Back to Journals » International Journal of General Medicine » Volume 17
The Prevalence of Depressive Symptoms in Patients with Idiopathic Parkinson’s Disease: Cross-Sectional Study from Somalia
Authors Sheikh Hassan M , Mohamed NA , Yücel Y , Abdirisak Mohamed Y , Gökgül A
Received 26 August 2024
Accepted for publication 31 October 2024
Published 6 November 2024 Volume 2024:17 Pages 5059—5068
DOI https://doi.org/10.2147/IJGM.S493161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Redoy Ranjan
Mohamed Sheikh Hassan,1 Nur Adam Mohamed,2 Yavuz Yücel,3 Yusuf Abdirisak Mohamed,2 Alper Gökgül1
1Department of Neurology, Mogadishu Somalia Turkiye Training and Research Hospital, Mogadishu, Somalia; 2Department of Psychiatry and Behavioral Sciences, Mogadishu Somalia Turkiye Training and Research Hospital, Mogadishu, Somalia; 3Dicle University, Faculty of Medicine, Neurology Department, Diyarbakir, Turkiye
Correspondence: Mohamed Sheikh Hassan, Department of Neurology, Mogadishu Somalia Turkiye Training and Research Hospital, Mogadishu, Somalia, Email [email protected]
Introduction: Depression is one of the most common non-motor symptom of Parkinson’s disease (PD), with an increasing prevalence in recent years. It causes significant psychological consequences that affect the disease course, overall quality of life, and functioning. The objective of this study was to determine the prevalence of depression in outpatients with Parkinson’s disease evaluated in the neurology clinic at tertiary hospital in Mogadishu, Somalia.
Methods: A cross-sectional study was conducted among 50 PD patients without dementia to determine the prevalence of depression at the neurology clinic of the Mogadishu Somalia Türkiye Training and Research Hospital between February and November 2022. All eligible participants were interviewed by a team of doctors using a structured questionnaire that consisted of sociodemographics and clinical characteristics, the Beck Depression Inventory-II (BDI-II) for depression assessment, and the Modified Hoehn and Yahr Scale for PD staging.
Results: Of the 50 PD participants, 60% were male and 58% were older than 60 years. 20% of them had a family history of PD and HTN as comorbid conditions. The prevalence of depression among the participants was 46% (95% CI 31.8– 60.7). Of the patients with depression, 22% and 24% had mild and moderate depressive symptoms, respectively. The Mann–Whitney U-test revealed a statistically significant association between depression symptoms and the presence of comorbidity (χ 2 = 136.50, p< 0.01). The Kruskal–Wallis test revealed a statistically significant association between depression symptoms and a longer duration of PD (χ 2 (2) = 18.21, p< 0.01) and advanced stages of PD (χ 2 (2) = 13.74, p< 0.01).
Conclusion: This is the first study conducted on patients with PD in Somalia and found that a significant proportion of these patients experienced depressive symptoms. We also highlighted that factors such the presence of medical comorbidities, high monthly income, advanced PD stage, longer duration of PD, and use of multiple medications for PD were significantly associated with the presence of depressive symptoms.
Keywords: Parkinson’s disease, depressive symptoms, non-motor symptoms, Somalia
Introduction
Parkinson’s disease (PD) is the second most frequent progressive neurodegenerative disease of the elderly and is characterized by motor symptoms including bradykinesia, shuffling gait, resting tremor, muscle rigidity, and postural instability.1,2 PD is uncommon before the age of 50 and affects 1–2 per 1000 individuals at any given time and 1% of people over the age of 60, and this prevalence increases to 4% in the highest age groups.3–5 According to a recent review, approximately 6 million people were suffering globally from PD in 2015, and this prevalence is predicted to rise to an estimated 12 million individuals by 2040.6 Several studies have shown the increasing incidence trends of PD, which may be attributed to factors such as aging, the growing population, several environmental causes, and modern advancements in diagnostic techniques.4 PD not only has motor symptoms but is also associated with non-motor symptoms (NMS), including sensory, autonomic, cognitive, sleep, and psychiatric disturbances.2,7
Depression is one of the most prevalent psychiatric manifestations of PD, which can manifest at any stage of the illness8 or even occur before the development of motor symptoms, and it is now recognized as a risk factor in the diagnostic criteria for prodromal PD.9 In fact, having symptoms of both depression and anxiety disorders has been linked to a twofold increase in the likelihood of acquiring PD later in life.10,11 Studies have shown that the prevalence of depression has been rising notably in the past few years, with up to 50% of PD patients presenting with this disorder.12,13 Depression in PD patients has been linked to increased disability and mortality, rapid cognitive decline, and a greater burden on families and caregivers, as well as impaired patients’ quality of life, which has major ramifications for the patient’s psychological and physical well-being.14–16 Although the pathophysiology of depression in PD patients is still unclear, studies have shown the presence of changes in the limbic system, dysfunction in subcortical nuclei and the prefrontal cortex, striatal–thalamic–prefrontal, nucleus accumbens and brainstem monoamine, and indolamine (ie dopamine, serotonin, and norepinephrine) systems.17–20 Genetic factors have been implicated in the development of PD, and these factors may also influence the risk of depression in individuals with PD. Inherited monogenic and idiopathic PD cases are caused by mutations in SNCA, LRRK2, and VPS35. The instances of early-onset PD (age <40) are related to autosomal recessive variants like PARKIN, PINK1, and DJ-1. While the late-onset PD (age >50) is correlated with autosomal dominant variants like LRRK2 and GBA.21,22
The diagnosis of depression in the setting of PD has always been difficult due to the symptom overlap between the two disorders. According to a recent study, psychiatric conditions and traits may have multiple roles in the likelihood of developing PD, and PD may also be related to the possibility of developing mental illnesses.23
There is little information available on the prevalence of depression in patients with PD Africa and to our knowledge there is no data regarding this topic in Somalia. Therefore, the primary objective of this study was to determine the prevalence of depression in patients with PD who visited the outpatient clinic of the Mogadishu Somalia Turkiye Training and Research Hospital, Mogadishu, Somalia.
Methods and Materials
Study Design and Participants
This hospital-based prospective cross-sectional study was conducted to determine the prevalence of depression among PD patients at the neurology clinic of Mogadishu Somalia Türkiye Training and Research Hospital (a public, teaching, and tertiary referral hospital located in Mogadishu, Somalia) between February and November 2022. Fifty patients with PD without cognitive decline were invited to participate in the study, with the aim of screening for depressive symptoms. PD was diagnosed according to the clinical criteria of the UK Brain Bank. In addition, patients with PD were classified into five stages using the Modified Hoehn and Yahr Scale.
Sample Size
The convenience sampling approach, a non-random sampling method, was used to enroll the participants. Moreover, the inability to calculate the sample size from a non-random sample was the main reason why the sample size could not be computed.
Inclusion and Exclusion Criteria
The inclusion criteria included adult patients with PD diagnosed for more than a year according to the UK Brain Bank criteria who did not have cognitive decline, were ready to participate in the study, and provided informed consent. The exclusion criteria included patients with symptoms or signs of cognitive dysfunction, a history of one of the dementia disorders, or treatment for cognitive impairment, serious hearing or visual impairment, severe general medical conditions such as renal and liver failure and patients with pre-existing diagnosis of depression prior to their diagnosis of Parkinson’s disease.
Data Collection Tools and Methods
The data were collected from eligible participants through interviews by a team of neurologists and psychiatrists using a structured questionnaire that consisted of sociodemographics and clinical characteristics, including age, gender, education status, comorbidities, duration, and medication used for PD. Depressive symptoms were assessed using the Beck Depression Inventory-II (BDI-II), and PD stage severity was assessed using the Hoehn and Yahr scale.
The BDI-II is a 21-item self-administered psychometric scale developed by Aaron Beck24 for the evaluation of the severity of depressive symptoms based on DSM-IV criteria.25 Every item has a 4-point Likert scale response ranging in severity from 0 to 3, providing a total score ranging from 0 to 63. A higher score indicates more severe depressive symptoms. A score of 0–13 suggests absence or minimal depressive symptoms, whereas scores from 14–19 suggest mild depression, 20–28 suggest moderate depression, and 29–63 suggest the presence of severe depressive symptoms.24 As a result, a score <14 indicates the absence of depression symptoms, while a score ≥14 indicates the presence of depression symptoms. The BDI-II has been demonstrated to be a valid and reliable assessment tool for depressive symptoms in PD patients.26,27
Statistical Analysis
Statistical analysis was conducted using IBM Corp.’s Statistics for Windows, Version 26.0, the Statistical Package for the Social Sciences (SPSS) software. Histograms, Q-Q plots, box plots, and the Shapiro–Wilk test of normality were used to assess the data distribution before the data were analyzed. Continuous variables are represented by means and standard deviations, and frequencies and percentages are used to illustrate categorical variables. The Kruskal–Wallis H-test and Mann–Whitney U-test were used to examine the differences between variables with three or more groups and variables with dichotomous independent variables, respectively. Statistical significance was set at p <0.05.
Ethical Approval
The study was reviewed and approved by the ethics committee of the Mogadishu Somalia Turkiye Training and Research Hospital (reference number: MSTH/8132). This study was conducted in compliance with the Declaration of Helsinki and all hospital policies and procedures. Informed written consent was obtained from each patient or their next of kin before the start of data collection, and no personal data that could lead patient recognition were presented in the results.
Results
The study enrolled 50 Patients with idiopathic Parkinson’s disease without dementia to determine the prevalence of depression in these patients. All eligible participants were interviewed using a structured questionnaire that consisted of sociodemographics and clinical characteristics, the Beck Depression Inventory-II (BDI-II) for depression assessment, and the Modified Hoehn and Yahr Scale for PD staging.
Sociodemographics of the Study Subjects
As indicated in Table 1, 58% of the study participants were older than 60 years of age, with the majority being male. Almost half of the patients had low income (48%). Approximately 46% of the subjects had a PD duration of 5–10 years. One-fifth of the participants had a family member with PD. More than one-third of the study participants did not have any comorbid diseases. The most common comorbidities were hypertension and diabetes mellitus (20% and 18%, respectively). Other comorbidities included heart diseases, metabolic conditions, and epilepsy. Based on the findings of the Modified Hoehn and Yahr Scale, the median PD stage score was 2±1.8. Most of the study subjects had early stage PD (stage I or stage 2).
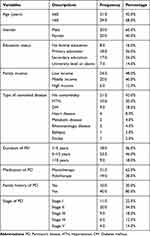 |
Table 1 Sociodemographic Characteristics of the Participants |
Prevalence of Depression Symptoms
As indicated in Table 2, twenty-three subjects had depressive symptoms according to the BDI-II. Thus, the prevalence of depression in this population was 46.0% (95% CI 31.8–60.7). The mean BDI-II score for the total sample was 12.60 (SD ± 7.13). In the depressed and non-depressed groups, the mean BDI scores were 19.30 (SD ± 3.44) and 6.89 (SD ± 3.47), respectively. Among the depressed patients, 11 (22.0%) had mild depressive symptoms, and 12 (24.0%) had moderate depressive symptoms when categorized based on the BDI-II score.
 |
Table 2 Prevalence of Depression Among Participants |
Depressive Symptom Level Comparison Among Participants
As indicated in Table 3, the Mann–Whitney U-test revealed no significant difference in depression levels between males and females (χ2 = 295, p = 0.92). Kruskal–Wallis test showed a statistically significant difference in depression levels across the three groups of PD duration (χ2 (2) = 18.21, p<0.01) and the five groups of PD stages (χ2 (4) = 13.74, p<0.01). The group with a disease duration of >10 years had a higher median score than the other two groups, and the groups with advanced stages of the disease had higher median scores. Patients with other comorbidities had more depressive symptoms than those without comorbidities (χ2= 136.50, p< 0.01). Patients with a higher income level had more depressive symptoms than those with low or middle income levels.
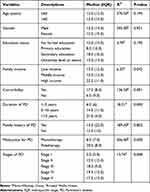 |
Table 3 Comparison of the Sociodemographic of the Subjects to the Depressive Symptom Levels |
Discussion
Patients who suffer from chronic medical conditions frequently experience psychiatric disorders, such as depression, and as a result of the negative effects these diseases have on their overall quality of life as well as on their occupational, physical, and emotional wellbeing.28–32 In the last few years, NMS, especially depression, has played a more significant role in the clinical definition of PD due to its frequent and incapacitating nature, which frequently affects the well-being of patients and caregivers.9 Although the prevalence of depression among people with PD is a well-known consequence, studies from developing countries are limited when compared to developed countries.33 Therefore, the primary aim of this study was to determine the prevalence of depressive symptoms in PD patients who visit the outpatient clinic of our Hospital in Mogadishu, Somalia.
In the present study, the prevalence of depression among PD patients reported is comparable with that observed in previous studies in Lebanon,34 Germany,35 and India.36 Our findings were a little lower than the results reported by several previous studies in Ethiopia37 and Sri Lanka,38 but higher than the findings of PD patients in Taiwan,39,40 Mexico,41 India,42 Pakistan,21 Egypt,31 and China.32 A recent systematic review and meta-analysis of 21 studies found that the pooled prevalence of depression among PD patients was 22.9%, which is lower than our results.43 Similarly, another recent systematic review and meta-analysis of 129 studies found that the pooled prevalence of depression among PD patients was 38%, which is again lower than what we reported in our study.44 According to several studies, the prevalence of depression in PD patients ranges between 11% and 61%.32,37,38,40 These differences may be due to the variabilities in sample size and sampling procedures, depression assessment tools, population genetic differences, and sociodemographic factors of the participants.
Various factors can contribute to the development of depressive symptoms in patients with PD, including dependence, physical changes, impaired balance, speed, agility, lack of spontaneity, and coexisting physical illnesses that are associated with the disease. Whether these factors alone can account for the higher rates of depressive symptoms in PD remains controversial. According to a recent study, PD patients may be more susceptible to experiencing depressive symptoms than healthy people due to anatomical brain abnormalities, such as abnormal amygdala function.45 Another study hypothesized that there is a link between depression in PD and compromised white matter integrity, particularly in the long contact fibers of the left hemisphere.46 In a recent review, it was found that there is significant proof linking the development of motor and non-motor symptoms such as tremors and depressive symptoms and the consequences associated with PD to the gradual degeneration of 5-HT terminals.17
Depression in patients with Parkinson’s disease is linked to increased disability and mortality, heightened cognitive decline, greater strain on families and caregivers, and reduced quality of life, significantly impacting the individual’s psychological and physical health. Depression affects quality of life both directly and indirectly by impairing daily activities, which subsequently diminishes overall quality of life.18,47 Eman M. Khedr et al found that depression exhibited a significant negative correlation with the quality of life in people with Parkinson’s disease. This demonstrates that depression is not a reactive response to the impairment but an intrinsic component of the Parkinson’s disease spectrum.48
Despite the high prevalence of depressive symptoms among PD patients, when managing the primary neurological disorder vigorously, it is common for the patient’s psychological health to be overlooked, unrecognized and untreated. In the present study, none of the PD patients with depression were taking antidepressants or had been previously diagnosed with a depression disorder. Comparable results have been reported from studies in the literature.31,37 Contrary to our results, several studies have shown higher rates of antidepressant medication use in PD patients.34,41,49 Several factors can make it challenging to diagnose depression in PD patients, such as the possibility that the characteristics of depression and PD coincide, the potential for an atypical symptom pattern in depression associated with PD, and the fact that a milder form of depression is frequent in PD and may go unrecognized. Another reason for underdiagnoses is that individuals with neurodegenerative diseases, their families, and even doctors themselves could view depressive symptoms as typical reactions to their condition rather than characteristics of a coexisting mood disorder.50
Parkinson’s disease-related depression and standard depression may have similar symptoms, but there are certain criteria that can help differentiate PD-related depression from conventional depression. First, the timing of onset: PD-related depression typically arises with the emergence of motor symptoms, while conventional depression may have a different onset pattern. Second, symptoms may overlap: Some symptoms of Parkinson’s Disease, such as fatigue, apathy, and cognitive decline, may coincide with symptoms of typical depression. However, depression related to Parkinson’s Disease may primarily exhibit symptoms such as anhedonia and feelings of worthlessness more than typical depression. Conversely, suicidal thoughts are less common in depression related to Parkinson’s disease than in typical depression. Third, antidepressants may be less effective in depression related to Parkinson’s disease, possibly due to distinct neurobiological alterations. Dopaminergic therapies may help alleviate depression symptoms by correcting dopamine deficiencies. The presence of motor symptoms in Parkinson’s disease patients (eg, tremors, stiffness, and bradykinesia) predisposes them to depression associated with Parkinson’s disease.
In the present study, most of the patients were older than the age of sixty, and we did not find any association between depression and the PD patient’s current age, which is similar to what a previous study reported.37 In contrast to our results, a study conducted among Lebanese PD patients reported an association between depression and younger age.34 Although being female may be a risk factor for the development of depression in PD patients, there is no agreement on that, and this current study does not find any correlation between gender and depression symptoms among the PD population, which contradicts several previous studies that have shown that females with PD experience depression more than males.31,34,41,42 In contrast to several previous studies41,42,44 which reported a relationship between education and depressive symptoms among this population, our current study did not find any association between depression levels and the education status of PD patients. We also found that, higher income levels were significantly associated with depressive symptoms among patients with PD. In contrast to our findings several studies have reported significant association between depressive symptoms and lower income levels38 and others reported no relationship between these variables,39,42,49 while Worku et al stated that all levels of income were significantly correlated with the presence of depressive symptoms among patients with PD.37
Our findings highlighted a significant positive association between comorbid medical conditions and the development of depressive symptoms in patients with PD. Contrary to our findings, several studies have shown no association between depression symptoms and comorbidities such as HTN and DM.37–39,42,49 Similarly, in contrast to our results, no association was found between medical comorbidity and depression symptoms in a recent one-year prospective study involving PD patients and their caretakers.40 It is logical to assume that the duration of PD will affect the levels of depression among these patients. Therefore, we have noticed that patients with a longer duration of disease experience more depressive symptoms, which is consistent with the literature32,51 but contradicts previous studies conducted among Ethiopian PD patients,37 Sri Lankan patients,38,49 and a recent Indian study.42 We also discovered that depressive symptoms were more common among PD patients with advanced stages of the disease, especially in stages III and IV, which is in line with previous studies.31,32,42,49,51
There are a few limitations to our study that should be mentioned. First, the study’s cross-sectional nature is a weakness that prevents us from establishing a cause-and-effect relationship. Second, the small sample size is another drawback of this study, and we cannot generalize the findings to the general population. Therefore, we recommend conducting larger cohort studies in the future that allow the results to be generalizable. Third, another limitation that affects the generalizability of the findings is the convenience sampling method adopted in this study because of the limited number of cases. Another limitation of this study is that we did not investigate other psychiatric comorbidities, which are known to frequently co-occur and may influence the overall mental health outcomes of the participants. Lastly, the evaluation of depressive symptoms was based on the BDI-II scale instead of the gold standard DSM-V criteria for depressive disorders. However, the BDI-II has good reliability and validity for screening and assessing depressive symptom levels in this population.26 In spite of these limitations, this is the first study to determine the prevalence of depressive symptoms among PD patients in Somalia.
Conclusions
In conclusion, this is the first study conducted in Somalia on PD patients and found that a significant percentage of these patients suffered from depressive symptoms, and none of them were diagnosed prior to screening for this study. Additionally, the factors that showed a significant association with the presence of depressive symptoms in our study were the presence of medical comorbidities, high monthly income, an advanced PD stage on the Hoehn and Yahr scale, longer duration of PD, and being on polytherapy for PD. Wider cohort studies with larger sample size is recommended in the future to further emphasize the clinical significance of our findings. In addition, we also recommend routine depression screening in patients with PD especially those with apathy) that may be mistaken for depression.
Abbreviations
PD, Parkinson’s Disease; NMS, Non-Motor Symptoms; BDI-II, Beck Depression Inventory-II; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Data Sharing Statement
All the required information is in the manuscript itself.
Informed Consent Statement
All study participants and their legal guardians provide informed written consent before the study recruitment.
Acknowledgments
The authors would like to thank the study participants for their contribution.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Rawat CS, Pandey S. Parkinson’s disease–an introduction. Techniques for Assessment of Parkinsonism for Diagnosis and Rehabilitation. 2022;1–24.
2. Hussein A, Guevara CA, Del Valle P, Gupta S, Benson DL, Huntley GW. Non-motor symptoms of parkinson’s disease: the neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist. 2023;29(1):97–116. doi:10.1177/10738584211011979
3. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi:10.1007/s00702-017-1686-y
4. Ou Z, Pan J, Tang S, et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9:776847. doi:10.3389/fpubh.2021.776847
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/S1474-4422(18)30499-X
6. Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3–S8. doi:10.3233/JPD-181474
7. Ahmad MH, Rizvi MA, Ali M, Mondal AC. Neurobiology of depression in Parkinson’s disease: insights into epidemiology, molecular mechanisms and treatment strategies. Ageing Res Rev. 2023;85(101840):101840. doi:10.1016/j.arr.2022.101840
8. Li Y, Huang P, Guo T, et al. Brain structural correlates of depressive symptoms in Parkinson’s disease patients at different disease stage. Neuroimaging. 2020;296:111029. doi:10.1016/j.pscychresns.2019.111029
9. Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470. doi:10.1002/mds.27802
10. Gustafsson H, Nordström A, Nordström P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology. 2015;84(24):2422–2429. doi:10.1212/WNL.0000000000001684
11. Wang S, Mao S, Xiang D, Fang C. Association between depression and the subsequent risk of Parkinson’s disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:186–192. doi:10.1016/j.pnpbp.2018.05.025
12. Weintraub D. Management of psychiatric disorders in Parkinson’s disease: neurotherapeutics. Movement Disord Therap Neurotherap. 2020;17(4):1511–1524.
13. Kay DB, Tanner JJ, Bowers D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018;8(6):e00967. doi:10.1002/brb3.967
14. Pontone GM, Bakker CC, Chen S, et al. The longitudinal impact of depression on disability in Parkinson disease. Int J Geriatr Psychiatry. 2016;31(5):458–465. doi:10.1002/gps.4350
15. Avanzino L, Lagravinese G, Abbruzzese G, Pelosin E. Relationships between gait and emotion in Parkinson’s disease: a narrative review. Gait Posture. 2018;65:57–64. doi:10.1016/j.gaitpost.2018.06.171
16. Santos-García D, de Deus Fonticoba T, Suárez Castro E, et al. Quality of life and non-motor symptoms in Parkinson’s disease patients with subthreshold depression. J Neurol Sci. 2020;418:117109. doi:10.1016/j.jns.2020.117109
17. Politis M, Niccolini F. Serotonin in Parkinson’s disease. Behav Brain Res. 2015;277:136–145. doi:10.1016/j.bbr.2014.07.037
18. Hu X, Song X, Li E, et al. Altered resting-state brain activity and connectivity in depressed Parkinson’s disease. PloS one. 2015;10(7):e0131133. doi:10.1371/journal.pone.0131133
19. Wei L, Hu X, Zhu Y, Yuan Y, Liu W, Chen H. Aberrant intra- and internetwork functional connectivity in depressed Parkinson’s disease. Sci Rep. 2017;7(1):2568. doi:10.1038/s41598-017-02127-y
20. Xu L, Nan J, Lan Y. The nucleus accumbens: a common target in the comorbidity of depression and addiction. Front Neural Circuit. 2020;14:37. doi:10.3389/fncir.2020.00037
21. Khan MF, Rezayee F, Shafique F. Prevalence of depression in patients of Parkinson disease at district Bannu. Tob Regul Sci. 2023;13:456–465.
22. Uwishema O, Onyeaka H, Badri R, et al. The understanding of Parkinson’s disease through genetics and new therapies. Brain and Behav. 2022;12(5):e2577. doi:10.1002/brb3.2577
23. Wu Q, Liu S, Huang X, et al. Bidirectional Mendelian randomization study of psychiatric disorders and Parkinson’s disease. Front Aging Neurosci. 2023;15:1120615. doi:10.3389/fnagi.2023.1120615
24. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; 1996.
25. American Psychiatric Association DS, American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American psychiatric association; 2013.
26. Torbey E, Pachana NA, Dissanayaka NN. Depression rating scales in Parkinson’s disease: a critical review updating recent literature. J Affect Disord. 2015;184:216–224. doi:10.1016/j.jad.2015.05.059
27. Maggi G, D’Iorio A, Aiello EN, et al. Psychometrics and diagnostics of the Italian version of the beck depression inventory-II (BDI-II) in Parkinson’s disease. Neurol Sci. 2023;44(5):1607–1612. doi:10.1007/s10072-023-06619-w
28. Li H, Ge S, Greene B, Dunbar-Jacob J.Depression in the context of chronic diseases in the United States and China. Int J Nurs Sci. 2018;6(1):117–122.
29. Mohamed NA, Eraslan A, Kose S. The impact of anxiety and depression on the quality of life of hemodialysis patients in a sample from Somalia. BMC Psychiatry. 2023;23(1):825. doi:10.1186/s12888-023-05312-8
30. Huang D, Wang J, Fang H, Fu Y, Lou J. Longitudinal association of chronic diseases with depressive symptoms in middle-aged and older adults in China: mediation by functional limitations, social interaction, and life satisfaction. J Glob Health. 2023;13:04119. doi:10.7189/jogh.13.04119
31. Khedr EM, Abdelrahman AA, Elserogy Y, et al. Depression and anxiety among patients with Parkinson’s disease: frequency, risk factors, and impact on quality of life. Egypt J Neurol Psychiatry Neurosurg. 2020;56(1):116. doi:10.1186/s41983-020-00253-5
32. Cui SS, Du JJ, Fu R, et al. Prevalence and risk factors for depression and anxiety in Chinese patients with Parkinson disease. BMC Geriatr. 2017;17(1):270. doi:10.1186/s12877-017-0666-2
33. Liu Y, Ding L, Xianyu Y, Nie S, Yang J. Research on depression in Parkinson disease: a bibliometric and visual analysis of studies published during 2012-2021. Medicine. 2022;101(31):e29931. doi:10.1097/MD.0000000000029931
34. Ghaddar A, Fawaz M, Khazen G, Abdallah J, Milane A. Prevalence of depression in Parkinson’s disease in a Lebanese tertiary clinic. J Clin Exp Neuropsychol. 2016;38(1):51–58. doi:10.1080/13803395.2015.1087466
35. Usnich T, Hauptmann B, Hanssen H, et al. Depressive symptoms in Parkinson’s disease are insufficiently but more often treated than in other chronic conditions. Parkinson’s Dis. 2023;9(1):113. doi:10.1038/s41531-023-00551-8
36. Sahu S, Mukherjee A, Biswas S, et al. Burden of nonmotor symptoms in Parkinson’s disease patients from eastern India. Ann Mov Disord. 2021;4(3):121–130. doi:10.4103/AOMD.AOMD_5_21
37. Worku DK, Yifru YM, Postels DG, Gashe FE. Prevalence of depression in Parkinson’s disease patients in Ethiopia. J Clin Mov Disord. 2014;1(1):10. (). doi:10.1186/s40734-014-0010-3
38. Herath TB, Withana M, Rodrigo C, Gamage R, Gamage C. Prevalence and associations for symptoms of depression in patients with Parkinson’s disease: a Sri Lankan experience. Int J Ment Health Syst. 2016;10(1):47. doi:10.1186/s13033-016-0079-1
39. Chang YP, Lee MS, Wu DW, et al. Risk factors for depression in patients with Parkinson’s disease: a nationwide nested case-control study. PLoS One. 2020;15(7):e0236443. doi:10.1371/journal.pone.0236443
40. Lee Y, Chang YY, Chen YF, et al. Prevalence and risk factors of depression between patients with parkinson’s disease and their caregivers: a one-year prospective study. Healthcare. 2022;10(7):1305. doi:10.3390/healthcare10071305
41. Rodríguez-Violante M, Cervantes-Arriaga A, Berlanga-Flores C, Ruiz-Chow A. Prevalence and determinants of depression in Mexican patients with Parkinson’s disease. Clin Neurol Neurosurg. 2012;114(10):1293–1296. doi:10.1016/j.clineuro.2012.03.035
42. Sujith P, Arjunan P, Iype T, Natarajan V. Depression in patients with Parkinson’s disease: a hospital-based cross-sectional study. Cureus. 2023;15(10). doi:10.7759/cureus.47214
43. Goodarzi Z, Mrklas KJ, Roberts DJ, Jette N, Pringsheim T, Holroyd-Leduc J. Detecting depression in Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;87(4):426–437. doi:10.1212/WNL.0000000000002898
44. Cong S, Xiang C, Zhang S, Zhang T, Wang H, Cong S. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta-analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
45. Huang P, Xuan M, Gu Q, et al. Abnormal amygdala function in Parkinson’s disease patients and its relationship to depression. J Affect Disord. 2015;183:263–268. doi:10.1016/j.jad.2015.05.029
46. Wu JY, Zhang Y, Wu WB, Hu G, Xu Y. Impaired long contact white matter fibers integrity is related to depression in Parkinson’s disease. CNS Neurosci Ther. 2018;24(2):108–114. doi:10.1111/cns.12778
47. Lawrence BJ, Gasson N, Kane R, Bucks RS, Loftus AM. Activities of daily living, depression, and quality of life in Parkinson’s disease. PLoS One. 2014;9(7):e102294. doi:10.1371/journal.pone.0102294
48. Khedr EM, Abdelrahman AA, Elserogy Y, Zaki AF, Gamea A. Depression and anxiety among patients with Parkinson’s disease: frequency, risk factors, and impact on quality of life. Eg J Neurol Psychiatry Neurosurg. 2020;56:1–9.
49. Ketharanathan T, Hanwella R, Weerasundera R, de Silva VA. Major depressive disorder in Parkinson’s disease: a cross-sectional study from Sri Lanka. BMC Psychiatry. 2014;14:278.
50. Richard IH, Kurlan R. The under-recognition of depression in Parkinson’s disease. Neuropsychiatr Dis Treat. 2006;2(3):349–353. doi:10.2147/nedt.2006.2.3.349
51. Zhu K, van Hilten JJ, Marinus J. Associated and predictive factors of depressive symptoms in patients with Parkinson’s disease. J Neurol. 2016;263(6):1215–1225. doi:10.1007/s00415-016-8130-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Relationship of Glymphatic Function with Cognitive Impairment, Sleep Disorders, Anxiety and Depression in Patients with Parkinson’s Disease
Gui Q, Meng J, Shen M, Feng H, Dong X, Xu D, Zhu W, Cheng Q, Wang L, Wu G, Lu Y
Neuropsychiatric Disease and Treatment 2024, 20:1809-1821
Published Date: 25 September 2024
Alternative Therapies for Non-Motor Symptoms in Parkinson’s Disease: A Mini Review
Liu H, Wang XP
Neuropsychiatric Disease and Treatment 2024, 20:2585-2591
Published Date: 21 December 2024

