Back to Journals » Infection and Drug Resistance » Volume 18
The Role of blaOXA-101 and blaOXA-573 in Extensively Drug-Resistant/ Pan Drug-Resistant (XDR/PDR) Pseudomonas aeruginosa Resistance to Ceftazidime-Avibactam
Received 16 December 2024
Accepted for publication 18 April 2025
Published 16 May 2025 Volume 2025:18 Pages 2547—2555
DOI https://doi.org/10.2147/IDR.S506452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Yixin Kang,1,2 Junchang Cui1,2
1Nankai University, Tianjin, People’s Republic of China; 2College of Pulmonary & Critical Care Medicine, The Eighth Medical Center, Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Junchang Cui, College of Pulmonary & Critical Care Medicine, The Eighth Medical Center, Chinese PLA General Hospital, 17A Heishanhu Road, Haidian District, Beijing, 100091, People’s Republic of China, Email [email protected]
Objective: To explore the association of the resistance of extensively drug-resistant/ pan-drug-resistant Pseudomonas aeruginosa (XDR/PDR-PA) to ceftazidime-avibactam (CZA) with various class D β-lactamase genes.
Methods: Twofold dilution was used to determine the minimum inhibitory concentration (MIC) of CZA against XDR/PDR-PA. Whole genome sequencing and bioinformatics analysis were used to determine the drug-resistant genes of each isolate. Pearson correlation coefficient and statistical analysis were used to assess the association of the resistance of XDR/PDR-PA to CZA and various class D β-lactamase genes.
Results: ST244 was the predominant type (34/68, 50%) among the 68 XDR/PDR-PA strains. Subsequently, ST357 was the second most prevalent type (5/68, 7.4%) strain. blaOXA-101 and blaOXA-573 genes were associated with resistance to CZA (p-value was 0.029 and 0.021, respectively) in the 68 XDR/PDR-PA isolates tested.
Conclusion: Our work found that blaOXA-101 and blaOXA-847 play a role in XDR/PDR-PA resistance to CZA.
Keywords: P. aeruginosa, β-lactamases, ceftazidime–avibactam
Introduction
Pseudomonas aeruginosa is an important pathogen responsible for many infections, particularly in immunocompromised and chronically ill patients.1 The intrinsic resistance of this bacterium to many antibiotics, coupled with its extraordinary ability to acquire other resistance mechanisms, makes it a significant challenge in the clinical setting.2 In recent years, one of the most worrying developments has been the emergence of extensively drug-resistant/ pan-drug-resistant Pseudomonas aeruginosa (XDR/PDR-PA).3 These strains are resistant to almost all available antibiotics, severely limiting treatment options and leading to increased morbidity and mortality.3 One of the key factors contributing to this resistance is the production of several β-lactamases that hydrolyze β-lactam antibiotics, rendering them ineffective.4 Among these enzymes, class D β-lactamases (particularly OXA-10 and OXA-488) have attracted attention because of their association with resistance to novel antibiotic combinations such as ceftolozane-tazobactam.5
Ceftazidime-avibactam (CZA) is a combination antibiotic of a third-generation cephalosporin (ceftazidime) and a novel beta-lactamase inhibitor (avibactam).6 This combination antibiotic has been recognized as a substantial breakthrough in treating infections caused by multi-drug-resistant gram-negative bacteria, such as Pseudomonas aeruginosa.7,8 Avibactam can effectively inhibit a wide range of β-lactamases (ie: TEM, KPC, CMY-1), thereby restoring the efficacy of ceftazidime against strains that have developed resistance.9–11 However, the effectiveness of CZA is compromised by certain class D β-lactamases (ie: OXA-48) that hydrolyze ceftazidime even in the presence of avibactam.
OXA (oxacillinase) enzymes represent a diverse family of β-lactamases that hydrolyze carbapenems and other β-lactam antibiotics, playing a major role in antibiotic resistance in PA. These enzymes are categorized into multiple families, including OXA-23-like, OXA-24/40-like, OXA-48-like, and OXA-58-like, exhibiting distinct substrate specificities and genetic backgrounds.12 Notably, the chromosome-encoded OXA-50 enzyme plays a pivotal role in mediating the intrinsic resistance of PA to β-lactams.13 Other resistance mechanisms in PA, such as the expression of endogenous AmpC β-lactamases and efflux pumps (eg, MexAB-OprM and other members of the resistance-nodulation-division [RND] family), can act synergistically with OXA enzymes to confer high levels of CZA resistance to PA.14
The widespread use of whole-genome sequencing technology has provided new opportunities to study drug resistance mechanisms in Pseudomonas aeruginosa. Whole-genome sequencing can comprehensively analyze the genomic structure and variation of Pseudomonas aeruginosa, reveal the drug resistance-related genes and their regulatory networks, and provide essential clues for an in-depth understanding of the drug resistance mechanism.15 Meanwhile, understanding the molecular mechanism of drug resistance generated by β-lactamases is crucial for developing effective therapeutic strategies and reducing the spread of XDR/PDR-PA. Based on the above research background, this study aimed to explore the association between beta-lactamase genes and CZAresistance in XDR/PDR-PA.
Materials and Methods
Bacterial Strains and Antimicrobial Agents
Between January 2016 and November 2021, 68 non-duplicated PA strains were isolated in the First Medical Centre of the General Hospital of the People’s Liberation Army.16 All included patients were diagnosed with XDR/PDR-PA respiratory infections by two or more experienced respiratory physicians. All samples were isolated from sputum or tracheal aspirates of the included patients. XDR isolates were defined as susceptible to at least one drug in all antimicrobial classes but not to two or fewer antimicrobial drugs. PDR isolates were defined as susceptible to all drugs in all antimicrobial classes.
Antimicrobial Susceptibility Tests
The twofold dilution method was used to determine the minimum inhibitory concentration (MIC) of CZA against 68 XDR/PDR-PA. A 96-well plate containing ceftazidime was prepared using the twofold dilution method, thoroughly mixed, and stored in a refrigerator at a temperature of −4°C for use on the same day. The concentration of avibactam was fixed at 4 mg/L. The concentrations of ceftazidime in the 96-well plates were as follows: 0.25, 0.5, 1, 2, 4, 8, 16, 32, and ≥64 mg/L.
The bacteria were removed from the refrigerator at a temperature of −80°C. After a recovery period of three generations, single colonies of bacteria were selected and inoculated in fresh Mueller-Hinton broth (MHB). They were then incubated in a shaking incubator for 6–8 hours. The cultured suspension was reduced to 0.5 McElroy units using turbidimetry, and approximately 106 colony-forming units (CFU) of bacteria were inoculated into 96-well plates containing the drug using a multipoint inoculator. The 96-well plates were then incubated at 37°C for 16–20 hours, and the results were observed and recorded. Quality control was established for each batch to guarantee the accuracy of the entire experiment.
The minimum inhibitory concentration (MIC) was defined as the lowest concentration of an antimicrobial drug that inhibited the growth of bacteria. The criteria for interpreting CZA against Pseudomonas aeruginosa were based on those set out by the Clinical and Laboratory Standards Institute (CLSI).17 These criteria define susceptibility as a minimum inhibitory concentration (MIC) of ≤8/4 mg/L, while resistance is indicated by a MIC of ≥16/4 mg/L. The susceptibility tests of all antimicrobials were repeated on three separate occasions by the CLSI standards. If the outcomes from a given replicate deviated considerably from those of the other replicates, additional tests were conducted to verify the results. When deemed necessary, the results from the three trials were averaged, or outlier data was excluded to ensure the reliability of the final susceptibility test. The quality control strains used in the experiments were Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922.
Whole-Genome Sequencing and Bioinformatics Analysis
Genomic DNA was extracted from 68 XDR/PDR-PA strains using the Gram-negative Bacterial Genomic DNA Extraction Kit (SolarBio) following the manufacturer’s instructions. Whole genome sequencing of 68 XDR/PDR-PA strains was performed using the Illumina MiSeq Short Fragment Sequencing System (Illumina, San Diego, CA, USA). Low-quality paired-end fragments were eliminated with Trimmomatic (version 0.39), resulting in high-quality sequencing data. The original contributions presented in the study are publicly available. Those data have been deposited into the NCBI repository under accession PRJNA967114. A multi-locus sequence typing (MLST) analysis was conducted on 68 XDR/PDR-PA strains using MLST software. The resistance genes of XDR/PDR-PA were analyzed using the Comprehensive Antibiotic Resistance Database (McMaster University, Hamilton, Ontario, v.1.2.0). Furthermore, the drug resistance genes of ST244 XDR/PDR-PA were correlated with the MLST typing results using Pearson correlation analysis (Pearson correlation coefficient P<0.05 r> 0.7).
Statistical Analysis
The association between class D β-lactamases and ceftazidime-avibactam resistance in XDR/PDR-PA was subjected to a comprehensive statistical analysis using the SPSS software (version 26.0). The Fisher Exact test was used to compare different groups within the dataset. A p-value < 0.05 (two-tailed) was considered statistically significant.
Results
Phylogenetic Analysis of 68 XDR/PDR-PA Strains
As shown in Figure 1, the 68 XDR/PDR-PA strains were grouped into 5 clusters. Each ST of XDR/PDR-PA is distributed in a specific cluster with a certain regularity. Among them, 34 strains of ST244 were concentrated in cluster5 (respectively: S76, S62, S39, S13, S42, S43, S42.2, S6, S7, S18, S86, S85, S14, S46, S99, S17, S19, S34, S45, S12, S15, S5, S4, S35, S24, S58, S26, S28, S61, S3, S81, S78, S57). Cluster4 (S65, S60, S40, S11, S67) contained all ST357 strains, Cluster3 (S87, S103, S98, S68) contained all ST253 strains, Cluster2 (S89, S84, S83, S82) contained all ST1200, ST316, and ST235 strains, while the rest of STs were concentrated in cluster1 (S41, S69, S70, S44, S97, S96, S91, S73, S54, S95, S49, S48, S29, S9, S8). S29, S9, S8, S100, S71, S47, S59).
 |
Figure 1 Phylogenetic tree of 68 XDR/PDR-PA strains. (S…is the serial number of each strain. Besides, different colors represent different clusters.). |
Minimum Spanning Tree of 68 XDR/PDR-PA Strains
Figure 2 depicts the distribution of ST types among the 68 XDR/PDR-PA strains, with ST244 being the predominant type (34/68). Subsequently, ST357 was the second most prevalent type, occurring in 5/68 (7.4%) strains. This was followed by ST235 and ST253 (4/68, 5.9%) and ST463 (3/68, 4.7%). Subsequently, there were also occurrences of ST270, ST385, and ST1290 (2/68 each), as well as instances of ST231, ST254, ST260, and ST316. Additionally, ST389, ST408, ST611, ST740, ST768, ST847, ST1123, and finally, one occurrence of ST1200 (1/68) were identified.
Heat Map of β-Lactamase Resistance Genes in 68 XDR/PDR-PA Strains
Figure 3 shows a heat map depicting the distribution of β-lactamase resistance genes in 68 XDR/PDR-PA strains. A total of 394 β-lactamase genes were identified across the 68 strains. Figure 3 reveals distinct distributions of β-lactamase resistance genes among STs. The vertical coordinates in Figure 3 represent clustered and processed β-lactamase genes, organized into three clades using a cut tree method. The genes depicted in the accompanying figure within clade 1 are present in all STs and exhibit darker colors. Therefore, we have identified the β-lactamase genes in clade 1 as the gene types common to all STs. The β-lactamase gene type is flexibly present in the respective ST types, while the β-lactamase gene type specific to each ST is designated as clade 3. Statistical analysis reveals that out of the 394 β-lactamase resistance genes, clade 1 accounted for 67.3% (265/394), clade 2 for 16.5% (65/394), and clade 3 for 16.2% (64/394) of the β-lactamase resistance genes.
In addition, a total of 146 β-lactamase genes were identified in ST244 strains, accounting for 37.1% of all β-lactamase resistance genes. Among these, 54 β-lactamase resistance genes were also found in other STs, while 10 resistance genes (blaCARB-10, blaIMP-48, blaLEN-6, blaNDM-6, blaOKP-A-10, blaOXA-127, blaOXA-18, blaOXA-441, and blaOXA-570) were unique to ST244 strains. In ST244 XDR/PDR-PA, a β-lactamase gene is in the core if it is present in more than 70% of strains and flexibly present if it is present in less than 70%. Among the 146 β-lactamases in ST244 strains, 87% were identified as core β-lactamase genes and 13% as flexible. Notably, blaCARB-10, blaLEN-6, and blaOXA-570 are specific core β-lactamases of ST244 strains. These findings suggest that these three β-lactamase genes may be unique to ST244 strains compared to other STs.
Correlation Analysis of Resistance Genes in ST244 XDR/PDR-PA
We used the Pearson correlation coefficient (p<0.05, r>0.7) to investigate the relationship between ST244 XDR/PDR-PA resistance genes, as shown in Figure 4. A total of 146 β-lactamase genes were identified in the ST244 strains and analyzed using Gephi (version 0.9.2) for Pearson correlation analysis. Based on their frequency, the analysis revealed that 18 β-lactamase genes significantly correlated with the ST244 strains. Additionally, classification by Gephi grouped these 18 β-lactamase genes into three distinct classes: class 1 (purple) containing the highest number of β-lactamase genes at 14. Class 2 (green) and class 3 (orange) contained two β-lactamase genes each. The three β-lactamases specific to the ST244 strain identified in our previous analysis, blaCARB-10, blaLEN-6, and blaOXA-570, are also classified within class 1. Therefore, it can be hypothesized that these 14 β-lactamase genes may contribute to the distinct resistance profiles exhibited by ST244 strains compared to other STs.
The Association between the OXA-Type β-Lactamase Genes and the Minimum Inhibitory Concentration of CZA Against XDR/PDR-PA
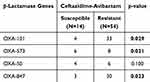
As shown in Table 1, blaOXA-101 and blaOXA-573 were associated with resistance to CZA (p-value < 0.05) in the 68 XDR/PDR-PA isolates tested. (p-value was 0.029 and 0.021, respectively). Figure S1 shows the MIC distribution of 68 XDR/PDR-PA against CZA. In addition, we selected a representative strain of PA-100 and listed the β-Lactamases produced by PA-100 in Table S1.
Discussion
The prevalence of hospital-acquired infections caused by XDR/PDR-PA is increasing, leading to a high mortality rate.18,19 This presents a significant global threat to public health and healthcare systems. Managing XDR/PDR-PA infections poses a challenge for clinicians due to the limited availability of effective antibiotics against these high-risk strains.20 Chromosomal mutations and the production of β-lactamases (ESBLs) are key factors contributing to drug resistance in XDR/PDR-PA, further complicating the treatment of these infections.21 In addition, the spread of these highly resistant bacteria within healthcare settings is a significant concern because they can easily infect vulnerable patients with compromised immune systems. Efforts to prevent the spread of XDR/PDR-PA include strict infection control measures, antibiotic stewardship programs, and research into new antimicrobial agents that can effectively target these pathogens. It is crucial to prioritize efforts toward preventing the further emergence and spread of these dangerous pathogens to safeguard patient safety and preserve the effectiveness of existing antibiotics for future generations.
In this study, we conducted whole-genome sequencing of 68 XDR/PDR-PA strains and performed bioinformatics analysis on the sequencing data. Our analysis revealed that the collected XDR/PDR-PA strains could be categorized into 20 STs, with the highest proportion being ST244 PA. Furthermore, a Pearson correlation analysis was conducted on ST244 PA, revealing three potential resistance patterns among these strains. Additionally, we identified 14 β-lactamases showing a strong correlation with resistance in ST244 PA. Drug susceptibility experiments using CZA were also conducted on the collected PAs. Statistical methods were employed to analyze the association between class D β-lactamases and CZA resistance in XDR/PDR-PA.
A study conducted by Chen et al collected 368 non-duplicated PA strains from various cities in southern China, with ST244 PA identified as the predominant clone during 2010–2011. This finding suggests that ST244 PA may have contributed to the widespread dissemination of PA in southern China, raising global concern due to its classification as a high-risk clone. Building on this research, our study at the First Medical Centre of the General Hospital of the People’s Liberation Army in Beijing, China, found that ST244 PA remained the predominant clonal strain from 2016 to 2021. These results are consistent with previous findings by Chen et al, indicating extensive dissemination of ST244 PA within our hospital over an extended period. The persistence and prevalence of these strains raise concerns about their potential impact on patient outcomes and healthcare-associated infections. Further investigation into the mechanisms driving its spread and strategies for containment is warranted to mitigate its effects on public health.
ST244 PA, the origin of the CC244 clonal complex, displays the exoS+ (exoU-) T3SS genotype and is associated with O antigen serotype O2.22 In a study conducted by Cabot et al, it was discovered that, in contrast to other high-risk clones, ST244 is prevalent in non-MDR isolates of PA, indicating that this type is widely distributed globally. However, it should be noted that the presence of ST244 PA does not necessarily correlate with antibiotic resistance.23 In our study, ST244 was common in XDR/PDR-PA, suggesting that ST244 PA may be associated with antibiotic resistance. The differences in the correlation between ST244 PA and drug resistance found in different studies may be related to the various sources of the collected strains.
In the study by Sid Ahmed et al, the susceptibility rate of ST244 PA to CZA was 50%.5 Furthermore, a wide range of horizontally acquired β-lactamases have been identified in ST244 PA. According to Fang et al, blaVIM-2 and blaIMP-6 were identified as prevalent resistance genes in ST244 PA from China.24 Similarly, our study found that the cause of antibiotic resistance in ST244 PA may be associated with 14 β-lactamases: blaCARB-5, blaOKP-A-10, blaCARB-10, blaOXA-433, blaTHIN-B, blaLEN-6, blaOXA-570, blaPER-1, blaMUS-2, blaLRA-2, blaRm3, blaRSA-1, blaGES-22, blaOXA-459. Therefore, we can develop new antimicrobial drugs according to the resistance background of ST244 PA.
In addition to the 14 β-lactamases mentioned in our study, further research has indicated that other factors, such as efflux pumps and outer membrane permeability, may also contribute to antibiotic resistance in ST244 PA. Understanding these resistance mechanisms is crucial for developing effective antimicrobial drugs. Furthermore, it is vital to consider the genetic diversity within ST244 PA when developing new antimicrobial drugs. Different strains of this bacterium may have varying resistance profiles, and a personalized approach to drug development could lead to more successful treatment outcomes. Furthermore, exploring alternative treatment options such as combination therapy or phage therapy could be beneficial in combating antibiotic resistance in ST244 PA. By diversifying our approach to treatment, we can increase the likelihood of successfully overcoming this challenge. Overall, our study highlights the complexity of antibiotic resistance in ST244 PA and emphasizes the continued need for research and innovation in antimicrobial drug development.
Sid Ahmed et al demonstrated a potential association between MDR-PA resistance to CZA and blaOXA-10, blaOXA-488.5,25,26 Furthermore, blaOXA-101 is a beta-lactamase like blaOXA-10, belonging to the AMR Gene Family. blaOXA-10 and blaOXA-101 may be involved in mediating PA resistance. We will clarify in the future whether these OXA enzymes are wild-type or contain amino acid changes to determine their potential role in conferring resistance to CZA in PA. We will also conduct further extensive studies on the mechanisms of resistance contributed by various β-lactamases.
In summary, our work found that blaOXA-101 and blaOXA-573 play a role in XDR/PDR-PA resistance to CZA.
Ethical Approval Statement
This work did not involve ethical issues. The study does not include patients’ privacy. No ethical approval was required.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Do Rego H, Timsit JF. Management strategies for severe Pseudomonas aeruginosa infections. Curr Opin Infect Dis. 2023;36(6):585–595. doi:10.1097/QCO.0000000000000981
2. Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4). doi:10.1128/CMR.00031-19
3. Reynolds D, Kollef M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: an update. Drugs. 2021;81(18):2117–2131. doi:10.1007/s40265-021-01635-6
4. Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi:10.1038/s41392-022-01056-1
5. Sid Ahmed MA, Khan FA, Hadi HA, et al. Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 beta-lactamase genes with resistance to ceftazidime-avibactam and ceftolozane-tazobactam in multidrug-resistant Pseudomonas aeruginosa. Antibiotics. 2022;11(2):130. doi:10.3390/antibiotics11020130
6. (Product information) ceftazidime and avibactam sodium for injection. Available from: https://reference.medscape.com/drug/avycaz-ceftazidime-avibactam-999985.
7. Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772. doi:10.1186/s12879-019-4409-1
8. Barber KE, Pogue JM, Warnock HD, Bonomo RA, Kaye KS. Ceftazidime/avibactam versus standard-of-care agents against carbapenem-resistant Enterobacteriaceae harbouring blaKPC in a one-compartment pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother. 2018;73(9):2405–2410. doi:10.1093/jac/dky213
9. Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs. 2013;73:159–177. doi:10.1007/s40265-013-0013-7
10. Stachyra T, Péchereau MC, Bruneau JM, et al. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother. 2010;54(12):5132–5138. doi:10.1128/AAC.00568-10
11. Lagacé-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of gram-negative bacterial infections. Core Evid. 2014;9:13–25. doi:10.2147/CE.S40698
12. Jean SS, Lee WS, Lam C, Hsu CW, Chen RJ, Hsueh PR. Carbapenemase-producing Gram-negative bacteria: current epidemics, antimicrobial susceptibility and treatment options. Future Microbiol. 2015;10(3):407–425. doi:10.2217/fmb.14.135
13. Mack AR, Hujer AM, Mojica MF, et al. β-Lactamase diversity in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2025;69:e0078524. doi:10.1128/aac.00785-24
14. Lorusso AB, Carrara JA, Barroso CDN, Tuon FF, Faoro H. Role of efflux pumps on antimicrobial resistance in Pseudomonas aeruginosa. Int J Mol Sci. 2022;23(24):15779. doi:10.3390/ijms232415779
15. Peykov S, Strateva T. Whole-genome sequencing-based resistome analysis of nosocomial multidrug-resistant non-fermenting gram-negative pathogens from the Balkans. Microorganisms. 2023;11(3):651. doi:10.3390/microorganisms11030651
16. Kang Y, Xie L, Yang J, Cui J. Optimal treatment of ceftazidime-avibactam and aztreonam-avibactam against bloodstream infections or lower respiratory tract infections caused by extensively drug-resistant or pan drug-resistant (XDR/PDR) Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2023;13. doi:10.3389/fcimb.2023.1023948
17. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow aerobicallyApproved Standard M7.
18. Palavutitotai N, Jitmuang A, Tongsai S, Kiratisin P, Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS One. 2018;13(2):e0193431. doi:10.1371/journal.pone.0193431
19. Mendes Pedro D, Paulo SE, Santos CM, et al. Extensively drug-resistant Pseudomonas aeruginosa: clinical features and treatment with ceftazidime/avibactam and ceftolozane/tazobactam in a tertiary care university hospital center in Portugal - A cross-sectional and retrospective observational study. Front Microbiol. 2024;15:1347521. doi:10.3389/fmicb.2024.1347521
20. PÉrez-VÁzquez M, Sola-Campoy PJ, Zurita ÁM, et al. Carbapenemase-producing Pseudomonas aeruginosa in Spain: interregional dissemination of the high-risk clones ST175 and ST244 carrying bla(VIM-2), bla(VIM-1), bla(IMP-8), bla(VIM-20) and bla(KPC-2). Int J Antimicrob Agents. 2020;56(1):106026. doi:10.1016/j.ijantimicag.2020.106026
21. Oliver A, Mulet X, Lopez-Causape C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat. 2015;21-22:41–59. doi:10.1016/j.drup.2015.08.002
22. E DB-T, Lopez-Causape C, Oliver A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired beta-lactamases: 2020 update. Int J Antimicrob Agents. 2020;56(6):106196. doi:10.1016/j.ijantimicag.2020.106196
23. Cabot G, Ocampo-Sosa AA, Domínguez MA, et al. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob Agents Chemother. 2012;56(12):6349–6357. doi:10.1128/AAC.01388-12
24. Fang Y, Baloch Z, Zhang W, et al. Emergence of carbapenem-resistant ST244, ST292, and ST2446 Pseudomonas aeruginosa clones in burn patients in Yunnan Province. Infect Drug Resist. 2022;15:1103–1114. doi:10.2147/IDR.S353130
25. Arca-Suárez J, Lasarte-Monterrubio C, Rodiño-Janeiro BK, et al. Molecular mechanisms driving the in vivo development of OXA-10-mediated resistance to ceftolozane/tazobactam and ceftazidime/avibactam during treatment of XDR Pseudomonas aeruginosa infections. J Antimicrob Chemother. 2021;76(1):91–100. doi:10.1093/jac/dkaa396
26. Fraile-Ribot PA, Mulet X, Cabot G, et al. In vivo emergence of resistance to novel cephalosporin-β-lactamase inhibitor combinations through the duplication of amino acid D149 from OXA-2 β-lactamase (OXA-539) in sequence type 235 Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61(9). doi:10.1128/AAC.01117-17
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.