Back to Journals » Journal of Pain Research » Volume 18
The Relationship Between Psychosocial Factors and Response to Epidural Steroid Injection for Chronic Lumbosacral Radicular Pain: A Prospective Pilot Study
Authors Stensland M , Sanford E, Houle TT, McGeary C, Cobos BA, Lugosi S, Lehman L, Nabity PS, Covell C, Fitzgerald E, Mojallal M , Reed DE, Pangarkar S, Eapen BC, Nanda U, McCormick ZL, McGeary D
Received 23 October 2024
Accepted for publication 19 February 2025
Published 11 April 2025 Volume 2025:18 Pages 1991—2002
DOI https://doi.org/10.2147/JPR.S496290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Meredith Stensland,1,2 Elizabeth Sanford,1,2 Timothy T Houle,3 Cindy McGeary,1,2 Briana A Cobos,1 Selena Lugosi,4 Luke Lehman,5 Paul S Nabity,1,2 Caleigh Covell,1 Elizabeth Fitzgerald,1 Mahsa Mojallal,1 David E Reed,6,7 Sanjog Pangarkar,8,9 Blessen C Eapen,8,9 Udai Nanda,8,9 Zachary L McCormick,10 Donald McGeary1,2,11
1Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA; 2South Texas Veterans Health Care System, Department of Veterans Affairs, San Antonio, TX, USA; 3Department of Anesthesia, Massachusetts General Hospital, Boston, MA, USA; 4School of Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA; 5Department of Rehabilitation Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA; 6Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA; 7Seattle-Denver Center of Innovation (COIN) for Veteran-Centered and Value-Driven Care, U.S. Department of Veterans Affairs, Seattle, WA, USA; 8Department of Physical Medicine & Rehabilitation, VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA; 9Department of Medicine, UCLA, Los Angeles, CA, USA; 10Department of Physical Medicine and Rehabilitation, University of Utah School of Medicine, Salt Lake City, UT, USA; 11Department of Psychology, University of Texas at San Antonio, San Antonio, TX, USA
Correspondence: Meredith Stensland, Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at San Antonio, 5788 Eckhert Road, San Antonio, TX, 78240, USA, Tel +1-515-681-0459, Email [email protected]
Purpose: Chronic lumbosacral radicular pain is a disabling condition commonly treated with epidural steroid injections (ESIs). Extant research suggests that psychosocial factors impact clinical outcomes among patients with back pain. The purpose of this study is to examine the relationship between psychosocial variables and post-injection pain intensity.
Setting: Interventional pain management clinic.
Methods: A prospective longitudinal cohort study with repeated within-subject measures. Assessment timepoints included a pre-injection baseline, immediately post-injection, 6 weeks, and 12 weeks, and 6 months; patients completed a battery of self-report assessments at each point. The primary outcome was pain intensity (numeric rating scale 0– 10). Data were analyzed using principal component analysis and generalized linear mixed-effects modeling.
Results: A total of 40 patients (age 52 ± 13.05) participated in this study. Higher pre-injection pain was predictive of higher post-injection pain at all time points (p< 0.001). Controlling for baseline pain and demographics, those with Negative Affect 1 standard deviation higher at baseline reported a 1.12-point mean higher pain rating at 12 weeks than those with lower Negative Affect (95% CI: 0.18– 2.07; p=0.020), while those with Cognitive Resilience 1 standard deviation higher at baseline had a 1.12-point mean lower pain rating at 6 months post-injection (95% CI: − 2.09 − 0.05, p=0.040).
Conclusion: Patients with higher negative affect and lower cognitive resilience achieve less pain improvement after ESIs for low back pain. Future research with a larger sample should focus on deepening our understanding of the role of psychosocial functioning as a potential mechanism of treatment response in patients undergoing ESI procedures. Findings point to the importance of multidisciplinary chronic pain care.
Keywords: epidural steroid injection, chronic low back pain, radiculopathy, psychosocial
Introduction
Chronic pain is a leading cause of disability worldwide1 and contributes to significant economic costs and health care spending in the United States.2 Compared to patients that only have low back pain, those with concomitant radiculopathy experience greater disability and healthcare utilization.3 Epidural steroid injections (ESIs) are the most frequently utilized outpatient pain management intervention for individuals with low back pain and radiculopathy who have failed to respond to other treatments such as oral medications, manual therapies, and exercise therapies.4 The use of ESIs has grown substantively in the United States, including a 665% increase in utilization among Medicare beneficiaries between 2000 and 2011.5 Understanding the factors that predict favorable treatment outcomes is especially important for patients with chronic rather than acute pain, who must, at minimum, periodically undergo repeat injections to reinstate pain relief.6 In an attempt to inform clinicians about how to best select patients for ESI, prior studies have evaluated a variety of factors, including procedural (eg, route of administration, agents injected),7 historical, and imaging factors. While there is evidence of favorable short-term outcomes for appropriately selected patients based on these factors,8,9 psychological factors that predict treatment success or failure have not been adequately evaluated in the outcome literature to date. Given the mixed research on durability of pain relief and treatment response predictions,6,8,10–17 further investigation of predictive factors is needed.
Psychosocial factors are increasingly being recognized for playing a pivotal role in patients’ chronic pain experiences and treatment responses, including post-surgical pain intensity, pain chronification, and pain duration.18,19 For example, pain catastrophizing20 is associated with poor pain outcomes after surgery18,21 A study of 529 individuals undergoing surgery for lumbar spinal stenosis revealed that those with depression before surgery were more likely to demonstrate poor outcome trajectories after surgery and, notably, demographic and clinical factors (like depression) made stronger contributions to surgical response than surgery-related procedural factors.22 A literature review summarizing 21 studies of patient-related factors affecting development of persistent pain after spine surgery found that psychosocial factors like pain catastrophizing, depression, and anxiety are strongly supported as risk factors for poor outcomes.23
Despite evolving research on psychosocial factors in other pain procedure research, there are relatively few published studies that primarily assess the contribution of known psychosocial risk factors on ESI response. Our recent scoping review confirms the relatively minimal amount of attention directed to understanding if and how psychosocial factors influence ESI treatment outcomes (under review). Prevalent conditions like depression24 and anxiety,25 however, have been shown to predict less favorable treatment outcomes. Based on the scoping review and evidence from surgical studies, it is likely that psychosocial variables are associated with response to ESI and may contribute significantly to models of risk and improvement after ESI. The present study addresses this significant research gap through a deliberate and focused assessment of non-procedural factors that may influence ESIs for chronic lumbosacral radicular pain. Understanding how psychosocial factors are associated with ESI response can improve extant research by adding a heretofore unexplored dimension of injection response variance that could lead to improved outcomes through treatment tailoring and pre-injection modification of psychosocial variables that diminish ESI response.
To evaluate the relationship between non-procedure influences (ie, baseline pain and psychosocial functioning) and post-ESI pain intensity, we completed a prospective study of a consecutive cohort of 40 patients in a metropolitan pain service who were referred for ESI to identify dimensions of physical and psychological functioning related to pre-injection pain intensity. Second, we aimed to test each dimension in association with post-injection pain intensity, followed by the development of an exploratory comprehensive model using baseline psychosocial and procedural factors to predict pain intensity longitudinally. We expected that these non-procedural predictors would at least partially explain a significant proportion of variance in post-injection pain intensities. This study aligns with the goals of precision medicine, as we aim to direct patients to the therapy to which they are most likely to respond favorably, which should be based not only on technical or imaging factors but also psychosocial factors.
Methods
Participants
All participants were recruited from a university-affiliated outpatient pain clinic. Patients were eligible if they met the following inclusion criteria: 1) chronic low back pain defined as pain lasting longer than 3 months; 2) lumbosacral radiculopathy lasting longer than 3 months; 3) referred and medically cleared for an epidural steroid injection (ESI); 4) aged 18+ years old. Exclusion criteria included clinical presentation with a condition that was a contraindication for ESI, active suicidal ideation, and active/uncontrolled psychosis. Patients were prospectively enrolled and provided informed consent to participate in this study, which complied with the Declaration of Helsinki. IRB approval was provided by the University of Texas Health Science Center at San Antonio (Approval number: 20180573HU).
Measures
Pain and psychosocial measurements were obtained via self-report questionnaire at the following timepoints: immediately before the injection (baseline), immediately after the injection, 6-weeks, 12-weeks, and 6-months post-injection; follow-up assessments were completed either by telephone or in-person. The primary outcome of interest, pain intensity, was measured using the 11-point Numeric Rating Scale (NRS),26 assessing pain from zero (ie, “No pain”) to 10 (ie, “Worst imaginable severe pain”). Pain interference and pain catastrophizing were measured by the Pain, Enjoyment of life, and General activity (PEG)27 and Pain Catastrophizing Scale (PCS),28 scales respectively. Pain avoidance and pain acceptance were measured by the Fear Avoidance Beliefs Questionnaire (FABQ)29 and the Chronic Pain Acceptance Questionnaire, Revised (CPAQ-R). Patient Reported Outcomes Measurement Information System (PROMIS) instrument for Pain intensity-short form was also administered.30
To evaluate functional disability due to back pain, the 10-item Oswestry Low Back Disability Questionnaire was used (ODI).31 The Credibility and Expectancy Questionnaire (CEQ)32 was used to measure treatment expectancy and rationale credibility. This measure was administered only once, at baseline (week 0), before ESI injection. The seven-item Insomnia Severity Index (ISI)33 was used to evaluate sleep dysfunction. The Generalized Anxiety Disorder Scale, Seven Items (GAD-7)34 and the Patient Health Questionnaire, Nine Items (PHQ-9)35 were administered to assess for depression and anxiety symptoms. Lastly, age, sex assigned at birth, ESI medical history, and health provider information were collected via abstraction from electronic medical record.
Procedure
All ESIs were performed by a physician who was board certified in Pain Medicine. Patients received one of the three injection types: interlaminar (ILESI), transforaminal (TFESI), or caudal (CESI). Patients were placed in a prone position, and the skin was prepared with chlorhexidine and draped utilizing sterile technique. All injections were performed under fluoroscopy. Most TFESI procedures were performed using an injection of 1.0 mL of Omnipaque (iohexol) 240 mgI/mL and then 1.5 mL of 0.5% bupivacaine and 5 mg of Decadron (dexamethasone). For ILESI, most injections used 1.0 mL of Omnipaque (iohexol), 240 mgI/mL and 10 mg of Decadron (dexamethasone). For CESI, 2.0 mL of 0.5% bupivacaine, 2 mL Omnipaque, 5 mL PFNS, and 40 mg of Depo-Medrol (methylprednisolone) were injected. There were a few differences noted; all injection details for N = 40 patients are summarized in Table S7, along with other patient information (demographic, clinical, imaging details).
Analysis
Descriptive statistics were run for age, sex, and relevant procedural, psychological, and medical data. The proportion of patients who achieved 50% reduction from baseline pain after ESI (ie, “responders”) at each time point was calculated. A multivariate regression model was planned to assess the combined contribution of all psychosocial components on long-term pain, after ESI injection. To avoid overfitting a predictive model with too many variables, a data reduction was performed using principal component analysis (PCA) for baseline psychosocial variables and pain. Assuming a high degree of correlation among psychosocial variables, PCA was used to generate composites or cohesive psychosocial indices (ie components), thus transforming a large set of variables into a smaller set that still contains most of the information in the large set.
After baseline psychosocial components were identified, we examined the relationship between each component and long-term numeric pain ratings, using a series of multivariate models. First, a “null” model was run to account for the possible influence of patient demographic characteristics and procedural variables on patient pain trajectory. Long-term pain was thus modeled using pre-injection pain, post-injection pain, age and the effect of time, plus random effects participant, sex, and type of ESI injection (using maximum likelihood estimation): 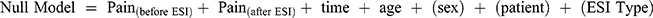 (See Supplemental Materials for a fuller description).
(See Supplemental Materials for a fuller description).
Long-term pain intensity was then modeled as a function of each psychosocial component, after controlling for patient demographic characteristics and procedural variables: each component was appended to the null model to isolate its unique relationship with long-term pain after ESI:  (See Supplemental Materials for a fuller description). Analyses were performed using the R version 4.3.1 (2023–06-16 ucrt).
(See Supplemental Materials for a fuller description). Analyses were performed using the R version 4.3.1 (2023–06-16 ucrt).
Results
Patient Characteristics
The average age of the 40 patients enrolled in this prospective study was 52 years (range, 30–80 years; SD=13.05); 18 (45.0%) were women, and 26 (65.0%) were Hispanic. Participants received the following injections: transforaminal (n=25; 62.5%), interlaminar (n=11, 27.5%), and caudal (n=4, 10%). Based on chart review, participants had received an average of 2 ESIs (SD=1.45) and 3 interventional pain procedures (SD=2.43) prior to their enrollment, respectively; 21 participants (52.5%) were naïve to ESI. See Table 1 for descriptive statistics (see Table S1 for correlations among variables). Immediately after ESI, 50% of patients responded; at 6 weeks, of patients who provided a pain rating (n=30), 16.7% were responders; at 12 weeks (n=31), it was 16.1%; and at 6 months (n=27), 22.2% (Figure 1).
 |
Table 1 Sample Description |
 |
Figure 1 Observed pain immediately before ESI procedure, then immediately after, and again at 6 weeks, 12 weeks, and 6 months. |
Components
Principal component analysis of baseline predictor variables using oblique cluster rotation and eigenvalues greater than 1 revealed three key components (see Tables S2 and S3). Variables that are loaded under Component 1, “Negative Affect”, encompassed pain helplessness (PCS-Help subscale), anxiety (GAD-7), pain magnification (PCS-Mag), depression (PHQ-9), pain rumination (PCS-Rum subscale), insomnia (ISI), work limitations (FABQw subscale), and physical limitations beliefs (FABQp). Component 2, “Disruption from Pain” encompassed pain interference (PROMIS and PEG), pain-related disability (ODI), baseline NRS, and activity (CPAQpw subscale). Component 3, “Cognitive Resilience” encompassed clinical expectations (CEQ) and activity engagement (CPAQa). Pearson correlation analyses showed that Negative Affect and Disruption from Pain were significantly correlated with one another (r=0.689, p<0.001); Negative Affect correlated with Cognitive Resilience (r=−0.336*p<0.05). Disruption from Pain and Cognitive Resilience were not correlated (see Table S4).
Predicting Post Injection Pain
Procedural variables (type of ESI, experience of the provider, and the epidural chemical agent) and socio-demographics (sex and age) and pre-injection pain were evaluated as predictors of pain intensity immediately after the ESI procedure, as well as at 6-weeks, 12-weeks, and 6-months, after controlling for baseline (pre-injection) pain. Initial modeling revealed that provider experience and injectate were non-significant. To maximize power, those variables were removed from the model, leaving ESI type as the sole procedural variable modeled. Age and sex, Negative Affect, Disruption from Pain, and Cognitive Resilience were modeled individually as fixed effects predictors of pain intensity over time, ie, immediately after ESI procedure, 6 weeks, 12 weeks and 6 months (Table 2). Higher pre-injection pain was predictive of higher post-injection pain at all time points (p<0.001). Even after controlling for baseline pain and demographics, Negative Affect significantly predicted pain intensity at 12 weeks, such that those with higher Negative Affect at baseline had 1.12 (95% CI: 0.18, 2.07; p=0.020) points higher pain at 12 weeks after injection on average. Higher baseline Cognitive Resilience significantly predicted lower pain at 6 months, such that patients with higher Cognitive Resilience at baseline had an average pain rating of 1.12-points lower than those with lower Cognitive Resilience at baseline (95% CI: −1.12, −0.05, p=0.040).
 |
Table 2 Predictive Values of Psychosocial Composite Variables on Post-Injection Pain at 6 weeks, 12 weeks, and 6 Months, Partial Table |
Impacts of Psychosocial Functioning
Unadjusted
Participants experienced significant relief initially after ESI, followed by a significant return of pain intensity (ps<0.001). By 6 weeks, the pain plateaued around 2.4–2.6 points higher than it was immediately after ESI injection (Table 2, Figure 2A).
 |
Figure 2 Pain trajectory predictions. (A). Using pain measurements taken immediately post-injection as the reference, unadjusted for psychosocial influences, the predicted pain over 6 months roughly approximated observed pain (see Figure 1). When the influence of psychosocial composite variables scores are factored, long term pain estimates are affected. (B). Elevated Negative Affect increases pain rating at 12 weeks. (C). Disruption from Pain does not appear to influence pain intensity at any point after ESI procedure. (D). Increased Cognitive Resilience significantly lowers pain intensity at 6 weeks and 6 months. |
Negative Affect
Participants with low Negative Affect at baseline experienced some initial pain returning (ie, average 2.4 points increase in pain intensity) over the first 6 weeks, but they experienced substantial overall pain relief over time, particularly at 12 weeks (p=0.020). By contrast, participants with high Negative Affect experienced pain returning to close to pre-injection pain intensity by 12 weeks (Figure 2B). At 12 weeks, pain in participants with high baseline Negative Affect is estimated to be about 2.3 points (SD=0.79) higher than pain in those with low baseline Negative Affect (p=0.005; Table 3).
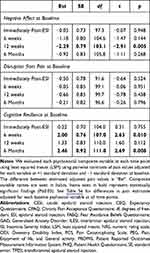 |
Table 3 Difference in Pain Estimates Adjusted for Each Baseline Psychosocial Variable, Partial Table |
Disruption from Pain
Disruption from Pain had no substantial impact on pain at any post-injection time point after ESI.
Cognitive Resilience
Participants low in baseline Cognitive Resilience had pain return to near pre-injection level. By contrast, those with high baseline Cognitive Resilience had only a minor increase in pain intensity over the first 6 weeks, remaining relatively low through 6 months, p=0.040 (Figure 2D). At 6 weeks, pain in participants with low baseline Cognitive Resilience was estimated to be 2 points (SD=0.76) higher than pain in those with high baseline Cognitive Resilience (p=0.010); and at 6 months, pain in patients with low baseline Cognitive Resilience was estimated to be 2.5 points (SD=0.92) higher than pain in patients with high baseline Cognitive Resilience (p=0.010).
Discussion
The present study represents one of the first purposive studies of the relationship between psychosocial factors and pain outcomes after ESI. We derived a structure of three psychosocial components, representing Negative Affect (characterized by high anxiety and depression), Disruption from Pain (characterized by greater disability and pain interference), and Cognitive Resilience (characterized by high pain acceptance and low fear avoidance). Results from the analysis of 40 consecutive patients who completed comprehensive assessments of pain and psychosocial domains both before and up to 6 months after ESI revealed that even after accounting for baseline pain, demographics, and route of ESI administration, both baseline Negative Affect and Cognitive Resilience were significantly associated with pain outcomes weeks to months after injection. It is remarkable to note that within our set of predictive models, the first unadjusted model very closely resembles the plot of observed data; it was not until the psychosocial composite variables were adjusted for that the predictive models then took on a notably different distribution.
This novel finding highlights how patients’ psychological functioning at the time they present to the clinic for injection is linked to their treatment response months after the injection is administered. Knowing the predictive value of factors before the injection occurs is highly valuable information, as identification of potentially modifiable factors related to Negative Affect, along with Cognitive Resilience, is an important first step towards understanding how to achieve clinically significant post-ESI outcomes. Pre-injection psychological interventions designed to enhance patient resilience and lower distress may be an effective approach for increasing the treatment responder rate. It also may serve as an impetus for timely referral to psychiatric services or pharmacological treatments for clinical depression, anxiety, and insomnia.
In the present study, Negative Affect was defined by data related to pain catastrophizing, anxiety, depression, insomnia, and avoidance of activity due to pain (ie, fear avoidance). Prior studies of ESI outcomes have shown a correlation between pre-injection psychosocial variables and post-injection pain, including significant findings for depression.25,36 In a study of 57 patients who received ESI for cervical spondylosis, patients with depression were significantly less likely to achieve a 50% reduction in arm or neck pain after ESI compared to those without depression.37 Though many acknowledge pre-injection anxiety as a notable concern in ESI,36 there appears to be mixed evidence of its role in ESI pain outcomes. Studies of patient preference for sedation (often with anti-anxiety benzodiazepine medications) prior to diagnostic or therapeutic spinal injections generally show that routine sedation is not necessary, though patients with high levels of pre-injection anxiety are more likely to request sedation).38,39 In counterpoint, studies designed to address anxiety through education or reassurance found little benefit for pain outcomes,38,40 and some studies have found pre-injection anxiety to be indicative of better ESI pain outcomes.25 The inclusion of anxiety as part of the Negative Affect component of the present study may suggest that anxiety’s relationship to pain outcomes is due to accompanying stress or an attentional bias to perceived negative aspects of the injection, similar to findings on the role of anxiety in COVID-19 stress.41 Indeed, the Cognitive Resilience component of the present study included positive credibility and expectancy of ESI as well as engagement in activity despite pain, both of which could suggest a benign or positive attentional bias to spinal injection. Future studies should explore the roles of stress and attentional bias to ESI outcomes to further examine these outcomes.
Findings from the present study align with both spinal surgery42–45 and spinal cord stimulator46–49 literatures, which have made strides in demonstrating the important role played by psychosocial factors in pain outcomes. For example, a large review paper that analyzed 96 studies on spinal surgery outcomes reported that patients with psychological disorders (eg, depression, anxiety, etc.) are at significantly greater risk for a host of poor post-operative outcomes including, higher rates of hospital readmission, longer hospital stays, and increased narcotic use.50 Additionally, an early study examining the relationship between childhood trauma and lumbar spine surgery among 86 patients found that those with three or more psychologically traumatic childhood experiences (eg, sexual and physical abuse, neglect, etc.) had an 85% likelihood of an unsuccessful surgical outcome, and the patients with no childhood trauma had only a 5% incidence of surgical failure.51 Future studies examining the efficacy of ESI should attempt to utilize comprehensive models that take into account both traditional factors (procedural, clinical, imaging) and psychosocial variables, which will aid in the proper identification of patients most likely to respond positively to the procedure.
Findings point to the critical importance of multidisciplinary chronic pain care, specifically involving behavioral health professionals to help address psychological factors impacting pain. A critical implication of psychosocial variables’ impact on patients’ ESI response is that many of these psychosocial variables are modifiable to an extent and, with proper assessment and intervention, could be improved, thereby enhancing patients’ likelihood of achieving a positive ESI response. As such, the pool of patients who stand to achieve clinically meaningful pain relief from this widely used intervention can be widened. Furthermore, a study of 2272 chronic pain patients demonstrated that those who expect significant positive outcomes as a result of undergoing treatment are the patients most likely to achieve superior clinical outcomes.52 Thus, if we utilize psychologists and social workers to effectively engage patients in pre-injection behavioral interventions and bring awareness to their successful improvement in relevant psychosocial issues, this may instill in patients a greater belief that they will benefit.
Strengths of the present study include an effective data reduction strategy to derive the greatest benefit from numerous psychosocial variables in a relatively small sample, assessment of ESI pain outcomes immediately before and after injection with follow-up to 6 months after injection, and accounting for patient-level demographic and pain variables as well as procedural variables in the univariate and multivariate models. Univariate models returned significant results for multiple psychosocial dimensions, as described above. However, examination of supplemental data confirms that the multivariate model is likely over-fit to the small sample used in this study, so future multivariate studies should include larger samples. Lastly, post-treatment follow-up allowed for assessment of the stability of ESI treatment effects, which was an additional strength of this study.
Limitations
Findings from the present study may be interpreted in light of several limitations. First, the study was conducted at a single pain clinic with a sample of 40 patients. Future research utilizing a multi-site design and a larger sample size for representativeness is needed. Second, while the study included several timepoints extending out to 6 months post-injection, follow-up beyond 6 months may reveal changes in effects that have important implications for clinical decision-making. Third, the present study focused solely on patients with chronic back pain; patients with acute back pain differ in meaningful ways, both physically and psychologically, so incorporating this patient population in future studies is necessary.53,54 Additionally, causal inferences may not be designed due to the fact that a randomized controlled design was not implemented. Lastly, we did not statistically control for certain physical, clinical, and imaging factors.
Conclusion
The present study evaluated the relationship between psychosocial variables and pain intensity after ESI. The findings show individuals who are Cognitively Resilient tend to experience greater pain improvement after ESI, while those who have worse Negative Affect also have more pain after ESI. Future research should focus on examining the nature of this relationship and any evidence of causality. Also, research aimed at modifying these predictors and evaluation for corresponding improvements in pain ratings after ESI is an important next step to advance our understanding of prehabilitation options for ESI.
Data Sharing Statement
Data are available upon request.
Acknowledgments
We wish to sincerely thank the patients who participated in this research study for their time and effort. We also extend a special thanks to the administrative and clinical staff at UT Pain Consultants who supported the successful implementation of the study.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
Dr Zachary McCormick reports grants and/or personal fees from Avanos, Boston Scientific/ Relievant, Saol Therapeutics, Spine Biopharma, SPR Therapeutics, Stratus Medical, OrthoSon, and Stryker, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ferreira ML, de Luca K, Haile LM, et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumat. 2023;5(6):e316–e329.
2. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863–884. doi:10.1001/jama.2020.0734
3. Konstantinou K, Hider SL, Jordan JL, Lewis M, Dunn KM, Hay EM. The impact of low back-related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clin J Pain. 2013;29(7):644–654. doi:10.1097/AJP.0b013e31826f9a52
4. Manchikanti L, Soin A, Mann DP, Bakshi S, Pampati V, Hirsch JA. Comparative analysis of utilization of epidural procedures in managing chronic pain in the medicare population: pre and post affordable care act. Spine. 2019;44(3):220–232. doi:10.1097/BRS.0000000000002785
5. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Phys. 2013;16(4):E349–E364.
6. McCormick ZL, Cushman D, Marshall B, et al. Pain reduction and repeat injections after transforaminal epidural injection with particulate versus nonparticulate steroid for the treatment of chronic painful lumbosacral radiculopathy. PM&R. 2016;8(11):1039–1045.
7. Kwak SG, Choo YJ, Kwak S, Chang MC. Effectiveness of transforaminal, interlaminar, and caudal epidural injections in lumbosacral disc herniation: a systematic review and network meta-analysis. Pain Phys. 2023;26(2):113.
8. Smith CC, McCormick ZL, Mattie R, MacVicar J, Duszynski B, Stojanovic MP. The effectiveness of lumbar transforaminal injection of steroid for the treatment of radicular pain: a comprehensive review of the published data. Pain Med. 2020;21(3):472–487. doi:10.1093/pm/pnz160
9. Park DY, Kang S, Park JH. Factors predicting favorable short-term response to transforaminal epidural steroid injections for lumbosacral radiculopathy. Medicina. 2019;55(5):162. doi:10.3390/medicina55050162
10. Yang S, Kim W, Kong HH, Do KH, Choi KH. Epidural steroid injection versus conservative treatment for patients with lumbosacral radicular pain: a meta-analysis of randomized controlled trials. Medicine. 2020;99(30).
11. Luijsterburg PA, Verhagen AP, Ostelo RW, Van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J. 2007;16:881–899. doi:10.1007/s00586-007-0367-1
12. Bresnahan BW, Rundell SD, Dagadakis MC, et al. A systematic review to assess comparative effectiveness studies in epidural steroid injections for lumbar spinal stenosis and to estimate reimbursement amounts. PM&R. 2013;5(8):705–714. doi:10.1016/j.pmrj.2013.05.012
13. Curatolo M, Rundell SD, Gold LS, et al. Long‐term effectiveness of epidural steroid injections after new episodes of low back pain in older adults. Eur J Pain. 2022;26(7):1469–1480. doi:10.1002/ejp.1975
14. Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010;11(8):1149–1168. doi:10.1111/j.1526-4637.2010.00908.x
15. Kennedy DJ, Plastaras C, Casey E, et al. Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med. 2014;15(4):548–555. doi:10.1111/pme.12325
16. Rahatli FK, Harman A, Boyvat F, Zararsiz G. Comparison of transforaminal and interlaminar epidural steroid injection in managing lumbar radiculopathy. Biomed Res. 2017;28:2204–2208.
17. Tecer D, Adiguzel E, Koroglu O, Tan AK, Taskaynatan MA. Can epidural contrast dispersal pattern help to predict the outcome of transforaminal epidural steroid injections in patients with lumbar radicular pain. World Neurosurg. 2018;116:e394–e398.
18. Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21(5):439–445. doi:10.1097/01.ajp.0000135236.12705.2d
19. Pavlin DJ, Sullivan MJ, Freund PR, Roesen K. Catastrophizing: a risk factor for postsurgical pain. Clin J Pain. 2005;21(1):83–90. doi:10.1097/00002508-200501000-00010
20. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. doi:10.1586/ern.09.34
21. Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468(3):798–806. doi:10.1007/s11999-009-0963-y
22. Hébert JJ, Abraham E, Wedderkopp N, et al. Preoperative factors predict postoperative trajectories of pain and disability following surgery for degenerative lumbar spinal stenosis. Spine. 2020;45(21):E1421–E1430. doi:10.1097/BRS.0000000000003587
23. Costelloe C, Burns S, Yong RJ, Kaye AD, Urman RD. An analysis of predictors of persistent postoperative pain in spine surgery. Curr Pain Headache Rep. 2020;24(1):1–6. doi:10.1007/s11916-020-0834-5
24. Cohen SP, Doshi TL, Kurihara C, et al. Multicenter study evaluating factors associated with treatment outcome for low back pain injections. Reg Anesth Pain Med. 2022;47(2):89–99. doi:10.1136/rapm-2021-103247
25. Bahar-Ozdemir Y, Sencan S, Ercalik T, Kokar S, Gunduz OH. The effect of pre-treatment depression, anxiety and somatization levels on transforaminal epidural steroid injection: a prospective observational study. Pain Phys. 2020;23(3):E273.
26. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short‐form McGill pain questionnaire (SF‐MPQ), chronic pain grade scale (CPGs), short form‐36 bodily pain scale (SF‐36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63(S11):S240–S252.
27. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733–738. doi:10.1007/s11606-009-0981-1
28. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psycholl Assess. 1995;7(4):524. doi:10.1037/1040-3590.7.4.524
29. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi:10.1016/0304-3959(93)90127-B
30. Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103–111. doi:10.1016/j.jclinepi.2015.08.035
31. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine. 2000;25(22):2940–2953. doi:10.1097/00007632-200011150-00017
32. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi:10.1016/S0005-7916(00)00012-4
33. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/S1389-9457(00)00065-4
34. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
35. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
36. Fish DE, Nguyen J, Pham Q. The prognostic value of the hospital anxiety and depression scale and the catastrophizing scale in functional outcome after epidural steroid injection for low back pain. Arch Phys Med Rehabil. 2006;87(11):e36.
37. Kim EJ, Chotai S, Schneider BJ, Sivaganesan A, McGirt MJ, Devin CJ. Effect of depression on patient-reported outcomes following cervical epidural steroid injection for degenerative spine disease. Pain Med. 2018;19(12):2371–2376. doi:10.1093/pm/pny196
38. Kim SH, Lee ES. The effects of comprehensive education program on anxiety, uncertainty and athletic performance of patients undergo spinal nerve block. Korean J Adult Nurs. 2017;29(2):143–153. doi:10.7475/kjan.2017.29.2.143
39. Cucuzzella TR, Delport EG, Kim N, Marley J, Pruitt C, Delport AG. A survey: conscious sedation with epidural and zygapophyseal injections: is it necessary? Spine J. 2006;6(4):364–369. doi:10.1016/j.spinee.2005.09.005
40. Özdemir Y B, Şencan S, Erçalık T, Kokar S, Gündüz OH. Do informative leaflets affect pre-procedural anxiety and immediate pain after transforaminal epidural steroid injections? A prospective randomized controlled study. Agri. 2021;33(1):1–6. doi:10.14744/agri.2020.27048
41. Feng Y-C, Krahé C, Koster EH, Lau JY, Hirsch CR. Cognitive processes predict worry and anxiety under different stressful situations. Behav Res Ther. 2022;157:104168.
42. Park C, Garcia AN, Cook C, Gottfried ON. Effect of change in preoperative depression/anxiety on patient outcomes following lumbar spine surgery. Clin Neurol Neurosurg. 2020;199:106312. doi:10.1016/j.clineuro.2020.106312
43. Schoell K, Wang C, D’Oro A, et al. Depression increases the rates of neurological complications and failed back surgery syndrome in patients undergoing lumbar spine surgery. Clin Spine Surg. 2019;32(2):E78–E85. doi:10.1097/BSD.0000000000000730
44. Mancuso CA, Stal M, Duculan R, Girardi FP. Physical and psychological comorbidity independently associated with spine-related disability. Spine. 2014;39(23):1969–1974. doi:10.1097/BRS.0000000000000569
45. Lee C-H, Liu J-T, Lin S-C, Hsu T-Y, Lin C-Y, Lin L-Y. Effects of educational intervention on state anxiety and pain in people undergoing spinal surgery: a randomized controlled trial. Pain Manag Nurs. 2018;19(2):163–171. doi:10.1016/j.pmn.2017.08.004
46. Modak A, Jani R, Jani S, Mammis A. Psychiatric screening for spinal cord stimulation for complex regional pain syndrome: a literature review and practical recommendations for implementation. Interdiscip Neurosurg. 2023;31:101633. doi:10.1016/j.inat.2022.101633
47. Block AR, Ben-Porath YS, Marek RJ. Psychological risk factors for poor outcome of spine surgery and spinal cord stimulator implant: a review of the literature and their assessment with the MMPI-2-RF. Clinl Neuropsychol. 2013;27(1):81–107. doi:10.1080/13854046.2012.721007
48. Beletsky A, Liu C, Alexander E, et al. The association of psychiatric comorbidities with short-term and long-term outcomes following spinal cord stimulator placement. Neuromodulation. 2023;26(5):1081–1088. doi:10.1016/j.neurom.2022.12.010
49. Davis CE III, Kyle BN, Thorp J, Wu Q, Firnhaber J. Comparison of pain, functioning, coping, and psychological distress in patients with chronic low back pain evaluated for spinal cord stimulator implant or behavioral pain management. Pain Med. 2015;16(4):753–760. doi:10.1111/pme.12526
50. Jackson KL, Rumley J, Griffith M, Agochukwu U, DeVine J. Correlating psychological comorbidities and outcomes after spine surgery. Global Spine J. 2020;10(7):929–939. doi:10.1177/2192568219886595
51. Schofferman J, Anderson D, Hines R, Smith G, White A. Childhood psychological trauma correlates with unsuccessful lumbar spine surgery. Spine. 1992;17(6):S138–S144. doi:10.1097/00007632-199206001-00013
52. Cormier S, Lavigne GL, Choinière M, Rainville P. Expectations predict chronic pain treatment outcomes. Pain. 2016;157(2):329–338. doi:10.1097/j.pain.0000000000000379
53. Gatchel RJ, Bernstein D, Stowell AW, Pransky G. Psychosocial differences between high‐risk acute vs. chronic low back pain patients. Pain Pract. 2008;8(2):91–97. doi:10.1111/j.1533-2500.2008.00176.x
54. Hüllemann P, Keller T, Kabelitz M, et al. Clinical manifestation of acute, subacute, and chronic low back pain in different age groups: low back pain in 35,446 patients. Pain Pract. 2018;18(8):1011–1023. doi:10.1111/papr.12704
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

