Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
The Role of Lactate Dehydrogenase in Exploring the Immune Evasion in HCC Patients Who Underwent TACE: Implications for Clinical Application
Authors Xie Y , Sun X, Xie F, Jian W, Wang Q, Ma X, Li C, Zhang K
Received 27 May 2024
Accepted for publication 11 September 2024
Published 29 September 2024 Volume 2024:11 Pages 1823—1833
DOI https://doi.org/10.2147/JHC.S480090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Imam Waked
Yang Xie, Xiangyang Sun, Fubo Xie, Wencheng Jian, Qingliang Wang, Xiaochen Ma, Caixia Li, Kai Zhang
Department of Radiology, Qilu Hospital of Shandong University, Shandong, People’s Republic of China
Correspondence: Kai Zhang, Department of Radiology, Qilu Hospital of Shandong University, Shandong, People’s Republic of China, Tel +8618560081593, Email [email protected]
Purpose: To examine the relationship between lactate dehydrogenase (LDH) levels and soluble programmed cell death-ligand 1 (sPD-L1) levels in hepatocellular carcinoma (HCC) patients undergoing transarterial chemoembolization (TACE).
Methods: A total of 83 hCC patients participated in this study. Patients were categorized into subgroups based on their alpha-fetoprotein (AFP) levels, presence or absence of extrahepatic metastasis, vascular invasion, Barcelona Clinic Liver Cancer (BCLC) stage, tumor response, tumor size, and number LDH and sPD-L1 levels were compared before and after TACE (3, 7, and 30 days post-TACE).
Results: LDH and sPD-L1 levels were significantly higher at 3 and 7 days post-TACE than at baseline. Positive correlations were observed between changes in LDH levels and sPD-L1 levels at 3 and 7 days post-TACE. LDH levels were higher in patients with elevated AFP compared to those in the normal AFP group at 3 and 7 days post-TACE, in the stable disease (SD) group compared to complete response (CR) and partial response (PR) groups at 7 days post-TACE, and in those with tumor > 5 cm compared with those with tumor ≤ 5 cm at 3 and 7 days after TACE (all P < 0.05). sPD-L1 levels were higher in patients with vascular invasion than those without vascular invasion at 3 and 7 days post-TACE, in the SD group compared to CR and PR groups at 3 and 7 days post-TACE, and in those with tumor > 5 cm compared to those with tumor < 5 cm at 3 and 7 days after TACE (all P < 0.05).
Conclusion: A positive correlation was found between LDH expression and sPD-L1 levels, suggesting LDH as a potential biomarker for assessing immune status in HCC patients following TACE.
Keywords: hepatocellular carcinoma, immunotherapy, lactate dehydrogenase, soluble programmed cell death ligand-1, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the third most common cause of cancer-related death globally.1 Surgical resection is the primary treatment approach for individuals with early-stage HCC. However, owing to the subtle onset of HCC, the majority of patients receive diagnoses at intermediate or advanced stages, thereby missing the opportunity for radical treatment.2 Moreover, almost 70% of HCC patients develop tumor recurrence within 5 years after surgery.3 Consequently, non-radical treatments, including transarterial chemoembolization (TACE), chemotherapy, radiotherapy, multi-tyrosine kinase inhibitors (TKIs), and immunotherapy, assume pivotal roles in HCC management.
Immune checkpoint inhibitors (ICIs), such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors, have garnered global approval for clinical use and are anticipated to enhance treatment outcomes in HCC. These agents can effectively counteract the immunosuppressive tumor microenvironment (TME) and reinstate the antitumor activity of the immune system by obstructing the interaction between checkpoint proteins and their ligands, thereby thwarting T cell inactivation. Recent endeavors have focused on augmenting the efficacy of ICIs by integrating them with locoregional therapies like TACE and ablation.4,5 TACE is the most commonly employed treatment for unresectable HCC. However, the emergence of immune suppressive conditions post-TACE,6,7 which tends to correlate with adverse prognoses, has also been suggested.8 Consequently, administering ICIs after TACE to rectify the aberrant tumor immune environment appears logical. Nevertheless, HCC exhibits high heterogeneity with intricate biological and physiological features within the TME, and the immune status following TACE is complex and diverse.9 The impact of ICIs on HCC may vary, with only a subset of patients benefitting from this treatment.10 Hence, there is an urgent need to identify a dependable and readily detectable biomarker capable of delineating immune status within tumors post-TACE, which could aid in discerning patients who are responsive to ICIs.
Lactate dehydrogenase (LDH) is a significant enzyme involved in carbohydrate metabolism. Elevated serum LDH levels are commonly observed in individuals with malignant tumors. Research indicates a close association between LDH and various tumor-related biological processes, including drug resistance, angiogenesis, and metastasis.11 Furthermore, recent studies have linked increased LDH expression with exacerbating immune suppression within tumors.12
In this study, we examined changes in serum LDH and sPD-L1 levels in HCC patients before and after TACE, and investigated the correlation between serum LDH and sPD-L1 post-TACE. Our objective was to evaluate whether LDH could serve as a potential biomarker to assess the modulation of tumor immune status following TACE and to identify TACE-treated patients who may benefit from ICI treatment.
Materials and Methods
Patients
This study received approval from the hospital’s ethics committee. Clinical data of patients who underwent TACE between March 2019 and February 2021 were collected and analyzed. HCC diagnosis was established through histological examination or clinical features, following the diagnostic criteria outlined in the Diagnostic and Treatment Practices for Hepatocellular Carcinoma (2019 edition, People’s Republic of China). Inclusion criteria were the following: patients suitable for TACE, without prior antitumor treatment, with liver function classified as Child-Pugh grade A/B, presenting measurable lesions, and an ECOG score of 0–2. Enrolled patients refused systemic therapy, including targeted and immune treatment, due to personal reasons (including health and economic reasons, etc). Exclusion criteria were: individuals with obvious arterioportal or arteriovenous shunts, hypovascular tumors, widespread metastases with a projected survival < 3 months, liver function classified as Child-Pugh level C, occlusion of the second hepatic hilum or inferior vena cava, anemia, cachexia, or multiple organ failure.
Clinical characteristics recorded included age, gender, Child-Pugh scores, Barcelona Clinic Liver Cancer (BCLC) stage, history of hepatitis B virus (HBV) or history of hepatitis B virus (HCV) infection, alpha-fetoprotein (AFP) levels, presence of vascular invasion, and extrahepatic metastasis. Tumor response was assessed 30 days post-TACE following mRECIST criteria (Modified Response Evaluation Criteria In Solid Tumors criteria).
LDH and sPD-L1 Assessment
LDH and sPD-L1 levels were assessed one day before and 3, 7, and 30 days after TACE. Peripheral vein blood samples were collected for analysis. LDH levels were determined using the IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) method. The assay was conducted in Institution Laboratories certified for Quality control according to European standards (ISO 9001:2008). Serum sPD-L1 was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) Kit (Abcam Plc, Cambridge, UK), following the manufacturer’s recommendations. Contrast-enhanced magnetic resonance imaging (MRI) scans were performed before and 30 days after TACE to evaluate the antitumor effect in accordance with the mRECIST criteria.
Tace
All procedures were conducted under the guidance of a digital angiographic system (GE3100). A dose of 60–80 mg doxorubicin was loaded into drug-eluting beads (CalliSpheres, Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China), with the procedure typically lasting 30 minutes. The size of the CalliSpheres ranged from 100–300 or 300–500 µm, based on tumor size and blood supply. Following a successful femoral artery puncture, a 4F RH catheter was selectively catheterized into the celiac trunk and superior mesenteric artery, and angiography was performed to gather information about the location, size, and feeding artery of the tumor. After assessing this information, the tumor-feeding artery was catheterized selectively using a microcatheter (2.7Fr). Another angiography was conducted to confirm the placement of the microcatheter in the target artery of the tumor. Under X-ray monitoring, doxorubicin-loaded CalliSpheres were slowly injected into the tumor-feeding artery until near-stasis of blood flow was achieved. Subsequent angiography was performed to confirm the effectiveness of embolization.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as rates. The mean LDH and sPD-L1 levels at each time point were compared using the Wilcoxon signed-rank test or repeated measures analysis of variance. Differences in LDH and sPD-L1 levels before and after TACE were analyzed using the Wilcoxon–Mann–Whitney or Kruskal–Wallis test. Correlations between sPD-L1 and LDH were assessed using Pearson or Spearman correlation analysis. All statistical tests were two-sided, with significance defined as P < 0.05. Data analysis was performed using IBM SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
The Baseline Characteristics
A total of 83 patients with HCC were enrolled in the study. Among them, 70 (84.3%) were male and 13 (15.%) were female, with an average age of 58.9 years (range 47–79). Among those patients, 65 (78.3%) were classified as Child-Pugh stage A, while the remaining 18 (21.7%) were stage B. Regarding BCLC staging, 14 (16.9%) patients were at stage A, 28 (33.7%) at stage B, and 41 (49.4%) at stage C. Vascular invasion was observed in 34 (41.0%) patients and extrahepatic metastases were detected in 39 (47.0%) patients. Elevated AFP levels were found in 58 (69.9%) patients. The median maximum diameter of tumor of all patients were 7.9 cm (range from 1.4 to 18.7 cm). The proportion of patients with maximum diameter of tumor < 5cm was 8.4% (7 cases), three to five cm were 24.1% (20 cases), and > 5 cm were 67.5% (56 cases). The number of patients with single tumor was 30 (36.1%), and 53 (63.9%) patients were detected with multiple tumor. 69 (83.1%) patients had the history of cirrhosis. Patients with monolobar tumors accounted for 59.0% (49/83). Detailed baseline characteristics are summarized in Table 1.
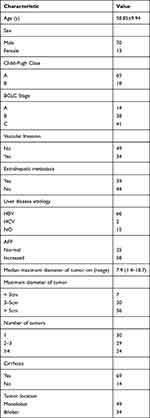 |
Table 1 Baseline Clinical Characteristics of HCC Patients |
Comparison of LDH and sPD-L1 Levels Before and After TACE
The levels of LDH and sPD-L1 were compared 1 day before and 3, 7, and 30 days after TACE. Compared to 1 day pre-TACE, LDH levels significantly increased on day 3 (524.9± 335.9 U/L vs 232.4± 66.6 U/L, P = 0.012) and day 7 (415.1±220.2 U/L vs 232.4 ± 66.6 U/L, P = 0.004) after TACE, while no significance was seen at day 30 post-TACE (241.1 ± 79.7 U/L vs 232.4 ± 66.6 U/L, P = 0.787) (Figure 1). Moreover, the sPD-L1 level on day 3 and 7 post-TACE was significantly higher compared to day 1 pre-TACE (day 3: 159.3±77.6 pg/mL vs 123.7 ± 37.6 pg/mL, P = 0.020; day 7: 151.7 ± 63.0 pg/mL vs 123.7 ± 37.6 pg/mL, P = 0.040), while no significance was seen at day 30 post-TACE (99.3 ± 36.4 pg/mL vs 123.7 ± 37.6 pg/mL, P = 0.381) (Figure 2).
The Correlation Between the Change of LDH and sPD-L1 Level
The fluctuation in LDH and sPD-L1 levels at 3, 7, and 30 days after TACE compared to those before TACE was calculated, and the correlation between LDH and sPD-L1 levels at each time point was analyzed. LDH levels were positively correlated with sPD-L1 levels at day 3 (R2 = 0.62 P < 0.05, Figure 3) and day 7 (R2 = 0.56, P < 0.05, Figure 4) after TACE. No significant correlation was seen at day 30 post-TACE (R2 = −0.23, P > 0.05, Figure 5).
 |
Figure 3 LDH and sPD-L1 levels showed a significantly positive association on day 3 post-TACE (R2 = 0.63, P < 0.05). |
 |
Figure 4 LDH and sPD-L1 levels showed a significantly positive correlation on day 7 post-TACE (R2 = 0.56, P < 0.05). |
 |
Figure 5 LDH and sPD-L1 levels did not significantly correlate on day 30 post-TACE (R2 = −0.23, P > 0.05). |
The Comparison of LDH Levels After TACE in Different Subgroups
Patients were categorized into subgroups based on AFP level, vascular invasion, BCLC stage, tumor response, tumor size, and tumor number. LDH levels were then compared among these subgroups on days 3, 7, and 30 post-TACE. The LDH level in the elevated AFP group was higher than that in the normal AFP group at day 3 and 7 after TACE (day 3: 572.7± 362.8 U/L vs 414.1 ± 233.7 U/L, P= 0.20; day 7: 447.7 ± 238.3 U/L vs 339.6 ± 149.2 U/L, P = 0.039), while no changes were seen for day 30 (245.6 ± 78.6 U/L vs 230.7 ± 82.9 U/L, P = 0.448) (Figure 6A). Also, there was no significant difference in LDH levels between patients with and without vascular invasion on day 3 (595.4± 424.8 U/L vs 476.0 ± 250.6 U/L, P = 0.112), day 7 (463.2 ± 279.0 U/L vs 381.8 ± 162.8 U/L, P = 0.098), and day 30 (241.6 ± 88.9 U/L vs 240.5 ± 73.6 U/L, P = 0.961) after TACE (Figure 6B). LDH levels among BCLC stage A, B, and C groups on day 3, 7, and 30 post-TACE showed no significant differences (P = 0.423, P = 0.319, P = 0.283, respectively) (Figure 6C).
Moreover, based on tumor response at day 30 post-TACE, 16 cases were defined as CR, 33 cases as PR, and the rest as SD. LDH levels in the SD group were higher than those in the CR and PR groups at day 7 post-TACE (SD vs CR: 489.7 ± 221.4 U/L vs 341.1 ± 117.3 U/L, P = 0.024; SD vs PR: 489.8 ± 221.4 U/L vs 374.2 ± 238.4 U/L, P = 0.029). However, no statistical difference in LDH levels was observed on days 3 and 30 post-TACE among CR, PR, and SD groups (P = 0.53, P = 0.085, respectively) (Figure 6D).
In addition, the LDH levels in patients with tumor > 5 cm were higher than in those with tumor ≤ 5 cm on day 3 and 7 post-TACE (day 3: 598.2± 382.7 U/L vs 372.9 ± 102.3 U/L, P = 0.002; day 7: 453.4 ± 243.7 U/L vs 335.8 ± 132.6 U/L, P = 0.006), while no changes were seen on day 30 post-TACE (248.8 ± 87.8U/L vs 225.2± 57.7 U/L, P = 0.208) (Figure 6E). Also, the LDH level in the single tumor group was higher than that in the multiple tumor group at day 3 post-TACE (579.3± 369.4 U/L vs 428.8 ± 243.8 U/L, P = 0.029) but similar on day 7 and 30 post-TACE (day 7: 444.3 ± 246.2 U/L vs 363.5 ± 155.0 U/L, P = 0.108; day 30: 244.7 ± 89.1U/L vs 234.8 ± 60.4 U/L, P = 0.591) (Figure 6F).
The Comparison of sPD-L1 Level After TACE in Different Subgroups
Like LDH, the sPD-L1 levels were compared among these subgroups on days 3, 7, and 30 post-TACE. There was no significant difference in sPD-L1 levels between patients with elevated or normal AFP levels on day 3 (164.8± 85.4 pg/mL vs 146.7 ± 54.8 pg/mL, P = 0.332), day 7 (156.2 ± 68.4 pg/mL vs 141.2 ± 47.7 pg/mL, P = 0.322), and day 30 (101.9 ± 36.9 pg/mL vs 93.2 ± 35.1 pg/mL, P = 0.318) after TACE (Figure 7A). The sPD-L1 level in the group with vascular invasion was higher than that in the group without vascular invasion on days 3 and 7 after TACE (day 3: 182.9± 98.2 pg/mL vs 142.9 ± 54.7 pg/mL, P = 0.036; day 7: 171.1 ± 84.6 pg/mL vs 138.2 ± 37.5 pg/mL, P = 0.039), but similar on day 30 (101.9 ± 34.1 pg/mL vs 97.6 ± 38.2 pg/mL, P = 0.593) (Figure 7B). sPD-L1 levels among BCLC stage A, B, and C groups on days 3, 7, and 30 showed no significant differences (P = 0.478, P = 0.962, P = 0.680, respectively) (Figure 7C).
sPD-L1 levels in the SD group were higher than those in the CR and PR groups on day 3 post-TACE (SD vs CR: 192.1 ± 89.0 pg/mL vs 122.6 ± 44.4 pg/mL, P = 0.002; SD vs PR: 192.1 ± 89.0 pg/mL vs 143.4 ± 65.1 pg/mL, P = 0.008) and day 7 (SD vs CR: 177.0 ± 78.7 pg/mL vs 129.2 ± 40.9 pg/mL, P = 0.007; SD vs PR: 177.0 ± 78.7 pg/mL vs 136.4 ± 42.8 pg/mL, P = 0.01). No difference in sPD-L1 levels was observed on day 30 among CR, PR, and SD groups (P = 0.269) (Figure 7D).
The sPD-L1 level in the group with tumor > 5 cm was higher than that in the group with tumor ≤ 5 cm on days 3 and 7 post-TACE (day 3: 171.3± 85.6 pg/mL vs 134.5 ± 50.4 pg/mL, P = 0.016; day 7: 161.1 ± 69.9 pg/mL vs 132.1 ± 40.0 pg/mL, P = 0.042), but similar on day 30 post-TACE (103.8 ± 34.3 pg/mL vs 90.0 ± 39.4 pg/mL, P = 0.105) (Figure 7E). The sPD-L1 level between the single tumor group and multiple tumor group was similar on day 3 (165.7 ± 77.4 pg/mL vs 148.0 ± 77.9 pg/mL, P = 0.321), day 7 (152.7 ± 56.3 pg/mL vs 150.0 ± 74.4 pg/mL, P = 0.849), and day 30 (97.7 ± 35.8 pg/mL vs 102.1 ± 37.9 pg/mL, P = 0.602) after TACE (Figure 7F).
Discussion
PD-1, an immunosuppressive receptor, is expressed on the cell membrane of various immune cells, including tumor cells, T cells, B cells, natural killer cells, and antigen-presenting cells.13 Upon binding with PD-L1, PD-1 becomes activated and inhibits key molecules in the TCR and CD28 pathways. This inhibition results in the suppression of T cell proliferation, activation, and cytotoxic T lymphocyte (CTL) functions, ultimately leading to activated T cell apoptosis.14 Increased PD-L1 expression, as the ligand of PD-1, has been associated with accelerated T cell apoptosis and immune evasion within the tumor.15 Moreover, previous studies on PD-L1 expression in HCC patients following TACE have demonstrated that increased PD-L1 levels impair immune surveillance of tumor cells, promoting tumor progression; also, increased PD-L1 levels have been correlated with poor prognosis.6,16 And in the present study, higher PD-L1 level after TACE were more likely found in the patients with heavier tumor burden and worse treatment response. PD-L1 molecules can exist as cell membranes or soluble forms. Previous studies suggested that sPD-L1, like PD-L1, can bind to PD-1 on the cytomembrane to deliver the message of immunosuppression. Among those, sPD-L1 levels have been associated with tumor staging, response to treatment, and prognosis and are considered potential biomarkers to assess the candidates for ICI therapy. In this study, increased sPD-L1 expression was observed following TACE. The administration of cytotoxic drugs and embolization of the feeding artery affected TME, remodeling it towards immune evasion.
The mechanism behind the increased PD-L1 expression after TACE may be attributed to several factors. First, TACE treatment can lower the intratumoral pH by enhancing glycolytic activity in both tumor and immune cells. The acidification of the intratumoral environment, caused by the accumulation of lactate produced during glycolysis, then induces the upregulation of PD-L1 expression.12,17 Additionally, tumor necrosis caused by TACE can trigger an inflammatory response, exposing tumor-associated antigens to antigen-presenting cells, stimulating tumor-specific immune responses, and ultimately promoting the recruitment and infiltration of immune cells into the tumor microenvironment.18 Among these infiltrating immune cells, CD8 cells, in particular, have been associated with the upregulation of PD-L1.19
Similarly to sPD-L1, elevated LDH levels after TACE were also observed in this study, with patients exhibiting better tumor response showing lower LDH levels post-TACE. LDH, acting as a pivotal enzyme in glycolysis, catalyzes the conversion of pyruvate to lactate.20 Tumor cells are known for their characteristic of aerobic glycolysis, a metabolic model wherein glycolysis persists even in oxygen-rich environments. LDH has a crucial role in sustaining efficient glycolytic metabolism within tumor cells. Previous studies have explored the role of LDH in tumors, identifying it as a potential biomarker for predicting the prognosis of HCC patients.21. Higher LDH level after TACE have demonstrated to be associated with larger tumor size and worse therapeutic effect in the present study. In the other researches, for instance, Scartozzi et al found better clinical outcomes (OS and TTP) in patients with decreased LDH levels after TACE compared to those with increased LDH.22 Furthermore, tumor-derived lactate has been implicated in promoting endothelial cell activation and angiogenesis. High initial LDH levels have shown a strong correlation with upregulated expression of vascular endothelial growth factor (VEGF) in tumors, suggesting that HCC patients with elevated LDH levels may be ideal candidates for multiple treatment approaches, including TACE and anti-VEGF inhibitors.22,23 Recent research has also delved into the impact of LDH on the antitumor immune response. For example, animal experiments utilizing lung and melanoma cancer models with knockdown of LDH-A have revealed that LDH-A deletion leads to decreased PD-L1 expression, underscoring the potential role of LDH in immune suppression.12
Considering that many HCC patients are not eligible for surgical resection, this study measured LDH levels in peripheral vein samples rather than tumor tissue. Moreover, relying solely on percutaneous puncture biopsy for quantitative analysis may introduce bias due to the limited amount of tumor tissue obtained. Conversely, measuring LDH levels in peripheral vein samples is a practical approach for monitoring LDH fluctuations, as serum LDH levels have been established to correspond to intra-tumor LDH levels.24,25 Hence, numerous previous studies have opted to monitor LDH levels in peripheral vein samples instead of tumor tissue. Moreover, as a routine biochemical index applied in the clinic, detecting the level of LDH in the blood is easily accessible and inexpensive.
The findings of this study revealed a close association between changes in LDH levels and sPD-L1 after TACE, with the high LDH level group post-TACE exhibiting elevated sPD-L1 expression. Several potential mechanisms may underlie the observed correlation between LDH and sPD-L1. Firstly, previous research has indicated that elevated LDH levels are linked to increased glycolytic activity post-TACE.26,27 Secondly, it is widely assumed that LDH can be released during tumor destruction following TACE, leading to higher LDH levels in peripheral blood.28,29 Moreover, a larger extent of tumor necrosis will likely result in higher LDH levels.30 After TACE, the elevation in LDH levels primarily arises from enhanced glycolysis and extensive tumor destruction, sharing many similarities with sPD-L1. This may elucidate the close correlation observed in LDH and sPD-L1 expression changes after TACE.
There are several limitations to the present study. Firstly, being a retrospective study, there is a potential for biased results. Secondly, the exploration of immune evasion status after TACE was limited to sPD-L1 alone. Given the complex nature of the immune environment of the tumor, this may not provide a comprehensive and unbiased reflection of the immune status.
It is widely believed that the immune evasion of tumors induced by TACE serves as the theoretical basis for considering ICI treatment following TACE as a promising strategy to improve the prognosis in HCC patients, as indicated by previous studies.7 However, not all HCC patients may benefit from ICI treatment following TACE due to the complex tumor environment post-TACE.31 Identifying HCC patients who underwent TACE and exhibit a better response to ICIs could be crucial; this could be achieved by exploring the immune status within the tumor post-TACE.
In conclusion, the objective of our study was to ascertain whether LDH could serve as a feasible biomarker capable of reflecting the immune status in HCC patients who have undergone TACE. The results of our study suggest that LDH may serve as a biomarker capable of reflecting the status of immune evasion within the tumor post-TACE, and HCC patients with higher LDH levels after TACE may be suitable candidates for ICI treatment.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics Approval and Consent to Participate
All procedures involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee, as well as the 1964 helsinki Declaration and its later amendments or comparable ethical standards. This work was approved by the Bioethics Committee of Qilu Hospital of Shandong University (No. 2018(140). Informed consent was obtained from all individual participants included in the study, and consent for publication was obtained for every individual person’s data included in the study.
Funding
This study received support from the China Health Promotion Foundation Kelly intervened in the scientific research fund (No. 6010120079).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
3. Xiao Y, Li W, Wan H, Tan Y, Wu H. Central hepatectomy versus major hepatectomy for patients with centrally located hepatocellular carcinoma: a meta-analysis. Internat J Surg. 2018;52:297–302. doi:10.1016/j.ijsu.2018.02.059
4. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70(5):999–1007. doi:10.1016/j.jhep.2019.01.027
5. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
6. Montasser A, Beaufrère A, Cauchy F, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79(1):36–46. doi:10.1111/his.14317
7. Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immuno Therap Can. 2021;9(9):e003311. doi:10.1136/jitc-2021-003311
8. Xiaochen M, Xiangyang S, Fubo X, et al. The influence of transarterial chemoembolization on serum levels of soluble programed cell death ligand-1 in advanced hepatocellular carcinoma patients. Asia-Pac J Clini Oncol. 2022;18(5):e515–e523. doi:10.1111/ajco.13687
9. He Q, Yang J, Jin Y. Development and validation of TACE refractoriness-related diagnostic and prognostic scores and characterization of tumor microenvironment infiltration in hepatocellular carcinoma. Front Immunol. 2022;13:869993. doi:10.3389/fimmu.2022.869993
10. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Anna Oncol. 2016;27(8):1492–1504. doi:10.1093/annonc/mdw217
11. Faloppi L, Bianconi M, Memeo R, et al. Lactate dehydrogenase in hepatocellular carcinoma: something old, something new. Biomed Res Int. 2016;2016:7196280. doi:10.1155/2016/7196280
12. Seth P, Csizmadia E, Hedblom A, et al. Deletion of lactate dehydrogenase-a in myeloid cells triggers antitumor immunity. Cancer Res. 2017;77(13):3632–3643. doi:10.1158/0008-5472.CAN-16-2938
13. Mahipal A, Tella SH, Kommalapati A, Lim A, Kim R. Immunotherapy in hepatocellular carcinoma: is there a light at the end of the tunnel? Cancers. 2019;11(8):1078. doi:10.3390/cancers11081078
14. Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol. 2020;1248:33–59.
15. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452.
16. Calderaro J, Rousseau B, Amaddeo G, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64(6):2038–2046. doi:10.1002/hep.28710
17. Lacroix R, Rozeman EA, Kreutz M, Renner K, Blank CU. Targeting tumor-associated acidity in cancer immunotherapy. Can Immunol Immunoth. 2018;67(9):1331–1348.
18. Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Molecul Med Rep. 2015;12(4):6065–6071. doi:10.3892/mmr.2015.4171
19. Naruse T, Yanamoto S, Okuyama K, et al. Immunohistochemical study of PD-1/PD-L1 axis expression in oral tongue squamous cell carcinomas: effect of neoadjuvant chemotherapy on local recurrence. Pathol Oncol Res. 2020;26(2):735–742. doi:10.1007/s12253-019-00606-3
20. Markert CL. Lactate dehydrogenase isozymes: dissociation and recombination of subunits. Science. 1963;140(3573):1329–1330. doi:10.1126/science.140.3573.1329
21. Zhuang G, Xie Y, Hong J, Lin S, Chen T, Fang W. Arterial chemoembolization for patients with hepatocellular carcinoma and elevated lactate dehydrogenase is associated with low survival: a cohort study. Infect Agent Cancer. 2022;17(1):31. doi:10.1186/s13027-022-00443-1
22. Scartozzi M, Faloppi L, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One. 2012;7(3):e32653. doi:10.1371/journal.pone.0032653
23. Faloppi L, Scartozzi M, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. doi:10.1186/1471-2407-14-110
24. Goldman RD, Kaplan NO, Hall TC. LACTIC dehydrogenase in human neoplastic tissues. Cancer Res. 1964;24:389–399.
25. Wood DC, Varela V, Palmquist M, Weber F. Serum lactic dehydrogenase and isoenzyme changes in clinical cancer. J Surg Oncol. 1973;5(3):251–257. doi:10.1002/jso.2930050308
26. Doemel LA, Santana JG, Savic LJ, et al. Comparison of metabolic and immunologic responses to transarterial chemoembolization with different chemoembolic regimens in a rabbit VX2 liver tumor model. Eur Radiol. 2022;32(4):2437–2447. doi:10.1007/s00330-021-08337-3
27. Serganova I, Rizwan A, Ni X, et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res. 2011;17(19):6250–6261. doi:10.1158/1078-0432.CCR-11-0397
28. Cui J, Xiong J, Zhang Y, et al. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J Crit Care. 2017;41:161–165. doi:10.1016/j.jcrc.2017.05.001
29. Green H, Tobar A, Gafter-Gvili A, et al. Serum lactate dehydrogenase is elevated in ischemic acute tubular necrosis but not in acute rejection in kidney transplant patients. Progr Transplantat. 2017;27(1):53–57.
30. Milross CG, Tucker SL, Mason KA, Hunter NR, Peters LJ, Milas L. The effect of tumor size on necrosis and polarographically measured pO2. Acta Oncol. 1997;36(2):183–189. doi:10.3109/02841869709109228
31. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi:10.1186/s12943-021-01489-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





