis and monitoring, making it a practical choice for resource-limited settings. To address this, a retrospective cohort study was conducted to analyze the prognostic significance of serum LDH levels in achieving viral suppression among PLWH.
Materials and Methods
Data Source and Study Population
This retrospective cohort study was conducted between November 2018 and April 2024. Newly diagnosed PLWH who visited the outpatient department of Ningbo Yinzhou No.2 hospital were included. The inclusion criteria were as follows: (1) PLWH who were ART-naive, meaning they had not previously received ART; (2) HIV viral load exceeding 200 copies/mL; (3) Age between 18–70 years. The exclusion criteria included overt infection, severe liver and kidney insufficiency, skeletal muscle injury, pulmonary embolism, myocardial infarction, a history of malignancy, prior use of ART or other antiviral drugs, and hospital admission for any reason within 30 days before cohort entry (including opportunistic infection or AIDS conditions). Ultimately, 393 participants met the inclusion and exclusion criteria (Figure 1).
 |
Figure 1 Flow chart of sample size. |
Study Variables
Eligible patients underwent detailed medical history taking and laboratory examination at study entry. Demographic, clinical, and biochemical data, including age, gender, transmission category, ART regimen, CD4 and CD8 counts, HIV viral load, white blood cell (WBC) count, lymphocyte (LY) count, hemoglobin (HGB) concentration, platelet (PLT) count, and LDH levels, were obtained from medical records. Transmission categories were classified into three groups: men who have sex with men (MSM), heterosexuals, and “Unknown” for those unwilling to disclose their status. LDH levels were measured using an LDH assay kit (MedicalSystem Biotechnology, China), following the manufacturer’s instructions. The intra-assay coefficient of variation (CV) was 3.6%, and the inter-assay CV was 5.5%. Blood samples were collected in EDTA tubes and processed within two hours. Plasma LDH levels were measured spectrophotometrically by assessing the reduced forms of nicotinamide adenine dinucleotide at 340 nm. PLWH were divided into three groups based on tertile LDH levels: group A (LDH≤178 U/L), group B (178 U/L < LDH≤213 U/L), and group C (LDH>213 U/L).
Treatment
All subjects received a rapid initiation of ART containing two nucleoside reverse transcriptase inhibitors (NRTIs) with a third agent, which could be a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an integrase strand transfer inhibitor (INSTI). To streamline analyses, ART regimens were categorized into four types based on whether they were single-tablet regimens (STRs) or multi-tablet regimens (MTRs) and whether they included INSTIs. INSTI-based STR regimens included elvitegravir/cobicistat emtricitabine/tenofovir, alafenamide/emtricitabine/bictegravir, and lamivudine/dolutegravir. INSTI-based MTR regimens included tenofovir alafenamide fumarate + lamividine + dolutegravir and tenofovir disoproxil fumarate + lamividine + dolutegravir. Non-INSTI-based STR regimens included tenofovir disoproxil fumarate/lamividine/ainuovirine. Non-INSTI-based MTR regimens included zidovudine + lamivudine + efavirenz, tenofovir alafenamide fumarate + lamividine + efavirenz, tenofovir disoproxil fumarate + lamividine + efavirenz, tenofovir disoproxil fumarate + lamividine + ainuovirine, tenofovir disoproxil fumarate + lamividine + nevirapine, zidovudine + lamividine + lopinavir/ritonavir, tenofovir alafenamide fumarate + lamividine + lopinavir/ritonavir, and tenofovir disoproxil fumarate + lamividine + lopinavir/ritonavir.
Endpoint
The study endpoint was defined as successful viral suppression, with follow-up time measured from ART initiation to either the first occurrence of viral suppression or ART replacement due to failure in achieving suppression. HIV viral load was assessed at baseline, one month after treatment initiation, every three months thereafter, and during clinical disease progression. As the sole designated hospital for HIV in the region, our institution managed the diagnosis, treatment, and follow-up of all HIV patients in this area. Additionally, medical case managers were specifically responsible for follow-up activities. This management approach ensured the highest level of stability in follow-up care. Definitions of viral suppression varied globally, largely due to differences in healthcare infrastructure, resources, and ART availability. Based on our laboratory testing conditions, viral suppression was defined as HIV plasma RNA levels < 200 copies/mL throughout overall survival, indicating that PLWH cannot transmit HIV to their sexual partners.3 To obtain the required endpoints, we conducted a comprehensive review of the hospital database. PLWH were categorized into a successful viral suppression group or a failed viral suppression group based on the different endpoint events.
Ethics Approval and Consent to Participate
The Institutional Review Board of Ningbo Yinzhou No.2 hospital approved this study (2023–050), and written informed consent was obtained from all the participants. The preservation of data confidentiality was rigorously maintained through the complete exclusion of any personal identifiers. Furthermore, neither the raw data nor the derived data were disseminated to any third party. This study complied with the Declaration of Helsinki.
Statistical Analysis
IBM SPSS Statistics version 24.0 for Mac was used for statistical analysis. No formal sample size estimation was performed due to the lack of published data on similar research. All p-values were two-tailed, with significance set at p < 0.05. Categorical variables were described as numbers and frequencies (%), and continuous variables as medians (IQR). χ²-tests or Fisher’s exact test were used to compare qualitative variables, and the Mann–Whitney U-test or Kruskal–Wallis H-test to was applied to compare continuous variables, as appropriate. Kaplan-Meier survival analysis was conducted to generate survival curves, with group comparisons performed using the Log rank test. HIV viral load was normalized by log10 transformation. Univariable and multivariable Cox proportional hazards regression models were used to identify potential risk factors associated with the endpoints, with hazard ratios and 95% confidence intervals reported.
Results
Baseline Characteristics
In this study, we analyzed data from 393 eligible PLWH received ART, with a median age of 33 (25–49) years old, comprising 350 males and 43 females (Table 1). Of these, 180 (45.8%) PLWH were transmitted through MSM, 181 (46.1%) through heterosexual transmission, and 32 (8.1%) did not disclose their transmission category due to privacy protection. The most common ART regimen was non- INSTI-based MTRs (70.2%), followed by INSTI-based STRs (23.9%), INSTI-based MTRs (4.6%), and non- INSTI-based STRs (1.3%). As shown in Figure S1, no significant differences were observed in the number of censoring events across LDH tertiles (375 vs 397 vs 413, P > 0.05). Although repeated measurements of HIV viral load increased with LDH tertiles, the differences were not statistically significant (1.9 [2.98, 4.42] vs 2.08 [2.98, 4.61] vs 2.11 [2.94, 4.62], P > 0.05).
 |
Table 1 Baseline Characteristics of PLWH According to LDH Tertiles in Different Outcomes |
PLWH were categorized based on tertile LDH levels in different outcomes (Table 1). In the successful viral suppression group, statistical analysis showed that LDH levels were significantly associated with age, CD4 count, log HIV viral load, and time to first viral suppression (P < 0.05). However, no significant differences were found in gender, transmission category, ART regimen, CD8 count, WBC, LY, HGB, or PLT (P>0.05). In the failed viral suppression group, no significant differences were observed in any variable, probably due to the small sample size. Additionally, apart from the transmission category (P < 0.05), no other variables showed statistical significance between the successful and failed viral suppression groups (P > 0.05).
Viral Suppression Rate
After a median time to first viral load suppressed of 84 (28–141.5) days, the overall viral suppression rate was 94.1%. Although viral suppression rates varied among the three groups, no statistically significant differences were observed due to the uniformly high suppression rates in PLWH (Figure 2A) (131/135 vs 120/129 vs 119/129, P = 0.187). However, the time to first viral load suppressed differed significantly among the three groups (Figure 2B). Compared to the low LDH tertile, the middle and high LDH tertiles exhibited longer times to first viral load suppressed (38 [28, 91] vs 84 [29, 154] vs 88 [30, 175] days, respectively, P < 0.001).
 |
Figure 2 Viral suppression rate(A) and time to first viral load suppressed(B) in different LDH tertiles in PLWH. Notes: Group A (LDH≤178U/L); Group B (178 U/L < LDH≤213U/L); Group C (LDH>213U/L). |
Kaplan-Meier Analysis
Kaplan-Meier survival analysis was performed to compare viral suppression across the three LDH tertiles and different ART regimens. As shown in Figure 3A, viral suppression was significantly lower in the middle and high LDH tertiles compared to the low LDH tertile (Log rank test, P < 0.001). As shown in Figure 3B, INSTI-based STRs demonstrated the best prognosis for viral suppression compared to non-INSTI-based MTRs, followed by INSTI-based MTRs and non-INSTI-based STRs (Log rank test, P < 0.001).
 |
Figure 3 The cumulative rate of viral suppression in different LDH tertiles (A) and ART regimens (B) in PLWH. Notes: Group A (LDH≤178U/L); Group B (178 U/L<LDH≤213U/L); Group C (LDH>213U/L). |
Prognostic Analyses of Endpoint
Cox regression models were constructed to evaluate the endpoint of viral suppression after ART (Table 2). According to univariate regression analysis, the ART regimen, log HIV viral load, and LDH tertile were identified as significant predictors of viral suppression. Compared to the low LDH tertile, the middle (HR = 0.635, CI = 0.494–0.816, P < 0.001) and high LDH tertiles (HR = 0.555, CI = 0.431–0.716, P < 0.001) were significant predictors of viral suppression. Multivariate Cox regression analysis further confirmed that LDH tertile remained a significant predictor of viral suppression (HR = 0.714, 95% CI = 0.553–0.922, P = 0.010 for middle vs low tertile, and HR = 0.575, 95% CI = 0.443–0.747, P < 0.001 for high vs low tertile), along with the ART regimen and log HIV viral load.
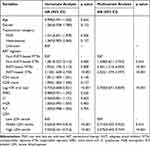 |
Table 2 Cox Regression Analysis for Viral Suppression of PLWH on ART |
Discussion
According to the UNAIDS report, 71% (60–83%) of PLWH worldwide achieved viral suppression in 2022 (https://www.unaids.org/en/resources/fact-sheet). In our study, the viral suppression rate among PLWH was 94.1%. Our results exceeded the global average, while a gap remains in achieving the “95–95-95” target set by UNAIDS to combat the AIDS epidemic. Maintaining viral suppression remains a significant challenge in efforts to end the HIV epidemic. Variability in the duration of viral suppression is observed among individuals receiving the same treatment regimen. Therefore, identifying improved prognostic indicators is of great clinical significance in the current environment. In this study, we enrolled 393 ART-naive patients in a retrospective cohort and investigated the association between LDH levels, time to viral suppressed and viral suppression outcomes using Cox regression analysis. Although no difference was observed in the viral suppression rate, LDH levels proved to be a reliable predictor of time to first viral load suppressed in PLWH, which was independent of ART regimen, baseline viral load, or other indicators.
In our study, INSTI-based STRs showed the highest efficacy in achieving viral suppression compared with other ART regimens. STRs, which combine several antiretroviral drugs into a single dose, offer a simplified dosing schedule. In contrast, MTRs often require a more complex treatment schedule but may offer greater flexibility in drug selection. Several studies have reported the association between STRs vs MTRs use and viral suppression, but the results are somewhat controversial. Five studies reported that the use of STRs positively affects viral suppression.17–21 Conversely, two studies found no significant relationship between STRs use and viral suppression.22,23 The positive effect of STRs may be attributed to improved adherence. A systematic review illustrated that PLWH show greater adherence to STRs than to MTRs.24 The outcomes indicated that augmented adherence substantially heightens the probability of achieving viral suppression in observational contexts. INSTIs have been shown to achieve faster viral suppression compared to other antiretroviral drugs, such as PIs and NNRTIs.25,26 Studies have also highlighted that INSTI-based regimens are highly effective across diverse populations, including pregnant individuals,27 those with drug-resistant HIV strains and suboptimal adherence.28 In our study, the use of STRs and INSTI can lead to faster viral suppression. When choosing between these regimens, factors such as patient preferences, potential drug interactions, and long-term treatment adherence should be considered, all of which contribute to the overall success of viral suppression in PLWH.
Elevated LDH levels were frequently observed in HIV-related complications, such as Pneumocystis pneumonia (PCP), lymphoma, and histoplasmosis, and are associated with poor prognosis. Among PLWH with non-Hodgkin lymphoma, elevated LDH levels strongly predicted an unfavorable prognosis following chemotherapy.29 Wu et al developed a predictive model for assessing in-hospital mortality risk among PLWH with PCP in China. They identified LDH > 350 U/L as an integral component, indicating that elevated LDH levels correlate with poor prognosis in PLWH with PCP.30 Additionally, LDH levels served as a predictor of mortality in PLWH with disseminated histoplasmosis.31 Similarly, case reports have shown that LDH levels are typically elevated during the acute phase of HIV infection, often accompanied by increased viral load.32–35 In our study, the differences in viral suppression rates across different LDH tertiles were statistically insignificant, indicating effective viral suppression in all PLWH. However, times to first viral load suppressed varied significantly, with lower LDH tertiles indicating faster viral suppression and better prognosis.
The elevation of serum LDH levels in PLWH occur through multiple mechanisms. In the context of opportunistic infections, the elevation of serum LDH levels are attributed to pathogen invasion, which causes direct tissue damage and releases LDH into the bloodstream.36 Although our study excluded patients with opportunistic infections, elevated LDH levels were observed, likely through a mechanism distinct from that of opportunistic infections. This mechanism may be mediated by glycolysis. LDH functions as a key enzyme in the glycolytic pathway, responsible for converting pyruvate to lactate.37 An enhanced glycolytic pathway can lead to elevated LDH levels. Since viruses lack their own metabolic pathways, they infect quiescent cells and induce metabolic activation to support genome replication and virion packaging. During infection, viral proteins interact with various cellular glycolytic enzymes, significantly increasing the glycolytic rate of the host cell.38 HIV enhances host cell glycolysis, raising LDH levels. The mTOR pathway, a central regulator of cellular processes, plays a crucial role in HIV’s regulation of glycolytic enzyme expression through its downstream effectors.39 In summary, inhibiting the glycolytic pathway could be a therapeutic strategy against viral infections, with targeted LDH activity inhibition offering potential management for PLWH.
There were some limitations in this study. First, due to laboratory constraints, the minimum detectable HIV viral load in our study was 200 copies/mL, which was in line with WHO requirements to the best extent possible.40 However, a more precise lower detection limit would improve the predictive accuracy for viral suppression in PLWH. Second, this cohort study was conducted on a Chinese population, so caution is necessary when extrapolating the findings to populations with diverse genetic backgrounds, considering potential variations and unique genetic factors. Additionally, this study did not explore other factors related to viral suppression, such as adherence and the emergence of drug resistance. Therefore, further prospective studies that incorporate treatment adherence and drug resistance are required to conclusively determine the predictive value of LDH in viral suppression.
Conclusion
Our study demonstrates that LDH levels serve as a predictor of the timing of viral suppressed in PLWH. A comprehensive evaluation of LDH levels is beneficial in establishing risk stratification in the context of rapid ART initiation.
Data Sharing Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Acknowledgments
We thank all of the participants of the study for their participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Medical Health Science and Technology Project of Zhejiang Province (Grant No. 2024KY1608).
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Bekker LG, Beyrer C, Mgodi N, et al. HIV infection. Nat Rev Dis Primers. 2023;9(1):42. doi:10.1038/s41572-023-00452-3
2. Wong CS, Wei L, Kim YS. HIV late presenters in Asia: management and public health challenges. AIDS Res Treat. 2023;2023:9488051. doi:10.1155/2023/9488051
3. Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of hiv infection: undetectable equals untransmittable. JAMA. 2019;321(5):451–452. doi:10.1001/jama.2018.21167
4. Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181. doi:10.1001/jama.2016.5148
5. Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–e447. doi:10.1016/S2352-3018(18)30132-2
6. Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi:10.1016/S0140-6736(19)30418-0
7. Michienzi SM, Barrios M, Badowski ME. Evidence regarding rapid initiation of antiretroviral therapy in patients living with HIV. Curr Infect Dis Rep. 2021;23(5):7. doi:10.1007/s11908-021-00750-5
8. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. doi:10.1371/journal.pmed.1002015
9. Mateo-Urdiales A, Johnson S, Smith R, Nachega JB, Eshun-Wilson I. Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database Syst Rev. 2019;6(6):Cd012962. doi:10.1002/14651858.CD012962.pub2
10. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA. 2023;329(1):63–84. doi:10.1001/jama.2022.22246
11. SeyedAlinaghi S, Afsahi AM, Moradi A, et al. Current ART, determinants for virologic failure and implications for HIV drug resistance: an umbrella review. AIDS Res Ther. 2023;20(1):74. doi:10.1186/s12981-023-00572-6
12. Di Stefano G, Manerba M, Di Ianni L, Fiume L. Lactate dehydrogenase inhibition: exploring possible applications beyond cancer treatment. Future Med Chem. 2016;8(6):713–725. doi:10.4155/fmc.16.10
13. Grywalska E, Roliński J, Pasiarski M, et al. High viral loads of Epstein-Barr virus DNA in peripheral blood of patients with chronic lymphocytic leukemia associated with unfavorable prognosis. PLoS One. 2015;10(10):e0140178. doi:10.1371/journal.pone.0140178
14. Liu J, Zhao F, Lu J, et al. High mycoplasma pneumoniae loads and persistent long-term mycoplasma pneumoniae DNA in lower airway associated with severity of pediatric mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2019;19(1):1045. doi:10.1186/s12879-019-4667-y
15. Nishijima H, Ogawa T, Shirasawa H. Diagnostic significance of lactate dehydrogenase in measles virus reinfection cases. Microbiol Immunol. 2022;66(11):519–528. doi:10.1111/1348-0421.13021
16. Ataee Z, Rahmani Fard A, Amel Jamehdar S, Khadem-Rezaiyan M, Ziaee M. Relationship of viral load with the laboratory markers and prognosis in COVID-19 patients. Med J Islam Repub Iran. 2023;37:67. doi:10.47176/mjiri.37.67
17. Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(5):587–596. doi:10.1097/QAI.0000000000000082
18. Chakraborty H, Weissman S, Duffus WA, et al. HIV community viral load trends in South Carolina. Int J STD AIDS. 2017;28(3):265–276. doi:10.1177/0956462416642349
19. Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17. doi:10.1186/s12981-018-0204-0
20. Carr A, Richardson R, Liu Z. Success and failure of initial antiretroviral therapy in adults: an updated systematic review. Aids. 2019;33(3):443–453. doi:10.1097/QAD.0000000000002077
21. Gallien S, Massetti M, Flandre P, Leleu H, Descamps D, Lazaro E. Comparison of 48-week efficacies of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide and nucleoside/nucleotide reverse transcriptase inhibitor-sparing regimens: a systematic review and network meta-analysis. HIV Med. 2018;19(8):559–571. doi:10.1111/hiv.12643
22. DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, Phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–2438. doi:10.1016/S0140-6736(12)60918-0
23. Tennant SJ, Hester EK, Caulder CR, Lu ZK, Bookstaver PB. Adherence among rural HIV-infected patients in the deep south: a comparison between single-tablet and multi-tablet once-daily regimens. J Int Assoc Provid AIDS Care. 2015;14(1):64–71. doi:10.1177/2325957414555228
24. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490. doi:10.2147/PPA.S192735
25. Jacobson K, Ogbuagu O. Comparison of time to viral suppression among treatment-naïve HIV-infected adults initiating combination antiretroviral therapy by antiretroviral regimen class. Open Forum Infect Dis. 2017;4(suppl_1):S432–S432. doi:10.1093/ofid/ofx163.1090
26. Jacobson K, Ogbuagu O. Integrase inhibitor-based regimens result in more rapid virologic suppression rates among treatment-naïve human immunodeficiency virus-infected patients compared to non-nucleoside and protease inhibitor-based regimens in a real-world clinical setting: a retrospective cohort study. Medicine. 2018;97(43):e13016. doi:10.1097/MD.0000000000013016
27. Rahangdale L, Cates J, Potter J, et al. Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. Am J Obstet Gynecol. 2016;214(3):385.e381–387. doi:10.1016/j.ajog.2015.12.052
28. Andreatta K, D’Antoni ML, Chang S, et al. High efficacy of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in Black adults in the United States, including those with pre-existing HIV resistance and suboptimal adherence. J Med Virol. 2024;96(10):e29899. doi:10.1002/jmv.29899
29. Clayton A, Mughal T. The changing face of HIV-associated lymphoma: what can we learn about optimal therapy inl the post highly active antiretroviral therapy era? Hematol Oncol. 2004;22(3):111–120. doi:10.1002/hon.735
30. Wu L, Zhang Z, Wang Y, et al. A model to predict in-hospital mortality in HIV/AIDS patients with pneumocystis pneumonia in China: the clinical practice in real world. Biomed Res Int. 2019;2019:6057028. doi:10.1155/2019/6057028
31. Boigues BCS, Paniago AMM, Lima GME, Nunes MO, Uehara SNO. Clinical outcomes and risk factors for death from disseminated histoplasmosis in patients with AIDS who visited a high-complexity hospital in Campo Grande, MS, Brazil. Rev Soc Bras Med Trop. 2018;51(2):155–161. doi:10.1590/0037-8682-0369-2017
32. Manji F, Wilson E, Mahe E, Gill J, Conly J. Acute HIV infection presenting as hemophagocytic lymphohistiocytosis: case report and review of the literature. BMC Infect Dis. 2017;17(1):633. doi:10.1186/s12879-017-2732-y
33. Sarmiento M, Balcells ME, Ramirez P. Thrombotic microangiopathy as first manifestation of acute human immunodeficiency virus infection: a case report and review of the literature. J Med Case Rep. 2016;10(1):152. doi:10.1186/s13256-016-0938-z
34. Vishnu P, Dorer RP, Aboulafia DM. Immune reconstitution inflammatory syndrome-associated Burkitt lymphoma after combination antiretroviral therapy in HIV-infected patients. Clin Lymphoma Myeloma Leuk. 2015;15(1):e23–29. doi:10.1016/j.clml.2014.09.009
35. Salzer HJF, Schäfer G, Hoenigl M, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with pneumocystis jirovecii pneumonia. Respiration. 2018;96(1):52–65. doi:10.1159/000487713
36. Tweedell RE, Malireddi RKS, Kanneganti TD. A comprehensive guide to studying inflammasome activation and cell death. Nat Protoc. 2020;15(10):3284–3333. doi:10.1038/s41596-020-0374-9
37. Zhou Y, Qi M, Yang M. Current status and future perspectives of lactate dehydrogenase detection and medical implications: a review. Biosensors. 2022;12(12):1145.
38. Goyal P, Rajala MS. Reprogramming of glucose metabolism in virus infected cells. mol Cell Biochem. 2023;478(11):2409–2418. doi:10.1007/s11010-023-04669-4
39. Crater JM, Nixon DF, Furler O’Brien RL. HIV-1 replication and latency are balanced by mTOR-driven cell metabolism. Front Cell Infect Microbiol. 2022;12:1068436. doi:10.3389/fcimb.2022.1068436
40. WHO. Guidelines approved by the guidelines review committee. In: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Vol. 2016. Geneva: World Health Organization Copyright © World Health Organization; 2016.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

