Back to Journals » Cancer Management and Research » Volume 17
The Value of Integrating Hormone Receptors into Immunohistochemistry-Based Simplified Molecular Classification in Endometrial Cancer
Authors Zhao S, Yan Y, Wang T, Zhang J, Zheng X, Li X, Zhao J, Yang E, Zhao X, Tian L, Xue F, Tian W, Wang Y
Received 21 January 2025
Accepted for publication 9 April 2025
Published 24 April 2025 Volume 2025:17 Pages 869—880
DOI https://doi.org/10.2147/CMAR.S514680
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shuangshuang Zhao,1,2,* Ye Yan,1,2,* Tianqi Wang,1,2,* Jingying Zhang,1,2 Xingyu Zheng,1,2 Xianxian Li,1,2 Jianzhen Zhao,1,2 Eryan Yang,1,2 Xue Zhao,1,2 Lina Tian,1,2 Fengxia Xue,1,2 Wenyan Tian,1,2 Yingmei Wang1,2
1Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 2Tianjin Key Laboratory of Female Reproductive Health and Eugenics, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingmei Wang, Tianjin Medical University General Hospital, No. 154 Anshan Street, Heping District, Tianjin, 300052, People’s Republic of China, Tel +86-22-60362255, Email [email protected] Wenyan Tian, Tianjin Medical University General Hospital, No. 154 Anshan Street, Heping District, Tianjin, 300052, People’s Republic of China, Tel +86-22-60362255, Email [email protected]
Purpose: To explore the clinical utility of immunohistochemistry (IHC)-based molecular classification and evaluate the distribution patterns and clinical implications of hormone receptor (HR) expression across different molecular classifications in endometrial cancer (EC).
Patients and Methods: This study retrospectively conducted simplified molecular classification based on IHC analysis of mismatch repair (MMR) and p53 protein from 322 EC patients admitted to the Obstetrics and Gynecology Department of Tianjin Medical University General Hospital from March 2017 to April 2024. 121 patients underwent WHO molecular classification by gene sequencing and IHC analysis. The application value of IHC-based simplified molecular classification was evaluated. The association between HR expression and molecular classification, and their combined value in predicting survival were analyzed.
Results: In IHC-based simplified molecular classification, 23.3% (75/322), 59.9% (193/322), and 16.8% (54/322) patients were included in the MMR deficient (MMRd) group, MMR proficient (MMRp) group, and p53-abnormal (p53abn) group, respectively. This classification correlated significantly with various clinicopathological features such as age (p=0.001), body mass index (p=0.016), FIGO stage (p=0.002), histological subtype (p< 0.001), and tumor differentiation (p< 0.001). Furthermore, differences in disease-free survival (DFS) among these groups were statistically significant (p=0.002). Subgroup analyses revealed that HR expressions significantly affected DFS within molecular classification groups. Patients with positive estrogen receptor (ER) or progesterone receptor (PR) expression demonstrated better DFS than those with negative expression in these groups (ER in MMRp: p< 0.001, PR in MMRp: p< 0.001, ER in MMRd: p< 0.001, PR in MMRd: p=0.032, ER in p53abn: p=0.052, PR in p53abn: p=0.019).
Conclusion: IHC-based simplified molecular classification is an economically viable and clinically applicable method that effectively stratifies patients by clinicopathological features and prognosis. Moreover, this approach allows stratification into different prognostic risk groups based on HR expression in molecular classification subgroups.
Keywords: endometrial cancer, molecular classification, immunohistochemistry, hormone receptor, hormonal therapy
Introduction
Endometrial cancer (EC) is among the most prevalent gynecological malignancies, showing a rising incidence globally. In addition, the incidence in patients younger than 40 years has been consistently increasing.1 In 1983, Bokhman divided EC into two subtypes according to clinical and endocrinological characteristic.2 Type I, constituting 80–90% of EC cases, is linked to unopposed estrogen stimulation and exhibits higher levels of estrogen receptor (ER) and progesterone receptor (PR). This subtype often affects younger women with metabolic or reproductive risk factors.1,3 Histopathologically, it is primarily composed of grade I (G1) or grade II (G2) endometrioid carcinoma (EEC), which is responsive to progesterone therapy and generally has a favorable prognosis. Conversely, type II makes up 10–20% of EC cases, features lower ER and PR expression, and typically affects older patients not influenced by estrogen. This subtype includes histopathological forms such as grade III (G3) EEC, endometrial serous carcinoma (ESC), endometrial clear cell carcinoma (ECCC), uterine carcinosarcoma (UCS), and undifferentiated/dedifferentiated endometrial carcinoma (UDEC), which are less responsive to progesterone therapy and associated with a poorer prognosis.3,4
ER and PR have long been established as traditional biomarkers for prognostic evaluation and hormonal therapy (HT) in EC.5,6 HT has been applied for EC since the 1950s7,8 with low toxicity but it is primarily reserved for fertility-sparing interventions in young patients with early-stage EC and as systemic palliative care for advanced recurrent cases of the disease.9,10 For advanced recurrent EC, studies report an overall response rate (ORR) of 30% (95% CI 25–36), which increases to 55.4% in PR-positive EC.11 However, these studies are dated, and there is significant heterogeneity among them, largely due to variations in the types of progestin drugs and dosages used. Consequently, the evidence remains inadequate to conclusively determine that relying solely on hormone receptor (HR) expression can effectively and accurately identify patients who would benefit from HT.
Recent years have seen significant advancements in the genomic and molecular research of EC, enhancing our understanding and aiding in the development of diagnostic classifications and tailored therapies.12,13 In addition to conventional HR, an increasing number of studies have demonstrated the prognostic value of other molecular biomarkers in EC, such as POLE, CTNNB1, TP53, L1CAM, and ARID1A.14,15 Molecular classifications based on immunohistochemistry (IHC) and genetic sequencing have been employed to guide prognostication and treatment recommendations. However, sequencing tests are not universally available across all regions due to financial and technological constraints. For instance, Perrone et al have demonstrated that a simplified molecular classification based on MMR and p53 IHC, without POLE sequencing, effectively categorizes EC according to clinical characteristics and prognostic outcomes, accurately reflecting the risk stratification associated with EC.16 This supports the utility and reliability of IHC for molecular classification of EC in everyday clinical settings.17–20
Moreover, within this updated framework of molecular classification for EC, the relevance of traditional prognostic markers like HR continues to be a subject of debate, primarily due to the scarcity of conclusive evidence.21,22 To address this gap in the literature, we initiated this retrospective study with the goal of assessing the prognostic value of molecular classification using IHC. Additionally, we incorporated HR expression into the IHC stratification to enhance both prognostic and predictive accuracy.
Materials and Methods
Patients
We conducted a retrospective analysis of all EC patients who underwent surgical staging at the Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, from March 2017 to April 2024. The following inclusion criteria were applied: 1) patients who underwent EC staging surgery, with postoperative pathological confirmation of EC, 2) availability of complete clinical, pathological, and treatment records. The exclusion criteria include: 1) patients who received preoperative HT, 2) patients who received primary non-surgical treatments or incomplete surgical staging. We collected data on various variables including baseline demographic characteristics, perioperative details, final pathology findings, IHC results, types of adjuvant therapy, and oncologic outcomes (disease-free survival, DFS). DFS was defined as the period from the date of surgery (diagnosis) to the first recurrence of the disease. The overall observation period extended from the date of diagnosis to the last follow-up on May 1st, 2024.
Immunohistochemical Staining and Evaluation
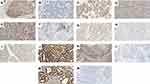
Tumor samples, collected from surgical specimens, including endometrial biopsies and/or hysterectomies, were sectioned into 0.5 cm slices, promptly fixed in 10% formalin, and subsequently embedded in paraffin. Coronal sections of 3 μm thickness were then prepared from these paraffin blocks. For immunohistochemical staining, formalin-fixed, paraffin-embedded (FFPE) tissue sections were processed using the IHC Protocol F Program on the Leica BOND-MAX™ Detection System (Leica Biosystems, Wetzlar, Germany), following the manufacturer’s guidelines. The antigen retrieval step involved heating the samples in a microwave for 20 minutes in EDTA buffer (pH 9.0). The primary mouse monoclonal antibodies including MLH1 (clone ES05), MSH2 (clone FE11), MSH6 (clone EP49), PMS2 (clone EPS1), p53 (clone DO-7), ER (clone EP1), PR (clone PgR636), and programmed cell death ligand-1 (PD-L1) (clone 73–10) (purchased from DakoCytomation, Glostrup, Denmark) were applied individually and incubated overnight at 4°C.
All sections were evaluated independently by two pathologists. To prevent false-negative outcomes, normal endometrial tissues adjacent to the tumor were used as an internal positive control. In cases where a consensus was not reached, a third pathologist was consulted. The MMR proteins were evaluated in accordance with the published guidelines.23 p53 expression was considered wild-type if 1%-80% of the tumor cell nuclei showed positive staining of varying intensities. Abnormal p53 expression was defined by the complete absence of nuclear staining in tumor cells with a positive internal control or when more than 80% of tumor cell nuclei were positively stained.20 ER and PR were considered positive when nuclei were stained in ≥1% of the tumor cells21 (Figure 1).
All patients were divided into 3 groups according to IHC-based simplified molecular classification: (1) MMR deficient (MMRd) group: Patients showing deficiency in one or more MMR proteins (MLH1, MSH2, MSH6, or PMS2), irrespective of p53 protein expression, were placed in the MMRd group; (2) MMR proficient (MMRp) group: Patients with no deficiencies in MMR proteins and no abnormal p53 protein expression were assigned to the MMRp group; (3) p53-abnormal (p53abn) group: Patients exhibiting abnormal expression of p53 protein, but without any MMR protein deficiencies, were classified into the p53abn group. The co-expressing abnormal p53 protein and MMRd EC were considered MMRd group according to this literature.
Next-Generation Sequencing (NGS)
Genomic DNA (gDNA) was isolated from the FFPE samples using a QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, USA). The quality and size of the DNA fragments were evaluated using a Qubit dsDNA High Sensitivity Assay Kit (Life Technologies, Carlsbad, USA). After validation, gDNAs were randomly fragmented, and their quality and fragment size were assessed using a LabChip GX Tough Nucleic Acid Analyzer (PerkinElmer, USA). These samples were then prepared into libraries and sequenced following the manufacturer’s guidelines. Whole exome sequencing (WES) was conducted using the xGen™ Exome Hyb Panel V2 (OrigiMed, Shanghai, China), and the sequenced libraries were processed on a NovaSeq 6000 sequencer (Illumina, San Diego, CA, USA) achieving a mean depth of 500×. Library construction and sequencing activities took place at the Molecular Diagnostics Service Laboratory at OrigiMed, Shanghai, China, which complies with Clinical Laboratory Improvement Amendments/College of American Pathologists standards. Sequencing data were aligned to the human genome reference sequence hg19 (GRCh37). The various duplicate reads were removed by Picard (https://broadinstitute. github. io/picard/) and recalibrated by the BaseRecalibrator tool from GATK (https://software.broadinstitute.org/gatk/). Genomic alterations (GAs) including Single nucleotide variants (SNVs), insertion-deletion polymorphisms (Indels), copy number variation (CNV), gene fusions, and gene rearrangements were identified by using MuTect (v1.7), PINDEL (v0.2.5), and Control-FREEC (v9.7), respectively.24–26 The functional impact of the GAs was annotated by SnpEff3.0.25 The results were annotated to several databases, including the Reference Sequence, 1000 Genomes (https://www.internationalgenome.org/), Genome Aggregation Database, the Exome Aggregation Consortium, Sorting Intolerant from Tolerant, PolyPhen, NHLBI GO Exome Sequencing Project 6500 (ESP6500), and Catalog of Somatic Mutations in Cancer (COSMIC) databases (https://cancer.sanger.ac.uk/cosmic). By comparing tumor tissues with matched blood samples, germline mutations were excluded, focusing analysis on somatic mutations. Patients who underwent NGS and IHC were classified into four groups according to the WHO molecular classification.27
Microsatellite Instability (MSI) and Tumor Mutational Burden (TMB)
MSI status was assessed using the Microsatellite Analysis for Normal Tumor InStability (MANTIS) tool28 and microsatellite regions were confirmed through manual inspection with the Integrated Genomics Viewer (IGV).
The TMB for each sample was calculated by totaling the number of somatic SNVs and Indels per megabase (Mb) of the targeted coding area, following previously established methods. A threshold of 10 was used to distinguish between TMB-high (TMB-H) and TMB-low (TMB-L).29
Statistical Analysis
Approximately-normally distributed continuous variables were summarized using means and standard deviations and compared using analysis of variance (ANOVA) tests. Variables not adhering to normality were summarized using medians and interquartile ranges and compared using the Kruskal–Wallis test. Categorical variables were described using frequencies and percentages and assessed using Pearson’s chi-square tests or Fisher’s Exact tests when appropriate. Bonferroni adjustments were applied to control for multiple comparisons in pairwise tests. Survival outcomes were evaluated using Kaplan–Meier methods, with differences between groups tested using the Log rank test. Cox proportional hazards models were employed to assess the prognostic significance of clinicopathological and molecular features across the entire cohort and within specific subgroups. The concordance between IHC and NGS methods was also quantified. Significance for all statistical tests was set at a two-sided P value of 0.05. All statistical analyses were conducted using SPSS software, version 27.0 (IBM Corporation, New York).
Ethical Approval
The study received approval from the Ethics Committee at Tianjin Medical University General Hospital. All patients provided written informed consent for the use of their biospecimens and clinical data for research purposes.
Results
Clinicopathologic Characteristics in All Study Populations
During the study period from March 2017 to April 2024, a total of 322 patients were enrolled in the study and underwent IHC-based simplified molecular classification. The classification grouped 23.3% (75/322) of patients as MMRd, 59.9% (193/322) as MMRp, and 16.8% (54/322) as p53abn. Table 1 illustrates the distribution of clinical and pathological parameters for each group. Significant differences were observed among the three groups in terms of age (p=0.001), BMI (p=0.016), FIGO stage (p=0.002), histological subtype (p<0.001), and differentiation (p<0.001). The p53abn group showed a higher percentage of adverse clinic-pathological features, including advanced stages, poor differentiation, and aggressive histological subtypes. In contrast, the MMRp group typically exhibited the most favorable clinic-pathological profile, characterized by early-stage cancer, high differentiation, and less aggressive histological subtypes.
 |
Table 1 Distribution of Clinicopathologic Characteristics in All Study Population |
During the study, 121 patients were classified according to the WHO molecular classification using gene sequencing and IHC analysis. The classifications included 5.8% (7/121) in the POLE mutation (POLEmut) group (Table S1), 23.1% (28/121) in the MMRd group, 20.7% (25/121) in the p53abn group, and 50.4% (61/121) in the non-specific molecular profile (NSMP) group. Table S2 presents the distribution of clinical and pathological parameters across these groups. There were significant differences observed in age (p<0.001), histological subtype (p<0.001), histological differentiation (p<0.001), TMB status (p<0.001), MSI status (p<0.001), and PD-L1 expression (p=0.004) among the four groups. Notably, the p53abn group displayed a higher proportion of unfavorable clinicopathological characteristics, such as poor histological differentiation and aggressive histological subtypes.
Distribution of HR Expression
Table 2 displays the distribution of HR expression within the subgroups of the IHC-based simplified molecular classification. Significant variations in ER and PR expressions were observed across the three groups (p<0.001 for both). The highest frequencies of ER and PR expression were found in the MMRp group (95.9% and 93.8%, respectively), while the lowest were in the p53abn group (68.5% and 57.4%, respectively).
 |
Table 2 Distribution of HR Expression Among IHC-Based Simplified Molecular Classification Subgroups |
Table S3 shows the distribution of HR expression across the WHO molecular classification subgroups. Significant differences in ER and PR expressions were noted among the four groups (p<0.001 for both).
Survival Analysis
Survival analysis was conducted using Kaplan-Meier survival curves based on IHC-based simplified molecular classification, to evaluate the prognostic differences among the three subgroups. Significant variations in DFS were observed among the three groups (p=0.002, Figure 2), with the p53abn group exhibiting the poorest DFS prognosis.
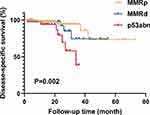 |
Figure 2 Estimated DFS for subgroups based on the IHC-based simplified molecular classification within the overall study population. |
Survival analysis was also conducted to assess the impact of HR expression on DFS across different subgroups. In both the MMRd and MMRp groups, significant associations were found between the expression of ER and PR with DFS (MMRp: p<0.001, p<0.001, MMRd: p<0.001, p=0.032, Figure 3A-D). Patients with positive ER or PR expression demonstrated better DFS than those with negative expression in these groups. Additionally, in the p53abn group, PR expression was significantly associated with DFS (p=0.019, Figure 3F), with patients exhibiting positive PR expression having improved DFS compared to those with negative PR expression, while ER expression was not significantly associated with DFS (p=0.052, Figure 3E).
Survival analysis based on the WHO molecular classification revealed that within the NSMP group, the expression of ER or PR was significantly related to better DFS (p<0.001, p=0.015, Figure S1). Patients with positive ER or PR expression exhibited improved DFS compared to those with negative expression.
Table S4 lists factors that influence DFS, including age, BMI, histological subtype, FIGO stage, LVSI, LNM, involvement of the lower uterine segment, myometrial invasion, and the expression of ER and PR. The multivariate analysis identified an aggressive histological subtype and involvement of the lower uterine segment as significant predictors of poor DFS (HR=24.89, 95% CI=2.05–301.97, p=0.012; HR=4.64, 95% CI=1.00–21.52, p=0.050; Table S4).
MMR-IHC versus MSI-NGS Concordance
Concordance between MMR status determined by MMR-IHC and MSI-NGS was evaluated in 117 out of 322 cases (Table S5). Among 90 cases identified as MMRp via IHC, 86 were found to have microsatellite stability (MSS) when assessed by NGS. Conversely, of the 27 cases labeled as MMRd by IHC, 21 were classified as MSI-high (MSI-H) by NGS (Figure S2A). The overall agreement between MMR-IHC and MSI-NGS reached 91.5% (107/117), which increased to 92.2% (106/115) when MMRd cases with POLE mutations were excluded (Figure S2B). Among the 6 discordant cases, where MMRd status by IHC did not match MSS status by NGS, four cases exhibited loss of MLH1 and PMS2, one case showed loss of MSH6, and one displayed loss of PMS2 along with a non-hotspot POLE mutation (A1946Sfs*4, exon43, InDel).
p53-IHC versus TP53-NGS Concordance
The alignment between p53 protein expression assessed by p53-IHC and TP53 gene mutations assessed by TP53-NGS was examined in 117 out of 322 cases. Among these, 30 cases showing abnormal p53 expression by IHC had corresponding TP53 mutations identified by NGS in 28 cases. For the 87 cases exhibiting wild-type p53 expression by IHC, 76 cases showed no TP53 mutations by NGS (Figure S3A). The agreement between p53-IHC and TP53-NGS was noted to be 88.9% (104/117). This concordance rate improved to 91.6% (98/107) when cases with TP53 mutations that also had either a concurrent POLE mutation and/or were MMRd were excluded (Figure S3B). Among 13 discordant cases, eight involved TP53 missense mutations, and in three of these cases, the variant allele frequency (VAF) was less than 10% (Table 3).
 |
Table 3 Detailed Information of Discordant p53-IHC versus TP53-NGS Cases in the Study |
Discussion
IHC-Based EC Risk Stratification in Clinical Practice
In clinical settings, widespread routine genomic profiling for all EC patients is not readily available. This is due to the labor-intensive, time-consuming, and costly nature of genomic methods, compounded by the lack of standardized procedures across different institutions and laboratories. Alternatively, IHC-based simplified molecular classification offers a more reproducible, cost-effective, and scalable testing option with accuracy comparable to genomic profiling in EC. Consistent with our findings, Perrone et al16 assessed the utility of IHC-based simplified molecular classification by examining histological characteristics and clinical outcomes. Their study found that this classification method was significantly correlated with FIGO stage, grade, histotype, presence of LVSI, myometrial invasion, and tumor dimension, and reflected the EC risk stratification, which is confirmed in overall survival (OS) and DFS. Specifically, the p53abn group displayed the poorest prognosis, characterized by an older median age, a higher incidence of advanced stages (III–IV), aggressive histological types, the presence of LVSI, and deep myometrial invasion. Conversely, the MMRp group showed the best prognosis, featuring a younger median age, a lower incidence of advanced stages, less aggressive histological types, and lesser extents of LVSI and myometrial invasion.
However, the short duration of follow-up in our study has not yet allowed for the collection of OS data, necessitating further follow-up to confirm the prognostic value of the IHC-based simplified molecular classification. Overall, our findings, along with previous studies, suggest that IHC-based simplified molecular classification can initially categorize the clinical and histological features, as well as the prognosis of EC patients. This classification provides valuable clinical insights, particularly when POLE sequencing is not available.
Potential Implications for HR in Molecular Classification
HR is crucial in the development of EC. Studies have shown that the absence of ER and PR in EC is associated with poorer survival outcomes.5,6 The expressions of ER and PR in EC tissues are frequently used as predictive biomarkers to guide HT.9 Hence, Exploring the role of HR within the framework of molecular classification could enhance risk stratification in EC, highlighting the importance of low-toxicity HT.
Perrone et al identified ER status as a prognostic marker for both DFS and OS in the MMRp group.16 In this study, we explored the distribution and clinical relevance of ER and PR expressions under both the IHC-based simplified molecular classification and WHO molecular classification. Statistically, the IHC-based simplified molecular classification was strongly associated with positive ER and PR expressions. Interestingly, positive ER and PR expressions were less common in the p53abn group and more prevalent in the MMRp group, suggesting variability in HT efficacy across different EC patient groups. Our subgroup analysis indicated that HR expression could enhance the risk stratification provided by the IHC-based simplified molecular classification. Similarly, the WHO molecular classification was statistically associated with positive ER and PR expressions. The lower frequency of positive ER and PR expressions in the p53abn group aligns with current evidence and guidelines, which advise against conservative therapy in p53abn EC cases.9
The NSMP group is the most prevalent molecular subtype, representing 39.0–50.4% of cases. It is categorized as a diagnosis of exclusion, lacking any of the three key molecular features: POLEmut, p53abn, or MMRd. This group encompasses a variety of clinicopathologic types, molecular characteristics, and clinical prognoses. The majority of cases within the NSMP group are characterized by non-aggressive behavior and a moderate prognosis, making them suitable candidates for HT30–32. However, there remains a small fraction of cases that are aggressive. NSMP EC constitutes 42%, 25%, 14%, 28%, and 36% of ECCC, UDEC, UCS, G3 EEC, and neuroendocrine ECs, respectively.31 Recently, some studies have explored the diverse prognostic outcomes within the NSMP group, attributed to variations in HR expression, which reflect differing molecular signatures.
Vermij et al highlighted the importance of ER expression for better prognostication in NSMP EC, including specific stages and grades such as stage Ia G3 EEC with LVSI, stage Ib G3 EEC, stage II–IIIc EEC, Ia stage with myometrial invasion, and Ib-III stage non-EEC. In high-risk NSMP EC, there is a significant difference in 5-year recurrence-free survival (RFS) between ER-positive and ER-negative groups within this category (80.9% vs 45.3%, p<0.001).33 Jamieson et al identified the low-risk (G1-G2, ER-positive [>1%]) and high-risk (G3, and/or ER-negative) subgroups of NSMP EC based on grade and ER expression, demonstrating a significant difference in 5-year progression-free survival (PFS) between these groups (6.1% vs 24.6% p<0.001).34 Additionally, the ongoing RAINBO trial is exploring the efficacy of HT as an adjuvant treatment in the NSMP group (ClinicalTrials.gov identifier: NCT05255653).
There are still some limitations with current studies. First, there is a lack of validated and standardized cutoff values for ER or PR expression in EC. Additionally, tumors often exhibit spatial and temporal heterogeneity, which complicates diagnosis and treatment. For some patients undergoing HT, HR expression data are derived from tumor biopsies rather than definitive hysterectomy specimens. This can lead to discrepancies in HR expression across different tumor sites, potentially misguiding HT decisions. Furthermore, as treatment progresses or the tumor evolves, HR expression may decrease, thereby reducing the sensitivity to HT.35 Collectively, previous research and our findings suggest that molecular classification could develop into a more dynamic tool for therapeutic and prognostic applications by incorporating HR markers for subgroups in routine clinical practice. Future studies involving larger populations are necessary to substantiate these observations.
The Concordance Between IHC and NGS in MMR and TP53 Mutation Status
Previous studies demonstrated the reliability of IHC. Liu et al. Liu et al found a high overall agreement of 94.3% between MMR-IHC and MSI-NGS, based on 200 out of 212 cases aligning, although 12 cases were discordant, including instances of 3 POLE mutations and 6 cases with MLH1 gene promoter methylation.17 Vermij et al observed that the concordance between p53-IHC and TP53-NGS was 90.7%, which increased to 94.5% when cases with MMRd and POLEmut cases were excluded. Notably, the VAF of variants included in their study was at least 10%.20 Thiel et al also evaluated the agreement between p53-IHC and TP53-NGS in the GOG-86P cohort, finding an overall concordance of 88%, which increased to 92% when cases with TP53 mutations that also had concurrent POLE mutations and/or were MMRd were excluded.19 In our study, we noted that among the discordant cases, which constituted 23.1% (3/13), the VAF was less than 10%, potentially explaining the lower concordance rate between p53b-IHC and TP53-NGS compared to other studies.
Several factors may contribute to the discrepancies between IHC and NGS. First, the increasing recognition of intratumoral heterogeneity and subclonal deletions, particularly with driver mutations like POLE mutations, offers a potential explanation, although there is currently limited clinical evidence to support this.36,37 It could also be associated with the differences in the tumor cellularity in the sections used for IHC and sequencing. Additionally, changes in non-coding regulatory regions, MDM2 amplification, or unidentified mechanisms might lead to the loss of p53 protein without an accompanying TP53 mutation.18 Other factors, such as redundant pathways and MLH1 gene promoter methylation, can also contribute to the discrepancies observed between MMR-IHC and MSI-NGS.17,38
Although our study highlights the utility of simplified molecular classification based on IHC and the integration of HR expression to enhance prognostic and predictive value, several limitations should be acknowledged. First, this is a retrospective, single-center study with a relatively short follow-up period and a limited sample size, which may lead to potential biases. Additionally, while HR expression was incorporated into the IHC-based stratification, other molecular biomarkers, such as CTNNB1, L1CAM, and ARID1A, were not evaluated. The introduction of these markers could provide further prognostic insights and refine risk stratification.
Conclusion
In conclusion, our study has demonstrated that a simplified molecular classification based on IHC can effectively provide an initial stratification of clinical and histological features, as well as the prognosis of patients with EC. Additionally, the integration of HR expression into this classification allows for more detailed subgrouping within the molecular categories. This refined stratification holds promise for emphasizing the importance of HT in treating EC, suggesting that HR expression could play a critical role in tailoring treatment plans to better meet the individual needs of patients, potentially enhancing therapeutic outcomes and personalizing care strategies.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The participating studies were performed in compliance with applicable laws, regulations, and guidelines for the protection of human participants, including obtaining consent, and were in accordance with the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tianjin medical university general hospital (IRB2024-YX-549-01).
Consent for Publication
All authors gave their final approval of the version to be published; have agreed on the journal to which the article has been submitted; agree to be accountable for all aspects of the work.
Acknowledgments
We express our gratitude to the Key Laboratory of Female Reproductive Health and Eugenics at Tianjin Medical University General Hospital for their support in the preparation of this article.
Funding
This work was funded by the National Natural Science Foundation of China (Program Nos. 82172626), the Health Technology Project of Tianjin Municipal Health Commission (Program Nos.TJWJ2022XK009), China Health Promotion Foundation, (Program Nos.XH-C034), the Tianjin Key Medical Discipline (Specialty) Construction Project (Program Nos. TJYXZDXK-031A), the Natural Science Foundation of Tianjin (Program Nos. 23JCQNJC00530).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–1428.
2. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi:10.1016/0090-8258(83)90111-7
3. Pijnenborg JMA, van Weelden WJ, Reijnen C, Xanthoulea S, Romano A. Redefining the position of hormonal therapy in endometrial cancer in the era of molecular classification. J Clin Oncol. 2024;42(1):8–12. doi:10.1200/JCO.23.00470
4. Makker V, MacKay H, Ray-Coquard I, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7(1):88. doi:10.1038/s41572-021-00324-8
5. Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol. 2008;199(5):543e1–7.
6. Smith D, Stewart CJR, Clarke EM, et al. ER and PR expression and survival after endometrial cancer. Gynecol Oncol. 2018;148(2):258–266. doi:10.1016/j.ygyno.2017.11.027
7. Kistner RW. Histological effects of progestins on hyperplasia and carcinoma in situ of the endometrium. Cancer. 1959;12(6):1106–1122. doi:10.1002/1097-0142(195911/12)12:6<1106::AID-CNCR2820120607>3.0.CO;2-M
8. Kelley RM, Baker WH. Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med. 1961;264(5):216–222. doi:10.1056/NEJM196102022640503
9. Rodolakis A, Scambia G, Planchamp F, et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Hum Reprod Open. 2023;2023(1):hoac057.
10. Gordhandas S, Zammarrelli WA, Rios-Doria EV, Green AK, Makker V. Current evidence-based systemic therapy for advanced and recurrent endometrial cancer. J Natl Compr Canc Netw. 2023;21(2):217–226. doi:10.6004/jnccn.2022.7254
11. van Weelden WJ, Birkendahl PB, Lalisang RI, et al. The effect of progestin therapy in advanced and recurrent endometrial cancer: a systematic review and meta-analysis. BJOG. 2023;130(2):143–152. doi:10.1111/1471-0528.17331
12. Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
13. Oaknin A, Bosse TJ, Creutzberg CL, et al. Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(9):860–877. doi:10.1016/j.annonc.2022.05.009
14. Giannini A, D’Oria O, Corrado G, et al. The role of L1CAM as predictor of poor prognosis in stage I endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2024;309(3):789–799.
15. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383(21):2053–2064. doi:10.1056/NEJMra1514010
16. Perrone E, De Felice F, Capasso I, et al. The immunohistochemical molecular risk classification in endometrial cancer: a pragmatic and high-reproducibility method. Gynecol Oncol. 2022;165(3):585–593. doi:10.1016/j.ygyno.2022.03.009
17. Liu Y, Wang YX, Sun XJ, et al. Comprehensive assessment of mismatch repair and microsatellite instability status in molecular classification of endometrial carcinoma. Zhonghua Fu Chan Ke Za Zhi. 2023;58(10):755–765. doi:10.3760/cma.j.cn112141-20230711-00316
18. Singh N, Piskorz AM, Bosse T, et al. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol. 2020;250(3):336–345. doi:10.1002/path.5375
19. Thiel KW, Devor EJ, Filiaci VL, et al. TP53 sequencing and p53 immunohistochemistry predict outcomes when bevacizumab is added to frontline chemotherapy in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2022;40(28):3289–3300. doi:10.1200/JCO.21.02506
20. Vermij L, Leon-Castillo A, Singh N, et al. p53 immunohistochemistry in endometrial cancer: clinical and molecular correlates in the PORTEC-3 trial. Mod Pathol. 2022;35(10):1475–1483. doi:10.1038/s41379-022-01102-x
21. Guan J, Xie L, Luo X, et al. The prognostic significance of estrogen and progesterone receptors in grade I and II endometrioid endometrial adenocarcinoma: hormone receptors in risk stratification. J Gynecol Oncol. 2019;30(1):e13. doi:10.3802/jgo.2019.30.e13
22. Perrone E, Capasso I, De Felice F, et al. Back to the future: the impact of oestrogen receptor profile in the era of molecular endometrial cancer classification. Eur J Cancer. 2023;186:98–112. doi:10.1016/j.ejca.2023.03.016
23. Bartley AN, Mills AM, Konnick E, et al. Mismatch repair and microsatellite instability testing for immune checkpoint inhibitor therapy: guideline from the College of American Pathologists in collaboration with the association for molecular pathology and fight colorectal cancer. Arch Pathol Lab Med. 2022;146(10):1194–1210. doi:10.5858/arpa.2021-0632-CP
24. Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–219.
25. Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865–2871. doi:10.1093/bioinformatics/btp394
26. Boeva V, Popova T, Bleakley K, et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics. 2012;28(3):423–425. doi:10.1093/bioinformatics/btr670
27. Organisation IAfRo CWH. WHO Classification of Tumors Editorial Board. Female Genital Tumours.
28. Kautto EA, Bonneville R, Miya J, et al. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget. 2017;8(5):7452–7463. doi:10.18632/oncotarget.13918
29. Osipov A, Lim SJ, Popovic A, et al. Tumor mutational burden, toxicity, and response of immune checkpoint inhibitors targeting PD(L)1, CTLA-4, and combination: a meta-regression analysis. Clin Cancer Res. 2020;26(18):4842–4851. doi:10.1158/1078-0432.CCR-20-0458
30. Da Cruz Paula A, DeLair DF, Ferrando L, et al. Genetic and molecular subtype heterogeneity in newly diagnosed early- and advanced-stage endometrial cancer. Gynecol Oncol. 2021;161(2):535–544.
31. Santoro A, Angelico G, Travaglino A, et al. New pathological and clinical insights in endometrial cancer in view of the updated ESGO/ESTRO/ESP guidelines. Cancers. 2021;13(11):2623. doi:10.3390/cancers13112623
32. Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29(5):1180–1188. doi:10.1093/annonc/mdy058
33. Vermij L, Jobsen JJ, Leon-Castillo A, et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br J Cancer. 2023;128(7):1360–1368. doi:10.1038/s41416-023-02141-0
34. Jamieson A, Huvila J, Chiu D, et al. Grade and estrogen receptor expression identify a subset of no specific molecular profile endometrial carcinomas at a very low risk of disease-specific death. Mod Pathol. 2023;36(4):100085. doi:10.1016/j.modpat.2022.100085
35. Tangen IL, Werner HM, Berg A, et al. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer. 2014;50(17):3003–3010. doi:10.1016/j.ejca.2014.09.003
36. Leon-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020;250(3):312–322. doi:10.1002/path.5373
37. Haradhvala NJ, Kim J, Maruvka YE, et al. Distinct mutational signatures characterize concurrent loss of polymerase proofreading and mismatch repair. Nat Commun. 2018;9(1):1746. doi:10.1038/s41467-018-04002-4
38. Marsischky GT, Filosi N, Kane MF, Kolodner R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996;10(4):407–420. doi:10.1101/gad.10.4.407
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Assessment of Immune Status in Patients with Mismatch Repair Deficiency Endometrial Cancer
Ma J, Lin J, Lin X, Ren Y, Liu D, Tang S, Huang L, Xu S, Mao X, Sun P
Journal of Inflammation Research 2024, 17:2039-2050
Published Date: 2 April 2024