Back to Journals » Drug Design, Development and Therapy » Volume 19
The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Subcutaneous Administration of a Novel Anti-NGF Monoclonal Antibody (AK115) in Healthy Participants: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Phase I Clinical Trial
Authors Zhang J, Fan YX, Huang Y, Guan R, Li R, Long S, Yang M, Yu B, Wang GQ, Chen P, Gong X, Li B, Xia M, He J
Received 11 November 2024
Accepted for publication 25 March 2025
Published 24 April 2025 Volume 2025:19 Pages 3225—3235
DOI https://doi.org/10.2147/DDDT.S500902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Juan Zhang,1,2,* Yu-Xin Fan,1,* Yu Huang,1 Runfang Guan,1 Ruixia Li,1 Shuxian Long,1 Mei Yang,1 Binge Yu,3 Guo Qin Wang,3 Peng Chen,3 Xia Gong,3 Baiyong Li,3 Michelle Xia,3 Jianchang He1
1Research Center of Clinical Pharmacology, the First Affiliated Hospital of Yunnan University of Chinese Medicine, Yunnan, People’s Republic of China; 2Research Center for Early Clinical Trials of Drugs (Vaccines), the Affiliated Anning First People’s Hospital, Kunming University of Science and Technology, Kunming, People’s Republic of China; 3Akeso Biopharma, Inc, Zhongshan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianchang He, The First Affiliated Hospital of Yunnan University of Chinese Medicine, 1 huachen Road, Kunming, 650103, People’s Republic of China, Email [email protected]
Objective: This study aimed to investigate the safety, tolerability, pharmacokinetics (PKs), and pharmacodynamics (PDs) of a novel anti-nerve growth factor (NGF) monoclonal antibody (mAb) (AK115) in healthy Chinese participants.
Methods: A randomized, double-blind, placebo-controlled, dose-escalation phase I clinical study was conducted as follows: eligible participants were divided into 6 dose groups, among which 0.5 mg group was administrated with AK115 injection and the remaining 5 groups were randomly assigned to AK115 injection or accompanying placebo at a ratio of 3:1. Adverse events (AEs), PKs, PDs, and anti-drug antibodies (ADAs)/neutralizing antibody were monitored throughout the study.
Results: A total of 42 participants completed the study. Twenty-seven (64.3%) participants occurred treatment emergent AEs (TEAEs), and 2 (4.80%) participants experienced treatment-related TEAEs. The TEAEs among the different dose groups were comparable. No significant differences were observed between the combined AK115 and the placebo group. It was demonstrated that the median Tmax was 4.50– 14.0 days, the mean Cmax and AUC0-t of different doses groups were 30.8– 5500 ng/mL and 792~181010 Day*ng/mL, respectively. The elimination half-life (t1/2) did not differ among the different dose groups and was calculated to be 7.60– 17.7 days. In addition, the total NGF concentration and percentage change from baseline increased with an increase in the AK115 dose. No ADA positivity was detected in the healthy participants.
Conclusion: The favorable safety and tolerability of AK115 in healthy Chinese participants, as well as the predictable PK and PD profiles, will provide sufficient support for future dose exploration studies of AK115 in patients with analgesia.
Trial Registration: This study was registered in the Chinese Clinical Trial Registry (CTR20220431) and the official website of the US Department of Health and Human Services, National Institutes of Health, with Clinical Trials. gov (NCT05286970) on March 2022.
Keywords: nerve growth factor, mAb, pain, phase I clinical trial
Introduction
Pain is an unpleasant sensory and emotional experience associated with or resembling actual or potential tissue damage, and can be classified as acute and chronic.1,2 It is defined as chronic when it lasts or recurs for longer than three months and persists beyond the normal healing time.3 Chronic pain is reported to affect more than 30% of people worldwide and represents one of the main causes of human suffering and disability, profoundly influencing patients’ quality of life and exerting an enormous personal and economic burden.4–6 It is generally categorized as chronic cancer-related pain or chronic non-cancer-related pain (CNRP), and CNRP affects approximately 30–41% of adults worldwide.3,7 Although numerous studies have elucidated the mechanisms underlying the occurrence and development of chronic pain, only a few currently available clinical therapeutic strategies effectively alleviate pain symptoms in patients owing to the side effects of some therapeutic options and individual responses.6
Nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and analgesic adjuvants used for the treatment of other therapeutic conditions (anticonvulsants, antidepressants, cannabinoids, and others) are widely used to relieve pain.2,8 NSAIDs are generally effective for mild or moderate pain but do not completely relieve moderate-to-severe pain.2,8 Traditional NSAIDs have been reported to have significant side effects on the gastrointestinal tract and stomach, including ulcers and bleeding, and can cause liver and kidney problems.9 Furthermore, although opioids still represent one of the main choices for moderate-to-severe pain in both cancer10 and non-cancerous patients,11 chronic opioid use is often linked to a series of adverse effects. The most common symptom is opioid-induced bowel dysfunction such as nausea, vomiting, and constipation.12 Moreover, the use of therapeutic doses of opioids is limited by the development of respiratory depression, tolerance and addiction.2,13 Actually, these dose-limiting adverse effects, such as respiratory depression, itching, gastrointestinal side effects, physical dependence, and abuse liability, are mainly related to μ-opioid receptor (MOR) activation, which is also related to opioid administration or to an increase in opioid dosage to maintain adequate analgesia as the main mechanism by which opioids provide analgesia.2 Therefore, it is necessary to explore and develop innovative analgesics with better tolerability and non-addictive properties to satisfy unmet medical needs for pain relief.
Nerve growth factor (NGF), one of the earliest identified nerve growth nutrition factors, is secreted by various immune and inflammatory cells and plays an important role in the generation, differentiation, and functional maintenance of neurons in organisms.14 As a mediator of persistent and chronic pain, numerous experiments and clinical studies have shown that NGF plays a key role in the generation of acute and chronic pain, as well as hyperalgesia in diverse pain states, and it is therefore considered to be a potential target molecule for developing more effective analgesics.15–17 Furthermore, increasing evidence has also indicated that selective NGF antagonists may help block pain signals generated by muscles, skin, and organs from entering the spinal cord and brain, thereby effectively relieving pain without the typical side effects of NSAIDs and opiates drugs.18–20 Thus, from the perspective of its unique mechanism of action, the anti-NGF monoclonal antibody (mAb) is considered to be one of the most promising next-generation analgesics in the field of pain, with long-term safety, non-addiction, non-drug resistance, and other superior characteristics.
Recently, several humanized anti-NGF mAbs (eg, Fasinumab, Fulranumab, etc.) have entered clinical trials as potential analgesics for the treatment of a variety of chronic pain conditions, most of which are associated with inflammatory pain, including osteoarthritis pain and chronic low back pain.21–23 AK115, a new anti-NGF antibody independently developed by Akeso Biopharma, Inc., is a humanized IgG1 mAb that targets NGF with more extensive indications including cancer pain. AK115 binds to human NGF with a high affinity and blocks its interaction with receptors, thus blocking the signals sent by nociceptors responsible for sensing pain and achieving pain relief. Furthermore, effective biological activity and a good safety profile of AK115 were observed in nonclinical pharmacological and toxicity tests. Therefore, we performed this phase I clinical trial in healthy Chinese adult participants to further explore the safety and tolerability of AK115 injection in humans, as well as its pharmacokinetic (PK) and pharmacodynamic (PD) characteristics, looking forward to provide more data for subsequent Phase II clinical trials and the development of AK115.
Materials and Methods
Study Design and Participants
This randomized, double-blind, placebo-controlled, dose-escalation phase I study was designed to evaluate the safety, PK, PD, and immunogenicity of a single-dose AK115 injection in healthy adult participants (the Chinese Clinical Trial Registry number was CTR20220431 and the registration number of the National Institutes of Health with Clinical Trials on the official website of the US Department of Health and Human Services was NCT05286970, March 2022). This trial was implemented in March 2022 and completed in September 2022, and each enrolled participant received a single dose of only one of these study dose levels. Subcutaneous injection was performed under fasting conditions, and participants in the same dose group were randomly administered the same dose volume of AK115 or matching placebo.
The eligible participants were healthy male and female aged 18–55 years (inclusive) with a body mass index (BMI) of 19.0–26.0 kg/m2. The weight of the men was 50.0 kg or greater, and that of the women was 45.0 kg or greater. Additional detailed inclusion and exclusion criteria are presented in Supplementary Table S1.
The study protocol was approved by the Ethics Committee of the First Affiliated 002–02). The study was conducted in accordance with the principles outlined in Good Clinical Practice, the Declaration of Helsinki, and the relevant domestic laws and regulations. All participants provided written informed consent before participating in the study.
Study Procedure
As shown in Figure 1, the dose-escalation trial of a single-dose subcutaneous injection was carried out according to the sequence of drug doses from low to high (0.5, 1, 3, 10, 30, and 60 mg) in the dose increment mode. Two eligible participants were included in the 0.5 mg dose group, and all were injected with AK115 subcutaneously into the abdomen. Starting from the second dose group, the first 2 participants in the sentry group were randomly assigned to receive the same volume of AK115 or placebo in a 1:1 ratio, and after safety follow-up and evaluation within 24 hours post administration, the remaining participants were assigned to receive AK115 or placebo in a 5:1 ratio. A trial of the next dose group should only be performed after a full review of the data from participants in the previous dose group and confirmation of the safety of that dose. The study duration included a screening period, dosing visit, and 12-week follow-up period. The total trial duration (including the screening period) for each participant was approximately 16 weeks.
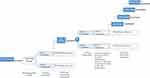 |
Figure 1 The overall design of the study. |
Safety Assessments
Safety evaluation was based on physical examination, 12-lead electrocardiography, and laboratory tests. The adverse events (AEs) were coded and summarized using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0, based on the System Organ Class (SOC) and Preferred Term (PT). The corresponding severity was graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
PK, PD and Immunogenicity Analysis
The PK analysis was performed using the PK analysis set (PKS). Blood samples for PK analysis were collected at baseline (pre-dose), at 5 min, 2 h, and 8 h post-administration, and on days 2, 4, 6, 8, 15, 22, 29, 43, 57, 71, and 85 post-administrations to measure the concentration of AK115 in serum. Serum AK115 was detected using validated methods based on the MERCK SHARP DOHME (MSD) platform, and the precision and accuracy of in-batch and inter-batch analyses met the requirements of the relevant regulations for the quantitative analysis of biological samples. The detection lower limit and standard curve quantitative range was 0.6 ng/mL and 0.6–729 ng/mL. The mean concentration-time (C-T) curves were drawn according to the concentrations measured by each participant. The PK parameters were calculated based on non-compartmental analysis, including the maximum observed concentration (Cmax), time to Cmax (Tmax), area under the curve from 0 to the time of the last quantifiable concentration (AUC0-t), terminal elimination half-life (t1/2), and volume of distribution (Vz/F).
PD analysis was based on the PD analysis set (PDS), and blood samples were collected at the following time points: baseline (pre-dose) and days 2, 8, 29, 57, and 85 post-administrations. NGF concentration was also detected using a validated electro chemiluminescent immunoassay based on the MSD platform, and the precision and accuracy of in-batch and inter-batch assays met the requirements of relevant regulations for the quantitative analysis of biological samples. The detection limit and quantitative range of the standard curve were 5 pg/mL and 5–12500 pg/mL. Changes in serum total NGF from baseline over time were analyzed, and the relationship between AK115 exposure and PD is also discussed.
Immunogenicity analysis was based on the immunogenicity analysis set (IMS) and performed only in the AK115 group. The ADA and NAb conditions were summarized and the number and percentage of participants with ADA/Nab positivity were calculated. The clinical immunogenicity analysis of AK115 was performed by the detection of anti-AK115 antibody in human serum by electrochemiluminescence after PEG precipitation and acidification of quality control samples/validation samples/test samples. The ADA analysis included screening, immunosuppression confirmation and titer determination.
Sample Size Calculation and Statistical Analyses
The sample size was determined for the preliminary safety and PK evaluation. Forty-two participants were enrolled and divided into 6 dose groups. Two participants were enrolled in the starting dose group (0.5 mg, both AK115) as safety entries and eight participants were enrolled in each of the other dose groups (six in the AK115 group and two in the placebo group). SAS Version 9.4 (SAS Institute Inc.) was used for statistical analysis, and WinNonlin 8.3 software (Pharsight Corporation) was used to estimate PK parameters of the non-atrioventricular model.
Results
Demographics and Baseline Characteristics
A total of 66 participants were screened and 42 were enrolled. All enrolled participants (100%) were included in the full analysis set (FAS) and SS, and 32 (76.20%) were included in the IMS group. As depicted in Table 1, the mean age of the 42 participants who received AK115 or placebo was 26.7 years (range: 18–42 years), 73.8% were males, and the mean BMI (standard deviation) was 21.90 (±1.78) kg/m2 based on FAS. Furthermore, the baseline values of each dose group were relatively balanced.
 |
Table 1 Demographic and Baseline Characteristics of the Included Participants |
Safety Profile
All 42 participants completed a 12-week safety follow-up period. No TEAE ≥ grade 3, dose-limiting event (DLE), serious adverse event (SAE), or death was observed. No TEAE leading to early withdrawal were observed.
As shown in Table 2, among the 42 participants receiving AK115 or placebo, 27 (64.3%) experienced treatment-emergent adverse events (TEAE), including 24 (75.0%) in the AK115 group and 3 (30.0%) in the placebo group. Two (4.8%) participants developed treatment-related adverse events (TRAE), all of which occurred in the AK115 group, including one case of elevated γ-glutamyl transferase level (AK115 3 mg group) and one case of elevated blood bilirubin level (AK115 10 mg group). The percentages of TEAE and TRAE in the AK115 group were higher than those in the placebo group, but no dose correlation was observed.
 |
Table 2 Summary of AEs of AK115 in Healthy Participants |
PK Results
As presented in Table 3 and Figure 2, a single subcutaneous injection of 0.5–60 mg AK115 in the healthy participants. The inter-individual variation in the exposure parameters (Cmax and AUC) of AK115 was low, with the coefficient of variation in the percentage (CV%) of Cmax being 12.7–31.0% and CV% of AUC being 13.8%–35.9%. The Cmax (CV%) were 30.8 (12.7) ng/mL, 76.5 (26.8) ng/mL, 246 (28.9) ng/mL, 910 (18.1) ng/mL, 3020 (31.0) ng/mL and 5500 (19.9) ng/mL in 0.5 mg, 1 mg, 3 mg, 10 mg, 30 mg and 60 mg groups, respectively. Furthermore, the AUC0-t (CV%) was 792 (35.9) days *ng/mL, 2040 (23.5) days *ng/mL, 7990 (13.8) days *ng/mL, 29230 (21.8) days *ng/mL, 101580 (31.2) days *ng/mL, and 181010 (21.0) days *ng/mL in the 0.5, 1, 3, 10, 30, and 60 mg groups, respectively. Moreover, the median Tmax, the mean t1/2, the mean Vz/F and CL/F was 4.50–14.0 days, 7.60~17.7 days, 5.33~8.35 L and 0.303~0.665 L/Day, respectively. The Cmax and AUC of AK115 increased with the dose, and AK115 exhibited an approximately linear PK characteristic in the dose range of 3–60 mg.
 |
Table 3 Results of the PK Parameters of AK115 in Chinese Healthy Participants |
 |
Figure 2 Mean (SD) serum concentration - time curve profiles of AK115 in healthy participants. |
PD Results
After a single subcutaneous injection of 0.5–60 mg AK115, the serum total NGF concentration and percentage change of total NGF from baseline began to gradually increase from day 2, and the extent of the mean increase in total NGF and percentage change from baseline was dose-dependent (Figure 3A and B). The mean serum total NGF of AK115 0.5–1 mg group reached the maximum value on day 29 and began to decline after day 29. The mean serum total NGF level of the AK115 3–60 mg group reached the maximum value on day 57 and began to decline thereafter. In the placebo group, the total serum NGF concentration was 5 pg/mL lower than the lower limit of detection.
 |
Figure 3 Change in actual serum total NGF concentration (A) and percentage change from baseline (B) after a single subcutaneous injection of AK115 or placebo in healthy participants. |
Immunogenicity Analysis
All 32 healthy participants who received AK115 were enrolled in the IMS study. One of them (3 mg group) tested positive for ADA at baseline, and none tested positive after administration. Detailed information is provided in Supplementary Table S2.
Discussion and Conclusion
This study was performed with 42 healthy Chinese adult participants to evaluate the safety, tolerability, PK, and PD characteristics of a single dose of AK115. The dose escalation study was based on six doses (0.5, 1, 3, 10, 30, and 60 mg) of AK115. According to our findings, AK115 was demonstrated to be generally safe and well tolerated in Chinese population. Meanwhile, the PK parameters of AK115 were consistent with the PK characteristics of typical mAbs, and the Cmax and AUC increased in a dose-dependent manner, showing an approximately linear relationship in the dose range of 3–60 mg. Furthermore, the average increase in total NGF in each dose group also showed a dose-dependent relationship and the increase of total NGF was highly positively correlated with the serum concentration of AK115.
It’s well acknowledged that NGF, the first discovered member of the neurotrophic family that signals through both tyrosine kinase receptors of the tropomyosin-related kinase (Trk) family and the unrelated p75 receptor, has been regarded as a potential target molecule for development as more effective analgesics.24 AK115 is a humanized mAb that tightly binds NGF with high selectivity and specificity, thereby preventing NGF from interacting with the receptors, neurotrophic TrkA and low-affinity NGF receptor (p75).
The PK and PD results showed that the Cmax and AUC of AK115 increased with increasing dose, with an approximately linear relationship in the 3–60 mg dose range, which was consistent with the limited distribution of mAbs in the intercellular space and in line with the PK characteristics of typical mAbs. Furthermore, it was found that the extent of the average increase in total NGF showed a linear dose-dependent relationship, and the increase in total NGF was highly positively correlated with the concentration of AK115, suggesting that AK115 may bind to its target NGF with high affinity in human.
For the safety results, AK115 was administered via subcutaneous injection, and no injection site-related adverse events were observed in our study. According to the safety report of products of the same target, following the administration of investigational products, participants were more likely to experience peripheral paranesthesia AEs (such as bradyesthesia, paresthesia, and abnormal pain, etc.) than the placebo,25,26 suggesting a good safety profile of AK115 in the current study setting.
Although we obtained the expected trial results, this study has some limitations. The small sample size and limited follow-up duration, which are inherent to most phase I trials, limit the generalizability of the results. Furthermore, although the correlation results between magnitude of increase of total NGF and AK115 concentration suggested that AK115 may bind to its target NGF with a high affinity, further confirmatory evidence will be required in the future studies. Additionally, a major concern with anti-NGF mAbs is the increased risk of rapidly progressing osteoarthritis (RPOA). As the occurrence of this AE is time- and exposure-related, the probability of its occurrence is very low in the phase I trial following a single-dose administration, which is consistent with the findings from early phase I trials of the same drug target. However, although no RPOA was observed in this study, it needs to be closely monitored in future multidose long-term studies.
In conclusion, this phase I study further demonstrated the favorable safety and tolerability of AK115 in healthy Chinese participants as well as its predictable PK and PD profiles. These findings will provide sufficient data for future dose-exploration studies of AK115 in patients and further support its continuing clinical development in the field of analgesia.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all participants, investigators, and people who contributed to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was sponsored by Akeso Biopharma Inc. This study was supported by the Construction of the Research Platform for Early Clinical Trials of Innovative Drugs from the Yunnan Province Science and Technology Department (No. 202302AA310007).
Disclosure
Binge Yu, Guoqin Wang, Peng Chen, Xia Gong, Baiyong Li, and Michelle Xia were employed by Akeso Biopharma, Inc. All authors declare no potential conflicts of interest relevant to this article.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
2. Laev SS, Salakhutdinov NF. New small-molecule analgesics. Curr Med Chem. 2021;28(30):6234–6273. doi:10.2174/0929867328666210614122444
3. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
4. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi:10.1016/S0140-6736(21)00393-7
5. Coluzzi F, Rullo L, Scerpa MS, et al. Current and future therapeutic options in pain management: multi-mechanistic opioids involving both MOR and NOP receptor activation. CNS Drugs. 2022;36(6):617–632. doi:10.1007/s40263-022-00924-2
6. Li Z, Li X, Jian W, Xue Q, Liu Z. Roles of long non-coding RNAs in the development of chronic pain. Front mol Neurosci. 2021;14:760964. doi:10.3389/fnmol.2021.760964
7. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–130. doi:10.1037/a0035514
8. Vardanyan R, Hruby V. Chapter 34 – antiviral drugs. In: Synthesis of Best-Seller Drugs. Amsterdam: Elsevier; 2016:687–736.
9. Piekielna-Ciesielska J, Wtorek K, Janecka A. Biased agonism as an emerging strategy in the search for better opioid analgesics. Curr Med Chem. 2020;27(9):1562–1575. doi:10.2174/0929867326666190506103124
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi:10.1016/S1470-2045(12)70040-2
11. Ambrosio F, Finco G, Mattia C, et al. SIAARTI recommendations for chronic noncancer pain. Minerva Anestesiol. 2006;72(11):859–880.
12. Coluzzi F, Alvaro D, Caraceni AT, et al. Common clinical practice for opioid-induced constipation: a physician survey. J Pain Res. 2021;14:2255–2264. doi:10.2147/JPR.S318564
13. Coluzzi F, Bifulco F, Cuomo A, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017;13:1163–1173. doi:10.2147/TCRM.S141332
14. Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27(2):85–91. doi:10.1016/j.tips.2005.12.001
15. Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int. 2012;61(8):1266–1275. doi:10.1016/j.neuint.2012.10.008
16. Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs. 2008;22(6):349–359. doi:10.2165/0063030-200822060-00002
17. McKelvey L, Shorten GD, O’Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem. 2013;124(3):276–289. doi:10.1111/jnc.12093
18. Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci. 2017;40:307–325. doi:10.1146/annurev-neuro-072116-031121
19. Oo WM, Hunter DJ. Nerve Growth Factor (NGF) inhibitors and related agents for chronic musculoskeletal pain: a comprehensive review. BioDrugs. 2021;35(6):611–641. doi:10.1007/s40259-021-00504-8
20. Sánchez-Robles EM, Girón R, Paniagua N, Rodríguez-Rivera C, Pascual D, Goicoechea C. Monoclonal antibodies for chronic pain treatment: present and future. Int J mol Sci. 2021;22(19):10325. doi:10.3390/ijms221910325
21. Sanga P, Katz N, Polverejan E, et al. Long-term safety and efficacy of fulranumab in patients with moderate-to-severe osteoarthritis pain: a phase II randomized, double-blind, placebo-controlled extension study. Arthritis Rheumatol. 2017;69(4):763–773. doi:10.1002/art.39943
22. Berenbaum F, Blanco FJ, Guermazi A, et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised Phase III study with a 24-week follow-up period. Ann Rheum Dis. 2020;79(6):800–810. doi:10.1136/annrheumdis-2019-216296
23. Markman JD, Bolash RB, McAlindon TE, et al. Tanezumab for chronic low back pain: a randomized, double-blind, placebo- and active-controlled, Phase 3 study of efficacy and safety. Pain. 2020;161(9):2068–2078. doi:10.1097/j.pain.0000000000001928
24. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi:10.1146/annurev.neuro.29.051605.112929
25. Schnitzer TJ, Easton R, Pang S, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. 2019;322(1):37–48. doi:10.1001/jama.2019.8044
26. Tive L, Bello AE, Radin D, et al. Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase III clinical trials in patients with osteoarthritis pain of the knee or Hip. J Pain Res. 2019;12:975–995. doi:10.2147/JPR.S191297
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

