Back to Journals » Nature and Science of Sleep » Volume 17
The Role of Mature Brain-Derived Neurotrophic Factor and Its Precursor in Predicting Early-Onset Insomnia in Stroke Patients Experiencing Early Neurological Deterioration
Authors Shi G , Yu P , Wang Z, Xu M, Guo M, Wang X, Zhou R
Received 11 October 2024
Accepted for publication 10 January 2025
Published 12 February 2025 Volume 2025:17 Pages 315—327
DOI https://doi.org/10.2147/NSS.S500052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Guomei Shi,1,2 Peng Yu,2,3 Ziru Wang,2,4 Mingyang Xu,2,3 Minwang Guo,2,3 Xiaorong Wang,2,3 Rujuan Zhou2,3
1Department of Neurology, Taixing Clinical College of Bengbu Medical College, Taixing, Jiangsu, People’s Republic of China; 2Stroke Center, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 3Department of Neurology, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 4Department of Rehabilitation, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China
Correspondence: Rujuan Zhou, Taixing People’s Hospital, No. 1 Changzheng Road, Taixing, Jiangsu Province, 225400, People’s Republic of China, Tel +86-13951158499, Email [email protected]
Background: The investigation and management of early-onset insomnia (EOI) in patients undergoing early neurological deterioration (END) appear to be insufficiently prioritized in clinical practice. Brain-derived neurotrophic factor (mBDNF) and its precursor, proBDNF, play essential roles in neuroplasticity and may be involved in the pathophysiological mechanisms underlying EOI. This study aimed to investigate the associations of serum mBDNF, proBDNF, and the mBDNF/proBDNF ratio with EOI in stroke patients experiencing END.
Methods: In a prospective cohort study from October 2021 to December 2023, 232 stroke patients with END and 56 healthy controls (HCs) were enrolled. Serum levels of mBDNF and proBDNF were quantified using enzyme-linked immunosorbent assays. EOI was diagnosed according to the International Classification of Sleep Disorders, Third Edition (ICSD-3). Patients with END were categorized into subgroups based on the presence or absence EOI.
Results: Serum levels of mBDNF, proBDNF, and the mBDNF/proBDNF ratio were significantly lower in END patients compared to those in HCs (all p < 0.05). Among the 232 END patients, 82 (35.3%) developed EOI. Those with EOI had significantly lower levels of mBDNF and the mBDNF/proBDNF ratio compared to those without EOI (all p < 0.001). Multivariate logistic regression analysis revealed that male gender (p = 0.026), Hamilton Depression Rating Scale (HAMD) scores (p < 0.001), mBDNF (p = 0.009), and the mBDNF/proBDNF ratio (p < 0.001) were independent predictors of EOI in END patients. The areas under the curve (AUC) for mBDNF and the mBDNF/proBDNF ratio were 0.686 and 0.778, respectively.
Conclusion: Our study identified a correlation between reduced mBDNF levels and a decreased mBDNF/proBDNF ratio with the development of EOI in END patients. In addition, the mBDNF/proBDNF ratio may provide greater insight as a promising biomarker for EOI than mBDNF or proBDNF alone.
Keywords: brain-derived neurotrophic factor, ischemic stroke, insomnia, early neurological deterioration, biomarker
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Yang has been published for this article.
Introduction
Stroke represents a serious global health challenge, being a leading cause of mortality and morbidity in China.1,2 Early neurological deterioration (END) is relatively common within the first 48 hours after acute ischemic stroke (AIS) and has garnered increasing attention in recent years.3 Post-stroke individuals frequently experience deficits in mobility, cognition, language, and emotion regulation. In addition, self-reported insomnia has a considerable frequency among stroke survivors, affecting an estimated 52.4% to 70.2% of them, with nearly half experiencing insomnia for the first time.4–6 Insomnia in the early stage of stroke is reported to be strongly associated with worse cognitive performance, increased risks of fatigue, depression and anxiety, greater disability and mortality, as well as decreased quality of life.7–9 Insomnia is a critical factor in the recovery process of stroke survivors; however, current clinical practices exhibit substantial deficiencies in addressing early-onset insomnia (EOI) among patients experiencing END. Despite its significance, EOI in this patient cohort appears to be inadequately investigated and insufficiently prioritized. The prompt identification and effective management of EOI are essential for enhancing stroke rehabilitation and promoting neurological recovery following END, underscoring the necessity to rectify these shortcomings in clinical practice.
The pathophysiology of insomnia following END is complex and involves multiple factors. Alterations in neurotransmitters, inflammatory cytokines, and neurotrophins may be proposed as potential biomarkers for EOI. However, due to the limited focus on post-stroke insomnia, research on biomarkers associated with insomnia following a stroke remains scarce. Only a limited number of small-sample studies, primarily from Chinese populations, have investigated the predictive value of biomarkers such as cholecystokinin-8 (CCK-8), substance P (SP), serotonin (5-HT), γ-aminobutyric acid (GABA), tumor necrosis factor (TNF), and gut microbiota for post-stroke insomnia, yielding results with limited statistical power.10–13 To date, no studies have yet investigated the potential of neurotrophins as biomarkers for EOI, indicating a significant gap in the literature.
Brain-derived neurotrophic factor (BDNF), a pivotal component of the neurotrophin family, is bounteously expressed across the mammalian brain and is capable of crossing the blood-brain barrier.14 Initially released as precursor BDNF (proBDNF), it undergoes intracellular or extracellular cleavage to mature BDNF (mBDNF). Mature BDNF is a neuroprotective neurotrophin that plays essential role in promoting neuronal survival, neurite growth, synaptic plasticity, and synaptic transmission by binding to tyrosine kinase receptor B (TrkB).15 In contrast, proBDNF initiates apoptosis and inhibits neurite growth via p75 neurotrophin receptor (p75NTR).16 Hence, the relative levels of mBDNF and proBDNF, which should determine the balance between survival and apoptotic neuron, are key regulators in modulating the structure and function in various neurological and psychiatric disorders including stroke and insomnia.
Previous studies have consistently demonstrated that mBDNF levels were significantly lower in AIS patients compared to healthy individuals.17 Within the AIS cohort, lower mBDNF levels correlated with cognitive and emotional disorders, as well as poor functional prognosis.17–19 Besides, in a rat model of AIS, BDNF showed the capacity to enhance the therapeutic efficacy of neural stem cell-derived exosomes, underscoring a close affinity between BDNF and AIS.20 However, the association between BDNF and insomnia has yielded inconsistent results, with increased BDNF levels observed in patients with acute sleep deprivation, yet decreased in those with chronic insomnia.21,22 The relationship between BDNF and EOI in AIS patients with END remains unexplored. Furthermore, little is known about the role of proBDNF in insomnia or stroke. Recently, the ratio between mBDNF and proBDNF has been proposed as a potential biomarker for neuroplasticity in several neurological disorders, such as Parkinson’s disease, mild cognitive impairment, intracerebral hemorrhage, as well as autism spectrum disorders.23–26 To our knowledge, no studies up to date have appraised the levels of mBDNF, proBDNF, or the mBDNF/proBDNF ratio in the context of EOI in patients with END.
Herein, the study aimed to investigate the associations between neurotrophin levels and EOI within a clinical cohort of patients diagnosed with END. Additionally, it aimed to assess whether the mBDNF/proBDNF ratio could serve as a more precise predictive biomarker for EOI compared to the individual levels of mBDNF and proBDNF.
Materials and Methods
Study Participants
This was a prospective cohort study on the correlations between neurotrophin levels and EOI after END. Stroke patients with END were enrolled at Taixing People’s Hospital from October 2021 to December 2023. Inclusion criteria: (1) age ≥ 18 years; (2) admitted within 48 hours with a diagnosis of AIS corroborated by neuroimaging; (3) an increase of at least 4 points in the total National Institutes of Health Stroke Scale (NIHSS) score or an increase of at least 1 point in limb-movement-related NIHSS items within 72 hours following AIS.27–29 Exclusion criteria: (1) pre-stroke insomnia or hypnotics treatment prior to admission; (2) pre-existing psychiatric disorders or cognitive impairments; (3) receipt of intravenous thrombolysis or endovascular treatment; (4) severe aphasia or other conditions interfering with scale assessment; (5) other severe, life-threatening conditions such as malignant tumor, heart failure, renal failure, or hepatic failure. Healthy controls (HCs) without a history of stroke or insomnia were recruited from the hospital’s Physical Examination Center. The study protocol was evaluated and approved by the Ethics Committee of Taixing People’s Hospital (ethical number: LS2021017), and written informed consent was obtained from all participants or their legal representatives.
Data Collection
Sociodemographic information, including age, gender, education years, and body mass index (BMI), was collected, along with past medical history, subtype of stroke, lesion location, NIHSS scores as well as laboratory data, as previously described.30 Lesion location, including telencephalon, diencephalon, cerebellum, and brainstem, were ascertained by neurologists based on clinical information. The assessments of mood status were also conducted for END patients using 17-item Hamilton Depression Rating Scale (HAMD-17) and 14-item Hamilton Anxiety Rating Scale (HAMA-14). For the individuals in the control group, sociodemographic information, past medical history, and laboratory data were collected.
Definition of EOI
Early onset insomnia (EOI) was defined as the absence of insomnia symptoms prior to the stroke with the subsequent development of insomnia within two weeks post-stroke. The diagnosis of insomnia adhered to the diagnostic criteria outlined in the International Classification of Sleep Disorders (ICSD-3).31 Besides, sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI), a well-validated scale consisting of seven components, each ranging from 0 to 3, and the total score is ranging from 0 to 21.32 Patients with END were further divided into two subgroups base on with EOI or without EOI.
Serum mBDNF and proBDNF Levels Measurements
Fasting blood samples were collected from both HC subjects and patients with END. Blood specimens were centrifuged at 1000 rpm for 20 minutes at 4°C within 1 hour of collection. The derived serum was aliquoted into cryotubes and stored at −80°C for subsequent analysis. Enzyme-linked immunosorbent assay (ELISA) was utilized to quantify serum mBDNF and proBDNF levels. The Human mBDNF ELISA Kit (Catalog No. EH0043) and Human proBDNF ELISA Kit (Catalog No. EH4255) from FineTest, China, were employed according to the manufacturer’s guidelines. The assays had a sensitivity of 18.75 pg/mL for mBDNF and 9.375 pg/mL for proBDNF, with results expressed in ng/mL. The mean inter-assay and intra-assay coefficients of variation were 5.2% and 5.5% for mBDNF, and 3.6% and 5.3% for proBDNF, respectively.
Statistical Analysis
Variables were expressed as medians accompanied by quartiles, or means accompanied by standard deviations, or numbers accompanied by percentages. Univariate analyses were performed using t-test or Mann–Whitney U-test for continuous variables, and Pearson’s Chi-square test or Fisher’s exact test for categorical variables, as deemed suitable Multivariate logistic regression analysis was performed to identify the independent risk factors for EOI. Variables that demonstrated a statistically significant association (p-value < 0.1) in univariate analysis, as well as those deemed potentially relevant to EOI based on clinical experience and previous research, were considered. Age, gender, education years, snore, lesion location, NIHSS score, HAMA score, HAMD score, FBG, mBDNF and mBDNF/proBDNF were included in the multivariate logistic regression analysis. Receiver operating characteristic (ROC) curves were utilized to evaluate the predictive value of neurotrophin levels for EOI. In addition, subgroup analyses were conducted to evaluate potential interactions of confounding factors (age, gender, NIHSS, and HAMD) on the associations between neurotrophin levels and EOI. A two-tailed p-value < 0.05 was deemed to be statistically significant, and statistical analyses were conducted using R 4.2.3 (http://www.R-project.org/) and GraphPad Prism 9.3.1.
Results
Baseline Characteristics
A total of 232 stroke patients with END (average age, 68.6 ± 12.2 years; 150 males and 82 females) were included in this study. Main vascular risk factors were hypertension (69.4%), current smoker (39.7%), current drinker (28.9%), and diabetes (24.1%). Snoring, which can interrupt sleep, was prevalent in 66.8% of the participants. Large-artery atherosclerosis (44.0%) was the most common stroke subtype, and telencephalon (76.3%) was the most common lesion location. The median levels of mBDNF, proBDNF, and the mBDNF/proBDNF ratio among END patients were 16.20 (IQR 9.32–25.32) ng/mL, 8.93 (IQR 5.03–11.62) ng/mL, and 1.88 (IQR 1.68–2.22), respectively (Table 1).
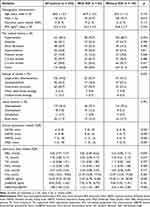 |
Table 1 Clinical Characteristics of END Patients With or Without EOI |
In addition, 56 HCs were enrolled (37 males and 19 females), with an average age of 66.2 ± 10.8 years. The baseline characteristics of HC individuals and stroke patients with END were described in Table 2. There were no significant differences between the HC group and the END group regarding age, gender, education years, BMI, or past medical history (p > 0.05). However, when compared to HCs, END patients exhibited a tendency towards lower levels of mBDNF, proBDNF, and the mBDNF/proBDNF ratio (p < 0.05).
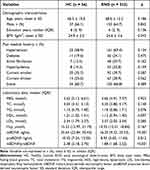 |
Table 2 Clinical Characteristics of the HC Individuals and the Stroke Patients With END |
Figure 1 depicted the serum levels of mBDNF, proBDNF, and the mBDNF/proBDNF ratio in both END patients and HCs. The serum concentrations of mBDNF (16.20 [IQR 9.32–25.32] ng/mL versus 25.64 [IQR 22.84–30.43] ng/mL, p < 0.001), proBDNF (8.93 [IQR 5.03–11.62] ng/mL versus 10.42 (IQR 7.54–13.05) ng/mL, p = 0.012), and the mBDNF/proBDNF ratio (1.88 [IQR 1.68–2.22] versus 2.38 [2.18–2.75], p < 0.001) were decreased in END group.
Correlations Between Neurotrophins and EOI
Among the END patients engaged in this study, 82 patients (35.3%) were identified with EOI. As displayed in Table 1, patients with EOI were more likely to be female (p = 0.001), had a lower prevalence of snore (p = 0.048), exhibited higher scores on the NIHSS (p < 0.001), HAMA (p < 0.001), HAMD (p < 0.001), and PSQI (p < 0.001). They also had higher levels of FBG (p = 0.040), lower levels of mBDNF (p < 0.001), and a lower mBDNF/proBDNF ratio (p < 0.001) compared to those without EOI. However, there was no significant difference in proBDNF between END patients with and without EOI (p > 0.05; Figure 2).
After adjusting for age, gender, and potential confounders with p < 0.1 in the univariate analysis, mBDNF (OR 0.935, 95% CI 0.889–0.983, p = 0.009), mBDNF/proBDNF ratio (OR 0.074, 95% CI 0.016–0.340, p < 0.001), HAMD (OR 1.429, 95% CI 1.184–1.725, p < 0.001), as well as male gender (OR 0.351, 95% CI 0.139–0.885, p = 0.026) were independently associated with EOI (Table 3).
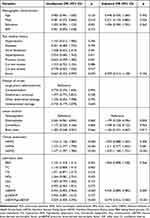 |
Table 3 Logistic Regression Analyses for the Related Factors Associated With EOI in END Patients |
Subgroup Analyses for Neurotrophins and EOI
Subgroup analyses, stratified by age (< 60 versus ≥ 60), gender (male versus female), NIHSS (< 10 versus ≥ 10), and HAMD (< 7 versus ≥ 7), indicated that higher levels of mBDNF and mBDNF/proBDNF ratio were associated with a decreased risk of EOI across most categories (Table 4). No significant interactions between neurotrophins and these subgroup factors were observed in relation to EOI risk (p for interaction > 0.05 for all). Moreover, the association between the mBDNF/proBDNF ratio and EOI was more pronounced than that of mBDNF alone.
 |
Table 4 Subgroup Analyses of the Associations of mBDNF and mBDNF/proBDNF With EOI |
Predictive Values of mBDNF and mBDNF/proBDNF Ratio for EOI in END
ROC curve analysis revealed that the optimal cutoff value of mBDNF for predicting EOI was projected to be 18.20 ng/mL, which yielded a sensitivity of 56.0% and a specificity of 79.3%, with the AUC of 0.686 (95% CI 0.615–0.757; p < 0.001; Figure 3A). The mBDNF/proBDNF ratio exhibited a significantly greater discriminatory ability for predicting EOI compared to mBDNF alone, with an AUC of 0.778 (95% CI 0.719–0.837; p < 0.001; Figure 3B). Subsequently, an ROC curve was generated by combining mBDNF with the mBDNF/proBDNF ratio. This curve had an AUC of 0.783 (95% CI 0.725–0.841; p < 0.001; Figure 3C). However, the combination of mBDNF and the mBDNF/proBDNF ratio did not show superior discriminative value compared to the mBDNF/proBDNF ratio alone (Figure 3D).
Discussion
This study may represent the first prospective study reporting the associations of neurotrophins including mBDNF, its precursor (proBDNF), and the mBDNF/proBDNF ratio, with early-onset insomnia (EOI) in END patients. The current study revealed significant reductions of both mBDNF levels and the mBDNF/proBDNF ratio among individuals with EOI, with the ratio exhibiting superior predictive value over mBDNF alone. The AUC for the mBDNF/proBDNF ratio in predicting EOI was 0.778. Typically, a biomarker with an AUC between 0.70 and 0.90 is considered to have moderate predictive value. Therefore, the mBDNF/proBDNF ratio could be proposed as a reliable biomarker for EOI.
As reported by previous published studies, as high as approximately 70% of stroke survivors may present with insomnia or insomnia symptoms during the first few months, with up to half patients presenting with insomnia for the first time.4,5 In our cohort, patients with previously diagnosed insomnia were excluded and the prevalence of EOI was identified to be 35.3%, which was concordant with Xie et al (36.1%),13 but slightly higher than that reported by Joa et al (26.9%)33 and Matas et al (28.7%).7 This discrepancy may be primarily ascribed to differences in the definition and timing of assessing insomnia, variances in the characteristics and regions of participants, and discrepancies in stroke severity. Meanwhile, our research uncovered that EOI patients tend to be female and had higher HAMD score, which were in line with literature data, suggesting a bidirectional relationship with insomnia and affective disorders.4,6
As one of the most abundant neurotrophin in the brain, mBDNF is an essential determinant of neuronal survival, neuronal differentiation and synaptic plasticity. Meanwhile, it also participates in the pathophysiological process of learning and memory.15 Prior studies have noted a closely relationship between mBDNF and ischemic stroke. Low levels of mBDNF were associated with an increased risk of stroke, and poor prognosis and recovery after stroke.17 In addition, data from a systematic review showed poststroke exercise can increase BDNF levels, which may contribute to increased neuroplasticity and enhance functional recovery.34 However, the literature on BDNF changes associated with insomnia has produced inconsistent findings.21,22 For instance, elevated BDNF levels have been observed in healthy adults with acute sleep deprivation,21 while decreased BDNF concentrations have been observed in patients with chronic sleep deprivation.22 Stroke patients, particularly those exhibiting severe neurological deficits, are often accompanied by insomnia during the initial phase of their condition.6 To date, prior research has not yet elucidated the correlation between BDNF and EOI in stroke patients with END. Our study is the first to report that serum levels of mBDNF are significantly lower in patients with END compared to HCs. Furthermore, a pronounced reduction in mBDNF levels was noted in patients who experienced EOI compared to those who did not. Multivariate analysis confirmed that serum mBDNF levels are independently associated with the occurrence of EOI, with an OR of 0.935 (95% CI 0.889–0.983, p = 0.009). Our results would contribute novel clinical insights into the alterations of mBDNF in individuals experiencing insomnia following a stroke.
ProBDNF, the precursor form of mBDNF, preferentially binds to p75NTR, thereby forming a complex that triggers neuronal degeneration, impairs synaptic transmission, and elicits neuronal apoptosis.16 The balance between proBDNF and mBDNF is critical for maintaining neuronal health and modulating synaptic function. Recent attention has been paid regarding the impact of the imbalance between proBDNF and mBDNF covering a spectrum of neuropsychiatric and neurodegenerative disorders. Higher proBDNF/mBDNF ratio was associated with lower cognitive performance in the pre-clinical stages of Alzheimer’s disease.24 In the diagnosis of Parkinson’s disease, the study by Yi et al suggest that mBDNF/proBDNF ratio has better diagnostic value than mBDNF and proBDNF alone.23 It has also been reported that the mBDNF/proBDNF ratio has demonstrated clinical utility in differentiating between children with autism spectrum disorders and those with intellectual disabilities.26 However, the dysregulation of mBDNF/proBDNF ratio has not been examined in the context of stroke or insomnia. In our study, proBDNF levels also decreased in END patients. Despite no significant association being observed between proBDNF levels and EOI, the mBDNF/proBDNF ratio exhibited a markedly enhanced predictive capacity for EOI. Furthermore, in pre-clinical animal models, the injection of ATP into corpus striatum could alleviate cerebral hemorrhage-induced injury by increasing the mBDNF/proBDNF ratio,25 aerobic exercise could improve depression and increase mBDNF/proBDNF ratio in the ischemic hippocampus,35 and the function of neural stem cell-derived exosomes could be improved by BDNF in the treatment of ischemic stroke.20 Our research built upon the prior discoveries by suggesting that mBDNF/proBDNF ratio could also serve as a promising biomarker for EOI in patients suffering END. Thus, we hypothesize that modulating the balance of mBDNF and proBDNF may represent an efficacious and innovative therapeutic approach for managing insomnia and stroke.
Research on the biomarkers associated with insomnia following a stroke remains limited. Zhang et al investigated the relationship between the serum expression levels of CCK-8, SP, and 5-HT and post-stroke insomnia. However, their findings did not substantiate the involvement of CCK-8, SP, or 5-HT in the pathogenesis of insomnia after stroke.10 Additionally, Zhang et al examined the role of glutamatergic hypofunction in post-stroke insomnia, proposing a negative correlation between GABA and post-stroke insomnia.11 Nevertheless, due to the limited sample size, the study was unable to assess the predictive value of GABA for post-stroke insomnia. Research conducted by Geng et al have demonstrated TUR may be severe as a biomarker for post-stroke insomnia, with an AUC of 0.703.12 Furthermore, Xie et al explored the role of gut microbiota in post-stroke sleep disorders and found that a predictive model utilizing eight operational-taxonomic-unit-based biomarkers achieved a high accuracy in predicting post-stroke sleep disorders, with an AUC of 0.768.13 It is important to note that these studies have primarily focused on insomnia occurring 1–3 months after a stroke. In contrast, our study is the first to investigate the predictive value of neurotrophic factors for EOI within two weeks in patients with END, achieving an AUC of 0.778 for the mBDNF/proBDNF ratio.
The pathogenesis of EOI following stroke is likely multifactorial, involving physiological, psychological, socioeconomic, and environmental factors. Our study showed that patients with EOI exhibit reduced levels of neurotrophins, including mBDNF, its precursor (proBDNF), and the mBDNF/proBDNF ratio, thereby underscoring the significant role of BDNF in the etiology and progression of EOI. Several potential hypotheses may elucidate this phenomenon. Firstly, in a rat model of AIS, zolpidem treatment demonstrated beneficial effects on behavioral recovery, as evidenced by an increase in BDNF-stained cells, indicating an upregulation of neuroplasticity.36 Besides, another rat model revealed that zolpidem treatment ameliorated circadian rhythm disruptions, alleviated sleep fragmentation, and enhanced sleep depth by restoring suprachiasmatic nucleus activity.37 Current evidence suggests that BDNF exhibits circadian rhythmicity.38,39 We hypothesize that decreased BDNF levels in patients with END may contribute to disturbances in circadian rhythms, increased sleep fragmentation, reduced sleep depth, and ultimately, EOI. Secondly, patients experiencing END often exhibit emotional responses, such as increased tension and stress. Jeanneteau et al reported that the expression of BDNF/TrkB was downregulated in the corticolimbic system but upregulated in the mesolimbic system following the cessation of stressor exposure.40 We propose that this reduction in BDNF expression may enhance neuronal plasticity in the mesolimbic region, thereby increasing the susceptibility of END patients to stress and tension, and potentially contributing to the pathophysiology of EOI.41 Thirdly, the presence of TrkB receptors on GABAergic and glutamatergic neurons in the midbrain and brainstem indicates a potential role for the BDNF-TrkB signaling pathway in sleep regulation.42 In patients with END, decreased levels of BDNF may influence the rapid eye movement (REM) and non-rapid eye movement (NREM) phases,43 potentially leading to EOI. Moreover, proBDNF can activate apoptotic signaling pathways, resulting in neuronal cell death, particularly in brain regions responsible for sleep regulation, such as the hypothalamus.44 In addition, Yang et al have confirmed that proBDNF can upregulate the expression of pro-inflammatory cytokines,45,46 indicating that a predisposition towards proBDNF post-stroke may exacerbate the inflammatory response. This overproduction of pro-inflammatory cytokines, stimulated by higher levels of proBDNF, has the potential to disrupt sleep patterns. In general, a comprehensive understanding of the complex mechanisms that link serum levels of mBDNF and its precursor (proBDNF) to EOI could pave the way for the development of more efficacious intervention strategies for EOI.
The present study has several limitations that warrant consideration. Firstly, the samples were collected from AIS patients experiencing END. For patients receiving intravenous thrombolysis or endovascular treatment, the time frame for assessing END is more likely to be within 24 hours,47,48 which differs from the parameters of this study. Besides, both thrombolysis and endovascular treatment can increase the likelihood of hemorrhagic transformation,49 potentially affecting the expression of BDNF.50 Thus, patients undergoing intravenous thrombolysis or endovascular treatment were excluded from the study, which may introduce a selection bias. Secondly, as emphasized in the literature, factors such as social support, marital status, economic situation, and family violence may severe as potential confounders for EOI.8 These factors, along with environmental and lifestyle elements such as diet, physical activity, and changes in sleep patterns, are acknowledged for their potential influence on the development of EOI.51 The lack of investigation into these variables in the present study constrains the understanding of their contributions to EOI in stroke patients experiencing END. We recognize the significance of these variables and intend to incorporate them in a prospective manner in future research to better assess their effects on the development of EOI. Thirdly, in the current study, a constraint was the limited number of patients (11 out of 232 subjects) who underwent polysomnography due to its high cost and technical complexity. Consequently, the severity of EOI was primarily assessed using PSQI, which may introduce a potential bias into our findings. For future studies, more objective measures of EOI, such as polysomnography or actigraphy, are crucial for accurately characterizing sleep patterns and diagnosing sleep disorders. Fourthly, it is known that AIS patients are more susceptible to sleep apnea and restless leg syndrome compared to the general population.52,53 We have also to consider that EOI could be attributed to undiagnosed sleep apnea or restless leg syndrome, which may manifest as insomnia. Therefore, these conditions must be considered as potential bias in the study. Fifthly, the cross-sectional and observational design of our study limits the ability to draw definitive conclusions about the causal relationship between mBDNF, its precursor (proBDNF), and EOI. Furthermore, the study’s relatively small sample size and the exclusive inclusion of Chinese participants highlight a significant limitation. This limitation emphasizes the necessity for future research to incorporate larger and more diverse populations, particularly non-Chinese cohorts, to improve the generalizability of the findings. Finally, our study establishes a pioneering correlation between EOI and decreased levels of mBDNF and mBDNF/proBDNF ratio, which is both innovative and beneficial. However, it is imperative to conduct further longitudinal research to determine the long-term prognostic significance of neurotrophin levels on post-stroke insomnia outcomes.
Conclusions
Our study revealed an association between decreased levels of mBDNF and a diminished mBDNF/proBDNF ratio with the occurrence of EOI in patients with END. In addition, the mBDNF/proBDNF ratio may serve as a more informative biomarker for EOI than either mBDNF or proBDNF individually. Targeting the mBDNF/proBDNF imbalance through therapeutic interventions, such as augmenting mBDNF signaling or inhibiting proBDNF activity, could potentially alleviate EOI and influence the comprehensive rehabilitation trajectory of patients with END.
Data Sharing Statement
The data that support the findings of this study are available from Rujuan Zhou upon reasonable request.
Ethics Approval
The study was approved by the Ethics Committee of Taixing People’s Hospital (ethical number: LS2021017) and conducted according to the Declaration of Helsinki.
Author Contributions
Conception and study design: Rujuan Zhou and Guomei Shi; execution and investigation: Xiaorong Wang and Minwang Guo; acquisition of data: Peng Yu, Ziru Wang and Mingyang Xu; analysis and interpretation: Guomei Shi and Peng Yu; funding acquisition: Guomei Shi. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Natural Science Foundation of Bengbu Medical College (2020BYZD269), the Science and Technology Development Fund of Jiangsu University (JLY2021195), the Scientific Research Project of Jiangsu Provincial Health Commission (Z2023052), the Doctoral Science Foundation of Taixing People’s Hospital (TRYBS2022001) and the Excellent Talent Fund Project of Xuzhou Medical University Affiliated Hospital (XYFY202420).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Tu WJ, Wang LD. Special Writing Group of China Stroke Surveillance R. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
2. GBD 2021. Risk factors collaborators. global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2162–2203. doi:10.1016/S0140-6736(24)00933-4.
3. Zhang X, Zhong W, Xue R, et al. Argatroban in patients with acute ischemic stroke with early neurological deterioration: a randomized clinical trial. JAMA Neurol. 2024;81(2):118–125. doi:10.1001/jamaneurol.2023.5093
4. Fan XW, Yang Y, Wang S, et al. Impact of persistent poor sleep quality on post-stroke anxiety and depression: a national prospective clinical registry study. Nat Sci Sleep. 2022;14:1125–1135. doi:10.2147/NSS.S357536
5. Chen P, Wang W, Ban W, et al. Deciphering post-stroke sleep disorders: unveiling neurological mechanisms in the realm of brain science. Brain Sci. 2024;14(4):307. doi:10.3390/brainsci14040307
6. Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. doi:10.1016/j.smrv.2019.101222
7. Matas A, Amaral L, Patto AV. Is post-ischemic stroke insomnia related to a negative functional and cognitive outcome? Sleep Med. 2022;94:1–7. doi:10.1016/j.sleep.2022.03.022
8. Rangel MFA, Silva LC, Gonçalves EH, Silva A, Teixeira-Salmela LF, Scianni AA. Presence of self-reported sleep alterations after stroke and their relationship with disability: a longitudinal study. Neurorehabil Neural Repair. 2024;38(7):518–526. doi:10.1177/15459683241252826
9. Celikbilek A, Koysuren A, Konar NM. Role of vitamin D in the association between pre-stroke sleep quality and poststroke depression and anxiety. Sleep Breath. 2024;28(2):841–848. doi:10.1007/s11325-023-02894-1
10. Zhang XH, Zhang X, Feng HY, et al. An investigation on the changes of serum CCK-8, substance P, and 5-HT in patients with post-stroke insomnia. Technol Health Care. 2023;31(6):2355–2361. doi:10.3233/THC-230506
11. Zhang XH, Zhang X, Liu XW, et al. Examining the Role of GLU/GABA to GLN metabolic cycle in the pathogenesis of post-stroke depressive disorder and insomnia. Neuropsychiatr Dis Treat. 2023;19:2833–2840. doi:10.2147/NDT.S443844
12. Geng D, Wu B, Lin Y, et al. High total bilirubin-to-uric acid ratio predicts poor sleep quality after acute ischemic stroke: a prospective nested case-control study. Psychogeriatrics. 2023;23(6):897–907. doi:10.1111/psyg.12992
13. Xie H, Chen J, Chen Q, et al. The diagnostic value of gut microbiota analysis for post-stroke sleep disorders. Diagnostics (Basel). 2023;13(18). doi:10.3390/diagnostics13182970
14. Yan Q, Rosenfeld RD, Matheson CR, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78(2):431–448. doi:10.1016/s0306-4522(96)00613-6
15. Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J mol Sci. 2018;19(11):3650. doi:10.3390/ijms19113650
16. Yang J, Harte-Hargrove LC, Siao CJ, et al. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7(3):796–806. doi:10.1016/j.celrep.2014.03.040
17. Mojtabavi H, Shaka Z, Momtazmanesh S, Ajdari A, Rezaei N. Circulating brain-derived neurotrophic factor as a potential biomarker in stroke: a systematic review and meta-analysis. J Transl Med. 2022;20(1):126. doi:10.1186/s12967-022-03312-y
18. Chang X, He Y, Liu Y, et al. Serum brain derived neurotrophic factor levels and post-stroke depression in ischemic stroke patients. J Affect Disord. 2024;361:341–347. doi:10.1016/j.jad.2024.06.050
19. Chang X, You J, Yang P, et al. High-serum brain-derived neurotrophic factor levels are associated with decreased risk of poststroke cognitive impairment. Stroke. 2024;55(3):643–650. doi:10.1161/STROKEAHA.123.044698
20. Zhu ZH, Jia F, Ahmed W, et al. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen Res. 2023;18(2):404–409. doi:10.4103/1673-5374.346466
21. Xue J, Li H, Xu Z, et al. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via BDNF signaling-mediated descending facilitation in rats. Neurochem Res. 2018;43(12):2353–2361. doi:10.1007/s11064-018-2660-2
22. Ballesio A, Zagaria A, Curti DG, et al. Peripheral brain-derived neurotrophic factor (BDNF) in insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2023;67:101738. doi:10.1016/j.smrv.2022.101738
23. Yi X, Yang Y, Zhao Z, et al. Serum mBDNF and ProBDNF expression levels as diagnosis clue for early stage parkinson’s disease. Front Neurol. 2021;12:680765. doi:10.3389/fneur.2021.680765
24. Cechova K, Angelucci F, Markova H, et al. Ratio of serum proBDNF to BDNF and its association with cognitive performance and brain morphometry in mild cognitive impairment. Alzheimers Dement. 2020;16(S6). doi:10.1002/alz.046340.
25. He Q, Li Z, Li T, Zhang Z, Zhao J. ATP stimulation promotes functional recovery after intracerebral haemorrhage by increasing the mBDNF/proBDNF ratio. Neuroscience. 2021;459:104–117. doi:10.1016/j.neuroscience.2020.12.034
26. Cui T, Liu Z, Li Z, et al. Serum brain-derived neurotrophic factor concentration is different between autism spectrum disorders and intellectual disability children and adolescents. J Psychiatr Res. 2024;170:355–360. doi:10.1016/j.jpsychires.2024.01.001
27. Li Y, Chen X, Zhou R, et al. Correlation between cognitive impairment and homocysteine and S100B protein in patients with progressive ischemic stroke. Neuropsychiatr Dis Treat. 2023;19:209–217. doi:10.2147/ndt.S393624
28. Helleberg BH, Ellekjaer H, Rohweder G, Indredavik B. Mechanisms, predictors and clinical impact of early neurological deterioration: the protocol of the Trondheim early neurological deterioration study. BMC Neurol. 2014;14(1):201. doi:10.1186/s12883-014-0201-4
29. Yang H, Lv Z, Wang W, Wang Y, Chen J, Wang Z. machine learning models for predicting early neurological deterioration and risk classification of acute ischemic stroke. Clin Appl Thromb Hemost. 2023;29:10760296231221738. doi:10.1177/10760296231221738
30. Li M, Liu H, Xu M, et al. Glial fibrillary acidic protein as a potential indicator for symptomatic intracranial hemorrhage in acute ischemic patients undergoing endovascular thrombectomy. Clin Interv Aging. 2024;19:123–132. doi:10.2147/cia.S448180
31. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
32. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi:10.1016/j.smrv.2015.01.009
33. Joa KL, Kim WH, Choi HY, et al. The effect of sleep disturbances on the functional recovery of rehabilitation inpatients following mild and moderate stroke. Am J Phys Med Rehabil. 2017;96(10):734–740. doi:10.1097/PHM.0000000000000744
34. Ashcroft SK, Ironside DD, Johnson L, Kuys SS, Thompson-Butel AG. Effect of exercise on brain-derived neurotrophic factor in stroke survivors: a systematic review and meta-analysis. Stroke. 2022;53(12):3706–3716. doi:10.1161/strokeaha.122.039919
35. Luo L, Li C, Du X, et al. Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav Brain Res. 2019;362:323–331. doi:10.1016/j.bbr.2018.11.037
36. Oh MK, Yoon KJ, Lee YT, et al. Effect of zolpidem on functional recovery in a rat model of ischemic stroke. J Int Med Res. 2018;46(1):249–257. doi:10.1177/0300060517723799
37. Zhong ZG, Tao GJ, Hao SM, et al. Alleviating sleep disturbances and modulating neuronal activity after ischemia: evidence for the benefits of zolpidem in stroke recovery. CNS Neurosci Ther. 2024;30(2):e14637. doi:10.1111/cns.14637
38. D’Agostino Y, Frigato E, Noviello TMR, et al. Loss of circadian rhythmicity in bdnf knockout zebrafish larvae. iScience. 2022;25(4):104054. doi:10.1016/j.isci.2022.104054
39. Cain SW, Chang AM, Vlasac I, et al. Circadian rhythms in plasma brain-derived neurotrophic factor differ in men and women. J Biol Rhythms. 2017;32(1):75–82. doi:10.1177/0748730417693124
40. Jeanneteau F, Borie A, Chao MV, Garabedian MJ. Bridging the gap between brain-derived neurotrophic factor and glucocorticoid effects on brain networks. Neuroendocrinology. 2019;109(3):277–284. doi:10.1159/000496392
41. Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. doi:10.2147/NSS.S138823
42. Kaczmarski P, Sochal M, Strzelecki D, Białasiewicz P, Gabryelska A. Influence of glutamatergic and GABAergic neurotransmission on obstructive sleep apnea. Front Neurosci. 2023;17:1213971. doi:10.3389/fnins.2023.1213971
43. Garner JM, Chambers J, Barnes AK, Datta S. Changes in brain-derived neurotrophic factor expression influence sleep-wake activity and homeostatic regulation of rapid eye movement sleep. Sleep. 2018;41(2). doi:10.1093/sleep/zsx194
44. Li JY, Liu J, Manaph NPA, Bobrovskaya L, Zhou XF. ProBDNF inhibits proliferation, migration and differentiation of mouse neural stem cells. Brain Res. 2017;1668:46–55. doi:10.1016/j.brainres.2017.05.013
45. Yang CR, Ding HJ, Yu M, et al. proBDNF/p75NTR promotes rheumatoid arthritis and inflammatory response by activating proinflammatory cytokines. FASEB J. 2022;36(3):e22180. doi:10.1096/fj.202101558R
46. Yang CR, Liang R, Liu Y, et al. Upregulation of proBDNF/p75NTR signaling in immune cells and its correlation with inflammatory markers in patients with major depression. FASEB J. 2024;38(1):e23312. doi:10.1096/fj.202301140RR
47. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
48. Girot JB, Richard S, Gariel F, et al. Predictors of unexplained early neurological deterioration after endovascular treatment for acute ischemic stroke. Stroke. 2020;51(10):2943–2950. doi:10.1161/STROKEAHA.120.029494
49. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48(12):e343–e361. doi:10.1161/STR.0000000000000152
50. Guo YC, Song XK, Xu YF, Ma JB, Zhang JJ, Han PJ. The expression and mechanism of BDNF and NGB in perihematomal tissue in rats with intracerebral hemorrhage. Eur Rev Med Pharmacol Sci. 2017;21(15):3452–3458.
51. Hasan F, Tu YK, Lin CM, et al. Comparative efficacy of exercise regimens on sleep quality in older adults: a systematic review and network meta-analysis. Sleep Med Rev. 2022;65:101673. doi:10.1016/j.smrv.2022.101673
52. Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur Respir J. 2020;55(4):1901104. doi:10.1183/13993003.01104-2019
53. Shi GM, Wang XR, Xu W, Guo MW, Ding CQ, Zhou RJ. Pontine warning syndrome and restless legs syndrome secondary to paramedian pontine infarction: a case report. Int J Neurosci. 2022;132(9):881–884. doi:10.1080/00207454.2020.1849187
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Malnutrition and the Risk of Early Neurological Deterioration in Elderly Patients with Acute Ischemic Stroke
Bao Y, Zhang Y, Du C, Ji Y, Dai Y, Jiang W
Neuropsychiatric Disease and Treatment 2022, 18:1779-1787
Published Date: 20 August 2022
A Prediction Model for Rapid Identification of Ischemic Stroke: Application of Serum Soluble Corin
Lu Y, Wang W, Tang Z, Chen L, Zhang M, Zhang Q, Wu L, Jiang J, Zhang X, He C, Peng H
Journal of Multidisciplinary Healthcare 2022, 15:2933-2943
Published Date: 22 December 2022
Exploration of the Shared Gene Signatures and Molecular Mechanisms Between Ischemic Stroke and Atherosclerosis
Ban R, Huo C, Wang J, Zhang G, Zhao X
International Journal of General Medicine 2024, 17:2223-2239
Published Date: 19 May 2024
Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictors of Short- and Long-Term Outcomes in Ischemic Stroke Patients with Atrial Fibrillation
Guo J, Wang D, Jia J, Zhang J, Liu Y, Lu J, Zhao X, Yan J
Journal of Inflammation Research 2024, 17:6661-6672
Published Date: 23 September 2024
Predictive Value of Epicardial Adipose Tissue for Hemorrhagic Transformation and Functional Outcomes in Acute Ischemic Stroke Patients Undergoing Intravenous Thrombolysis Therapy
Liu L, Jia C, Xing C, Fu X, Liu Z, Ma A
Journal of Inflammation Research 2024, 17:11915-11929
Published Date: 31 December 2024




