Back to Journals » International Journal of Nanomedicine » Volume 20
Therapeutic Innovations in Nanomedicine: Exploring the Potential of Magnetotactic Bacteria and Bacterial Magnetosomes
Authors Yadav VK , Pramanik S, Alghamdi S , Atwah B, Qusty NF, Babalghith AO , Solanki VS, Agarwal N, Gupta N , Niazi P , Patel A, Choudhary N, Zairov R
Received 24 September 2024
Accepted for publication 7 December 2024
Published 11 January 2025 Volume 2025:20 Pages 403—444
DOI https://doi.org/10.2147/IJN.S462031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Virendra Kumar Yadav,1,* Sheersha Pramanik,2,* Saad Alghamdi,3 Banan Atwah,3 Naeem F Qusty,3 Ahmad O Babalghith,4 Vijendra Singh Solanki,5 Neha Agarwal,6 Nishant Gupta,7 Parwiz Niazi,8 Ashish Patel,9 Nisha Choudhary,9 Rustem Zairov10,11
1Marwadi University Research Center, Department of Microbiology, Faculty of Sciences, Marwadi University, Rajkot, Gujarat, 360003, India; 2Department of Biotechnology, Bhupat and Jyoti Mehta School of Biosciences, Indian Institute of Technology Madras, Chennai, Tamil Nadu, 600036, India; 3Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia; 4Medical Genetics Department, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 5Department of Chemistry, Institute of Science and Research (ISR), IPS Academy, Indore, India; 6Department of Chemistry, Navyug Kanya Mahavidyalaya, University of Lucknow, Lucknow, Uttar Pradesh, India; 7Department of Engineering and Medical Devices, River Engineering Pvt Ltd, Ecotech-III, Greater Noida, U.p., India; 8Department of Biology, Faculty of Education, Kandahar University, Kandahar, Afghanistan; 9Department of Lifesciences, Hemchandracharya North Gujarat University, Patan, Gujarat, 384265, India; 10Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center RAS, Kazan, Russian Federation; 11Aleksander Butlerov Institute of Chemistry, Kazan Federal University, Kazan, Russian Federation
*These authors contributed equally to this work
Correspondence: Virendra Kumar Yadav; Nisha Choudhary;, Email [email protected]; [email protected]
Abstract: Nanotechnology has emerged as a revolutionary domain with diverse applications in medicine, and one of the noteworthy developments is the exploration of bacterial magnetosomes acquired from magnetotactic bacteria (MTB) for therapeutic purposes. The demand for natural nanomaterials in the biomedical field is continuously increasing due to their biocompatibility and eco-friendly nature. MTB produces uniform, well-ordered magnetic nanoparticles inside the magnetosomes, drawing attention due to their unique and remarkable features. MTB and magnetosomes have gained popularity in cancer treatment and diagnosis, especially in magnetic resonance imaging. Distinctive features highlighted include advancements in extraction, characterization, and functionalization techniques, alongside breakthroughs in utilizing MTB-based magnetosomes as contrast agents in imaging, biocompatible drug carriers, and tools for minimally invasive therapies. The biocompatible nature, functionalizing of the surface of bacterial magnetosomes, and response to the external magnetic field make them a potential candidate for the theragnostic purpose of MTB and magnetosomes. In the present review, emphasis has been given to the foundation of magnetosomes at a genetic level, mass production of magnetosomes, etc. Further authors have reviewed the various functionalization methods of the magnetosomes for cancer treatment. Finally, the authors have reviewed the recent advancements in MTB and magnetosome-based cancer detection, diagnosis, and treatment. Challenges such as scalability, long-term safety, and clinical translation are also discussed, presenting a roadmap for future research exploiting MTBs and magnetosomes’ unique properties.
Keywords: magnetotactic bacteria, magnetosomes, hyperthermia, gene therapy, nanomedicine
Introduction
Over the last decade, nanoparticles (NPs) and nanotechnology have attained significant consideration because of their exceptional and distinctive features.1 The properties are acquired by considering their narrow-sized geometry and structural and surface modifications. Today, nanoparticles have potential importance in medicine, electronics, environmental clean-up, research, defences, aerospace, etc.2,3 Nanomedicine is the emerging branch of nanosciences that applies NPs in the biomedical field.4,5 Among all the types of NPs, magnetic nanoparticles (MNPs) have emerged as transformative tools in nanomedicine, leveraging their unique physicochemical properties for various biomedical applications.6,7 MNPs have emerged as potential candidates in nanomedicine due to their response to an external magnetic field (EMF), recycling nature, low cost and easy availability, etc. Recently, MNPs have shown emerging roles in medicine for treatment and diagnosis. For instance, MNPs are widely used for drug delivery8 and in magnetic resonance imaging (MRI) as a contrast agent.9,10
The different types of iron oxide nanoparticles (IONPs) are magnetite, maghemite, hematite, and greigite, which have different magnetic properties. Out of all these MNPs, magnetite has a stronger magnetic strength than the others, and it has gained huge attention in medicine and environmental clean-up.11 MNPs are extensively utilized in drug delivery, MRI, contrast agents, etc.12 due to their high responsiveness to EMF and their magnetic properties, which allow precise targeting and raise localized hyperthermia for cancer therapy.13 Depending on their size, composition, and environmental conditions, MNPs exhibit diverse magnetic properties, primarily categorized as paramagnetic, superparamagnetic, or ferromagnetic. Paramagnetic NPs possess unpaired electrons, leading to a weak attraction to EMFs. They do not retain magnetization once the external field is removed, making them less suitable for applications requiring persistent magnetism. Superparamagnetic NPs, such as superparamagnetic iron oxide nanoparticles (SPIONs), can be magnetized in the presence of an EMF but exhibit no remanence or coercivity when the field is removed.14 This property allows them to be used effectively in drug delivery and MRI contrast agents, as they can be manipulated without residual magnetism.15 SPIONs can be magnetized in an external field and return to a non-magnetic state, facilitating minimal side effects during treatments like hyperthermia.14 Ferromagnetic NPs retain their magnetization even after removing the EMF, exhibiting hysteresis in their magnetization curves.16 They are typically larger in size and can be utilized in applications requiring stable MNPs, such as data storage, magnetic separation, and biosensing.14,17 Their strong magnetic properties are advantageous in industrial settings where consistent magnetic behavior is crucial. Superparamagnetic NPs are usually chosen for biomedical applications due to their non-toxic and biocompatible properties, but ferromagnetic NPs may be more appropriate for industrial applications that require stable magnetization.
IONPs (MNPs) can be synthesized by all three methods, ie, chemical, physical, and biological (plants and microorganisms).18 All these synthesis methods have their own advantages and drawbacks. For instance, the chemical method uses harmful chemicals that threaten the environment. Moreover, the chemical route requires a capping agent (polyvinyl alcohol, polyethylene glycol, etc) and stabilizing agents (chitosan, amino acids, etc) to control the size of the MNPs. So, this further increases the cost of the synthesis method. Moreover, the chemically synthesized MNPs require strict conditions to obtain uniform MNP, which may reduce the effectiveness of such MNPs.19,20 The physical approach requires sophisticated instruments for the synthesis, which is expensive and energy-intensive, increasing their production cost. The biological method is eco-friendly as it requires the least chemicals, and also, if microorganisms are used for synthesis, uniformity in size will be maintained.21 So, by microbial method, the synthesized MNPs have uniform shapes and sizes and are well crystalline in nature.
The shortcomings of the chemically and physically synthesized MNPs could be overcome by using the magnetotactic bacteria (MTB) and their magnetosomes as the source of MNPs, which possess well-ordered MNPs. Magnetosomes are membrane-bound MNPs synthesized by MTB that exhibit biocompatibility, uniform size, and strong magnetic properties. The role of MTB and their magnetosomes in nanomedicine is increasingly recognized for their nano-dimension, recyclability, and response to an EMF.22 MTB and their magnetosomes can be natural nanocarriers for anticancer drugs, antibodies, and nucleic acids, enhancing therapeutics’ stability and targeted delivery to tumor sites.23,24 Due to their high importance in nanomedicine, investigators have termed the MTB and their magnetosomes as potential nanobots/nanorobots. These microscopic robots could be easily manipulated and guided externally inside human beings. These nanobots may navigate through the human body driven to particular sites, for instance, tumor locations, while retaining their therapeutic and imaging properties.25–27
Even though some of the MTB synthesizes magnetite and greigite28 but magnetosomes are primarily composed of magnetite (Fe3O4) and are produced by MTB such as Magnetospirillum gryphiswaldense.29 They possess a narrow size distribution and uniform morphology, which is crucial for their functionality in drug delivery and imaging.23 Studies have shown that magnetosomes can improve the efficacy of treatments like magnetic hyperthermia, where localized heating is applied to cancer cells.30 Magnetosomes are also being explored as contrast agents in magnetic resonance imaging (MRI), providing enhanced imaging capabilities due to their magnetic properties.23,24 Advanced imaging techniques, such as Magnetic Force Microscopy (MFM), have been developed to characterize individual magnetosome chains, facilitating their application in biological settings.31
Earlier, some of the investigators have used magnetosomes for precise drug delivery to cancerous cells through hyperthermia under EMFs, showing potential in progressing focused and efficient cancer therapies.32 Xie et al reviewed and concluded that MTB-based magnetite NPs have several advantages over synthetic chemically synthesized magnetite NPs.33
Even though numerous investigations have been done in this field where MTB have been used for the drug delivery review, none of them have in-depth studies of the formation of magnetosomes, mass production of magnetosomes, and descriptive biomedical applications. Here, emphasis was given to the magnetosome’s role in targeted drug delivery, advanced MRI contrast enhancement, and hyperthermia therapy for cancer treatment, where their precise magnetic control minimizes off-target effects. Moreover, the study explores the genetic mechanisms of magnetosome formation, particularly the role of the magnetosome genomic cluster (MGC), and discusses bioengineering advances for customized applications. An emphasis was given to the state-of-the-art extraction and functionalization methods alongside detailed characterization of the magnetosomes. Moreover, the article addresses the scalability, safety, and regulatory hurdles, calling for interdisciplinary efforts to advance magnetosome biomedical applications and highlighting their transformative potential in nanomedicine.
In the present review, investigators have emphasized the basic information of magnetotactic bacteria and their magnetosomes. Further investigators have emphasized the habitats and morphology of MTB. The authors have reviewed the detailed information about the extraction, purification, and characterization of MTB and magnetosomes has been provided. Further, current and emerging applications of MTB and magnetosomes in biomedical applications, especially contrast agents, imaging, and drug delivery, have been highlighted. Finally, the authors have reviewed the current clinical trials on MTB-based drug delivery.
Magnetotactic Bacteria (MTB)
MTB is mainly a Gram-negative, flagellated microorganism that swims and inactively orients on the influence of geomagnetic fields.34 MTB is usually categorized under different structures based on elemental and synthesized mineral nanocrystals, which involve such as Fe3O4, Fe3S4 (greigite), combined greigite with the pyrite (FeS2), and a mixture of magnetite and greigite. These distinct mineral compositions give rise to different magnetic properties and behaviors within MTB.35 MTB exhibit a remarkable morphological diversity crucial for their ecological roles and evolutionary adaptations. The morphological diversity is phylogenetic and functional, as different morphotypes are adapted to specific environmental niches.36 The primary morphological types identified include coccoid, rod, spirillum, and vibrio forms, with coccoid shapes being the most prevalent.37–39 The most abundant are identified as magnetotactic cocci, characterized by their spherical shape and significant species-specific magnetosome arrangements. For instance, a giant rod-shaped species (QR-1) dominated in certain environments, showcasing a unique arrangement of magnetosomes in chains.40 In addition to the above two, spirillum and vibrio shapes contribute to the morphological spectrum, indicating various adaptations to aquatic environments.40
The variation in the morphology of MTB has ecological implications. For instance, MTB are vital in biogeochemical cycles, influencing iron and sulfur dynamics in ecosystems.25 Their morphological adaptations allow them to thrive in diverse habitats, from freshwater to marine environments, enhancing their ecological significance.41 MTB are predominantly anaerobic or facultatively anaerobic.42 Scientists have efficiently cultivated at least twenty kinds of magnetite-synthesizing MTB in pure culture.
Discovery, Natural Habitats and Biological Characteristics of MTB
Numerous magnetosomes producing MTB have been identified over several decades from various parts of the globe. The first identification of MTB in 1975 was Magnetospirillum magnetotacticum,43 followed by Magnetococcus marinus in 1978.44 In 1981, M. magnetotacticum MS-1 was described;42 by 1988, Magnetovibrio MV-2 was identified,45 and in 1991, researchers recognized Magnetospirillum magneticum, M. magneticum AMB-1, and Magnetovibrio MV-1.46
The discovery of Magnetovibrio MV-4 was made in the year 1993,45 while Magnetotactic bacterium strain WD-1 and Magnetotactic bacterium strain HM-1 were identified in 1994 and 1996, respectively.25 In 1999, Magnetospirillum sp. MSM-4 was reported.47 By 2002, Desulfovibrio magneticus RS-1 was discovered,48 followed by Magnetotactic bacterium strain YN-1 in 2003.49 In 2007, M. gryphiswaldense was identified.50
A significant period of discoveries occurred in 2010 and 2016. In 2010, Magnetovibrio blakemorei was identified33 and in 2016, several new species were discovered, including Magnetogaba australis IT-1,51 Magnetospirillum QH-2,52 Gammaproteobacterium BW-2, Gammaproteobacterium SS-5, and Magnetospira thiophila MMS-1.53 Candidatus Desulfamplus magnetomortis BW-1, Delta proteobacterium ML-1, Delta proteobacterium ZZ-1, and δ-proteobacterium AV-1 were documented.53
Further discoveries in 2016 included Magnetoovoid strain MO-1,54 Magnetotactic bacterium strain TH-1,45 and Herbaspirillum sp. TK-2.55 Other identified strains were Magnetotactic bacterium strain MG-1,56 Magnetotactic bacterium strain MG-2,57 and Magnetotactic bacterium strain YSC-1.58 Table 1 shows the year-wise identification of MTB from various parts of the world.
 |
Table 1 Year-Wise Identification of MTB from Various Parts of the World |
The above milestones reflect the progression from initial observations to identifying specific species, showcasing the increasing understanding of MTB’s ecological roles and biological mechanisms.
Natural Habitats of the MTB
MTB exhibit diverse physiological adaptations and occupies various ecological niches. These range from oxygen-rich aerobic environments to oxygen-deprived anaerobic habitats, spanning marine to freshwater ecosystems.59 Most MTB require an anaerobic environment; typically, the oxic and anoxic transition area near the nethermost residues (sediments) is the best zone for MTB growth. The MTB growing in oxygen-rich regions of water bodies, such as lakes, ponds, oceans, etc., have magnetite inside them. While going into deeper parts of the ocean and beyond this zone, there is an abundance of magnetite and greigite-producing MTB, mainly microaerophiles and anaerobic. Due to the anaerobic condition, there is a chemical change in magnetite into maghemite. Finally, there is a high concentration of sulphide users at the deepest part of the ocean or lake.60
From the physiology, ecology, and phylogeny aspects, MTB are found in water columns or sediments due to vertical chemical stratification. MTB have long been limited to environments with pH values close to neutral and at room temperature. Nevertheless, a moderately thermophilic MTB, capable of thriving up to a potential upper growth limit of 63°C, has been observed in hot springs, which requires a pH of 9.0 to grow well.22
Some theories claim that finding low-oxygen environments is a one-dimensional task rather than a three-dimensional one, with the latter typically connected to different cell taxi mechanisms. Freshwater bacteria from the genus Magnetospirillum are among the most extensively investigated MTB.
Mechanism of Magnetotaxis
The mechanism of magnetotaxis in MTB involves a combination of magnetic sensing and chemotactic responses, enabling these microorganisms to navigate their environments effectively. This navigation is primarily facilitated by magnetosomes that align the bacteria with the Earth’s magnetic field.61 Magnetosomes are made up of either magnetite or greigite crystals, organized in chains that function like a compass needle.62 The arrangement of these magnetosomes allows MTB to line up with geomagnetic fields, helping in their directional movement toward optimal habitats. MTB exhibit magneto-chemotaxis, responding to chemical gradients in their environment, such as oxygen and other repellents. This dual response allows MTB to navigate complex redox gradients, optimizing their position in stratified aquatic environments.63 Their ability to adapt to various ecological niches highlights the evolutionary significance of magnetotaxis in diverse habitats.25
MTB display remarkable magnetotaxis characteristics that permit them to orient themselves with the Earth’s geomagnetic field and navigate utilizing their flagella. There are six known magneto-aerotaxis variations, and research on the nature of magneto-aerotaxis is still ongoing.59 This unique ability allows MTB to harness the Earth’s MF as a natural compass, aiding it in precise orientation and movement.42 Two categories—polar and axial—can be used to categorize the magnetotaxis of MTB. Axial MTB demonstrate a relatively unrestrained, back-and-forth swimming pattern in multiple directions. Conversely, polar MTBs tend to swim preferably in a definite direction associated with the local geomagnetic field, showing a more directed and oriented movement.
Relying on the magnetic crystal formations within MTB, there are two main classifications of polar MTB: south-seeking MTB and north-seeking MTB. South-seeking MTB are principally observed in the southern hemisphere and tend to swim in the north pole line at a magnetic field.61 Conversely, north-seeking MTB are more frequently found in the northern hemisphere and prefer swimming towards the south magnetic zone in a magnetic field. The north-seeking and south-seeking MTB are widely obtained around the geographical zone (equator) with migration in opposite directions.61 In this area, there are two zones, north and south, seeking MTB with almost the same numbers. Intriguingly, a remarkable discovery has contested conventional expectations. While south-seeking MTB are generally associated with the southern hemisphere, a population of these bacteria has been determined in the northern hemisphere. Their exceptional swimming behavior sets them apart, contradicting the traditional patterns in formerly known MTB. This breakthrough introduces a breathtaking area of research and desires a re-evaluation of the factors affecting the magnetic orientation of these MTB in various geographic locations.22
Applications in Biomedical Research
MTB and their magnetosomes have significant potential in biomedical research, where they are commonly used in drug delivery systems (targeted drug delivery and as nanocarriers), imaging and diagnostics, and pharmaceutical applications.64 MTB can be engineered to deliver anticancer drugs directly to tumor sites, enhancing treatment efficacy while reducing the side effects.65 Magnetosomes act as natural nanocarriers for various therapeutic agents, including antibodies and siRNA, improving stability and targeted delivery.23 Besides this, magnetosomes are also used in advanced techniques like Magnetic Force Microscopy (MFM), which allow for the characterization of magnetosome chains, facilitating their use in imaging applications.31 One major feature of the application of magnetosomes in diagnostics is their good biocompatibility and low cytotoxicity.66 The establishment of pharmaceutical cell banks for MTB enables large-scale production of high-purity magnetosomes, which can be utilized in nanomedicine.29
Bacterial Magnetosomes
The bacterial magnetosome, a genuine prokaryotic organelle, displays a complexity equivalent to its eukaryotic counterparts. Magnetosomes of MTB are lipid bilayer membranes enclosing nanosized crystals of either greigite or magnetite, with most MTB comprising the mineral magnetite.61 These magnetosomes have a potential impact on the magnetic characteristics of the geometry of MTB (Fe3S4).59 Chemical, metabolic, and genetic controls particular to each species affect morphology and content, including the structural morphology of magnetic-based solid bio-minerals.56 Moreover, it also depends on the amount of O2 and food available, pH, redox potential, source of carbon, temperature, and mode of Fe absorption via MTB.
Structure and Composition
MTB are unique microorganisms that produce magnetosomes, specialized organelles containing magnetic iron minerals. These magnetosomes are crucial for the bacteria’s navigation in geomagnetic fields.61 The structure and composition of magnetosomes involve several key components, including the magnetic crystals, associated proteins, and the surrounding membrane. Inside the bacteria, the magnetosomes are typically grouped in chains. Even following bacterial disruption for the extraction of magnetosomes, the structural integrity of the magnetosome configuration persists, displaying robust stability that permits preservation and further analysis. This configuration is desirable because it felicitates uniform distributions and enhanced internalization into human cells, characteristics which may typically preferred for medicinal purposes.31
Magnetic Crystals
The structure of magnetosomes mainly comprises magnetic crystals and membrane enclosure. Magnetosomes typically contain magnetite or greigite crystals, which are biomineralized within the bacteria.62 Each magnetosome is enclosed by a lipid bilayer membrane derived from the inner membrane (IM) of the bacteria, which helps maintain the integrity of the magnetic crystals.61 Although uncultured coccus bacteria can develop exceptional magnetite in bulk shapes and sizes (up to 250 nm), established solid magnetite structures usually come under the confined nanoscale around 35–120 nm. The magnetosome’s size range has physical implications mirrored in its magnetic pull. It has two cores: an organic membrane and an organized arrangement of inorganic magnetite. An examination of isolated magnetosomes through lipid studies has uncovered that the magnetosome membrane mainly comprises a combination of amino acids, lipids, or lipid polymers. On the contrary, vesicles formed by the IM are considered the organic inner core of magnetosomes, including phospholipids, acids, glycolipids, and sulfolipids. Active sites on the magnetosome surfaces were identified as carboxyl, -OH, and amino monomers. Using a microscope, magnetosomes of different shapes have been observed, varying from rectangular, cubooctahedral, bullet-shaped, and elongated prismatic morphologies.67 Magnetite, which can oxidize to maghemite (Fe2O3), typically makes up the core of magnetosomes. The magnetosome core often has high degrees of crystallinity and purity.
Proteins
In addition to this, several proteins are associated with the magnetosomes. For instance, proteins such as MamK and MamJ are critical in organizing magnetosomes into chains, facilitating their function as geomagnetic sensors.61,68 Nonetheless, “Mam” refers to the magnetosome membrane, and “Mms” denotes the membrane specific to magnetic particles. These protein structural units play an essential role in forming the magnetosome membrane. This biological covering produces negatively charged magnetosomes with good water dispersion. The magnetosome surface is decorated with several chemical groups, facilitating straightforward functionalization.69 Moreover, some of the novel proteins, like Mad28, have also been identified, which contribute to the structural integrity of magnetosome chains.68 Besides this, Magnetosome Gene Clusters (MGCs) regulate the formation of magnetosomes that encode proteins essential for biomineralization.62
Magnetotactic Bacteria’s Genetic Landscape
Concerning molecular biology, genes specific to MTB were discovered following the sequencing of the genomes of numerous MTB species, which regulate the formation of magnetosomes. These genes are located within the MGC region, which plays a valuable role in various functions encompassing magnetosome membranes’ development. Their responsibilities extend to developing iron transport into magnetosomes, contributing to the structural composition, core formation, and the controlled morphological growth of magnetite crystals. Molecular genetic research was originally conducted using M. magnetotacticum MS-1. According to a hypothesis, certain genes from M. magnetotacticum MS-1 could be found within Escherichia coli. This is feasible because both organisms’ transcription and translation machinery are compatible, assisting genetic manipulation at the molecular level. This organism’s recA gene was cloned and expressed in E. coli. M. magnetotacticum MS-1 strain cloned a 2 kb DNA fragment to complement the iron uptake deficits in Salmonella typhimurium and mutants of E. coli lacking a functioning gene aroD. The above investigation concluded that such a 2 kb DNA fragment could help regulate the uptake of Fe.70 It is now possible to test non-magnetic mutants that lack magnetosomes due to the advancements in MTB culture technique on agar plates. The chromosome of a non-magnetic mutant strain of M. magneticum AMB-1 bacterium grown on an agar plate contained Tn5 transposon. A gene called magA was discovered using transposon mutagenesis, expressed in E. coli. Cultivation of M. magneticum AMB-1 bacterial cells followed by Fe-deficient optimizations produced extra magnetosomes, and magA appeared higher. Recent research shows magA may be a contender for enhanced MRI impacts. The aor gene was discovered to be expressed within a cytoplasm of M. magneticum AMB-1 and restricted to microaerobic environments. The mam22 gene was successfully cloned in M. magnetotacticum via reverse genetics. A gene for the homologous protein mamA was discovered in M. gryphiswaldense MSR1, M. magneticum AMB-1, and Magnetococcus sp. MC-1. Because of a shorter magnetosome chain developed by M. magneticum AMB-1, when mamA was deleted, it was determined that mamA is necessary to generate functioning magnetosome vesicles.71,72
Four preserved gene clusters inside a sizable unsteady genomic area known as the magnetosome island contain the bulk genomic substantially involved in magnetosome development. Various forms of primary genes are carried to form magnetosomes where the putative iron transporter genes are mainly mamB and mamM among the discovered primary genes. The mamK gene for the bacteria encodes several protein units. In contrast, the mamJ gene produces an acidic protein crucial for assembling the magnetosome chain.71,72 A summary of all the genes involved in the MTB for producing magnetosomes and their role is provided in Table 2.
 |
Table 2 Summary of the Genes Involved in the MTB for Producing Magnetosomes and Their Role |
Biosynthesis of Magnetosomes
The formation of magnetosomes in Magnetospirillum species, such as M. magnetotacticum and M. gryphiswaldense, is a complex process extensively studied across various strains. The formation of magnetosomes varies from one MTB to another, but the overall process remains the same. The formation of magnetosomes involves a series of genetic, biochemical, and structural processes crucial for their function as geomagnetic sensors. This understanding is primarily derived from research on multiple strains of Magnetospirillum, which have provided insights into the genetic and molecular mechanisms underlying magnetosome formation.68,73 The key reason for this is that these microorganisms are more accessible to cultivate than the majority of other MTB, that have made it easier to analyze their physiological and metabolic processes.74 The following phases make up the magnetosome formation mechanism:
- Intrusion into cytoplasmic membrane and creation of the magnetosome vesicle.
- Uptake of Fe by MTB and its transportation into the magnetosome membrane vesicle.
- A partial oxidative process catalyzed by an oxidation-reduction enzyme guides the development of low-density hydrous ferric oxides resembling the mineral ferrihydrite.
- Hydrous oxides have reduced a third of their Fe3+, and additional dehydration produces Fe3O4.
During the initial phase, the inner membrane of MTB undergoes swelling, forming a vesicle this is followed by the uptake of Fe from the surroundings. The MTB have various transporters in the magnetosome membrane, which pumps in Fe2+/Fe3+, leading to the elevated concentration of Fe ion in the vesicle. The membrane of the magnetosome comprises a set of magnetosome-associated proteins (MAPs) that regulate the biomineralization environment, encompassing Fe concentration, redox state, and pH levels.35 In the final step, ie, in biomineralization, there is a high concentration of Fe ion, which is further crystallized by the magnetosome protein, guiding the creation of a magnetite crystal.75
The chronological order of the Fe intake, transport, and crystal biomineralization processes is well understood. However, whether the formation of magnetosome vesicles occurs before or after crystal biomineralization or if both processes occur simultaneously is unclear. The Fe-deficient cells of M. magnetotactic and M. gryphiswaldense possessed unfilled along the moderately full magnetosome vesicles, according to reported morphological studies. These findings suggested that magnetosome vesicles were present before the biomineralization of the mineral phase.74
In the first step of forming new cells, extracellular Fe2+/Fe3+ ions are generally obtained from cells. Subsequently, it penetrates the external membrane and enters the periplasm, where there is a potential for the formation of magnetite crystals within invaginations, leading to magnetite magnetosomes.76 On the other hand, particular details and definite steps in magnetosome biomineralization are yet unclear and may differ according to the MTB species. Nothing is known regarding the production process of greigite biomineralization in MTB outside the identification of genes and chemical precursors. It has been shown that many genes and proteins contribute to magnetosome development. The genes and proteins concerned in the biomineralization procedure are yet unclear.62 The species of Magnetospirillum were the principal organisms used to study the synthesis of bacterial magnetosomes. Figure 1 displays the scheme of the hypothetical mechanism of magnetite biomineralization.42
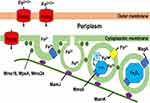 |
Figure 1 Schematic representation of the proposed magnetite biomineralization mechanism in MTB. Notes: Reproduced with permission from Yan L, Zhang S, Chen P, Liu H, Yin H, Li H. Magnetotactic bacteria, magnetosomes, and their application. Microbiol Res 0.2012; 167:507–19. Copyright © 2012 Elsevier GmbH. All rights reserved.42 |
Magnetosome Vesicle Formation
In this stage, membrane vesicles are created, and a cytoplasmic film obtains magnetosome membrane findings. Approximately 3–4 nm double-layered lipid film, composed of proteins, phospholipids, glycolipids, and fatty acids comparable to those found in cytoplasmic membranes, makes up the membrane of magnetosome in various Magnetospirillum species. This shows that the cytoplasmic membrane may be the source of the magnetosome vesicle. The creation of the magnetosome membrane in M. magneticum is brought about by the magnetosome proteins MamB, MamI, MamL, and MamQ. The magnetosome formation and the association of oligomeric units under a process of magnetosome biomineralization may be coordinated by the MamA gene, which functions as a scaffolding protein. For magnetite to maintain its thermodynamic stability, the magnetosome vesicle may be alkaline.77
Uptake of Iron in Magnetotactic Bacteria
In this process, siderophores and transport proteins internalize exogenous iron, accumulating through transmembrane iron transporters into the magnetosome vesicles. Magnetospirillum utilizes both Fe2+/Fe3+ to create ferrites.78 Since Fe2+ can dissolve by limiting 0.1 mol/L at around seven pH, MTB can absorb it using non-specific processes. Contrarily, because Fe (III) is insoluble, many bacteria rely on Fe chelators for holding, dispersing, and subsequently ingesting Fe3+. Low molecular weight siderophore produced by MTB has a strong propensity for iron complexation. Ligand- or proton-induced magnetic oxide dispersion processes can accelerate the dissolution of Fe (III). As a result, siderophore boosts the likelihood that MTB will come into touch with Fe (III), improving MTB’s ability to absorb iron. Compared to non-magnetotactic bacteria, MTB have more iron, about 3% of their dry weight. However, there is no proof that MTB have unique Fe uptake mechanisms. Although the specifics surrounding iron intake by MTB for magnetosome production are unknown, it appears that each magnetotactic bacterial species has several iron uptake systems in operation.75,79
Transportation of Fe Within a Membrane Vesicle
Irrespective of the timeline of vesicle formation, the commencement of nanocrystal formation begins upon the internalization of dissolved iron into the vesicle. For instance, M. magneticum AMB-1 cells had a 200-fold higher capacity to collect iron than conventional E. coli cells, up to 0.01 mol/L. To attain such high iron concentrations, MTB must have an effective iron transfer system, albeit its exact workings are still unknown. However, many membrane proteins control how iron gets into magnetosomes for biomineralization. For instance, proteins (MamB and MamM), the cationic distribution of the metal-based carriers, have been found in the identified MTB genomes.80–82 Only a few species have been reported to contain the protein MamV, which can carry iron to the membrane vesicle or invasion of the magnetosome.75,83,84
Controlled Biomineralization Within the Magnetosome Vesicle
Fe2+ and Fe3+ are liberated at the interface between the compartment and the magnetosome after being bound by organic substrates, where A remains unidentified, and B is identified as ferritin. Magnetite nanocrystals are formed in the final phase because of closely bound magnetosome proteins.85 The development of magnetite crystals in magnetosomes seems to be mediated by specific magnetosome proteins. Mms proteins control how nanocrystals form and become biomineralized in the vesicles of MTB. The development of magnetic nanocrystals is regulated by five Mms-proteins (Mms5, Mms6, Mms7, Mms12, and Mms13).
Magnetized solid structures with comparable characteristics to those generated in MTB cells are noticed when M. gryphiswaldense MSR-1 is exposed to Mms6. In the absence of Mms6, the nanocrystals displayed irregular size and shape. In M. gryphiswaldense, MamX, MamZ, and MamH are a factor in governing the biomineralization of magnetite via redox control, which is believed to impact the size and maturation of magnetosome Fe3O4 crystals. MamK and MamJ, two proteins found in M. gryphiswaldense, were involved in regulating the development of the magnetosome chain. A recent discovery uncovered that MamY acts as a membrane-anchored mechanical scaffold, performing a vital role in positioning the motility axis of the Magnetotactic spirilla. This alignment helped reconcile the procedure of magnetoreception on moving orientations, furnishing significant insights into how these microorganisms navigate employing the earth’s magnetic field.74
Gene Cluster Involved in Magnetosome Formation
In MTB, a gene cluster is involved in the magnetosome formation called Magnetosome Island (MAI). These are highly conserved in MTB, which have five operons, namely, mamAB, mamGFDC, mms6/mms36-48, mamXY/mag123, and feoAB operons.86 The mamAB operon plays a crucial role in the biogenesis of magnetosomes in MTB, particularly in M. gryphiswaldense. This operon consists of several genes essential for forming and maturing magnetite crystals. Table 3 provides a summary of the role of various genes within the mamAB operon.
 |
Table 3 A Summary of Roles of Various Genes Within the mamAB Operon in M. gryphiswaldense |
Kolinko et al inserted these 5 operons from M. gryphiswaldense into Rhodospirillum rubrum (photosynthetic bacteria). R. rubrum is phylogenetically nearest to Magnetospirillum sp. but does not produce magnetosomes. Once these genes were introduced, small vesicles were formed in R. rubrum. Further, investigators also revealed that it was possible to recollect vesicles containing R. rubrum using a strong permanent magnet. This step proved that this vesicle may act as a magnetosome.89
Schüler and their group showed the function of genes in MAI by knocking out each one of them. If any of the genes, ie, mamL, mamQ, or mamB, coming under the mamAB operon, is defeated, there will be no magnetosome formation in MTB. It is believed that the proteins of these three genes are associated with an internal membrane and trigger vesicle construction. Moreover, the exact function of these three genes is not yet known.90 Figure 2 illustrates the role of various genes of the mamAB operon in M. gryphiswaldense and the steps involved in forming bacterial magnetite.
 |
Figure 2 Role of various genes of mamAB operon in M. gryphiswaldense. Notes: Reproduced from IGEM Kyoto. Magnetosome formation. IGEM. Published 2014. https://2014.igem.org/Team:Kyoto/Project/Magnetosome_Formation. Accessed December 20, 2024. Creative Commons.91 |
Factors That Affect Magnetite Formation
Magnetosome composition in MTB is influenced by various environmental factors, including oxygen levels, nutrient availability, pH, redox potential, carbon sources, temperature, and iron absorption methods. These factors collectively impact the biomineralization method and the quality of magnetosomes produced. The formation of magnetosomes is a genetically controlled phenomenon, but environmental conditions play a significant role in modulating this method. Oxygen and redox potential play an important role in the composition of the magnetosomes of MTB. Magnetosome formation is favored in low-oxygen environments, as high oxygen levels can repress biomineralization. Redox modulation is critical, with specific redox proteins and pathways influencing magnetosome synthesis.92 The redox potential within magnetosomes is typically between −0.25 and −0.6 V, supporting magnetite formation.93 In addition to this, nutrient carbon sources also guide the composition of the magnetosomes. For instance, the availability of nutrients, particularly Fe, is essential for magnetosome formation. MTB can accumulate large pools of iron, which are distinct from the magnetite crystals, indicating efficient iron uptake and storage systems.93 Carbon sources such as Na-pyruvate have been shown to enhance magnetosome yield, with optimized culture conditions significantly increasing production.94 Some studies have shown that magnetosome synthesis is sensitive to pH and temperature. Optimal conditions for M. gryphiswaldense include a pH of 7 and a temp. of 28°C. Deviations from these conditions can limit magnetosome formation.95 High-resolution imaging has shown that variations in pH and temperature can lead to differences in magnetosome morphology and size.95 Some studies have proven that iron absorption and biomineralization directly impact magnetosome formation and composition. Iron uptake is a critical step in magnetosome formation, involving specific proteins and pathways. The process includes iron transport into magnetosomes, biomineralized into magnetite or greigite.71 The mode of iron absorption and the dissolved iron concentration significantly affect the efficiency of magnetosome production.94
No doubt environmental factors significantly influence magnetosome composition, but the genetic control of biomineralization remains a dominant factor. The interplay between genetic and environmental influences ensures magnetosomes’ high structural and compositional perfection, which are crucial for their applications in biotechnology and medicine.56,95 Understanding these dynamics can lead to improved methods for magnetosome production and potential applications in various fields.
Properties: Physicochemical and Magnetic and Their Relevance
MTB exhibit unique physicochemical and magnetic properties due to their ability to synthesize magnetosomes, which are intracellular MNPs. These properties facilitate magnetotaxis, allowing bacteria to navigate geomagnetic fields and hold significant potential for various applications, particularly in biotechnology and medicine.31 These magnetosomes, primarily composed of magnetite (Fe₃O₄) or greigite (Fe3S4), are arranged in chains that enhance the bacteria’s magnetic orientation and mobility. The magnetosomes have magnetite, which has ferromagnetic behavior.86 Besides this, it has a stable magnetic moment and adds physiological temperature. The MNPs formed inside the MTB are present in uniform chains coated with various biological molecules, which prevent their aggregation.
Physiochemical Properties
The concept of a chain of spheres is employed to demonstrate the magnetostatic interactions that underlie the magnetic features of MTB and isolated magnetosomes, principally composed of magnetite crystals. Magnetosomes are mainly 30–120 nm in size and are enclosed in a lipid bilayer, forming linear chains that behave like magnetic dipoles. Magnetosomes primarily comprise magnetite or greigite NPs, with high purity levels exceeding 99.9% in some strains.29
The magnetostatic interactions between grains organized in chains block the magnetic transitions, even avoiding the external magnetic field for crystals as small as 30 nm. The arrangement of magnetosomes into chains appears essential for their maturation, but its impact on MTB cell division is minimal, according to experiments and computational models. Early in the biomineralization process, researchers looked at alterations in the state of iron oxidation and magnetosome crystal size distribution as a function of time. Controlled magnetosome solids, including MGC genes, can be impacted by budding MTB in a magnetic field weaker than the geomagnetic field.71
Magnetic Properties of Magnetosomes
The magnetosomes are typically large nanoparticles with a single magnetic domain. Under physiological temperatures, this guides the development of a magnetic moment that remains stable against thermal fluctuations.86 Consequently, it produces a magnetism surpassing chemically induced magnetic NPs, which are generally superparamagnetic and hold a temperature-based magnetic moment. This results in a larger coercivity (Hc 20–40 mT) and the remanent to saturation magnetization ratio (Mr/Ms 0.4–0.5). These magnetic qualities result in superior MRI causes, including temperature-induced capabilities toward the magnetosomes under specific circumstances.30
Magnetosomes are organized in chains, functioning like a compass needle, which enhances the bacteria’s orientation in magnetic fields.29 Different crystal morphologies of magnetite within magnetosomes, such as cuboctahedron and bullet shapes, exhibit distinct magnetic behaviors, influencing their coercivity and magnetic moments. The magnetic features of MTB are influenced by the arrangement of magnetosomes into chains, which behave like compass needles. For instance, the coercivity of these chains can vary significantly, with some exhibiting low coercivity (<30 mT) and others high coercivity (>50 mT). Pie et al calculated that larger MTB cells with bullet-shaped magnetosome chains have higher magnetic moments, enhancing magnetic navigation by overcoming viscous resistance.96
Studies have shown that magnetosomes exhibit distinct hysteresis loops, with coercivity values ranging from 9.8 mT to over 50 mT, depending on the crystal morphology.96,97 Exposure to static magnetic fields can enhance cell growth but may inhibit magnetosome formation, indicating a complex interaction between environmental magnetic fields and bacterial metabolism.98 The magnetic parameters of various Magnetospirillum strains have been characterized, revealing stable single-domain states and superparamagnetic properties crucial for their functionality.99 Studies have shown that magnetic hysteresis and effective magnetic anisotropy are critical for the magnetotactic behaviour of these bacteria.29
Growth, Isolation, and Purification of Bacterial Magnetosomes
Growth of MTB in the Laboratory
MTB from their natural habitats could be isolated by various means, and the most widely accepted method is microfluidic system-based isolation, where the ferrofluids and the machinery are arranged so that MTB can be isolated with different amounts of magnetism.100
Despite numerous research attempts, only a few instances of this specific species of bacterium have been found since its discovery. MTB are extremely common and are found in considerable quantities in the sediments of various freshwater and marine ecosystems; however, their meticulous lifestyle makes MTB challenging to isolate and cultivate. Only a few MTB strains, primarily of the genus Magnetospirillum were purified and identified.25 Currently, γ-proteobacteria, δ-proteobacteria, Nitrospirae, and candidate phylum Omnitrophica are home to most MTB found. Magnetospirillum magnetotacticum strain MS-1, Magnetospirillium gryphiswaldense strain MSR-1, Magnetospirillum magneticum strain AMB-1, Magnetococcus marinus strain MC-1, and D. magneticus strain RS-1 were among the MTB strains that were successfully separated and cultured in the laboratory conditions.101
Isolation of Magnetosomes
Isolated magnetosomes resemble synthesized magnetic particles during the development of chain structures and variation in the rheological features in a suspension.56 The impact of the filament joining them in MTB and the power limitations of the magnetosome magnetostatic contact on particular sequences have been considered in mathematical models that detail the mechanical strength of magnetosome sequences. Immobilizing MTB engaged by the semisolid substrate of hydrated silica impacts the response of the magnetosome chain to shifts to align a particular magnetic zone, guiding sequencing units reorientation and structural disorder. However, applying MF (1 T) did not result in the disintegration of the chain for freeze-dried immobilized MTB.56
The first step involved in the isolation of magnetosomes from MTB is exposure to air, ultrasonic cell lysis or using a French press in a laboratory, agitation in a shaker, magnetic isolation of the magnetosomes or the elimination of the cellular debris by centrifugation followed by multiple times washing with distilled water and buffered solutions. The magnetic properties of magnetite can be controlled in a programmed MTB culture method by varying oxygen amounts to tailor them for the intended use. One mg of lyophilized MTB yields a mass of dry particles of only 20 g or less biomass. Under optimum conditions, the maximum daily dry biomass production can reach approximately 150 mg/L of culture.102
Purification of Bacterial Magnetosomes
It is well-studied that the magnetosomes made in the MTB have been isolated and purified by various techniques. Of all such purification techniques, one approach involves the growth of MTB under optimized conditions inside a bioreactor. Once the cells were grown completely, it was lysed by different methods like ultrasonication and organic solvent treatment. Once the cells were lysed, the magnetosomes were collected using an external magnet.103
Recently, Martinez et al extracted and purified the magnetosomes from M. gryphiswaldense MSR-1 by three approaches, ie, enzymatic treatment, probe sonication, and high-pressure homogenization. Further, the investigators studied a systemic comparison between all these three methods. In this experiment, the focus was to study the effect of the extraction techniques on the chain length, integrity, and aggregation state of magnetosomes isolated from M. gryphiswaldense MSR-1 cells. The cell disruption yield from all three methods was about (>89%).104
Mass Production of Magnetosomes
The mass production of magnetosomes from MTB is a promising area of research due to its exceptional features and potential applications in nanomedicine. Various approaches have been developed to enhance the yield and purity of magnetosomes, which focus on optimizing bacterial cultivation, cell disruption, and purification processes. These advancements aim to overcome the hurdles associated with the low natural yield of magnetosomes and the complexity of their extraction and purification. Earlier, some of the studies achieved a significant number of magnetosomes using a specific strain where the yield has reached up to 170 mg/L/day. The magnetosomes have good biocompatibility and low toxicity when manufactured under appropriate circumstances. Finally, magnetosomes can be produced by growing MTB in a non-toxic growth medium (for instance, ATCC medium 1653 for the AMB-1 species).69
In some of the cases, cultivation strategies have been applied, like high cell density fermentation. In this technique, a scalable platform using M. gryphiswaldense MSR-1 has been developed, achieving high cell density and magnetosome yield through a two-stage continuous fermentation process. This method allows for efficient cell disruption and magnetosome recovery, with the potential for continuous production.97 Chades et al also developed and established PCB for M. gryphiswaldense MSR1, enabling large-scale bacterial amplification under minimal growth conditions, producing highly pure magnetosomes with over 99.9% iron content.29 Corrêa et al, 2022 used a fed-batch strategy for M. blakemorei, supplemented with iron and nitrous oxide, followed by continuous culture. There was improved magnetosome production and productivity.105
In some of the cases, cell disruption and purification methods were applied to achieve efficient magnetosomes. For instance, Masó-Martínez et al, 2023 applied high-pressure homogenization as an effective method for preserving magnetosome chain integrity during cell disruption, compared to enzymatic treatment and probe sonication.104 Fernández-Castané et al, 2024 used high-gradient magnetic separation and magnetically enhanced density separation to purify magnetosomes, achieving high purity and yield without damaging the crystal structure.97
Some of the studies applied a genetic approach to increase the yield and purity of the magnetosomes. For instance, Dziuba et al, 2023 transferred the magnetosome biosynthesis pathways into non-magnetic bacteria. Further, the transformed bacteria have shown potential, although challenges remain in achieving high yields in new hosts.106 Some of the investigators used synthetic bacteria for the production of magnetosomes. For instance, Mickoleit et al, 2019 genetically modified R. rubrum to produce magnetosomes, with cultivation conditions optimized for high yield and potential for functionalization.66
Characterization of Magnetosomes
Characterization techniques for the magnetosomes of MTB involve a variety of advanced methodologies that enhance the understanding of their magnetic properties and structural integrity. These techniques are crucial for fundamental research and potential applications in biotechnology and medicine. The detailed investigation of the magnetosomes by various analytical techniques will help in their specific applications in nanomedicine.31 Various investigators have reported the electrical and physicochemical aspects of the bacterial magnets; for instance, it has been analyzed by spectral techniques like X-ray diffraction (XRD), Fourier transform infrared (FTIR), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), Mossbauer spectroscopy, vibrating sample magnetometry (VSM), microscopy such as Magnetic Force Microscopy (MFM), scanning electron microscope (SEM), transmission electron microscope (TEM), etc.107 All these instrumentation techniques revealed detailed morphological and elemental information about the bacterial magnetosomes. All these microscopic techniques helped confirm the shapes, spectroscopic techniques helped identify several functional groups on the surface of these magnetosomes, and the XRD technique helped determine the crystalline nature of the MTB and their magnetosomes and magnetite. The most crucial role of these instruments is Raman and Mossbauer spectroscopy, which helps identify and confirm iron oxides’ phases (magnetite, maghemite, or hematite) in the magnetosomes. Electron diffraction spectroscopy (EDS) is another technique that helps reveal various elements in the bacterial magnets, ensuring their purity. Besides this, since a biological membrane encloses bacterial magnets, their analysis could also be done using a carbon-hydrogen nitrogen and sulfur (CHNS) analyzer to detect carbon-hydrogen composition.108
The ultrastructure of MTB has been extensively examined through a range of electron microscopy procedures, with a specific emphasis on methods utilizing signals from inelastically scattered electrons. It is possible to map the scattered elements, their compositional ratios, and the binding energies of chemical bonds inside MTB using EDS and XRD techniques. New advancements in TEM enable high-resolution direct observation of the biomineralization procedure utilizing a liquid graphene cell with an encapsulated 1 µL sample of MTB.
Marqués-Marchán et al, 2024, have used MFM to characterize individual magnetosome chains, overcoming challenges posed by their low magnetic signal and the larger size of the MTB. Custom probes enhance sensitivity in various environments, allowing for quantitative data collection under in-situ magnetic fields.31 Some studies have used ultrasensitive torque magnetometry (UTM) to measure individual bacteria’s magnetic hysteresis, revealing magnetosome configurations and magnetic moments. This is complemented by transmission electron microscopy (TEM) to visualize the magnetosome structures. In some of the studies, dynamic light scattering (DLS) and nano-flow cytometry (nFCM) were used to provide insights into magnetosome size and quality.104
Crystallographic measurements of magnetosome structures are well explained, as uncovered by high-resolution TEM (HRTEM), which emerges to be influenced by a complicated, multistage biomineralization procedure. This procedure is governed by factors involving negatively charged ions and hydrocarbon-based moieties within the vesicle. Interestingly, this procedure could vary even among magnetosomes on similar units. It has been proposed that Fe2O3 forms in magnetosomes at a transitional stage of biomineralization. It only occurs in a small range of crystal sizes for this metastable polymorph of iron (III) oxide. Inorganic salts of manganese or cobalt, when directly introduced into the nutritional media of MTB, allow for the production of metal-replaced magnetite with altered morphologies and magnetic characteristics.97,109,110
In addition, some investigations have used three-dimensional finite-element micromagnetic models to link magnetosome morphology to magnetic properties, identifying distinct behaviours based on crystal forms. This modelling helps in understanding the magnetic orientation efficiency of various chains.96 Furthermore, some of the studies studied the magnetosomes by applying techniques like nonlinear longitudinal response measurements and electron magnetic resonance (EMR) spectra analysis to help assess the magnetic states of magnetosomes, which revealed their stability and anisotropy over time.99
Rosenfeldt et al studied the different stages of magnetosome biogenesis in the model organism M. gryphiswaldense using lab-based small-angle X-ray scattering (SAXS). The analyses confirm a narrow particle size distribution, indicating an overall magnetosome radius of 19 nm in M. gryphiswaldense. The average distance between individual magnetosomes is quantified, indicating a chain-like particle arrangement with a center-to-center distance of 53 nm. The data indicate that SAXS serves as an innovative stand-alone method for at-line monitoring of magnetosome biosynthesis, offering precise information regarding particle nanostructure.111
Magnetospirillum spp. produces a singular magnetosome chain for orientation inside the Earth’s magnetic field in the M. caucaseum SO-1 and M. marisnigri SP-1 strains. The quantity and dimensions of magnetosomes in the chain vary among different strains of Magnetospirillum spp. BB-1, SO-1, SP-1, MS-1, and LBB-42 generate approximately 25 magnetosomes per cell, averaging 40–50 nm in size; AMB-1 produces around 20 magnetosomes/cell, each measuring approximately 45 nm; MSR-1 produces about 30 magnetosomes, ranging from 32 to 45 nm in size.99
Pei et al developed 3D finite-element micromagnetic models to study intact and collapsed magnetosome chains in common MTB species. They modeled cuboctahedron, prism, and bullet-shaped biogenic magnetite crystals, revealing distinct magnetic properties for each shape.
Cuboctahedron and bullet crystals formed low (<30 mT) and high (>50 mT) magnetic clusters, respectively, while prismatic chains exhibited a wide range of hysteresis parameters due to their structure. These findings enable biogenic magnetite fingerprinting in geological samples using magnetic clustering, unmixing, and electron microscopy. Calculations showed that larger MTB cells with bullet-shaped magnetosome chains have higher magnetic moments, enhancing magnetic navigation by overcoming viscous resistance.96 Table 4 summarizes the techniques used for characterization of MTB and their magnetosomes, and their corresponding size measurements.
 |
Table 4 Techniques Used for Characterization of MTB and Magnetosomes and Their Corresponding Size Measurements |
Engineering of Magnetotactic Bacteria and Magnetosomes
The engineering of MTB and their magnetosomes has received significant attention due to their potential applications in nanomedicine and biotechnology. Various techniques like genetic engineering and surface functionalization strategies have been used to increase the properties and functionalities of these microorganisms and their MNPs.117
Genetic Engineering Approaches
Genetic manipulation of MTB offers a promising pathway to enhance the characteristics of magnetosomes for specific applications. Some of the major genetic engineering strategies include gene editing for magnetosome formation, introducing non-native functions, synthetic biology approaches, and heterologous expression.23
Modifications to genes involved in magnetosome biomineralization, such as mam and mms gene clusters, have enabled control over magnetosome size, shape, and magnetic properties. Genetic engineering has been used to express foreign proteins or enzymes on magnetosome surfaces, facilitating their use in biosensing or targeted drug delivery.117 Incorporating synthetic promoters, regulatory elements, and pathways enhances production yield and tailors magnetosome biosynthesis for industrial scalability. Efforts to transfer magnetosome synthesis pathways to more robust or faster-growing microbial hosts, like E. coli, are underway, aiming to overcome the slow growth of native MTB.
Surface Functionalization Strategies
Surface functionalization strategies of MTB and magnetosomes have obtained significant attention due to their potential applications in biomedicine and nanotechnology. These strategies leverage genetic engineering and chemical modifications to enhance the functionality of magnetosomes, enabling their use in various diagnostic and therapeutic contexts like imaging, therapy, and drug delivery. Strategies include chemical functionalization, bioconjugation, hybrid nanostructures, and natural surface modifications.118
Coating magnetosome surfaces with biocompatible polymers (eg, polyethylene glycol) or ligands for enhanced stability and reduced immunogenicity. Attaching targeting moieties such as antibodies, peptides, or aptamers to magnetosomes for enhanced specificity in targeting disease sites. Combining magnetosomes with other materials (eg, gold nanoparticles or silica) to develop multifunctional nanocomposites with improved optical, thermal, or catalytic properties. Exploiting the native biological machinery of MTB to express functional proteins or peptides directly on magnetosome membranes.23,119
There are certain investigations where genetic approaches were involved in the functionalization of the magnetosomes, for instance. Wu et al, 2021 genetically modified to display nanobodies, small, stable proteins that can bind specific targets, such as the insecticide fipronil. This method allows for developing sensitive immunoassays for environmental monitoring.120 A system has been developed where magnetosome membrane anchors are fused with SpyCatcher groups, facilitating the covalent attachment of proteins. This approach has shown improved biocatalytic performance compared to traditional methods.121 In addition, some of the studies used chemical modification techniques to functionalize the magnetosomes. For instance, Ren et al, 2018 altered the culture medium’s mineral concentration, which modified the magnetosome properties and enhanced their suitability for biomedical applications.122 In some of the studies, oxidative treatment was also used for the surface medication of the magnetosomes. For instance, the treatments with oxidizing agents can adjust the binding affinities of functionalized magnetosomes, allowing for tailored interactions with target molecules.120 Table 5 summarizes all the previous investigations where modification of MTBs has been done by genetic engineering and surface functionalization.
 |
Table 5 Studies of All the Previous Investigations Where Modification of MTB Has Been Done by Genetic Engineering and Surface Functionalization |
Applications of MTB and Magnetosomes in Therapeutic Nanomedicine
MTB’s magnetosome envelope’s surface can link the bioactive chemicals, which are significant for numerous medical applications. Moreover, MTB and their magnetosomes present promising applications in therapeutic nanomedicine, particularly in targeted drug delivery, hyperthermia and cancer treatment, genetic engineering, pharmaceuticals, pathogen detection, antigen retrieval, heating, imaging, and MRI contrast agents (Figure 3). These biogenic NPs demonstrate unique features, like high crystallinity and strong magnetization, making them appropriate for several biomedical applications. It can be utilized for IgG antibody quantitation, magnetic antibody production, enzyme immobilization, and many more. Additionally, bacterial magnetite particles served as carriers of nucleic acid recognition and elicited genes’ specific immunity against the antigen.64
 |
Figure 3 Multiple uses of bacterial magnetosomes and magnetite in the biomedical field. |
Magnetosomes from bacteria were utilized to immobilize certain enzymes (glucose oxidase, uricase), which led to improved catalytic action. Magnetosomes are also very useful tools for identifying biomolecular interactions in medical diagnostic examinations and could be utilized as a promising drug carrier for anti-tumor treatments and boosters for MRI signals. Applying magnetosomes in genetic engineering can provide various enzyme catalytic activity. Modification in the membrane of magnetosomes by using various organic linkers offers extensive utilizations in oncology and gene therapy.30,117
MTB or bacterial magnetite has been widely used for DNA/antigen analysis, especially for hepatitis B. Real-time FQ-PCR has been utilized for the analysis of food and foodborne pathogens like Salmonella and Vibrio spp., targeted delivery of drugs for breast cancer treatment like doxorubicin, as separation of cells like B lymphocytes, for hyperthermia, for image contrasting due to magnetosome proteins which bind predominantly to pancreatic and brain cells, xenograft tumors, and breast cancer and for immobilization of enzyme and bioremediation. Moreover, it has also been used for the bioremediation of paraoxon pesticides.126,127
Targeted Drug Delivery
In the ever-evolving scenery of medical science, the quest for highly potent and specific drug-delivery methods has directed researchers toward exploring innovative strategies.128,129 Among these, magnetosomes have appeared as an extraordinary candidate capable of revolutionizing the drug delivery domain.127 The theory of targeted drug delivery is established on the principle of electively delivering therapeutic compounds to particular regions within the body, either via local administration or systemic circulation.130,131 This accuracy is essential for improving treatment efficiency while reducing side effects and harm to healthy tissues. In this regard, magnetosomes furnish a favorable solution. Their capability to be regulated and guided to precise sites inside the body employing external MFs holds the potential to enhance the efficacy of drug delivery substantially. The innate characteristics of magnetosomes, linked with their biocompatibility, make them a powerful choice for encapsulating and delivering an extensive range of pharmaceutical compounds.132
Doxorubicin serves as an anti-tumor agent specifically administered for the treatment of hepatic cancers.133 Drugs like doxorubicin can be conjugated to the surface of magnetosomes by modifying the various active surface functionalities. Doxorubicin’s anti-tumor activity has been modestly increased by conjugating it to the magnetosomes, which were about 79%. The reduction in toxicity is the main benefit of employing magnetosomes. Doxorubicin is extremely hazardous when taken alone, with a mortality rate of 80%; however, it is far less harmful when attached to magnetosomes, with a mortality rate of 20%. Alphandery (2020) recently reported using MTB and their magnetosomes in various fields. The authors briefed about using magnetosomes in cancer treatment as an agent for carrying the anti-cancer drug (Figure 4).134 In Figure 4, the MTB loaded with the anticancer drug are introduced into the artery, where it senses chemicals or oxygen. Further, from the arteries, MTB moves to the tumor region, where there is a hypoxic condition. As a result, heat is generated when exposed to an external alternating magnetic field, triggering the release of the drug from the MTB.
 |
Figure 4 The mechanism involved in the targeting and destroying tumors by a MTB. Notes: Reproduced from Alphandéry E. Natural metallic nanoparticles for application in nano-oncology. Int J Mol Sci, 2020; 21:1–12. Copyright 2020, with permission from, Elsevier.135 |
Moreover, Raguraman et al studied the impact of magnetosome-mediated insulin delivery on diabetic-induced rat models orally. The investigation encompassed preparing Magnetosome-Insulin (MI) conjugates via direct and indirect coupling techniques employing polyethylene glycol (PEG). The authors investigated the efficacy of in vivo delivery of MI conjugates orally on rat models induced with streptozotocin-induced diabetes. It was observed that the administration of MI led to a noteworthy decrease in fasting blood glucose (FBG) levels, with a reduction of up to 65% in contrast to the standard insulin therapy. Additionally, essential serum parameters, which involve triglycerides (43.81%), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (39.4% and 57.2%, respectively), and total cholesterol (43.8%), demonstrated substantial progressions in comparison to the diabetic control group. Histological evaluations of the rats preserved with MI uncovered outcomes resembling those of the control group.136
In another investigation, the objective of Geng et al was to develop anthracycline-incorporated magnetosomes to improve their effectiveness in battling cancer while also shedding light on the procedure of cellular uptake. The successful preparation and characterization of drug-loaded BMs (DBMs) uncovered their potent growth-inhibiting effect on cancerous cells in controlled surroundings and living organisms without substantially harming healthy tissues. The investigation revealed that DBMs were integrated into cells via two different mechanisms: caveolae-mediated endocytosis and macropinocytosis. Moreover, the drugs encapsulated within DBMs were noted to be discharged in the cell cytoplasm and later migrated into the nucleus, where they performed their anticancer characteristics.137 Table 6 summarises the applications of MTB and their magnetosomes in targeted drug delivery.
 |
Table 6 Summary of the Applications of MTB and Their Magnetosomes in Targeted Drug Delivery |
Magnetosomes in Gene Therapy
In modern medicine, the escalating field of gene therapy holds the assurance of revolutionizing the healing of various genetic disorders and diseases. Gene therapy includes the delivery of therapeutic genes to particular target cells within the body, with the supreme objective of rectifying or replacing defective genes.139–141 For the prosperity of gene therapy, an effective and accurate delivery system is crucial. This is where magnetosomes, natural MNPs originating from MTB, emerge as an appealing candidate. Their unique characteristics, comprising biocompatibility, low toxicity, and responsiveness to EMFs, make them an ideal candidate for gene therapy applications. With their inherent capability to be regulated and guided to particular areas within the body employing EMFs, magnetosomes can substantially enhance gene therapy’s accuracy, safety, and efficiency.142
The existing chemical treatment exhibits a poor prognosis, particularly for patients with malignant and invasive gliomas. The tumor’s extremely invasive nature hinders complete resection, resulting in considerable neurological morbidity and mortality. So, recently, magnetosomes have shown potential for treating gliomas epidermal growth factor receptor (EGFR).143 Magnetosomes have been employed for significant treatment in gene therapy of gliomas, and most of the studies were performed in mice. Tat/BM/PAMAM-psiRNA-EGFR-transfected U251-MG xenografts revealed a reduction in tumor volume, and research on protein expression using immunohistopathology in situ coordinated the outcomes from in vitro. Gene therapy operates by suppressing oncogenes and regulating critical transcription factors that perform a vital role in the progression of tumors.144 However, this method has the drawback that presenting therapeutic constituents inside tumor cells is challenging. Magnetosomes are very important in gene therapy. The investigators developed magnetosome–plasmid combinations that enabled cecropin B and apoptin (pVAX1-VA) co-expression. The proteins exhibited the prevention of tumor development by inducing cell death, halting the cell membrane, and causing cell cycle arrest in the G2/M phases disintegration. In contrast, cells transfected with a lipofectamine-based plasmid displayed higher apoptin and cecropin B expressions, indicating a distinct pattern in controlled cells. These protein genes could be used in gene therapy to treat various cancers. Apoptin activity has also been demonstrated by cecropin B. The modifications in their biological membranes, which affect their stability and tissue dispersibility, are related to using magnetosomes to carry drug nanocarriers.145
Fu et al reported that the Mms6 gene, derived from MTB, has been found to help M2 macrophages in forming magnetic bio-nanoparticles (MBNs). Further, the investigator suggested this method is valuable as it helps prevent ferroptosis. Here, the investigators used the Mms6-transfected M2 macrophages in the spinal cord injury (SCI) in mice and found that the above macrophages effectively promote structural repair and recovery of locomotor function. Such type of a novel strategy in immune cell therapy supports the survival and strengthens the function of M2 macrophages based on MBNs. The investigators also suggested that such methods have the potential for cross-species applications for treating traumatic injury and inflammatory diseases. In addition, the Mms6 gene has also been utilized to protect stem cells from oxidative damage (OD), improving their survival rate in conditions of iron overload. Further, it has also been concluded that it could also be helpful for the treatment of strokes, where OD could seriously affect the survival and function of stem cells.146
Hypoxia-inducible factor-1 (HIF-1) has a pivotal impact on the progress of tumors and their resistance to chemotherapy. Hence, the suppression of HIF-1 has surfaced as a fascinating process in the combat against cancer. In this context, Lyu et al had prepared a highly adaptable system for delivering small interfering RNA (siRNA) that could efficiently target HIF-1 inside deep hypoxic tumors, surmounting numerous physiological impediments (Figure 5). This advanced system comprised a magnetic nanocluster of Fe3O4 at its core, a pre-fabricated chimeric membrane for its external layer, and hyaluronidase decorating its surface. These components together assisted extended circulation within the blood circulation, offered guidance through MRI, supported magnetic accumulation within the tumor area, enabled penetration into hypoxic sites, enhanced homotypic targeting of tumor, and ensured effective cytoplasmic delivery. The remarkable versatility and programmed delivery abilities of these engineered magnetosomes culminate in the magnificent silencing of HIF-1, causing an effective therapeutic impact and the betterment of chemoresistance, all while resulting in minimal abnormalities.147 Table 7 summarizes the MTB/magnetosomes and their application in gene therapy.
 |
Figure 5 The diagram presented an illustrative outline of a versatile magnetosome system prepared to effectively deliver HIF-1 siRNA for antitumor therapy. Notes: Reproduced from Lyu C, Lu G, Bao W et al. Engineering magnetosomes with chimeric membrane and hyaluronidase for efficient delivery of HIF-1 siRNA into deep hypoxic tumors 2020; 398:125453. Copyright 2020, permission from Elsevier.147 |
 |
Table 7 Summary of MTB/Magnetosomes and Their Application in Gene Therapy |
Hyperthermia Therapy
Magnetic hyperthermia is a therapeutic procedure concerning the controlled heating of MNPs to induce cell necrosis.149 The remarkable magnetic characteristics of magnetosomes make them propitious candidates for magnetic hyperthermia.150 In tumor hyperthermia therapy, the purpose is to subject tumor cells to intracellular heat stress within a temp. range of 41°C–46°C, resulting their destruction. Nonetheless, non-selective heating may potentially damage the nearby healthy tissues, resulting in serious side effects.151 Magnetosomes display excellent magnetic characteristics at elevated temperatures in contrast to synthetic MNPs. The benefits of employing magnetosomes in this context include their elevated coercivity values and capability for effective heating under definite conditions. This quality permits more accurate and controlled hyperthermia treatment with diminished collateral damage to healthful tissue.152
Magnetic hyperthermia is a beneficial treatment for patients.153–155 This is more beneficial with lower limitations than any other treatment, significantly limits less than radiotherapy and chemotherapy, and can even be combined to enhance healing success. In the magnetic hyperthermia process, ferrite NPs are primarily administered to tumors, subsequently undergoing heating when exposed to an alternating magnetic field. The NPs must, therefore, generate a lot of heat if they are to be effective at producing magnetic hyperthermia. The magnetosome’s enormous size, ferromagnetic activity at optimal temperatures, and strong crystalline nature are responsible for its heating qualities. By analyzing magnetosome losses per cycle, which are calculated as the magnetosome’s SAR (specific absorption rates) for employed MF, measuring how much heat the magnetosomes produce has been possible. The magnetosome losses per cycle increased from 0.1 to 0.2 j/kg with increasing magnetic field strength. Once magnetosomes are influenced by alternating MF, two mechanisms can cause heat to be produced. Orientation of MF is either reversed or the Magnetosome physically rotate as an alternating magnetic field is employed, which is responsible for these effects.156
About 100 mL of solutions comprising either separate magnetosomes or units of magnetosomes on a 10 mg/mL amount were injected into MDA-MB-231 breast tumors xenografted beneath the mice membrane to assess the anti-tumor efficacy of magnetosomes. The mice were subjected to a regulated MF for 20 minutes, three times, at 20 Mt MF average intensities and 198 kHz frequencies. The temperature rose to 43°C as a result of this. In some mice, the therapy with chains of magnetosomes resulted in the tumor completely disappearing 30 days after the treatment, although employing individual magnetosomes did not significantly increase anti-tumor efficacy.156
In another investigation, Chen et al explored the prospective of employing MTB AMB-1 to operate MRI-driven magnetic hyperthermia therapy for hypoxic tumors (Figure 6). The results demonstrated that AMB-1 bacteria exhibited a selective capacity to travel to the hypoxic zones within dense tumors, owing to their anaerobic attributes, thus proving vigorous and deep penetration into tumor tissue. In addition, AMB-1 bacteria displayed remarkable MRI contrast and magnetic heating abilities, principally attributed to the extraordinary magnetic attributes of their magnetosomes. The in vivo experiment provided convincing evidence of the efficacy of AMB-1 bacteria in this regard. Not only could AMB-1 produce T2-weighted contrast indications within tumor cells, but they also demonstrated a capability to competently induce the removal of hypoxic compact tumors via the effect of magnetic hyperthermia.157
 |
Figure 6 Schematic diagram showing magnetic hyperthermia in the treatment of hypoxic tumors by using MTB. Notes: Reproduced from Chen X, Lai L, Li X et al. Magnetotactic bacteria AMB-1 with active deep tumor penetrability for magnetic hyperthermia of hypoxic tumors. Biomater Sci. 2022; 10:6510–6. Copyright 2022, Royal Society of Chemistry.157 |
Kuzajewska et al emphasized the potential of MTB and magnetosomes as an innovative drug delivery strategy for tumor treatment. The researchers highlighted the utilization of these MTBs and associated magnetosomes as natural nanocarriers for conventional anti-cancer pharmaceuticals, antibodies, vaccine DNA, and siRNA. The use of MTBs and magnetosomes as transporters improves the stability of chemotherapeutics and facilitates the exact delivery of individual ligands or their combinations to malignant tumors. This accuracy allows drugs to target molecular sites more efficiently.158
With pharmaceutically compatible media, Nguyen et al developed very pure magnetosomes from MTB. These magnetosomes are rendered non-pyrogenic by extracting them from microorganisms and heating them above 400°C, a process that removes and denatures bacterial organic material, leaving behind inorganic magnetosome minerals. These magnetosomes were coated with citric acid to stabilize them and form (magnetosomes-citric acid) M-CA, restoring magnetosome chains. Moreover, the heating properties and anti-tumor effects of highly pure M-CA were investigated by exposing PC3-Luc tumor cells to M-CA. After subjecting the assembly to an alternating magnetic field (AMF) of 42 mT and 195 kHz for 30 minutes, the investigators observed that M-CA was non-cytotoxic in the absence of AMF. However, upon AMF application, a 35% decrease in cell viability was recorded. Finally, the researchers concluded that the efficacy of the treatment of magnetosomes could be linked with a specific absorption rate (SAR) value of M-CA, which is relatively high in the cellular environment, ie, SAR cell = 253 ± 11 W/g Fe. A lower efficacy was noted when the SAR value was measured in water (M-CA), ie, SAR water = 1025 ± 194 W/g Fe, which might be due to the decrease in the Brownian contribution to the SAR value in the cellular environment.159
Magnetic Resonance Imaging (MRI) Contrast Agents
MRI is based on the principle of nuclear magnetic resonance (NMR), widely used for contrast imaging and has proven to be an extremely dependable diagnostic tool. The signal of magnetic resonance produced by an object varies based on its position.160 MRI, along with modern improvements, could help acquire the resonance frequency of the object and play a vital role in establishing a three-dimensional (3D) image of an object. This technique is superior to conventional imaging as it involves high-resolution imaging, safety, and the capability for multidirectional and multiparameter assessments, etc. In short, MRI has shown a significant role in contrast images in biomedical detection.161
MRI tools have been significantly employed to diagnose cancer, and some recent investigations have utilized magnetosomes in MRI to have both positive and negative contrast instead of conventional iron oxides.22 MTB naturally predisposes to target tumors when given intravenously to mice.162,163 Magnetospirillum is utilized in a radiological examination, and it is considered a magnetic microbe with a micro-aerophilic nature. Magnetosomes are predominantly composed of magnetite, mainly crystalline iron substances, and are encased with double-layered membranes.164 The magnetotactic behavior of magnetosomes generated from micro-organisms was evaluated using a multimodal approach.165 When magnetosomes were exposed to an applied magnetic field, they generated heat, demonstrating a linear correlation between the magnetic field strength and the resulting temperature increase.166 In vivo, chains of MNs with high iron concentration can be easily distinguished when administered directly into live tissue via MRI. This suggested that these NPs could serve as magnetic tracers or contrast agents. MagA gene encodes an iron transporter controlled at low iron concentrations. Specifically, the functional sites of magnetosomes are induced by the resonating action of an applied magnetic field, with pronounced signals detected within a specific frequency band of MRI intensities.
Benoit et al used M. magneticum AMB-1 to enhance in vivo tumor visualization via positive MRI contrast, leveraging small magnetite particles for T1-weighted imaging. After intravenous injection of 64 Cu-labeled AMB-1, positron emission tomography (PET) imaging showed tumor colonization and reduced organ infection within 240 minutes. By the 6th day, the bacteria selectively colonized tumors and were cleared from other organs, with MRI showing a 1.22-fold increase in contrast by day 2 and 1.39-fold by day 6. The study highlighted MTB and its derivatives as promising tools for improved MRI visualization in cancer research.167
Ye et al extracted magnetosome-like structures (MSC) from M. magneticum AMB-1 as core components, which were further coated with gold via a reduction process using chloroauric acid (HAuCl₄), forming MSC-Au NPs. Further, these engineered NPs were administered to mice, and finally, the tumor-focused magnetic field was applied to enhance accumulation at the tumor site (Figure 7). The developed, engineered NPs were evaluated as a boosting candidate to enhance photoacoustic imaging in MRI, including their promising use in diverse anti-cancer treatments, such as starvation therapy, chemo dynamic healing, and photothermal treatment when guided by specific magnetic fields.168 Due to their enhanced imaging-quality impact, a single injection and a single laser irradiation approach yielded noteworthy healing in hindering tumor growth across numerous cell-derived xenograft tumor subjects. Significantly, these promising findings extended to patient-originated organoid and patient-derived xenograft tumor models, underscoring the platform’s versatility and capability for ground-breaking disease treatments in humans.169
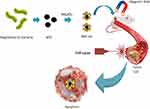 |
Figure 7 Therapeutic effect of surface functionalized magnetosomes guided by multimodal imaging, reproduced with permission from Ye P, Li F, Zou J et al. In Situ Generation of Gold Nanoparticles on Bacteria-Derived Magnetosomes for Imaging-Guided Starving/Chemodynamic/Photothermal Synergistic Therapy against Cancer. Adv Funct Mater. 2022; 32:2110063. Copyright 2022, Wiley and Sons.169 |
Apart from the extraordinary inherent features of MTB, diverse strategies have been tailored to confer these particles with added functionalities and enhance their overall capabilities as biomedical platforms for various applications. Gandarias et al 2023 reported the development of an MTB, which has enhanced diagnostic functionality due to the incorporation of Terbium (Tb) and Gadolinium (Gd) into MTB. The incorporation of Tb imparted luminescence properties to MTB, enabling their potential use as biomarkers, while the addition of Gd transformed MTB into dual-contrast agents for MRI by introducing T1 contrast alongside the inherent T2 contrast of unmodified MTB. Further, these modified MTBs were successfully tested in vitro in two cell models, which confirmed their suitability as fluorescent markers (Tb-MSR-1) and dual contrast agents for MRI (Gd-MSR-1).170
In a different study, a team led by Li prepared a cancer-originated magnetosome acting as a groundbreaking cancer vaccine, featuring a core composed of Fe3O4 magnetic nanoclusters (MNCs) and a protective cloak constructed from membranes of cancer cells adorned with anti-CD205. The exceptional characteristics of the MNCs, comprising superparamagnetics and magnetizations, permitted the vaccine’s precise magnetic retention within lymph nodes, guided by MRI. This strategy created a crucial time window for dendritic cells (DCs) to capture antigens. Concurrently, the disguised cancer cell membranes acted as a reservoir for a diverse extent of antigens, flooring the way for a subsequent multi-antigenic reaction. Furthermore, the anti-CD205 decorations directed more of the vaccine towards CD8+ DCs, assisting major histocompatibility complex (MHC) I cross-presentation. Such combined benefits resulted in substantial T cell proliferation, distinguished by remarkable clonal diversity and improved cytotoxic action. Thus, this cancer-originated magnetosome, integrating recent signs of progress in various nanotechnologies, holds great assurance for safe and high-performing cancer vaccination.171
Nan et al (2021) extracted magnetosomes from Magnetospirillum gryphiswaldense MSR-1 using an ultrasonic method and studied their distribution and clearance in mice up to 135 days post-intravenous injection. They identified the liver as the primary target organ for magnetosome accumulation, with additional accumulation in the spleen. The study revealed two main elimination pathways and estimated the clearance time of magnetosomes. The findings confirmed the high biocompatibility of magnetosomes, providing a long-term in vivo assessment that supports their potential use as MRI contrast agents.172
Nuschke et al (2023) proposed M. magneticum as an MRI contrast agent for in-vivo tracking of adoptively transferred immune cells, with mammalian cells utilizing the bacterium as magneto-endosymbionts (MEs). In a pilot study, ME labeling was tested in myeloid-derived suppressor cells (MDSCs), cytotoxic T lymphocytes (CTLs), and dendritic cells (DCs), examining its effects on cell purity, function, and MRI contrast. Iron loading varied by cell type: >0.6 pg Fe/cell for MDSCs, <0.5 pg Fe/cell for CTLs, and ~1.4 pg Fe/cell for DCs. MDSC functionality remained unaffected at 1000 MLR but was impacted at 2000 MLR, while CTL and DC markers showed no significant changes. In vivo, ME-labeled MDSCs produced detectable MRI contrast similar to SPIO-labeled cells. The study emphasized caution with higher ME concentrations, which may affect cell function or morphology, and concluded that this approach could be a valuable tool for tracking transplanted cells and advancing cancer therapy research.173
Magnetosomes have demonstrated substantial potential for MRI applications. Diverse MRI contrast mediators leveraging magnetosomes were considered to enable real-time attention for tumor treatment. The ongoing investigation in this field implies that magnetosomes may find practicable use in clinical fields as our knowledge digs deeper and their applications persist in advancing. Table 8 shows the application of MTB and their magnetosomes in MRI as a contrast agent.
 |
Table 8 Summarizes the Application of MTB and Their Magnetosomes in MRI as a Contrast Agent |
Magnetosomes in Magnetic Particle Imaging (MPI)
MPI, a developing molecular imaging method, depends upon the utilization of tracers in the form of superparamagnetic NPs. This approach assists as a bridge between traditional analytical techniques, offering an exceptional technique for imaging. In contrast to MRI, which employs a static gradient MF with lower magnetic potentials, MPI is established on the Langevin concept of paramagnetism and a nonlinear magnetization model. MPI furnishes several distinct benefits, including linear quantification, the absence of ionizing radiation, no limitations on permeation strength, positive contrast, and the absence of biological background signals, which have made considerable strides in molecular imaging.175
SPIONs have been established for their advantage as imaging agents, aiding in the differentiation between healthy and unhealthy tissues and facilitating the monitoring of cell movement. MPI leverages the magnetic characteristics of SPIONs to provide quantitative and extremely sensitive data of images. MTB generates magnetosomes that demonstrate properties akin to those of synthetic nanoparticles. Genetic mutations impacting biosynthesis could alter these biological magnetosomes (Figure 8). Employing M. gryphiswaldense MSR-1, highlighting a mamJ gene removal, and clustering magnetosomes instead of the usual linear chains enhanced MPI signal strength and resolution. Bioluminescent MSR-1 bacteria having deleted mamJ were introduced into mice with tumors and healthy mice. In vivo, bioluminescence imaging established MSR-1 feasibility, while MPI efficiently identified marks in the livers and tumors of the subjects.174
 |
Figure 8 The improvisation of magnetic particle imaging with an altered arrangement of magnetosomes in magnetotactic bacteria as living contrast agents. Notes: Reproduced from Makela A V, Schott MA, Madsen CS, Greeson EM, Contag CH. Magnetic Particle Imaging of Magnetotactic Bacteria as Living Contrast Agents Is Improved by Altering Magnetosome Arrangement. Nano Lett. 2022; 22:4630–9. Copyright © 2022, American Chemical Society.174 |
In another investigation, Tay et al, 2018, introduced a theragnostic platform incorporating measurable MPI for the accurate treatment design. This platform utilized MPI gradients to focus magnetic hyperthermia on particular zones spatially. This procedure addressed a substantial hurdle in traditional magnetic hyperthermia where systemically delivered SPIONs are inclined to accumulate in unintended organs. The complication in localizing hyperthermia often outcome in collateral impairment to the unintended organs. With the MPI magnetic hyperthermia workflow, the researchers demonstrated the capability to guide and pinpoint hyperthermia to the tumor while reducing harm in the vicinity of the liver (usually at a distance of 1 to 2 cm). This accurate localization of thermal treatment and its validation was affirmed via luciferase activity and histological evaluations. For the successful implementation of this strategy, it could be extended to confine the activation of drug release alongside specific biomechanical therapies and thermal treatment.176 Table 9 summarizes the applications of MTB and magnetosomes in Magnetic Particle Imaging (MPI).
 |
Table 9 Applications of MTB and Magnetosomes in MPI |
MTBs and Magnetosomes in Detection Assays
Protein detection techniques have exploited magnetosomes with effectiveness. The protein streptavidin was attached to magnetosomes by biotin groups connected to the membrane of magnetosomes. These semi-synthetic solid composites with biotin-binding properties helped combine the multiple functionalized biomoieties. In this technique, HBsAg (the hepatitis B antigen) was immobilized in human serum using antibody-functionalized magnetosomes, and the signal from the detecting complex was amplified using magnetic concentration. Unlike magneto-ELISA, which used artificial nano substances to improve antigen discovery in ELISA, magneto-immune PCR (M-PCR) was around 100 times more sensitive at detecting HBsAg. The production of antibodies attached to magnetosomes or magnetosome crystals has proven beneficial in various immunoassays: immunoglobulin, cancer cell detection, and allergen detection quantification.177
MTB and magnetosomes have also been used as photoacoustic (PA) and photothermal agents. There are several examples where investigators have utilized them for this application, such as Nima et al studied the biogenic magnetic NPs as multimodal PA, PT, and photomechanical contrast agents.6
Cancer Therapy
Numerous non-selective chemotherapy substances can harm a patient’s healthy cells during treatment, which can be easily overcome using MTB and bacterial magnetosomes.178,179 Due to their ease of recovery, magnetosomes were employed extensively in the immobilization process for particular enzymes.127 One significant advantage of utilizing MTB and magnetosomes for therapeutic application is their biological origin, where the magnetosomes have encapsulation of biomolecules that are not harmful to the patients.127 Moreover, their manipulation by an EMF further increases their potential as a therapeutic agent as it would deliver drugs and exhibit therapeutic effects at the specific site under an external magnet’s control, leaving the neighbour’s healthy cells unaffected. There are several applications where MTB can be used instead of magnetosomes as prospective agents for cancer treatment. While using the entire MTB for cancer treatment, their flagella play an essential role as they provide motility and self-propel them.180 These can be controlled inside a patient’s body under the influence of an EMF and are driven to hypoxic areas, such as tumor sites. Moreover, under the control of EMF, these magnetosomes do not lose their therapeutic and imaging characteristics.127,180
Photothermal therapy for cancer treatment has gained massive attention in the last decade due to its effectiveness. Mainly, NPs have been used for the photothermal therapy (PTT) of cancer but now MTB-based magnetosomes or MTB have also been used for this application. In an investigation by Chen, magnetosomes from M. magneticum AMB-1 (ATCC 700264) were developed and applied for the PTT of cancer under the guidance of MRI, both in vivo and in vitro conditions. The investigators revealed that the magnetosomes could rapidly convert the energy of 808 nm near-infrared (NIR) light into heat when these magnetosomes are internalized by the HepG2 tumor cells. It was observed that magnetosomes with excellent biocompatibility could effectively induce tumor cell death upon near-infrared (NIR) light irradiation. This effect was associated with alterations in mitochondrial membrane potential (ΔΨm) and increased intracellular reactive oxygen species (ROS) levels. In vivo experiments demonstrated that PTT using bacterial magnetic NPs could completely eliminate tumors in mice without causing detectable toxicity. Additionally, T2-weighted MRI revealed a well-defined tumor boundary and a 25% increase in negative contrast enhancement at the tumor site, highlighting the potential of bacterial magnetic NPs as effective MRI contrast agents for guiding PTT.181
Chen et al employed bacterial magnetosomes for PTT of cancer, guided by MRI, in both in vitro and in vivo studies. Their findings demonstrated that magnetosomes efficiently converted 808 nm near-infrared (NIR) light energy into heat. Additionally, in vivo experiments revealed magnetosomes exhibited a high retention rate in magnetically targeted regions, even under varying flow conditions. Due to the magnetic targeting, there was an increase of about 40% in BM retention in the tumor tissues of H22 tumor-bearing mice. Complete elimination of the tumor was observed when the magnetic targeting was done with the In vivo photothermal therapy with 808 nm laser irradiation. The investigation suggested that the systematically administrated magnetosomes with magnetic targeting would be promising for PPT cancer therapy.181
Chen et al reported an approach for targeting tumors by PTT, which involves generating magnetically targeted nanoparticles (IRFes) where the IRFes were guided to the tumor site under the influence of an EMF. The investigator utilized the strong NIR absorption properties of IR780, NIR fluorescence (NIRF) imaging, and effective magnetic targeting for the photothermal ablation of tumors. In vitro, cell viability assays, and in vivo antitumor studies demonstrated that IRFes could effectively ablate 4T1 cells or transplanted 4T1 tumors when exposed to 808 nm laser irradiation and a magnetic field. The in vivo experiments further confirmed that IRFes specifically targeted tumors without causing damage to other organs and enabled tumor imaging. The researchers concluded that this approach holds significant potential for localized PTT of cancer under EMF guidance, highlighting the promise of multifunctional NPs in tumor therapy.182 Table 10 summarizes the studies where MTB and their magnetosomes have been used for cancer treatment.
 |
Table 10 Summary of Applications of MTB and Their Magnetosomes for the Treatment of Cancer |
Magnetosomes in Pathogen Detection
Whole MTB and magnetosomes could identify numerous pathogenic bacteria, fungi, and toxins. These pathogens are mainly food and water-borne, like Salmonella, Shigella, Vibrio, and Listeria sps.
Xu et al, 2014 expressed the staphylococcal protein A (SPA), extracted from Staphylococcus aureus, on M. gryphiswaldense strain MSR-1 magnetosomes by fusion with MamC or MamF. Further, the investigators constructed recombinant plasmids pBBR-mamC-spa and pBBR-mamF-spa by fusing spa (the gene that encodes SPA) with mamC and mamF, respectively. Recombinant magnetosomes with surface expression of SPA were generated by introducing these fusion genes into wild-type MSR-1 or a mamF mutant strain. Studies revealed that the magnetosome colloids were relatively stable with a zeta potential value of −30 mV. Further, the recombinant magnetosome/Ab complexes were used to capture the Vibrio parahaemolyticus, which was measured by real-time fluorescence-based quantitative PCR and found that 1 mg of the complex could capture as many as 1.74×107 Vibrio cells.186
Sannigrahi et al extracted magnetosomes from Magnetospirillum sp. RJS1 and conjugated them with an anti-Listeriolysin O (LLO) antibody (0.25–1 µg/mL), as confirmed by spectroscopy. The resulting magnetosome–LLO antibody complex was 25% more cost-effective than traditional methods. Impedance spectroscopy revealed a significant increase in resistance (RCT value) on the electrode surface corresponding to higher concentrations of the LLO protein. The biosensor was successfully applied to detect Listeria monocytogenes in contaminated milk and water samples, with the extracted LLO protein serving as the detection target. Cross-reactivity assays confirmed the biosensor’s specificity against other foodborne pathogens, while a detection limit of 101 CFU/mL in both water and milk underscored its sensitivity. This biosensor represents a rapid, sensitive, specific, and cost-effective approach for detecting L. monocytogenes in food samples.187
Role of Magnetosomes and MTB in Biosensing
There are several investigations where MTB and magnetosomes have been used as biosensors for detecting pathogens, biomolecules, etc. The MTBs could be used directly in their natural form, or their engineered form could be used for these applications. Some of the examples are cited below. Roda et al used a bioengineered bioluminescent MTB (M. gryphiswaldense strain MSR-1) as a powerful tool to develop a robust and economical chip-based whole-cell biosensors where the bacterium constitutively expressed a red-emitting click beetle luciferase. The microfluidic chip was developed by using a multilayered black and transparent polydimethylsiloxane (PDMS) in which BL-MTB is incubated for 30 min with the sample, then moved by microfluidics, trapped, and concentrated in detection chambers by an array of neodymium–iron–boron magnets. Further, the investigator suggested that the bioluminescent signal from this luciferase is directly proportional to bacterial viability. The investigators have used the magnetic features of these MTBs as “natural actuators” to transfer the cells in the chip from the reaction to the detection area, optimizing the chip’s analytical performance. The investigator placed the chip in contact with a cooled CCD via a fiber optic taper to perform quantitative bioluminescence imaging after adding luciferin substrate. Further, the investigators have used dimethyl sulfoxide, (DMSO) and bile acid (taurochenodeoxycholic acid, TCDCA) to investigate the analytical performance of the developed whole-cell biosensor “MAGNETOX.” When the investigator incubated the chip with the DMSO, there was a drastic reduction in the bioluminescent signal in a dose-related manner. Finally, the investigators concluded that developing such bioengineered MTB with magnetic and luminescent properties has the advantage of easy 2D cell handling with ultra-sensitive detection.188
Dieudonné et al (2020) utilized M. magneticum AMB-1 and M. gryphiswaldense MSR-1 as cellular chassis to formulate sensitive magnetic bacterial biosensors. The ArsR-dependent regulation mechanism was validated through reverse transcription-PCR experiments. These biosensors, constructed by transcriptionally fusing arsenic-inducible promoters with the bacterial luciferase luxCDABE operon, exhibited element-specific responses within half an hour and achieved an arsenite detection limit of 0.5 μM. By optimizing the system, the sensitivity was enhanced 50-fold, reaching a detection threshold of 10 nM, which is more than an order of magnitude below the recommended arsenic limit in drinking water (0.13 μM).189 Applications of MTB and magnetosomes in pathogen detection and biosensing are shown in Table 11.
 |
Table 11 A Summary of All the Studies of MTB and Magnetosomes in Pathogen Detection and Biosensing |
Magnetosomes in Wound Healing
In an era where biomedical science constantly drives the boundaries of invention, the confluence of biotechnology and nanotechnology presents noteworthy opportunities for tackling complex medical difficulties.193–195 The work of Xu et al in developing a silver-magnetite hybrid magnetosome epitomizes the synergy between these two fields, offering a unique strategy for wound healing and infection management. In this investigation, they originally demonstrated that magnetosomes, when extracted, can experience a remineralization procedure in vitro upon exposure to a silver nitrate solution. The ionic Ag+ is introduced inside the biomembrane of the magnetosome, which further converts into crystalline silver following the specific miller indices, guiding the prompt synthesis of a magnetosome-Ag hybrid material (magnetosomes-Ag). The association among the biomembrane, crystals of Fe3O4, and the unmineralized iron constituent fostered the magnetosome remineralization, specifically when the concentration of Ag+ was equal to or exceeded 1.0 mg mL−1. Magnetosome-Ag demonstrated favorable biocompatibility and exhibited antibacterial characteristics. When utilized at 2.0 mg/mL concentration, both magnetosomes-Ag and the biomembrane efficiently suppressed Gram-negative and Gram-positive bacteria’s growth, emphasizing their capability as wound dressings to advance the healing of infected wounds.196
In a separate study, a team led by Revathy employed magnetosomes to bind with lemon grass extract (LGE) in an endeavor to hinder the formation of microbial biofilm on wound-dressing materials. The magnetosomes were acquired from Magnetospirillum sp. VITRJS-1 and experienced characterization through morphological and diffraction techniques. The extract’s phytochemical investigation demonstrated the existence of bioactive constituents recognized for their antimicrobial and anti-inflammatory characteristics. Gas chromatography-mass spectrometry (GCMS) analysis established the existence of citral and fernesal, which were antimicrobial elements. The extract was effectively linked to the magnetosomes, as corroborated by the FTIR study. The resulting magnetosome-lemon grass extract (MLGE) was assessed for its capability to combat biofilm creation by pathogenic microorganisms, for example, Bacillus subtilis, Pseudomonas spps, E. coli, Klebsiella spps, and Staphylococcus aureus on wound dressing materials. The findings of the plate assay uncovered a substantial decrease in the number of colonies developed on MLGE-coated wound dressings in contrast to those treated with LGE and the control group. Therefore, it can be concluded that MLGE-coated wound dressings efficiently avoid the establishment of microbial biofilms. Additional inquiry, comprising studies on animal models, carries the potential to pave the way for commercial applications of this favorable strategy.197
Miscellaneous Medical Application of Bacterial Magnetosomes
The cytostatic effect on human hepatocellular carcinoma was discovered in the case of in vivo and in vitro. The number of HepG2 cells that can specifically bind to magnetosomes is more significant than medications not attached to nanoparticles, which are modified with anthracycline pharmaceuticals. The magnetosomes are smaller than the magnetic particles, over the extremely magnetized ferrite spinel (spion) and beads, and have more robust magnetic properties and ferromagnetic characteristics.198–200 Due to this, they are excellent candidates for use in cell separation. The magnetosomes have been utilized in immunoassays to identify harmful, hormone-like, or polluting small compounds and detergents. Antibodies201–203 that connect with such molecular bodies have been affixed to the surface of the magnetosomes. The complexity of these chemicals and the magnetosomes that they produce has since been discovered. Finally, magnetosomes were used to obtain DNA molecules.203 They have been changed and coated in layers of DNA-linking aminosilanes. It is a complex created by the DNA, and magnetosomes have been joined to DNA obtained from a magnetic column after elution with a phosphate buffer.145
Mickoleit et al (2024) recently investigated the biocompatibility and toxicity of magnetosomes isolated from M. gryphiswaldense. The study tested magnetosomes with THP-1, Jurkat cell lines, and primary human-derived materials such as peripheral blood mononuclear cells (PBMCs). The findings revealed excellent biocompatibility and high blood compatibility across all tested magnetosome concentrations, highlighting their potential for biomedical applications. Minimal complement activation was observed without significant plasma coagulation or hemolysis. However, lipopolysaccharides from M. gryphiswaldense triggered PBMC activation and cytokine release, highlighting endotoxicity as a challenge for in vivo applications. Despite this, magnetosomes show strong potential for various biomedical uses, particularly in vitro diagnostics.204 Table 12 summarizes the IONPs and other similar NPs used for therapeutic applications.
 |
Table 12 The Comparative Investigation of All the IONPs and Other Similar NPs for Therapeutic Applications |
The above table reveals several approaches where MTB, their magnetosomes, or similar magnetosomes developed by chemical route have been applied for biomedical applications like cancer treatment, drug delivery, PTT, wound healing, etc.217 Some of the approaches are affordable and reliable for highly efficient targeting of tumor sites.
Preclinical and Clinical Studies
The advancement of MTB and magnetosomes toward clinical applications requires comprehensive evaluation through preclinical and clinical studies. These investigations are crucial for understanding their therapeutic potential, safety profiles, and effectiveness in real-world scenarios. In vitro studies are pivotal in exploring the biomedical applications of MTB and magnetosomes, particularly in targeted drug delivery, magnetic hyperthermia, and imaging. Magnetosomes exhibit superior biocompatibility and stability compared to synthetic MNPs due to their natural lipid bilayer and uniform magnetic properties. Cellular studies reveal efficient internalization of functionalized magnetosomes, enabling targeted therapeutic action and precise localization under magnetic fields for enhanced specificity. Cytotoxicity and immune cell interactions show generally favorable profiles influenced by surface modifications and concentrations.
Animal models have been crucial for evaluating the safety and efficacy of MTB and magnetosomes in vivo. Preclinical studies in rodents and larger animals have explored biodistribution, clearance mechanisms, and off-target effects, showing tissue-specific accumulation under magnetic guidance and clearance via the reticuloendothelial system, particularly in the liver and spleen. Safety assessments report low immunogenicity and systemic toxicity with functionalized magnetosomes. Therapeutic applications, such as cancer treatment through magnetic hyperthermia and targeted drug delivery, have demonstrated promising efficacy with minimal side effects.
The clinical translation of MTB and magnetosomes remains in its early stages. While preclinical studies provide a solid foundation, only a few clinical trials have explored their use in humans, focusing on cancer treatments like magnetic hyperthermia and drug delivery. These trials assess safety, tolerability, and preliminary efficacy in small patient cohorts. Regulatory approval poses challenges, requiring robust safety data and consistent production methods. Efforts are ongoing to address these through standardized protocols, scalable production, and thorough safety testing. Advancing trials are anticipated to offer key insights into their medical potential. MTBs are non-pathogenic like other bacteria, increasing their potential as a candidate for clinical trials.180 To date, three MNPs have been approved for clinical applications, ie, nanotherm (aminosilane-coated IONPs), Sienna+: dextran-coated IONPs, and Resovist: dextran-coated IONPs. The former is used for magnetic hyperthermia, the second is used as a tracer for cancer-locating lymph nodes, and the last is used for MRI imaging of the liver.218
Future Research Directives
Due to their physical and chemical features, MTBs and magnetosomes have recently gained huge attention in biomedicine and biotechnology. Since these magnetosomes are biocompatible in nature, it has drawn their attention mainly as new theragnostic agents and drug delivery, which are not present with the chemically synthesized MNPs. Moreover, studying these applications requires collaborative attempts among investigators in diverse fields, including genetic engineering, nanosystem chemistry, micromagnetism, and microfluidics. The detailed study of the biomineralization process of bacterial magnetite provides basic knowledge for developing methods aimed at synthesizing biomimetic magnetic NPs. Such a study will provide substantially more yield while preserving novel biological features. By resembling the natural phenomenon by which magnetite is developed in MTB, researchers could aim to develop laboratory-synthesized MNPs that reproduce the physical attributes of their natural counterparts and display enhanced production efficiency. Magnetosomes could track treatment progress while providing therapeutic advantages in the promising field of theragnostic (combining therapeutic and diagnostic functions). Moreover, the application of MTB and magnetosomes for magnetic hyperthermia treatment for tumors, biosensors with enhanced sensitivity, tissue engineering for controlled cell growth, and novel imaging processes reveal a diverse range of possibilities.
Conclusion
The present investigation reviewed the immense potential of magnetotactic bacteria and bacterial magnetosomes as transformative tools in nanomedicine, where they have acted as microbots or nanobots. Using magnetics in nature, both MTB and magnetosomes could be easily manipulated under an EMF in a patient with high efficiency and low toxicity to healthy cells, which makes them viable alternatives to synthetic nanoparticles. Moreover, the uniformity and ordered structure of the magnetosomes have proven valuable in biomedicine, where the nanoparticle size becomes very important. Studies confirmed that MTB’s natural magnetosome formation process is governed by specific gene clusters and their tunable properties for precision medicine. The biocompatible nature, low toxicity, easy functionalization and manipulation under EMF have been successfully applied in cancer treatment, nanocarriers, hyperthermia, gene therapy, contrast agents for MRI, photothermal therapy, wound healing agents, etc. The specificity and efficiency of these magnetosomes are further enhanced by surface functionalization by biomolecules or chemicals and genetic engineering approaches. The applications of MTB and magnetosomes in biotechnology and healthcare will open a new era in nanomedicine. Moreover, the confluence of diagnostics and therapeutics can substantially influence healthcare and biomedical investigation.
Declarations
All authors have read, understood, and complied as applicable, with the statement on “Ethical responsibilities of Authors”.
Data Sharing Statement
The data can be availed by requesting the corresponding author.
Consent to Publish
All the authors have given their consent to publish this article.
Acknowledgments
This research was funded by the state assignment of Arbuzov Institute of Organic and Physical Chemistry of FRC Kazan Scientific Center of RAS. Author SP would like to thank the Indian Institute of Technology, Madras, for providing financial assistance and resources.
Funding
There is no funding to report.
Disclosure
The authors declare no conflict of interest.
References
1. Talodthaisong C, Plaeyao K, Mongseetong C, et al. The decoration of ZnO nanoparticles by gamma aminobutyric acid, curcumin derivative and silver nanoparticles: synthesis, characterization and antibacterial evaluation. Nanomaterials. 2021;11(2):1–19. doi:10.3390/nano11020442
2. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem.2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
3. El-Hawwary SS, Abd Almaksoud HM, Saber FR, et al. Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of: sabal blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021;11(29):18009–18025. doi:10.1039/d1ra01725j
4. He X, Jiang Z, Akakuru OU, Li J, Wu A. Nanoscale covalent organic frameworks: from controlled synthesis to cancer therapy. Chem Commun 2021;57(93):12417–12435. doi:10.1039/D1CC04846E
5. Nikwade V, Choudhary N, Solanki R, et al. Fabrication and characterization of ConA conjugated Curcumin loaded solid lipid nanoparticles for theranostic applications in lung cancer. Nanoscale. 2024. doi:10.1039/D4NR03157A
6. Nima ZA, Watanabe F, Jamshidi-Parsian A, et al. Bioinspired magnetic nanoparticles as multimodal photoacoustic, photothermal and photomechanical contrast agents. Sci Rep. 2019;9(1). doi:10.1038/s41598-018-37353-5
7. Wang Y, Li C, Shen B, Zhu L, Zhang Y, Jiang L. Ultra-small Au/Pt NCs@GOX clusterzyme for enhancing cascade catalytic antibiofilm effect against F. nucleatum-induced periodontitis. Chem Eng J. 2023;466:143292. doi:10.1016/j.cej.2023.143292
8. Gan Y, Xu Y, Zhang X, et al. Revisiting supersaturation of a biopharmaceutical classification system IIB drug: evaluation via a multi-cup dissolution approach and molecular dynamic simulation. Molecules. 2023;28(19):6962. doi:10.3390/molecules28196962
9. Wang Y, Zhai W, Cheng S, Li J, Zhang H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 2023;11(8):1371–1394. doi:10.1007/s40544-022-0710-x
10. Yadav VK, Yadav KK, Gnanamoorthy G, et al. A novel synthesis and characterization of polyhedral shaped amorphous iron oxide nanoparticles from incense sticks ash waste. Environ Technol Innov. 2020;20:101089. doi:10.1016/j.eti.2020.101089
11. Nogueira V ME, Barbosa PF, Mayanna S, et al. Characterization of the crystallographic preferred orientation relationships of the magnetite-hematite-goethite phase transformation during martitization. Minerals. 2022;12(3). doi:10.3390/min12030326
12. Elwakeel KZ, Al-Bogami AS, Guibal E. 2-Mercaptobenzimidazole derivative of chitosan for silver sorption – contribution of magnetite incorporation and sonication effects on enhanced metal recovery. Chem Eng J. 2021;403:126265. doi:10.1016/j.cej.2020.126265
13. Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62(2):144–149. doi:10.1016/j.phrs.2010.01.014
14. Lamichhane N, Sharifabad ME, Hodgson B, Mercer T, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs) as therapeutic and diagnostic agents. Nanoparticle Ther. 2022:455–497. doi:10.1016/B978-0-12-820757-4.00003-X
15. Tegafaw T, Liu S, Ahmad MY, et al. Magnetic nanoparticle-based high-performance positive and negative magnetic resonance imaging contrast agents. Pharmaceutics. 2023;15(6):1745. doi:10.3390/pharmaceutics15061745
16. Onodera R, Kita E, Kuroiwa T, Yanagihara H. Effect of static magnetic field bias on dynamic hysteresis loops of a magnetic nanoparticle suspension. Jpn J Appl Phys. 2022;61(6):065003. doi:10.35848/1347-4065/ac6407
17. Sharma A, Thakur RC, Sharma R. Biomedical Applications of Ferrites. In: Sharma P, Bhargava GK, Bhardwaj S, Sharma I editors. Engineered Ferrites and Their Applications. Materials Horizons: From Nature to Nanomaterials. Springer; 2023:241–256. doi:10.1007/978-981-99-2583-4_13
18. Patel S, Desai R, Patel B, et al. Phytonanofabrication of iron oxide particles from the Acacia jacquemontii plant and their potential application for the removal of brilliant green and Congo red dye from wastewater. Front Bioeng Biotechnol. 2023:11. doi:10.3389/fbioe.2023.1319927
19. Priyadarshini M, Tamil Selvan S, Narayanan K,S, Fawzy A. Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material. Bioengineering. 2017. 4:59. doi:10.3390/bioengineering4030059
20. Zhao Q, Wang Y, Zhu Z, Zhao Q, Zhu L, Jiang L. Efficient reduction of β-lactoglobulin allergenicity in milk using Clostridium tyrobutyricum Z816. Food Sci Human Wellness. 2023;12(3):809–816. doi:10.1016/j.fshw.2022.09.017
21. Baumberger S, Green Chemistry SMC. Eco-friendly chemistry, biorefinery. In: Baumberger S editor. Green Chemistry and Agro-Food Industry: Towards a Sustainable Bioeconomy. Springer Nature Switzerland; 2024:3–22. doi:10.1007/978-3-031-54188-9_1
22. Barr CR, Bedrossian M, Lohmann KJ, Nealson KH. Magnetotactic bacteria: concepts, conundrums, and insights from a novel in situ approach using digital holographic microscopy (DHM). J Comp Physiol A. 2022;208(1):107–124. doi:10.1007/s00359-022-01543-4
23. Alsharedeh R, Alshraiedeh N, Aljabali AA, Tambuwala MM. Magnetosomes as Potential Nanocarriers for Cancer Treatment. Curr Drug Deliv. 2024;21(8):1073–1081. doi:10.2174/1567201820666230619155528
24. Alonso-Villegas R, González-Amaro RM, Figueroa-Hernández CY, Rodríguez-Buenfil IM. The Genus Capsicum: a Review of Bioactive Properties of Its Polyphenolic and Capsaicinoid Composition. Molecules. 2023;28(10):4239. doi:10.3390/molecules28104239
25. Goswami P, He K, Li J, Pan Y, Roberts AP, Lin W. Magnetotactic bacteria and magnetofossils: ecology, evolution and environmental implications. NPJ Biofilms Microbiomes. 2022;8(1). doi:10.1038/s41522-022-00304-0
26. David Soto Rodriguez PE, Sirinelli-Kojadinovic M, Rouzaud M, Faivre D. Azide click chemistry on magnetotactic bacteria: a versatile technique to attach a cargo. Mater Today Bio. 2023;19:100587. doi:10.1016/j.mtbio.2023.100587
27. Ying G, Zhang G, Yang J, et al. Biomineralization and biotechnological applications of bacterial magnetosomes. Colloids Surf B Biointerfaces. 2022;216:112556. doi:10.1016/j.colsurfb.2022.112556
28. Selvaraj R, Pai S, Vinayagam R, et al. A recent update on green synthesized iron and iron oxide nanoparticles for environmental applications. Chemosphere. 2022;308:136331. doi:10.1016/j.chemosphere.2022.136331
29. Chades T, Le Fèvre R, Chebbi I, Blondeau K, Guyot F, Alphandéry E. Set-up of a pharmaceutical cell bank of Magnetospirillum gryphiswaldense MSR1 magnetotactic bacteria producing highly pure magnetosomes. Microb Cell Fact. 2024;23(1):70. doi:10.1186/s12934-024-02313-4
30. Alonso J, Gandía D, Jefremovas EM, et al. Magnetotactic bacteria: biorobots for targeted therapies and model nanomagnetic systems. In:
31. Marqués-Marchán J, Jaafar M, Ares P, et al. Magnetic imaging of individual magnetosome chains in magnetotactic bacteria. Biomater Adv. 2024;163:213969. doi:10.1016/j.bioadv.2024.213969
32. Kianfar E. Magnetic nanoparticles in targeted drug delivery: a review. J Supercond Nov Magn. 2021;34(7):1709–1735. doi:10.1007/s10948-021-05932-9
33. Xie J, Chen K, Chen X. Production, modification and bio-applications of magnetic nanoparticles gestated by magnetotactic bacteria. Nano Res. 2009;2(4):261–278. doi:10.1007/s12274-009-9025-8
34. Mathuriya AS. Magnetotactic bacteria: nanodrivers of the future. Crit Rev Biotechnol. 2016;36(5):788–802. doi:10.3109/07388551.2015.1046810
35. Ben-Shimon S, Stein D, Zarivach R. Current view of iron biomineralization in magnetotactic bacteria. J Struct Biol X. 2021;5:100052. doi:10.1016/j.yjsbx.2021.100052
36. Matthews DG, Dial TR, Lauder GV. Genes, morphology, performance, and fitness: quantifying organismal performance to understand adaptive evolution. Integr Comp Biol. 2023;63(3):843–859. doi:10.1093/icb/icad096
37. Liu P, Liu Y, Zhao X, et al. Diverse phylogeny and morphology of magnetite biomineralized by magnetotactic cocci. Environ Microbiol. 2021;23(2):1115–1129. doi:10.1111/1462-2920.15254
38. Jogler C, Wanner G, Kolinko S, et al. Conservation of proteobacterial magnetosome genes and structures in an uncultivated member of the deep-branching Nitrospira phylum. Proc Natl Acad Sci. 2011;108(3):1134–1139. doi:10.1073/pnas.1012694108
39. Zhou K, Zhang WY, Yu-Zhang K, et al. A novel genus of multicellular magnetotactic prokaryotes from the Yellow Sea. Environ Microbiol. 2012;14(2):405–413. doi:10.1111/j.1462-2920.2011.02590.x
40. Teng Z, Zhang W, Chen Y, Pan H, Xiao T, Wu LF. Characterization of dominant giant rod-shaped magnetotactic bacteria from a low tide zone of the China Sea. J Oceanol Limnol. 2018;36(3):783–794. doi:10.1007/s00343-018-7072-2
41. Qian X, Liu J, Menguy N, et al. Identification of novel species of marine magnetotactic bacteria affiliated with Nitrospirae phylum. Environ Microbiol Rep. 2019;11(3):330–337. doi:10.1111/1758-2229.12755
42. Yan L, Zhang S, Chen P, Liu H, Yin H, Li H. Magnetotactic bacteria, magnetosomes and their application. Microbiol Res. 2012;167(9):507–519. doi:10.1016/j.micres.2012.04.002
43. Frankel RB. The discovery of magnetotactic/magnetosensitive bacteria. Chin J Oceanol Limnol. 2009;27(1):1–2. doi:10.1007/s00343-009-0001-7
44. Kirschvink JL. South-seeking magnetic bacteria. J Exp Biol. 1980;86(1):345–347. doi:10.1242/jeb.86.1.345
45. Lefèvre Christopher T, Bazylinski Dennis A. Diversity, and Evolution of Magnetotactic Bacteria. Microbiol Mol Biol Rev. 2013;77(3):497–526. doi:10.1128/mmbr.00021-13
46. Chen H, Zhang SD, Chen L, et al. Efficient genome editing of Magnetospirillum magneticum AMB-1 by CRISPR-Cas9 system for analyzing magnetotactic behavior. Front Microbiol. 2018;9. doi:10.3389/fmicb.2018.01569
47. Le Nagard L, Morillo-López V, Fradin C, Bazylinski DA. Growing Magnetotactic Bacteria of the Genus Magnetospirillum: strains MSR-1, AMB-1 and MS-1. J Visualized Exp. 2018;140. doi:10.3791/58536
48. Byrne ME, Ball DA, Guerquin-Kern JL, et al. Desulfovibrio magneticus RS-1 contains an iron- and phosphorus-rich organelle distinct from its bullet-shaped magnetosomes. Proc Natl Acad Sci. 2010;107(27):12263–12268. doi:10.1073/pnas.1001290107
49. Uzun M, Alekseeva L, Krutkina M, Koziaeva V, Grouzdev D. Unravelling the diversity of magnetotactic bacteria through analysis of open genomic databases. Sci Data. 2020;7(1):252. doi:10.1038/s41597-020-00593-0
50. Amann R, Peplies J, Schüler D. Diversity and taxonomy of magnetotactic bacteria. In: Schüler D editor. Magnetoreception and Magnetosomes in Bacteria. Microbiology Monographs. Springer; 2006:25–36. doi:10.1007/7171_037
51. Morillo V, Abreu F, Araujo AC, et al. Isolation, cultivation and genomic analysis of magnetosome biomineralization genes of a new genus of South-seeking magnetotactic cocci within the Alphaproteobacteria. Front Microbiol. 2014:5. doi:10.3389/fmicb.2014.00072
52. Lin W, Zhang W, Zhao X, et al. Genomic expansion of magnetotactic bacteria reveals an early common origin of magnetotaxis with lineage-specific evolution. ISME J. 2018;12(6):1508–1519. doi:10.1038/s41396-018-0098-9
53. Araujo A, Abreu F, Silva K, Bazylinski D, Lins U. Magnetotactic bacteria as potential sources of bioproducts. Mar Drugs. 2015;13(1):389–430. doi:10.3390/md13010389
54. Yang C, Chen C, Ma Q, Wu L, Song T. Dynamic model and motion mechanism of magnetotactic bacteria with two lateral flagellar bundles. J Bionic Eng. 2012;9(2):200–210. doi:10.1016/S1672-6529(11)60108-X
55. Marques ACQ, Paludo KS, Dallagassa CB, et al. Biochemical characteristics, adhesion, and cytotoxicity of environmental and clinical isolates of Herbaspirillum spp. J Clin Microbiol. 2015;53(1):302–308. doi:10.1128/JCM.02192-14
56. Gareev KG, Grouzdev DS, V KP, et al. Magnetotactic Bacteria and Magnetosomes: basic Properties and Applications. Magnetochemistry. 2021;7(6):86. doi:10.3390/magnetochemistry
57. Lefèvre CT, Wu LF. Evolution of the bacterial organelle responsible for magnetotaxis. Trends Microbiol. 2013;21(10):534–543. doi:10.1016/j.tim.2013.07.005
58. Jun G, Hongmiao P, Haidong Y, et al. Isolation and biological characteristics of aerobic marine magnetotactic bacterium YSC-1. Chin J Oceanol Limnol. 2006;24(4):358–363. doi:10.1007/BF02842850
59. Wang X, Li Y, Zhao J, et al. Magnetotactic bacteria: characteristics and environmental applications. Front Environ Sci Eng. 2020;14(4):56. doi:10.1007/s11783-020-1235-z
60. Kopp RE, Kirschvink JL. The identification and biogeochemical interpretation of fossil magnetotactic bacteria. Earth Sci Rev. 2008;86(1):42–61. doi:10.1016/j.earscirev.2007.08.001
61. Taoka A, Eguchi Y, Shimoshige R, Fukumori Y. Recent advances in studies on magnetosome‐associated proteins composing the bacterial geomagnetic sensor organelle. Microbiol Immunol. 2023;67(5):228–238. doi:10.1111/1348-0421.13062
62. Cui K, Pan H, Chen J, et al. A novel isolate of spherical multicellular magnetotactic prokaryotes has two magnetosome gene clusters and synthesizes both magnetite and Greigite crystals. Microorganisms. 2022;10(5):925. doi:10.3390/microorganisms10050925
63. Mao X, Egli R, Petersen N, Liu X. Combined response of polar magnetotaxis to oxygen and pH: insights from hanging drop assays and microcosm experiments. Sci Rep. 2024;14(1):27331. doi:10.1038/s41598-024-78946-7
64. Wang Q, Chen C, Chen H, et al. Comparison of aggregation effect of axial and polar magnetotactic bacteria for tumor therapy. J Magn Magn Mater. 2024;598:172038. doi:10.1016/j.jmmm.2024.172038
65. Zhang J, Wang C, Zhang Y, et al. Application of magnetotactic bacteria as engineering microrobots: higher delivery efficiency of antitumor medicine. Chin Chem Lett 2024;35(10):109420. doi:10.1016/j.cclet.2023.109420
66. Mickoleit F, Schüler D. Generation of nanomagnetic biocomposites by genetic engineering of bacterial magnetosomes. Bioinspired Biomimetic Nanobiomater. 2019;8(1):86–98. doi:10.1680/jbibn.18.00005
67. Vargas G, Cypriano J, Correa T, Leão P, Bazylinski DA, Abreu F. Applications of magnetotactic bacteria, magnetosomes and magnetosome crystals in biotechnology and nanotechnology: mini-review. Molecules. 2018;23(10):2438. doi:10.3390/molecules23102438
68. Awal RP, Müller FD, Pfeiffer D, et al. Experimental analysis of diverse actin-like proteins from various magnetotactic bacteria by functional expression in Magnetospirillum gryphiswaldense. mBio. 2023;14(5). doi:10.1128/mbio.01649-23
69. Curcio A, Van de Walle A, Serrano A, et al. Transformation cycle of magnetosomes in human stem cells: from degradation to biosynthesis of magnetic nanoparticles anew. ACS Nano. 2020;14(2):1406–1417. doi:10.1021/acsnano.9b08061
70. Berson AE, Hudson DV, Waleh NS. Cloning of a sequence of Aquaspirillum magnetotacticum that complements the aroD gene of Escherichia coli. Mol Microbiol. 1991;5(9):2261–2264. doi:10.1111/j.1365-2958.1991.tb02156.x
71. Barber-Zucker S, Zarivach R. A look into the biochemistry of magnetosome biosynthesis in magnetotactic bacteria. ACS Chem Biol. 2017;12(1):13–22. doi:10.1021/acschembio.6b01000
72. Uzun M, Koziaeva V, Dziuba M, Leão P, Krutkina M, Grouzdev D. Detection of interphylum transfers of the magnetosome gene cluster in magnetotactic bacteria. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.945734
73. Riese CN, Wittchen M, Jérôme V, et al. The transcriptomic landscape of Magnetospirillum gryphiswaldense during magnetosome biomineralization. BMC Genomics. 2022;23(1):699. doi:10.1186/s12864-022-08913-x
74. Basit A, Wang J, Guo F, Niu W, Jiang W. Improved methods for mass production of magnetosomes and applications: a review. Microb Cell Fact. 2020;19(1). doi:10.1186/s12934-020-01455-5
75. Matthieu A, Alejandro C, Juan W, et al. Magnetotactic bacteria accumulate a large pool of iron distinct from their magnetite crystals. Appl Environ Microbiol. 2020;86(22):e01278–20. doi:10.1128/AEM.01278-20
76. Leão P, Le Nagard L, Yuan H, et al. Magnetosome magnetite biomineralization in a flagellated protist: evidence for an early evolutionary origin for magnetoreception in eukaryotes. Environ Microbiol. 2020;22(4):1495–1506. doi:10.1111/1462-2920.14711
77. Zhao D, Yang J, Zhang G, et al. Potential and whole-genome sequence-based mechanism of elongated-prismatic magnetite magnetosome formation in Acidithiobacillus ferrooxidans BYM. World J Microbiol Biotechnol. 2022;38(7):121. doi:10.1007/s11274-022-03308-2
78. Taoka A, Umeyama C, Fukumori Y. Identification of iron transporters expressed in the magnetotactic bacterium Magnetospirillum magnetotacticum. Curr Microbiol. 2009;58(2):177–181. doi:10.1007/s00284-008-9305-7
79. Taveira I, Bazylinski DA, Abreu F. Release the iron: does the infection of magnetotactic bacteria by phages play a role in making iron available in aquatic environments? J Oceanol Limnol. 2021;39(6):2063–2069. doi:10.1007/s00343-021-1072-3
80. Sabrina S, Williams TJ, Gary X, et al. Complete genome sequence of the chemolithoautotrophic marine magnetotactic coccus strain MC-1. Appl Environ Microbiol. 2009;75(14):4835–4852. doi:10.1128/AEM.02874-08
81. Uebe R, Junge K, Henn V, et al. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome membrane assembly. Mol Microbiol. 2011;82(4):818–835. doi:10.1111/j.1365-2958.2011.07863.x
82. Zeytuni N, Uebe R, Maes M, et al. Cation diffusion facilitators transport initiation and regulation is mediated by cation induced conformational changes of the cytoplasmic domain. PLoS One. 2014;9(3):e92141. doi:10.1371/journal.pone.0092141
83. Monteil CL, Perrière G, Menguy N, et al. Genomic study of a novel magnetotactic Alphaproteobacteria uncovers the multiple ancestry of magnetotaxis. Environ Microbiol. 2018;20(12):4415–4430. doi:10.1111/1462-2920.14364
84. Monteil CL, Grouzdev DS, Perrière G, et al. Repeated horizontal gene transfers triggered parallel evolution of magnetotaxis in two evolutionary divergent lineages of magnetotactic bacteria. ISME J. 2020;14(7):1783–1794. doi:10.1038/s41396-020-0647-x
85. Peigneux A, Valverde-Tercedor C, López-Moreno R, Pérez-González T, Fernández-Vivas MA, Jiménez-López C. Learning from magnetotactic bacteria: a review on the synthesis of biomimetic nanoparticles mediated by magnetosome-associated proteins. J Struct Biol. 2016;196(2):75–84. doi:10.1016/j.jsb.2016.06.026
86. Fitak RR. The magneto-microbiome: a dataset of the metagenomic distribution of magnetotactic bacteria. Data Brief. 2024;53:110073. doi:10.1016/j.dib.2024.110073
87. Kolinko I. Homologous and Heterologous Expression of Magnetosome Operons from Magnetospirillum Gryphiswaldense. Ludwig-Maximilians-Universität München; 2014.
88. Lohße A, Ullrich S, Katzmann E, et al. Functional Analysis of the Magnetosome Island in Magnetospirillum gryphiswaldense: the mamAB Operon Is Sufficient for Magnetite Biomineralization. PLoS One. 2011;6(10):e25561. doi:10.1371/journal.pone.0025561
89. Kolinko I, Lohße A, Borg S, et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat Nanotechnol. 2014;9(3):193–197. doi:10.1038/nnano.2014.13
90. Schüler D. Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol Rev. 2008;32(4):654–672. doi:10.1111/j.1574-6976.2008.00116.x
91. IGEM Kyoto. Magnetosome formation. IGEM. Published 2014. Available from: https://2014.igem.org/Team:Kyoto/Project/Magnetosome_Formation.
92. Li Y. Redox control of magnetosome biomineralization. J Oceanol Limnol. 2021;39(6):2070–2081. doi:10.1007/s00343-021-0422-5
93. Amor M, Faivre D, Corvisier J, et al. Defining local chemical conditions in magnetosomes of magnetotactic bacteria. J Phys Chem B. 2022;126(14):2677–2687. doi:10.1021/acs.jpcb.2c00752
94. Jajan LHG, Hosseini SN, Ghorbani M, Mousavi SF, Ghareyazie B, Abolhassani M. Effects of environmental conditions on high-yield magnetosome production by magnetospirillum gryphiswaldense MSR-1. Iran Biomed J. 2019;23(3):209–219. doi:10.29252/.23.3.209
95. Moisescu C, Bonneville S, Staniland S, Ardelean I, Benning LG. Iron uptake kinetics and magnetosome formation by Magnetospirillum gryphiswaldense as a function of pH, temperature and dissolved iron availability. Geomicrobiol J. 2011;28(7):590–600. doi:10.1080/01490451.2011.594146
96. Pei Z, Chang L, Bai F, Harrison RJ. Micromagnetic calculation of the magnetite magnetosomal morphology control of magnetism in magnetotactic bacteria. J R Soc Interface. 2023;20(206). doi:10.1098/rsif.2023.0297
97. Fernández-Castané A, Li H, Ebeler M, Franzreb M, Overton TW, Thomas ORT. A scalable biomanufacturing platform for bacterial magnetosomes. Food Bioprod Process. 2024;144:110–122. doi:10.1016/j.fbp.2024.01.005
98. Chen H, Shi H, Chen C, et al. Effects of static magnetic field on the sulfate metabolic pathway involved in Magnetospirillum magneticum AMB-1 cell growth and magnetosome formation. J Appl Microbiol. 2023;134(12). doi:10.1093/jambio/lxad302
99. Ryzhov V, Deriglazov V, Grouzdev D, et al. Biogenic nanomagnetic carriers derived from magnetotactic bacteria: magnetic parameters of magnetosomes inside Magnetospirillum spp. Appl Sci. 2023;13(4):2431. doi:10.3390/app13042431
100. Tay A, Pfeiffer D, Rowe K, et al. High-throughput microfluidic sorting of live magnetotactic bacteria. Appl Environ Microbiol. 2018;84(17). doi:10.1128/AEM.01308-18
101. Sorty AM, Shaikh NR. Novel co-enrichment method for isolation of magnetotactic bacteria. J Basic Microbiol. 2015;55(4):520–526. doi:10.1002/jobm.201400457
102. Yan L, Xing W. Chapter 12 - methods to study magnetotactic bacteria and magnetosomes. In: Gurtler V, Trevors JT editors, Methods in Microbiology. Vol. 45. Academic Press; 2018:357–386. doi:10.1016/bs.mim.2018.05.003
103. Lai W, Li D, Wang Q, Ma Y, Tian J, Fang Q. Bacterial magnetosomes release iron ions and induce regulation of iron homeostasis in endothelial cells. Nanomaterials. 2022;12(22):3995. doi:10.3390/nano12223995
104. Masó-Martínez M, Fryer B, Aubert D, et al. Evaluation of cell disruption technologies on magnetosome chain length and aggregation behaviour from Magnetospirillum gryphiswaldense MSR-1. Front Bioeng Biotechnol. 2023:11. doi:10.3389/fbioe.2023.1172457
105. Correa T, Godoy MG, Bazylinski DA, Abreu F. Continuous production of biogenic magnetite nanoparticles by the marine bacterium Magnetovibrio blakemorei Strain MV-1T with a nitrous oxide injection strategy. Mar Drugs. 2022;20(11):724. doi:10.3390/md20110724
106. Dziuba MV, Müller FD, Pósfai M, Schüler D. Exploring the host range for genetic transfer of magnetic organelle biosynthesis. Nat Nanotechnol. 2024;19(1):115–123. doi:10.1038/s41565-023-01500-5
107. Smit BA, Van Zyl E, Joubert JJ, et al. Magnetotactic bacteria used to generate electricity based on Faraday’s law of electromagnetic induction. Lett Appl Microbiol. 2018;66(5):362–367. doi:10.1111/lam.12862
108. Rosenfeldt S, Mickoleit F, Jörke C, et al. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications. Acta Biomater. 2021;120:293–303. doi:10.1016/j.actbio.2020.07.042
109. Li J, Menguy N, Arrio MA, et al. Controlled cobalt doping in the spinel structure of magnetosome magnetite: new evidences from element- and site-specific X-ray magnetic circular dichroism analyses. J R Soc Interface. 2016;13(121):20160355. doi:10.1098/rsif.2016.0355
110. Amor M, Mosselmans JFW, Scoppola E, Li C, Faivre D, Chevrier DM. Crystal–chemical and biological controls of elemental incorporation into magnetite nanocrystals. ACS Nano. 2023;17(2):927–939. doi:10.1021/acsnano.2c05469
111. Rosenfeldt S, Riese CN, Mickoleit F, Schüler D, Schenk AS, Atomi H. Probing the nanostructure and arrangement of bacterial magnetosomes by small-angle X-ray scattering. Appl Environ Microbiol. 2019. 85. doi:10.1128/AEM
112. Uebe R, Schüler D. Magnetosome biogenesis in magnetotactic bacteria. Nat Rev Microbiol. 2016;14(10):621–637. doi:10.1038/nrmicro.2016.99
113. Li J, Menguy N, Roberts AP, et al. Bullet‐shaped magnetite biomineralization within a magnetotactic Deltaproteobacterium: implications for magnetofossil identification. J Geophys Res Biogeosci. 2020;125(7). doi:10.1029/2020JG005680
114. Amor M, Mathon FP, Monteil CL, Busigny V, Lefevre CT. Iron‐biomineralizing organelle in magnetotactic bacteria: function, synthesis and preservation in ancient rock samples. Environ Microbiol. 2020;22(9):3611–3632. doi:10.1111/1462-2920.15098
115. Jefremovas EM, Gandarias L, Marcano L, et al. Modifying the magnetic response of magnetotactic bacteria: incorporation of Gd and Tb ions into the magnetosome structure. Nanoscale Adv. 2022;4(12):2649–2659. doi:10.1039/D2NA00094F
116. Gareev KG, Grouzdev DS, V KP, et al. Magnetic properties of bacterial magnetosomes produced by magnetospirillum caucaseum so-1. Microorganisms. 2021;9(9):1854. doi:10.3390/microorganisms9091854
117. Tomoe R, Fujimoto K, Tanaka T, Arakaki A, Kisailus D, Yoshino T. Lipid membrane modulated control of magnetic nanoparticles within bacterial systems. J Biosci Bioeng. 2023;136(3):253–260. doi:10.1016/j.jbiosc.2023.06.007
118. Comanescu C. Recent advances in surface functionalization of magnetic nanoparticles. Coatings. 2023;13(10):1772. doi:10.3390/coatings13101772
119. Qi J, Wang Z, Wen X, Tan W, Yuan Y, Yue T. Nanosilver embedded in a magnetosome nanoflower to enhance antibacterial activity for wound dressing applications. ACS Appl Mater Interfaces. 2023;15(42):48882–48891. doi:10.1021/acsami.3c08483
120. Wu S, Ma F, He J, et al. Fusion expression of nanobodies specific for the insecticide fipronil on magnetosomes in Magnetospirillum gryphiswaldense MSR-1. J Nanobiotechnology. 2021;19(1):27. doi:10.1186/s12951-021-00773-z
121. Mittmann E, Mickoleit F, Maier DS, et al. A magnetosome-based platform for flow biocatalysis. ACS Appl Mater Interfaces. 2022;14(19):22138–22150. doi:10.1021/acsami.2c03337
122. Ren E, Lei Z, Wang J, Zhang Y, Liu G. Magnetosome modification: from bio‐nano engineering toward nanomedicine. Adv Ther. 2018;1(6). doi:10.1002/adtp.201800080
123. Sun J, Duan J, Dai S, et al. Preparation and anti‐tumor efficiency evaluation of doxorubicin‐loaded bacterial magnetosomes: magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Bioeng. 2008;101(6):1313–1320. doi:10.1002/bit.22011
124. Awal RP, Lefevre CT, Schüler D. Functional expression of foreign magnetosome genes in the alphaproteobacterium Magnetospirillum gryphiswaldense. mBio. 2023. doi:10.1128/mbio.03282-22
125. Yoshino T, Tayama S, Maeda Y, et al. Magnetosome membrane engineering to improve G protein-coupled receptor activities in the magnetosome display system. Metab Eng. 2021;67:125–132. doi:10.1016/j.ymben.2021.06.008
126. Ginet N, Pardoux R, Adryanczyk G, Garcia D, Brutesco C, Pignol D. Single-step production of a recyclable nanobiocatalyst for organophosphate pesticides biodegradation using functionalized bacterial magnetosomes. PLoS One. 2011;6(6):e21442. doi:10.1371/journal.pone.0021442
127. Kotakadi SM, Borelli DPR, Nannepaga JS. Therapeutic applications of magnetotactic bacteria and magnetosomes: a review emphasizing on the cancer treatment. Front Bioeng Biotechnol. 2022;10. doi:10.3389/fbioe.2022.789016
128. Umesh SS, Bera S, Moitra P, Bhattacharya S. A self-healable and injectable hydrogel for pH-responsive doxorubicin drug delivery in vitro and in vivo for colon cancer treatment. Mater Today Chem. 2023;30:101554. doi:10.1016/j.mtchem.2023.101554
129. Zhang H, Chen W, Wang J, et al. A novel ROS-activable self-immolative prodrug for tumor-specific amplification of oxidative stress and enhancing chemotherapy of mitoxantrone. Biomaterials. 2023;293:121954. doi:10.1016/j.biomaterials.2022.121954
130. Pramanik S, Mohanto S, Manne R, et al. Nanoparticle-based drug delivery system: the magic bullet for the treatment of chronic pulmonary diseases. Mol Pharm. 2021;18(10):3671–3718. doi:10.1021/acs.molpharmaceut.1c00491
131. Guo JW, Wang CF, Lai JY, Lu CH, Chen JK. Poly(N-isopropylacrylamide)-gelatin hydrogel membranes with thermo-tunable pores for water flux gating and protein separation. J Memb Sci. 2021;618:118732. doi:10.1016/j.memsci.2020.118732
132. Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization. toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112(11):5818–5878. doi:10.1021/cr300068p
133. Mallick S, Abouomar R, Rivas D, et al. Doxorubicin-loaded microrobots for targeted drug delivery and anticancer therapy. Adv Healthc Mater. 2023;12(28):2300939. doi:10.1002/adhm.202300939
134. Alphandéry E. Natural metallic nanoparticles for application in nano-oncology. Int J Mol Sci. 2020;21(12):1–12. doi:10.3390/ijms21124412
135. Alphandéry E. Applications of magnetotactic bacteria and magnetosome for cancer treatment: a review emphasizing on practical and mechanistic aspects. Drug Discov Today. 2020;25(8):1444–1452. doi:10.1016/j.drudis.2020.06.010
136. Raguraman V, Jayasri MA, Suthindhiran K. Magnetosome mediated oral Insulin delivery and its possible use in diabetes management. J Mater Sci Mater Med. 2020;31(8):75. doi:10.1007/s10856-020-06417-2
137. Geng Y, Wang J, Wang X, et al. Growth-inhibitory effects of anthracycline-loaded bacterial magnetosomes against hepatic cancer in vitro and in vivo. Nanomedicine. 2019;14(13):1663–1680. doi:10.2217/nnm-2018-0296
138. Feng F, Li Q, Sun X, Yao L, Wang X. Tumor microenvironment-responsive magnetotactic bacteria-based multi-drug delivery platform for MRI-visualized tumor photothermal chemodynamic therapy. Biology. 2024;13(9):658. doi:10.3390/biology13090658
139. Radloff K, Gutbier B, Dunne CM, et al. Cationic LNP-formulated mRNA expressing Tie2-agonist in the lung endothelium prevents pulmonary vascular leakage. Mol Ther Nucleic Acids. 2023:34. doi:10.1016/j.omtn.2023.102068
140. Rossi M, Steklov M, Huberty F, et al. Efficient shRNA-based knockdown of multiple target genes for cell therapy using a chimeric miRNA cluster platform. Mol Ther Nucleic Acids. 2023:34. doi:10.1016/j.omtn.2023.102038
141. Kong H, Zhuo C, Yi K, et al. Hepatocyte-confined CRISPR/Cas9-based nanocleaver precisely eliminates viral DNA for efficient and safe treatment of hepatitis B virus infection. Nano Today. 2023;53:102040. doi:10.1016/j.nantod.2023.102040
142. Sun J, Li Y, Liang XJ, Wang PC. Bacterial magnetosome: a novel biogenetic magnetic targeted drug carrier with potential multifunctions. J Nanomater. 2011;2011:1–13. doi:10.1155/2011/469031
143. Liang J, Lv X, Lu C, et al. Prognostic factors of patients with Gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. 2020;20(1):35. doi:10.1186/s12885-019-6511-6
144. Pálffy R, Gardlík R, Hodosy J, et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther. 2006;13(2):101–105. doi:10.1038/sj.gt.3302635
145. Wang X, gui WJ, yuan GY, et al. An enhanced anti-tumor effect of apoptin-cecropin B on human hepatoma cells by using bacterial magnetic particle gene delivery system. Biochem Biophys Res Commun. 2018;496(2):719–725. doi:10.1016/j.bbrc.2018.01.108
146. Fu C, Mao X, Jin X, et al. Magnetotactic bacteria-derived Mms6 Gene Helps M2 macrophages to form magnetic bio-nanoparticles to prevent ferroptosis and promote locomotor functional recovery after spinal cord injury in mice. Adv Funct Mater. 2023;2305325. doi:10.1002/adfm.202305325
147. Lyu C, Lu G, Bao W, et al. Engineering magnetosomes with chimeric membrane and hyaluronidase for efficient delivery of HIF-1 siRNA into deep hypoxic tumors. Chem Eng J. 2020;398:125453. doi:10.1016/j.cej.2020.125453
148. Dai Q, Long R, Wang S, et al. Bacterial magnetosomes as an efficient gene delivery platform for cancer theranostics. Microb Cell Fact. 2017;16(1):216. doi:10.1186/s12934-017-0830-6
149. Chang D, Lim M, Goos JACM, et al. Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. 2018:9. doi:10.3389/fphar.2018.00831
150. Périgo EA, Hemery G, Sandre O, et al. Fundamentals and advances in magnetic hyperthermia. Appl Phys Rev. 2015;2(4):041302. doi:10.1063/1.4935688
151. Zargar FA, Bhat HA, MohdA Z, Malik SA. Magnetic nanoparticles in cancer thermotherapy: a mathematical approach to optimal treatment design. MatSci Express. 2024;01(02):116–124. doi:10.69626/mse.2024.0116
152. Molcan M, Skumiel A, Timko M, Safarik I, Zolochevska K, Kopcansky P. Tuning of magnetic hyperthermia response in the systems containing magnetosomes. Molecules. 2022;27(17):5605. doi:10.3390/molecules27175605
153. Hao X, Jiang B, Wu J, et al. Nanomaterials for bone metastasis. J Control Release. 2024;373:640–651. doi:10.1016/j.jconrel.2024.07.067
154. Nie Y, Li D, Peng Y, et al. Metal organic framework coated MnO2 nanosheets delivering doxorubicin and self-activated DNAzyme for chemo-gene combinatorial treatment of cancer. Int J Pharm. 2020;585:119513. doi:10.1016/j.ijpharm.2020.119513
155. Zeng M, Guo D, Fernández-Varo G, et al. The integration of nanomedicine with traditional Chinese medicine: drug delivery of natural products and other opportunities. Mol Pharm. 2023;20(2):886–904. doi:10.1021/acs.molpharmaceut.2c00882
156. Jefremovas EM, Gandarias L, Rodrigo I, et al. Nanoflowers versus magnetosomes: comparison between two promising candidates for magnetic hyperthermia therapy. IEEE Access. 2021;9:99552–99561. doi:10.1109/ACCESS.2021.3096740
157. Chen X, Lai L, Li X, et al. Magnetotactic bacteria AMB-1 with active deep tumor penetrability for magnetic hyperthermia of hypoxic tumors. Biomater Sci. 2022;10(22):6510–6516. doi:10.1039/D2BM01029A
158. Kuzajewska D, Wszołek A, Żwierełło W, Kirczuk L, Maruszewska A. Magnetotactic bacteria and magnetosomes as smart drug delivery systems: a new weapon on the battlefield with cancer? Biology. 2020;9(5):102. doi:10.3390/biology9050102
159. Nguyen TN, Chebbi I, Le Fèvre R, Guyot F, Alphandéry E. Non-pyrogenic highly pure magnetosomes for efficient hyperthermia treatment of prostate cancer. Appl Microbiol Biotechnol. 2023;107(4):1159–1176. doi:10.1007/s00253-022-12247-9
160. Yousaf T, Dervenoulas G, Politis M. Chapter two - advances in MRI methodology. In Politis M editor, International Review of Neurobiology. Vol. 141. Academic Press; 2018:31–76. doi:10.1016/bs.irn.2018.08.008
161. Hussain S, Mubeen I, Ullah N, et al. Modern diagnostic imaging technique applications and risk factors in the medical field: a review. Biomed Res Int. 2022;2022:1–19. doi:10.1155/2022/5164970
162. Sim AJ, Kaza E, Singer L, Rosenberg SA. A review of the role of MRI in diagnosis and treatment of early stage lung cancer. Clin Transl Radiat Oncol. 2020;24:16–22. doi:10.1016/j.ctro.2020.06.002
163. Li L, Wang S, Zhou W. Balance cell apoptosis and pyroptosis of caspase-3-activating chemotherapy for better antitumor therapy. Cancers. 2022;15(1):26. doi:10.3390/cancers15010026
164. Wang Q, Wang X, Zhang W, et al. Physiological characteristics of Magnetospirillum gryphiswaldense MSR-1 that control cell growth under high-iron and low-oxygen conditions. Sci Rep. 2017;7(1):2800. doi:10.1038/s41598-017-03012-4
165. Wang Y, Xu Y, Song J, et al. Tumor cell-targeting and tumor microenvironment–responsive nanoplatforms for the multimodal imaging-guided photodynamic/photothermal/chemodynamic treatment of cervical cancer. Int J Nanomed. 2024;19:5837–5858. doi:10.2147/IJN.S466042
166. Li Q, Chen H, Feng X, et al. Nanoparticle‐regulated semiartificial magnetotactic bacteria with tunable magnetic moment and magnetic sensitivity. Small. 2019;15(15). doi:10.1002/smll.201900427
167. Benoit MR, Mayer D, Barak Y, et al. Visualizing implanted tumors in mice with magnetic resonance imaging using magnetotactic bacteria. Clin Cancer Res. 2009;15(16):5170–5177. doi:10.1158/1078-0432.CCR-08-3206
168. Han X, Zhao C, Wang S, Pan Z, Jiang Z, Tang X. Multifunctional TiO2/C nanosheets derived from 3D metal–organic frameworks for mild-temperature-photothermal-sonodynamic-chemodynamic therapy under photoacoustic image guidance. J Colloid Interface Sci. 2022;621:360–373. doi:10.1016/j.jcis.2022.04.077
169. Ye P, Li F, Zou J, et al. In situ generation of gold nanoparticles on bacteria-derived magnetosomes for imaging-guided starving/chemodynamic/photothermal synergistic therapy against cancer. Adv Funct Mater. 2022;32(17):2110063. doi:10.1002/adfm.202110063
170. Gandarias L, Jefremovas EM, Gandia D, et al. Incorporation of Tb and Gd improves the diagnostic functionality of magnetotactic bacteria. Mater Today Bio. 2023;20:100680. doi:10.1016/j.mtbio.2023.100680
171. Li F, Nie W, Zhang F, et al. Engineering magnetosomes for high-performance cancer vaccination. ACS Cent Sci. 2019;5(5):796–807. doi:10.1021/acscentsci.9b00060
172. Nan X, Lai W, Li D, Tian J, Hu Z, Fang Q. Biocompatibility of bacterial magnetosomes as mri contrast agent: a long-term in vivo follow-up study. Nanomaterials. 2021;11(5):1235. doi:10.3390/nano11051235
173. Nuschke A, Sobey-Skelton C, Dawod B, et al. Use of magnetotactic bacteria as an MRI contrast agent for in vivo tracking of adoptively transferred immune cells. Mol Imaging Biol. 2023;25(5):844–856. doi:10.1007/s11307-023-01849-y
174. Makela AV, Schott MA, Madsen CS, Greeson EM, Contag CH. Magnetic particle imaging of magnetotactic bacteria as living contrast agents is improved by altering magnetosome arrangement. Nano Lett. 2022;22(12):4630–4639. doi:10.1021/acs.nanolett.1c05042
175. Panagiotopoulos N, Duschka RL, Ahlborg M, et al. Magnetic particle imaging: current developments and future directions. Int J Nanomed. 2015;10:3097–3114. doi:10.2147/IJN.S70488
176. Tay ZW, Chandrasekharan P, Chiu-Lam A, et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano. 2018;12(4):3699–3713. doi:10.1021/acsnano.8b00893
177. Gheorghiu E. Detection of pathogenic bacteria by magneto-immunoassays: a review. J Biomed Res. 2021;35(4):277–283. doi:10.7555/JBR.34.20200123
178. Huang H, Liu L, Wang J, et al. Aggregation caused quenching to aggregation induced emission transformation: a precise tuning based on BN-doped polycyclic aromatic hydrocarbons toward subcellular organelle specific imaging. Chem Sci. 2022;13(11):3129–3139. doi:10.1039/D2SC00380E
179. Chen J, Li X, Liu H, et al. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabetic Med. 2023;40(7):e15031. doi:10.1111/dme.15031
180. Fdez-Gubieda ML, Alonso J, García-Prieto A, García-Arribas A, Fernández Barquín L, Muela A. Magnetotactic bacteria for cancer therapy. J Appl Phys. 2020;128(7):070902. doi:10.1063/5.0018036
181. Chen C, Wang S, Li L, et al. Bacterial magnetic nanoparticles for photothermal therapy of cancer under the guidance of MRI. Biomaterials. 2016;104:352–360. doi:10.1016/j.biomaterials.2016.07.030
182. Chen S, Huang B, Pei W, et al. Magnetically targeted nanoparticles for imaging-guided photothermal therapy of cancer. RSC Adv. 2019;9(65):38154–38164. doi:10.1039/C9RA08281F
183. Alphandéry E. Applications of magnetosomes synthesized by magnetotactic bacteria in medicine. Front Bioeng Biotechnol. 2014;2(MAR). doi:10.3389/fbioe.2014.00005
184. Chen X, Cao Q, Liang Z, Huang L, Wang J, Hu Y. Hollow magnetic nanocarrier-based microrobot swarms for NIR-responsive targeted drug delivery and synergistic therapy. ACS Appl Mater Interfaces. 2024;16(44):60874–60883. doi:10.1021/acsami.4c14062
185. Huang G, Zhu G, Lin R, et al. Magnetotactic bacteria AMB-1 with active deep tumor penetrability for NIR-II photothermal tumor therapy. ACS Omega. 2024;9(21):23060–23068. doi:10.1021/acsomega.4c02914
186. Xu J, Hu J, Liu L, et al. Surface expression of protein A on magnetosomes and capture of pathogenic bacteria by magnetosome/antibody complexes. Front Microbiol. 2014:5. doi:10.3389/fmicb.2014.00136
187. Sannigrahi S, Arumugasamy SK, Mathiyarasu J, Suthindhiran K. Development of magnetosomes-based biosensor for the detection of Listeria monocytogenes from food sample. IET Nanobiotechnol. 2020;14(9):839–850. doi:10.1049/iet-nbt.2020.0091
188. Roda A, Cevenini L, Borg S, Michelini E, Calabretta MM, Schüler D. Bioengineered bioluminescent magnetotactic bacteria as a powerful tool for chip-based whole-cell biosensors. Lab Chip. 2013;13(24):4881. doi:10.1039/c3lc50868d
189. Dieudonné A, Prévéral S, Pignol D. A sensitive magnetic arsenite-specific biosensor hosted in magnetotactic bacteria. Appl Environ Microbiol. 2020;86(14). doi:10.1128/AEM.00803-20
190. He J, Hou Y, Wu W, Li Y, Tang F. Development of a broad-spectrum one-step immunoassay for detection of glucocorticoids in milk using magnetosome-based immunomagnetic beads. Food Chem. 2024;441:138377. doi:10.1016/j.foodchem.2024.138377
191. Dobre AA, Ilie-Sandoiu AM, Morega AM, Gheorghiu E. Magnetic field control in an analytic platform for assessment of pathogenic bacteria. Revue Roumaine Des Sciences Techniques-serie Electrotechnique Et Energetique. 2023;68(3):317–322. doi:10.59277/RRST-EE.2023.3.12
192. Ren G, Zhou X, Long R, et al. Biomedical applications of magnetosomes: state of the art and perspectives. Bioact Mater. 2023;28:27–49. doi:10.1016/j.bioactmat.2023.04.025
193. Gajera G, Thakkar N, Godse C, DeSouza A, Mehta D, Kothari V. Sub-lethal concentration of a colloidal nanosilver formulation (Silversol®) triggers dysregulation of iron homeostasis and nitrogen metabolism in multidrug resistant Pseudomonas aeruginosa. BMC Microbiol. 2023;23(1):303. doi:10.1186/s12866-023-03062-x
194. Sarkar S, Moitra P, Bhattacharya S. Structure–activity relationship of drug conjugated polymeric materials against uropathogenic bacteria colonization under in vitro and in vivo settings. J Mater Chem B. 2024;12:187–201. doi:10.1039/D3TB01841E
195. Zhao J, Xu T, Sun J, et al. Multifunctional nanozyme-reinforced copper-coordination polymer nanoparticles for drug-resistance bacteria extinction and diabetic wound healing. Biomater Res. 2023;27(1):88. doi:10.1186/s40824-023-00429-z
196. Xu J, Ma S, Zhang W, et al. In vitro magnetosome remineralization for silver-magnetite hybrid magnetosome biosynthesis and used for healing of the infected wound. J Nanobiotechnology. 2022;20(1). doi:10.1186/s12951-022-01532-4
197. Revathy T, Jacob JJ, Jayasri MA, Suthindhiran K. Microbial biofilm prevention on wound dressing by nanobiocoating using magnetosomes-coupled lemon grass extract. IET Nanobiotechnol. 2017;11(6):738–745. doi:10.1049/iet-nbt.2016.0236
198. Zhao J, Zhang Q, Cheng W, et al. Heart–gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc Res. 2023;119(6):1390–1402. doi:10.1093/cvr/cvad023
199. Hu X, Zhou L, Wu X, Peng Y. Review on near-field detection technology in the biomedical field. Advanced Photonics Nexus. 2023;2(4):044002. doi:10.1117/1.APN.2.4.044002
200. Su YN, Wang MJ, Yang JP, et al. Effects of Yulin Tong Bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front Endocrinol. 2023:14. doi:10.3389/fendo.2023.1122709
201. Chen L, He Y, Zhu J, et al. The roles and mechanism of m6A RNA methylation regulators in cancer immunity. Biomed Pharmacother. 2023;163:114839. doi:10.1016/j.biopha.2023.114839
202. Wang Z, Ma R, Chen B, et al. A transcription factor-based bacterial biosensor system and its application for on-site detection of explosives. Biosens Bioelectron. 2024;244:115805. doi:10.1016/j.bios.2023.115805
203. Zhu Y, Huang R, Wu Z, Song S, Cheng L, Zhu R. Deep learning-based predictive identification of neural stem cell differentiation. Nat Commun. 2021;12(1):2614. doi:10.1038/s41467-021-22758-0
204. Mickoleit F, Pfister F, Friedrich B, et al. Assessing cytotoxicity, endotoxicity, and blood compatibility of nanoscale iron oxide magnetosomes for biomedical applications. ACS Appl Nano Mater. 2024;7(1):1278–1288. doi:10.1021/acsanm.3c05248
205. Li X, Wang Y, Shi L, et al. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology. 2020;18(1):113. doi:10.1186/s12951-020-00670-x
206. Pawlik P, Blasiak B, Depciuch J, et al. Application of iron-based magnetic nanoparticles stabilized with triethanolammonium oleate for theranostics. J Mater Sci. 2022;57(7):4716–4737. doi:10.1007/s10853-021-06244-y
207. Cristina Bunea M, Vasile E, Galateanu B, Hudita A, Serban M, Zaharia C, Silk Fibroin films decorated with magnetic nanoparticles for wound healing applications. Materiale plastice 2017;54(1):83–87. doi:10.37358/MP.17.1.4791
208. Alberti TB, Coelho DS, de Prá M, Maraschin M, Veleirinho B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J Mater Sci. 2020;55(23):9712–9727. doi:10.1007/s10853-020-04502-z
209. Fan JX, Peng MY, Wang H, et al. Engineered bacterial bioreactor for tumor therapy via Fenton-like reaction with localized H2O2 generation. Adv Mater. 2019;31(16):1808278. doi:10.1002/adma.201808278
210. Long R, Liu Y, Dai Q, Wang S, Deng Q, Zhou X. A natural bacterium-produced membrane-bound nanocarrier for drug combination therapy. Materials. 2016;9(11):889. doi:10.3390/ma9110889
211. Li X, Guan F, Li X, Guo J, Yang G. Ganglioside-magnetosome complex formation enhances uptake of gangliosides by cells. Int J Nanomed. 2015;6919. doi:10.2147/IJN.S92228
212. Cheng L, Ke Y, Yu S, Jing J. Co-delivery of doxorubicin and recombinant plasmid pHSP70-Plk1-shRNA by bacterial magnetosomes for osteosarcoma therapy. Int J Nanomed. 2016;11:5277–5286. doi:10.2147/IJN.S115364
213. Wu L, Gao B, Zhang F, Sun X, Zhang Y, Li Z. A novel electrochemical immunosensor based on magnetosomes for detection of staphylococcal enterotoxin B in milk. Talanta. 2013;106:360–366. doi:10.1016/j.talanta.2012.12.053
214. Li A, Zhang H, Zhang X, et al. Rapid separation and immunoassay for low levels of Salmonella in foods using magnetosome–antibody complex and real‐time fluorescence quantitative PCR. J Sep Sci. 2010;33(21):3437–3443. doi:10.1002/jssc.201000441
215. Hermann CA, Hofmann C, Duerkop A, Baeumner AJ. Magnetosomes for bioassays by merging fluorescent liposomes and magnetic nanoparticles: encapsulation and bilayer insertion strategies. Anal Bioanal Chem. 2020;412(24):6295–6305. doi:10.1007/s00216-020-02503-0
216. Yang M, Zhan Y, Zhang S, Wang W, Yan L. Biological materials formed by Acidithiobacillus ferrooxidans and their potential applications. 3 Biotech. 2020;10(11):475. doi:10.1007/s13205-020-02463-3
217. Thakare NR, Singh R, Talukdar H, et al. Functionalization of biogenic and biomimetic magnetic nanosystems for biomedical applications. Func Magn Nanosyst Diagn Tools Devices. 2024:229–255. doi:10.1016/B978-0-443-19012-4.00020-5
218. Chen Y, Hou S. Application of magnetic nanoparticles in cell therapy. Stem Cell Res Ther. 2022;13(1):135. doi:10.1186/s13287-022-02808-0
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

