Back to Journals » International Journal of Nanomedicine » Volume 19
Topical Application of Dipyridamole and Roflumilast Combination Nanoparticles Loaded Nanoemulgel for the Treatment of Psoriasis in Rats
Authors Maded ZK, Lassoued MA, Taqa GA, Fawzi HA , Abdulqader AA, Jabir MS , Mahal RK, Sfar S
Received 11 September 2024
Accepted for publication 26 November 2024
Published 7 December 2024 Volume 2024:19 Pages 13113—13134
DOI https://doi.org/10.2147/IJN.S492180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Zeyad Khalaf Maded,1 Mohamed Ali Lassoued,1 Ghada Abd Alrhman Taqa,2 Hayder Adnan Fawzi,3 Alaa Abdulelah Abdulqader,4 Majid S Jabir,5 Raffah Khamis Mahal,6 Souad Sfar7
1Laboratory of Pharmaceutical, Chemical, and Pharmacological Drug Development LR12ES09, Faculty of Pharmacy, University of Monastir, Monastir, Tunisia; 2Department of Dental Basic Sciences, College of Dentistry, University of Mosul, Mosul, Iraq; 3Department of Pharmacy, Al Mustafa University College, Baghdad, Iraq; 4Department of Pharmaceutics, College of Pharmacy, University of Tikrit, Tikrit, Iraq; 5Department of Applied Science, University of Technology, Baghdad, Iraq; 6Department of Pharmaceutics, College of Pharmacy, The University of Mashreq, Baghdad, 10023, Iraq; 7Laboratory of Chemical, Galenic and Pharmacological Development of Medicines (LR12ES09), Faculty of Pharmacy of Monastir, University of Monastir, Monastir, Tunisia
Correspondence: Hayder Adnan Fawzi, Email [email protected]
Background: Phosphodiesterase-4 is an enzyme that regulates immune responses and contributes to the development of psoriasis. Dipyridamole and roflumilast function as phosphodiesterase-4 inhibitors, reducing pro-inflammatory cytokine expression. The aim was to evaluate the anti-psoriatic effect of the topical administration of dipyridamole and roflumilast nanoemulgel combination on imiquimod-induced psoriasiform skin inflammation in rats.
Methods: Dipyridamole and roflumilast were formulated into nanoemulgel to enhance skin penetration and retention. The production of nanoemulgels involves a two-part process. A nanoemulsion is created (the aqueous phase titration method was employed to create nanoemulsions), which is then incorporated into the gelling agent during the second phase. The new formula was then tested in rats. The rats were divided into seven groups; all animals were treated for 16 days. Induction was achieved by 120 mg of 5% imiquimod cream, which was applied daily for 8 days. After induction, groups received one of the following: 0.05% clobetasol ointment, 1% dipyridamole nanoemulgel (D-NEG), 0.3% roflumilast nanoemulgel (R-NEG), 1% dipyridamole and 0.3% roflumilast gel combination (DR-gel), and 1% dipyridamole and 0.3% roflumilast nanoemulgel combination (DR-NEG). At the end of the experiment, all animals were euthanized, and their blood and skin tissue samples were obtained. Inflammatory markers, immunohistochemistry, and histopathology were measured.
Results: The DR-NEG group showed significantly lower levels of IL17, IL23, and TNF-α, while TGF-β showed higher levels than the clobetasol group. The expression of CK16 was significantly lower compared to the clobetasol group. DR-NEG showed a significantly lower PASI and Baker score than the clobetasol group.
Conclusion: The new DR-NEG’s topical combination administration showed better anti-inflammatory, tissue healing, and anti-psoriatic activity than each drug alone or topical clobetasol administration; this could be attributed to the possible synergic effects of both drugs and the enhanced skin penetration offered by the nanoemulgel formulation.
Keywords: dipyridamole, roflumilast, nanoemulgel, psoriasis, anti-inflammatory
Graphical Abstract:

Introduction
Psoriasis is a dermatological condition caused by an overactive immune system. It is marked by thickened skin due to excessive growth of skin cells and the presence of inflammatory cells in the skin layers.1 Approximately 125 million people worldwide are affected by psoriasis,2 the global prevalence rate is around 0.09% to 11.43% of the population.3
Phosphodiesterase (PDE)-4 is an enzyme that regulates the immune system and contributes to the development of psoriasis. PDE-4 enzymatically breaks down cyclic adenosine monophosphate (cAMP) into adenosine monophosphate (AMP), generating pro-inflammatory mediators.4 PDE-4 inhibitors function by impeding the breakdown of cAMP, hence diminishing inflammation.4 Psoriasis is characterized by a decrease in the expression of keratins K1 and K10 and an increase in the production of hyperproliferation-associated keratins K6, K16, and K17.5 The immunopathogenesis of psoriasis entails the activation of many inflammatory mediators, such as tumor necrosis factor (TNF)-α, various interleukin (IL) (12, 17, 22, and 23), and interferon (IFN)-γ.6
Roflumilast (RFL) is a specific inhibitor of PDE-4,7 roflumilast 0.3% cream once daily. It has gained recent FDA approval for treating plaque psoriasis in adolescents and adults. It is indicated for use on all body surfaces, including intertriginous areas.8 RFL exhibits anti-inflammatory properties by modulating the inflammatory signaling cascade in immune cells through the elevation of cAMP levels;9 RFL is recognized for its involvement in multiple inflammatory pathways, leading to a reduction in levels of TNF-α, IL-1b, IL-2, and IL-13.10,11 Dipyridamole (DP) is a PDE inhibitor that enhances the quantity of intracellular cAMP and cyclic Guanosine monophosphate (cGMP). DP possesses antioxidant characteristics that can stabilize platelet and vascular membranes, preventing the oxidation of low-density lipoproteins.12 By preventing adenosine reabsorption, it raises adenosine levels, widening the coronary blood vessels through its interaction with the A2 receptors.13 DP exhibits an anti-fibrotic activity and inhibits the expression of collagen genes induced by TGF-β in human peritoneal mesothelial cells.14 Moreover, DP hinders the process of attracting, stimulating, and releasing pro-inflammatory substances by lymphocytes.15,16 DP inhibits inflammation, stimulates the healing of mucosal tissue, and perhaps prevents immediate damage and gradual scarring in the lungs, heart, liver, and kidneys.17
Topical therapy is the initial therapeutic choice for mild to severe cases of localized psoriasis.18 Nano-drug-based topical therapy has shown enhanced delivery capabilities for insoluble drugs in the treatment of psoriasis, resulting in increased drug bioavailability and effectiveness;19 enabling the transportation of drugs through epidermal barriers to increase absorption and decrease the required drug dosage;20–22 regulating the administration of drugs at certain doses over a controllable duration.,23–25 protecting drugs from degradation along with increased solubility;26–28 and by including cell-specific ligands, this modification enhances the targeting capabilities and minimizes any negative consequences.29,30
Nanoemulsion refers to a stable mixture of two phases (oil and water) that are normally unable to mix but are merged using surfactant molecules. This mixture’s droplets have diameters ranging from 5 to 200 nm.31,32 The utilization of nanoemulsion as a carrier for anti-psoriatic drugs has several advantages, including the absence of creaming, flocculation, sedimentation, or coalescence, which are typically observed in macroemulsions.33 Nanoemulsions exhibit excellent characteristics for topical distribution, including a lack of skin irritation, a high penetration ability, and a large drug-loading capacity.34,35 Due to their low viscosity, nanoemulsions have limited retention capacity in the skin, which restricts their application in the pharmaceutical industry.36 In order to address this constraint and deliver a suitable topical application, the viscosity can be increased by using gelling agents such as carbopol 940, xanthan gum, sodium alginate, and similar substances.37 This therapy hinders the absorption of drugs into the bloodstream and enhances the accumulation of drugs in the skin, hence enabling more efficient effectiveness. The improved nanoemulsion formulation was incorporated into several gel bases to enhance the localized skin accumulation of the medicine while minimizing systemic absorption.38 Due to the solubility issue that arises during drug release, it is not feasible to directly incorporate most hydrophobic drugs into the gel basis.39
A gel-based nanoemulsion known as nanoemulgel is a desirable drug delivery system for nanolipoidal distribution through the skin; this is because of its good features such as easy spreadability, thixotropic behavior, lack of greasiness, easy removal, and biocompatibility. The topical route of drugs delivery is a practical method that offers both local and systemic benefits when selecting the site of action.40,41 The gelling agent is a key constituent of the nanoemulgel, providing texture to the formulation. Carbopol, poloxamer, tragacanth, hydroxypropyl methylcellulose (HPMC), xanthan gum, and other similar substances are utilized as gelling agents in the formulation of nanoemulgels. Utilizing a gelling agent to produce a nanoemulgel serves the objective of transforming its physical state from liquid to semi-solid. This alteration offers numerous benefits for patient adherence.42
The nanoemulsion formulation was converted into a gel by incorporating three distinct concentrations of gel, specifically 0.5%, 1%, and 1.5%, to generate various formulations. Therefore, the uniqueness of this system resides in the fact that the constituents (oil, surfactant, and particularly cosurfactant) of the nanoemulgel functioned as agents that increased penetration.43 Nanolipoidal formulations can create a nanoemulsion-based gel that is effective for delivering drugs through topical routes.44
In spite of the advantages of topical therapy, many data deficiencies persist in the topical management of psoriasis.45 Primary data deficiencies encompass the absence of evidence concerning the ideal frequency of treatment administration and the necessity for novel, more convenient, and effective formulations. The widespread corticosteroid phobia, characterized by apprehension over topical corticosteroid usage, must be considered due to its significant prevalence among dermatological patients, frequently resulting in diminished adherence rates. Furthermore, it is essential to account for tachyphylaxis with prolonged usage and the presence of challenging-to-treat locations, including the scalp, face, intertriginous regions (such as the genitals), and palmoplantar surfaces.46 Recent advancements in the topical management of psoriasis must be formulated considering patient preferences. Recent advancements in topical therapies have produced once-daily cream compositions that enhance cosmetic acceptance, potentially leading to enhanced patient adherence.47
The current study offered two novel approaches to treat psoriasis. First, the combination therapy of dipyridamole and roflumilast is a unique combination that was not reported in the literature previously. The second one involves using nanoemulgel as a topical formulation since gel, cream, and ointment are the only available formulations in the market. By combining these two novel approaches, we hypothesized that dipyridamole and roflumilast nanoemulgel would be more effective in managing psoriasis than either agent alone, compared to topical steroids and dipyridamole and roflumilast in gel formulation. Thus, we designed a pre-clinical (animal) study to assess the efficacy of the newly formulated dipyridamole and roflumilast nanoemulgel. The primary objective of this study was to assess the anti-inflammatory and immunomodulatory properties of a combination of dipyridamole and roflumilast nanoemulgel in rat models with produced psoriasis; this was done by examining the effects of the combination on inflammatory markers, histological alterations, and immunohistochemistry.
Methods
Materials
All materials used were pharmaceutical grade purity, dipyridamole powder purchased from Hyperchem (China), roflumilast purchased from Lupin Lab (India), 95% ethanol (J.T. Baker, China), oleic acid (CDH, India), Tween 80 (Polyoxyethylene Sorbitan Monooleate) (HiMedia, India), xanthan gum (HiMedia Lab, India), phosphate buffer saline (pH = 7.4) (PBS) (Titan Bio. Tech, India), chloroform (Sigma, Germany), clobetasol ointment (GlaxoSmithKline, England), distill water (Samara Drug industry, Iraq), formalin (Merck chemicals, Germany), hematoxylin and eosin stain solutions (Thermo Shandon, USA), imiquimod cream 5% (Aldara, Meda Pharmaceuticals, Germany), mercuric oxide (China), methylparaben powder (Samara Drug industry, Iraq), paraffin wax (BDH, England), triethanolamine (TEA) (Hopkins and Williams Ltd, England), and xylol (BDH Chemical Ltd., England).
Rat TNF-α ELISA (SunLong, China), rat IL-17 ELISA (SunLong, China), rat IL-23 ELISA (SunLong, China), rat TGF-β ELISA (SunLong, China), and CK-16 polyclonal antibody at a dilution of 1:30 (Catalog No. E-AB-12323) ELISA from (Elabscience, USA).
Instruments
Electric balance (KERN & SOHN GmbH, Germany), high-speed centrifuges (15,000 rpm) (Hermle Z216 MK, Germany), hotplate with a magnetic stirrer (CB 162 heat-Stir, Stuart Copley Scientific, UK), particle size analyzer (Malvern Zetasizer, UK), vortex mixer (Labinco L46, Netherland), water bath with shaker (G.F.L, Karl Kolb, Germany), beakers 100 mL (China), conical flasks (Erlynmir, Germany), cylinder test tube 5 mL (Barco-company, China), deep freeze (Hyper hanil, Korea), ELISA reader from (Diagnostic automation, USA), Eppendorf tube 1.5 mL (Biozek medical, China), light microscopic (Olympus, Japan), micropipette (Huawei, China), microscope slide (Alasera co, China), microtome (Leica, Germany), ordinary slides (Sail, China), oven (Panasonic, China), plain tube (China), qualitative filter paper 7.5 cm (Coster corning, USA), refrigerator (Samsung, Japan), scissors (China), shaving mechanical device (Kemei, China), spatula (China), surgical animals tools (Petsurgical, Pakistan), syringe 3–5 mL (Medico, UAE), and urine cap (China).
Preparation of Dipyridamole and Roflumilast Nanoparticles Loaded Nanoemulgel
The preparation and characterization of the D-NEG, R-NEG, DR-gel, and DR-NEG were described previously by the authors,48 which can be summarized as follows:
The aqueous phase titration method was employed to create nanoemulsions. The nanoemulsion composition was selected based on the pseudo-ternary phase diagram. A solution was prepared by dissolving 50 mg of dipyridamole (1%) and 15 mg of roflumilast (0.3%) powder in the selected oil. The desired concentration of surfactant and cosurfactant mixture was then added. Water was incrementally added while continuously swirling until a transparent nanoemulsion was created. The optimal combination of Smix and Oil was chosen based on the phase diagram produced. The drugs were then added to the Oil & Smix mixture and subjected to particle size analysis. A nanoemulsion was created by combining oleic acid as the oil phase with Tween80 as the surfactant and Ethanol as the cosurfactant in various ratios (1:2), as specified in the Tables. Dipyridamole and roflumilast were precisely measured and dissolved in an appropriate amount of oil. Then, the specified amount of Smix, as indicated in the respective formula, was added to the drug mixture. Using a vortexer, the mixture was vigorously mixed for 5 minutes at 100 rpm. Finally, purified water was gradually added to obtain a clear oil-in-water nanoemulsion.48
The production of nanoemulgels involves a two-part process. A nanoemulsion is created and incorporated into the gelling agent during the second phase. Nanoemulsions are formed by reducing the interfacial tension between oil/water interfaces or applying external energy to a heterogeneous mixture. In this case, the nanoemulsion formulation was transformed into a gel by adding three different concentrations of gel: 0.5%, 1%, and 1.5%. Therefore, the uniqueness of this system resides in the fact that the constituents (oil, surfactant, and particularly cosurfactant) of the nanoemulgel functioned as agents that increased penetration. Nanolipoidal formulations in nanoemulsion-based gel, also known as nanoemulgel, have been identified as an effective method for delivering drugs by topical administration. The suggested formula was transformed into a nano emulgel by incorporating 0.5% w/w of xanthan gum as a gelling agent. Xanthan gum possesses mucoadhesive properties and additional characteristics that enhance its suitability for pharmaceutical formulation, such as improving the bioavailability of poorly soluble drugs. To determine the precise amount of xanthan gum needed to achieve a concentration of 0.5% w/w, a 5 mL sample of formula 1 (composed of 50% Smix of 1:2 Tween 80: Ethanol, 10% oleic acid, and 40% water; all in v/v %) was mixed with 25mg of xanthan gum. The mixture was stirred with a vortex mixer for a few minutes, left for 1 hour, and then refrigerated overnight.48
The dipyridamole drug (1% w/w) and roflumilast (0.3% w/w) were dissolved in a hot mixture consisting of propylene glycol (20% w/w) and glycerin (10% w/w) as moistening agents. The polyacrylic acid polymer (carbopol 940) gel was then prepared by dispersing the appropriate amount of polymer in a calculated amount (60–70 mL) of warm water. This was done with constant stirring for 30 minutes using a magnetic stirrer at a moderate speed. Next, incorporate the prior solution containing the drugs. TEA was used to modify the pH of the carbopol gel. Ultimately, methyl and propylparaben, preservatives, were gradually and consistently incorporated into the mixture while continually stirring until a gel was formed. The manufactured gels were placed in a wide-mouth glass jar and topped with a plastic lid that could be screwed on. Before sealing the jar, the mouth was covered with aluminum foil. The jar was then stored in a dark and cool location.48,49
The nanoemulgel formula had a particle size of 172.7 nm, 0.121% PDI, and a −28.31 mV zeta potential, as seen in Figure 1. The viscosity of nanoemulgel formulations was measured at various rotational speeds (10, 12, 20, 30, 50, 60, 100, and 200 rpm) and a fixed temperature of 25°C, as seen in Table 1.
 |
Table 1 Viscosity of Nanoemulgel (Millipascal/Second) |
 |
Figure 1 The particle size, PDI, and zeta potential of nanoemulgel. |
Furthermore, the effect of storage conditions for the stability test was that the chosen samples were stored in airtight amber bottles under various storage circumstances. An unstable system exhibits obvious creaming or phase separation. After storage at 4 ± 2 and 40 ± 2 °C for 0, 30, and 60 days, the samples’ droplet size, PDI, sol-gel transition temperature, and gelation time values were evaluated.50 Various formulations of nanoemulsion were physically examined before combining with the gel solution. Detecting distinct preparations involved assessing their odor, color change, uniformity, and centrifugation. The formulation samples were stored at 8°C, 25°C, and 40°C with relative humidity for 28 days, as mentioned previously.51
In the present study, the Franz cell diffusion apparatus was used in this experiment to study the ex-vivo permeation of the selected nanogel compared with pure roflumilast and dipyridamole gel.52 The cumulative amount of roflumilast and dipyridamole nanogel permeated through rat skin was demonstrated in Figure 2.
 |
Figure 2 A study of permeability of pure roflumilast, pure dipyridamole, and nanogel of roflumilast and dipyridamole. |
Animal Housing
White male Albino rats, 8–11 weeks of age, and average body weight 200–250 g. The animals were left for 12 days to acclimate to animal house environments of exact temperature (25±2°C) and 40–60% humidity. Water and standard food pellets (Elazig Food Company, Turkey) were provided ad libitum in the ventilated room. Before and during the experimental period, all rats were assessed for their health status, including food and water intake.
Before commencing the experiment, the animals were examined to detect any skin lesions. Only rats with visibly healthy skin and coat were selected for the investigation. Two days before the work began, an automated machine shaved the hair on the dorsal skin. Subsequently, hair-removing cream was utilized to eliminate almost all residual hair (2 x 1 cm2).
At the conclusion of the study, all the animals were euthanized using anesthesia with a combination of ketamine and xylazine. Specifically, the rats were anesthetized intraperitoneally with 80 mg/kg of ketamine (Ketamine 10%, Alfasan Nederland BV, Holland) and 10 mg/kg of xylazine (XYL-M2, VMD® Livestock Pharma, Belgium). Once the animals were fully anesthetized, they passed away by exsanguination through cardiac puncture. This method was chosen as it allows for effective tissue harvest and preservation,53–56 blood and tissue samples were collected for further analysis (because of the need for tissue samples, euthanasia of the animal was deemed necessary). The study was conducted at the animal house in the College of Veterinary Medicine, University of Mosul, between the 1st of June 2023 and the 1st of April 2024.
Ethical Considerations
The study was approved by the Scientific Research Ethical Committee (SREC) of the College of Pharmacy, Tikrit University, approval number (SREC20231022), data (22/10/2023), the study carried out in accordance with the American Veterinary Association Guidelines (AVMA).54
Special Considerations to Minimize the Suffering and Distress of Animals
The animals in the study received treatment for any external injuries involving their skin using appropriate skin creams, such as Fusidic acid (Fucidin, Leo), to prevent potential secondary infections. Additionally, a low dose of xylazine (3 mg/kg, IP) was administered when the animals exhibited signs of stress.57
Imiquimod (IMQ) Induced Psoriasis
All treatment groups received the same induction by daily topical applications of 120 mg of 5% IMQ cream for 8 consecutive days on shaved back areas of rats.58–60 The animal model treated with IMQ closely mimics the characteristics of plaque-type psoriasis in humans, including skin redness, thickness, scaling, changes in the outer layer of the skin (acanthosis, parakeratosis), and the formation of new blood vessels (neo-angiogenesis). Additionally, it exhibits an inflammatory response characterized by the presence of T cells, neutrophils, and dendritic cells.61,62
Study Design
The study included 56 albino rats divided into 7 groups (n = 8), as illustrated in Figure 3 (Groups D, E, F, and G are the investigated treatment groups):
- Negative control group: Vaseline (Vaseline® Petroleum Jelly, GlaxoSmithKline, UK) was applied daily for 16 days.
- Induction group: 120 mg of 5% imiquimod cream was applied daily to the shaved back area for 8 days;58–60 after induction, the rats received Vaseline daily for 8 days (16 days total duration).
- Clobetasol group (CLO): IMQ induction was received for the first 8 days, then clobetasol (CLO) 0.05% ointment (0.05% Dermovate ointment®, GlaxoSmithKline, UK) was applied 8 days once daily (at a dosage of 0.25 g/kg) on the shaved area after the induction period.63–66
- Dipyridamole nanoemulgel group (DP): IMQ induction was received for the first 8 days, and DP nanoemulgel 1% was applied 8 days once daily after induction to the shaved back area.
- Roflumilast group (RFL): IMQ induction was received for the first 8 days, and RFL cream 0.3% was applied once daily to the shaved back area for 8 days after induction.
- Dipyridamole with roflumilast gel (DP-RFL gel): IMQ induction was received for the first 8 days, and DP 1% gel with RFL 0.3% gel was applied once daily after induction to the shaved back area for 8 days.
- Dipyridamole with roflumilast nanoemulgel (DP-RFL nanoemulgel): IMQ induction was received for the first 8 days, and DP 1% with RFL 0.3% nanoemulgel was applied once daily after induction to the shaved area for 8 days.
 |
Figure 3 Flow chart of the study. |
Samples Collection
Blood samples were collected from the animals through heart puncture.67 After collecting blood in a tube, the sample was spun at a speed of 5000 revolutions per minute for 10 minutes to separate the serum. The serum was collected in 2mL Eppendorf tubes and kept at a temperature of −20°C until the analysis day. Following the animals’ sacrifice, a skin sample from the dorsal region was obtained. The tissues were then stored in a neutral buffer formalin solution (10%) and underwent histological inspection.
Analytic Procedure
The stored samples were thawed and analyzed using the sandwich Enzyme-Linked immunosorbent assay (ELISA) technique using the ELISA Reader. Regarding the ELISA kits, the following markers were determined: TNF-α (pg/mL), IL-17 (pg/mL), IL-23 (pg/mL), and TGF-β (pg/mL).
Preparation of Histopathological Samples
Skin tissues were preserved in neutral buffer formalin (10%) and subjected to histopathological examination. The small parts of the tissue were taken and washed with tap water (2 mm of the dorsal skin tissue). The specimen was dehydrated using different ethanol concentrations (70%, 80%, 90%, 95%, and 100%) for 2 hours each. Then, xylol was added to the specimen before dipping it in liquid paraffin at 55–60°C. After that, paraffin blocks were made by embedding the tissue in paraffin on a cold plate. Sections with 5 μm were made by using a microtome. With the aid of a water bath, the slice section was placed on the slide (containing a very thin layer of egg albumin).68 Hematoxylin and eosin (H & E) stain-involved staining procedures were carried out by deparaffinization, rehydration, immersing in hematoxylin, discoloration of access stain, dipping in eosin stain, and dehydration. Several drops of di-N-butyl phthalate in xylene (DPX) were placed on the section and covered with a coverslip.68
Psoriasis Area Severity Index (PASI) Score
A back skin irritation severity assessment system was developed utilizing the PASI score. The assessment of scaling and thickening was conducted individually using a rating scale ranging from 0 to 4, where 0 represents no presence, 1 indicates a modest presence, 2 represents a moderate presence, 3 indicates a marked presence, and 4 represents a very marked presence. Erythema levels were evaluated using a scoring table that included shades of red (0= no lesion; 1= faintly pink; 2= pink; 3= red; 4= strong red).69
The main author and two expert consultant dermatologists independently evaluated the redness, thickness, and scaling of the rats’ skin during the study’s induction and therapy stages. This assessment utilizes high-resolution photographs of the rats’ skin, captured and documented regularly through photography. The evaluations were conducted blindly, and the results were examined and compared to the original reading using intra-class correlation as a measure of reliability. Any reading with a reliability score of 0.80 or above was deemed acceptable for the whole experiment.
Baker’s Score for Histopathology
The Backer score is a semi-quantitative scoring method used to evaluate the histopathology of rats’ models. Pathological alterations are assessed on a scale of 0 to 10 using Baker’s scoring system.70,71 The scoring process was carried out blindly, without prior knowledge of the exact regions being assessed, and the mean score was subsequently reported.72 The scoring methods are capable of evaluating psoriasiform inflammation in a mouse model. These systems evaluate features such as the presence of lymphocytic infiltrate, papillary congestion, Munro abscess, acanthosis, parakeratosis, and hyperkeratosis.68,73,74
Immunohistochemistry
The tissue sections were subjected to immunohistochemistry by undergoing dewaxing in xylene, rehydration in ethanol, and washing in phosphate-buffered saline (pH = 7.4). A hydrogen peroxide-methanol solution (3%) was administered for 30 minutes to suppress the activity of the naturally occurring peroxidase. Subsequently, tissue samples were subjected to cryopreservation for 60 minutes at a temperature of 25°C. Subsequently, the sections were overnight incubated with primary antibodies at 4°C. The primary antibodies used in this study were CK-16 polyclonal antibodies (Thermo Scientific, Wilmington, DE, USA). The antibodies were diluted at a ratio of 1:30 (Catalog No. E-AB-12323) and 1:100, specifically targeting the CK16 protein.75 The quantification of IHC was conducted using semiquantitative scores, which were determined based on the percentage of cells that showed positive staining (H-score or Histoscore):75
Statistical Analysis
Descriptive and inferential statistics were performed using GraphPad Prism 10.3; the normality test (Anderson Darling test) was performed initially to determine the continuous variable’s adherence to a normal distribution. The parametric variable was analyzed using the ANOVA and post hoc Tukey’s tests. In contrast, the non-parametric variable was analyzed using the Kruskal–Wallis and post hoc Dunn tests. 0.05 was considered the level of significance for the current study.
The software program G.Power was employed to calculate the sample size.76,77 Random numbers were employed to generate groups in an Excel spreadsheet. The rats were placed in containers that were labeled and assigned tail tags to minimize confusion.78
Results
Assessment of Serum Biomarkers
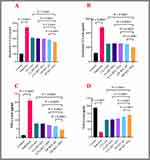
The induction group exhibited a considerable increase in serum levels of IL-17, IL-23, and TNF-α, while TGF-β levels were dramatically decreased compared to the control groups. The IL-17, IL-23, and TNF-α levels were substantially lower in the treatment groups compared to the induction groups. Additionally, the TGF-β values were greater than the treatment groups. The serum levels of IL-17, IL-23, TNF-α, and TGF-β did not differ significantly between the D-NEG (dipyridamole 0.3% nanoemulgel) and CLO (clobetasol) groups. The serum IL-17 and IL-23 levels of the R-gel group do not differ significantly from those of the CLO group. The levels of TNF-α and TGF-β in the serum are significantly different compared to the CLO group, as depicted in Figure 4.
The group treated with DR-gel (dipyridamole 1% and roflumilast 0.3% gel) exhibited significantly reduced levels of IL17 and TNF-α, although TGF-β levels were higher compared to the CLO group. The IL23 levels of the experimental groups did not show any noteworthy differences compared to the control groups. The DR-NEG (dipyridamole 1% and roflumilast 0.3% nanoemulgel) group had significantly reduced IL17, IL23, and TNF-α levels. However, TGF-β levels were higher compared to the CLO group, as depicted in Figure 4.
Assessment of PASI Score
The induction group showed significantly higher PASI scores in the induction group compared to the control. Treatment groups (CLO, R-NEG, DR-gel, and DR-NEG) showed statistically lower PASI scores than the induction group. There was statistical indifference in the PASI between the D-NEG, R-NEG (roflumilast 1% nanoemulgel), and DR-gel groups compared to the CLO group (DR-gel showed the lowest numerical value). DR-NEG showed the lowest numerical value of PASI, which was statistically lower than the CLO group, as seen in Figure 5.
Histopathological Assessment
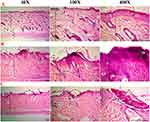
The histopathologic findings suggest that the control group displayed usual features, such as an appropriate granular layer, a typical corneal (basket weave) layer distribution, and an appropriate epidermis thickness, as depicted in Figure 6A. The histological portion of rat skin in the positive control group (psoriasis induction) exhibited parakeratosis, sub-corneal pustule, an inflammatory crust containing inflammatory cells, epidermal thickening, absence of hair follicles in the dermis, and a limited number of sweat glands and sebaceous glands, as depicted in Figure 6B. The histological characteristics of the CLO group showed notable changes, including a decrease in the thickness of the outermost layer of the skin, a modest rise in keratin production, the presence of inflammatory cells in the upper layer of the dermis, and the observation of expanded blood vessels in the top layer of the dermis, as illustrated in Figure 6C.
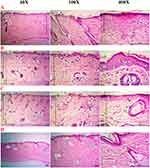
The histological analysis of rat skin subjected to D-NEG revealed a decrease in the thickness of the outermost layer of the skin, a slight increase in the generation of keratin, an abundance of inflammatory cells in the upper layer of the dermis, and the observation of enlarged blood vessels in the upper layer of the dermis, as shown in Figure 7A. Similar findings were seen in the R-NEG, as shown in Figure 7B. The DR-NEG group showed an intact epidermis with mild hydropic degeneration of the epithelial cells and an intact dermis with high numbers of hair follicles, sweat glands, and sebaceous glands, as seen in Figure 7C. The DR-gel group shows a sub-corneal pustule, an inflammatory crust containing inflammatory cells, a dermis with multiple sebaceous glands, and mild focal inflammatory cells, as seen in Figure 7D.
Baker’s Score
The induction group showed a significantly higher Baker score than the control group. Treatment groups (CLO, DR-gel, and DR-NEG) showed significantly lower Baker scores than the induction group. There was no significant difference in the Baker score between the D-NEG, R-NEG, and DR-gel groups compared to the CLO group (DR-gel showed the lowest numerical value). DR-NEG showed the lowest numerical value of Baker, which was significantly lower than the CLO group, as seen in Figure 8.
Immunohistochemical (IHC) Findings
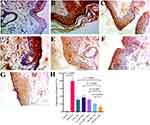
Modifications in protein expression (upregulated or downregulated) were detected as the brown pigment in the section. The extent of expression was measured using a scoring system (H-score) based on the extent of staining and the proportion of positive cells, as depicted in Figure 9A to 7G.
The IHC revealed upregulation in CK16 expression in the induction group compared to the control group; all treatment groups showed a significant reduction in the expression of CK16 compared to the induction group. There was no significant difference in the expression of CK16 between the D-NEG and R-NEG groups compared to the CLO group. At the same time, both DR-gel and DR-NEG showed significantly lower expression of CK16 than CLO groups (DR-NEG also had a lower expression of CK16 than DR-gel), as seen in Figure 9H.
Discussion
In the present study, 5% imiquimod (IMQ) cream successfully induced psoriatic lesions in rats, which was evidenced by significantly higher levels of IL-17, IL-23, TNF-α, PASI score, Baker score, CK17 protein expression, and significantly lower levels of TGF-β compared to the control group. IMQ cream induces an immune response that stimulates numerous inflammatory mediators, IL-1, IL-17, IL-23, and TNF-α.79 The occurrence of erythema caused by IMQ typically happens within three days of treatment. This is primarily due to the activation of many mast cells, which can be triggered either directly through IgE-dependent or non-dependent pathways. The elevated levels of mast cells and neuropeptides in the skin tissue, along with the dilation of blood vessels, are responsible for the erythema observed in psoriasis induced by IMQ.80 The scaling observed in rats due to IMQ is caused by activating the IL-17A and IL-23 axis, which stimulates Th17 and Th1 cells. This leads to a rapid turnover of keratinocytes and sebum depletion, resembling psoriasis pathophysiology in humans;61 the thickening and excessive growth of the outermost layer of the skin caused by IMQ is due to the excessive multiplication of keratin-producing cells and an increased number of these cells in the deepest layer of the skin.81
The current study showed that the topical use of CLO ointment substantially inhibited disease activity in the imiquimod-induced psoriasis-like rats model. CLO is classified as a corticosteroid, a group of drugs that exhibit vasoconstriction, anti-proliferative, anti-inflammatory, and immunosuppressive properties. In addition, they control the process of gene transcription for multiple genes, specifically those responsible for producing pro-inflammatory cytokines. As a result, they decrease the expression of interleukins and TNF-α.82
In the present study, the combination of dipyridamole 1% and roflumilast 0.3% NEG showed superior anti-psoriatic activity compared to CLO ointment and each drug alone. Even better activity than dipyridamole 1% and roflumilast 0.3% gel formulation was evidenced by improved PASI score, Baker score, IL17, IL23, TNF-α, TGF-β, CK-16 expression, and histopathologically.
Topical application of numerous anti-psoriatic drugs is common; however, their clinical efficacy is restricted due to their inadequate absorption. The nanoemulgel delivery technology shows promising potential for effectively delivering anti-psoriatic drugs and achieving positive therapeutic outcomes. A novel nanoemulgel drug delivery method was recently developed, utilizing betamethasone dipropionate (BD) as an anti-psoriatic agent.83 Swati Pund et al developed a nanoemulgel containing leflunomide (LFD) to treat psoriasis in a separate proof-of-concept investigation. LFD is a newly licensed, very effective, and beneficial drug for treating psoriatic arthritis.84,85 Kaur et al utilized CLO propionate and calcipotriol as the preferred pharmaceutical agents in their formulation. The researchers employed an imiquimod-induced psoriatic model in BALB/c mice and formulated a nanoemulsion with superior skin penetration into the stratum corneum compared to commercially available drugs.86 Several studies have indicated that Th17 cells produce effector cytokines, including IL-17 and IL-23, which can be found in the peripheral blood of individuals with psoriasis. These cytokines are believed to play a role in the development of psoriasis.87
TGF-β1, a crucial mediator in numerous illnesses, stimulates the production of pro-inflammatory cytokines like TNF-α.5 In their study, Ahmed et al determined that dipyridamole gel may have a potential impact on the anti-inflammatory efficacy of skin homogenate parameters in a mouse model of imiquimod-induced psoriasiform skin inflammation.88 In the present study, topical DR-NEG significantly reduced IL-17, IL-23, and TNF-α levels compared with the induction group.
TGF-β, a multifunctional cytokine, plays a crucial role in maintaining the stability of tissues and organs by regulating many cellular processes.89 Han et al (2010) demonstrated that levels of TGF-β are elevated in both the epidermis and serum of individuals with psoriasis.90 The increase in TGF-β level may be attributed to the enhanced production of TGF-β by activated endothelial cells, fibroblasts, or inflammatory cells in patients with psoriasis.91 In a separate investigation, the concentrations of IL-17, IL-23, and TNF-α were found to be reduced; on the other hand, the level of TGF-β increased when treated with dipyridamole gel and dipyridamole gel plus oral drug, compared to the induction group. Dipyridamole was discovered to decrease the activation and multiplication of T cells, resulting in the production of pro-inflammatory cytokines IL-17 in mice .92 Dipyridamole functions as an anti-inflammatory agent by enhancing cAMP mediators. This results in elevated amounts of TGF-β and reduced levels of the pro-inflammatory cytokines TNF-α, IL-23, and IL-17.93 Additional research has shown that dipyridamole inhibited the heightened production of the cytokine TNF-α in astrocytes and decreased the levels of pro-inflammatory substances .94
Different studies have demonstrated roflumilast’s efficacy and safety in treating plaque and intertriginous psoriasis.95–97 In the treatment of psoriasis, the roflumilast cream with a greater concentration (0.3%) showed superior results compared to the cream with a lower dose (0.15%).98 The activity of PDE-4 is higher in psoriatic skin than healthy skin.99 The PDE-4 inhibitors in psoriasis prevent the breakdown of cAMP, which results in a decrease in inflammation,100 roflumilast inhibits the activity of PDE4, resulting in the buildup of cAMP in specific cells and a consequent elevation in cAMP signaling. The buildup of cAMP in specific immune cells is believed to play a crucial role in avoiding inflammation,101,102 and blocking PDE-4 leads to a decrease in the activity of immune modulators, such as TNF-α, interferon-γ, IL-17, and IL-23.103 An IHC study verified the presence of IL-23 in the skin affected by psoriasis. IL-23R was notably expressed in immune cells and keratinocytes in psoriatic skin.104
Roflumilast is a pharmacological agent that exhibits anti-inflammatory properties and is recognized for its involvement in multiple inflammatory pathways. It reduces the concentrations of leptin, TNF-a, IL-1b, IL-2, IL-13, IFN-g, and ROS.10,11 Roflumilast decreases the concentration of TNF-a and IL-1b in both the plasma blood and sputum and decreases oxidative stress.105 Roflumilast increases cAMP levels by blocking the activity of PDE4, an enzyme that specifically breaks down and deactivates cAMP.106 These elevated amounts of cAMP inhibit the release of different cytokines (particularly TNF-a, IL-1b, TGF-b, and IFN-g) in multiple cell types.107 Furthermore, the mRNA levels of TNF-a, TGF-b, IL-1b, and IFN-g saw a substantial increase, which the administration of roflumilast notably diminished. Roflumilast showed a considerable reduction in TNF-a, TGF-b, and IL-1b.11,108 The investigators determined that the anti-inflammatory properties of roflumilast were attributed to its capacity to inhibit the recruitment of neutrophils and eosinophils.101,109–111 Various cell types, including macrophages, neutrophils, lymphocytes, endothelial, and mast cells, produced pleiotropic cytokines such as TNF-α.112 TNF-α is widely recognized for its significant involvement in vascular inflammation and the infiltration of immune cells.113 TNF-α was upregulated in the perivascular dermis and epidermal keratinocytes of psoriatic plaque skin. In the skin around the lesion, immunostaining was mainly observed in the basal layer of keratinocytes. However, in the psoriatic plaque, all layers of the skin were positively stained, with a more intense expression at the base.114
The present study examined the effects of tested drugs on IHC Findings. Figure 9 summarizes the net data regarding each group and the immunoreactivity against CK16. Furthermore, an actual picture clarifies the expression of CK16 in each group, as shown in the illustrated immunohistochemistry panel. Both combination groups showed significantly lower CK16 protein expression, and DR-NEG showed the lowest reduction, indicating that DR-NEG resulted in the best anti-inflammatory effect by inhibiting CK16 overexpression.
The present investigation demonstrated an elevation in psoriatic skin characteristics, including acanthosis, Munro micro-abscess, hyperkeratosis, parakeratosis, thinning above papillae, lengthening and clubbing, and mild dermis lymphocytic in the induction group. Fanti et al demonstrated that imiquimod was topically administered to mouse skin to exhibit the characteristics of erythema, scaling, thickness, and inflammatory reactions caused by the excessive growth of keratinocytes.115 Administration of dipyridamole topically enhanced the histopathological alterations caused by IMQ. The cAMP is the primary regulator of cellular immune response. It controls the production of pro-inflammatory and anti-inflammatory cytokines and the activation of T cells and neutrophil degranulation. cAMP exhibits strong expression in the brain, cardiovascular tissues, smooth muscles, keratinocytes, and immunocytes.116
In the present study, the histological section of rat skin of the psoriasis induction group showed para-keratosis, sub-corneal pustule, inflammatory crust containing inflammatory cells, thickening epidermis, without hair follicles in the dermis, few sweat glands and sebaceous glands, these results are compatible with other studies.117 In this study, using DR-NEG through direct application on the skin resulted in enhanced histopathological alterations caused by imiquimod. This led to decreased acanthosis, which can be linked to reduced angiogenesis and basal cell proliferation. Pharmacological drugs that limit PDE activity increase the amount of cAMP inside cells, resulting in broad anti-inflammatory effects.118
Conclusion
The new dipyridamole 1% and roflumilast 0.3% nanoemulgel combination’s topical administration showed better anti-inflammatory, tissue healing, and anti-psoriatic activity than either drug alone or topical clobetasol administration. The combination gel formulation showed good anti-inflammatory and anti-psoriatic activity compared to clobetasol ointment. However, the nanoemulgel combination was superior to the gel formulation. This could be attributed to the possible synergic effects of both drugs and the enhanced skin penetration offered by the nanoemulgel formulation.
Future Perspectives
- Clinical Trials: Further research and clinical trials are needed to establish the efficacy and safety of this combination nanoemulgel in a larger patient population.
- Formulation Optimization: Continued optimization of the nanoemulgel formulation can lead to even better performance and patient outcomes.
- Broader Applications: The principles of this combination therapy could be applied to other inflammatory skin conditions, expanding its therapeutic potential.
- Regulatory Approval: Securing regulatory approvals will be key to bringing this innovative treatment to market and making it widely available to patients.
- Patient Compliance: The enhanced efficacy and improved healing properties may lead to better patient compliance and satisfaction with the treatment.
This combination nanoemulgel represents a promising advancement in the treatment of psoriasis and potentially other inflammatory skin conditions, offering improved efficacy and better patient outcomes.
Data Sharing Statement
Underlying data: Fawzi, H. A. (2024). DR-NEG biological study [Data set]. Zenodo. https://doi.org/10.5281/zenodo.13310632
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Disclosure
The authors declare that no funds, grants, or other support were received during the preparation of this paper.
References
1. An J, Li Z, Dong Y, Ren J, Huo J. Amentoflavone protects against psoriasis-like skin lesion through suppression of NF-κB-mediated inflammation and keratinocyte proliferation. Mol Cell Biochem. 2016;413(1–2):87–95. doi:10.1007/s11010-015-2641-6
2. Bu J, Ding R, Zhou L, Chen X, Shen E. Epidemiology of psoriasis and comorbid diseases: a narrative review. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.880201
3. WHO. Global report on psoriasis; 2016. Available from: https://www.who.int/publications/i/item/9789241565189?form=MG0AV3.
4. Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis. 2021;11:21–29. doi:10.2147/ptt.S303634
5. Di Fusco D, Laudisi F, Dinallo V, et al. Smad7 positively regulates keratinocyte proliferation in psoriasis. Br J Dermatol. 2017;177(6):1633–1643. doi:10.1111/bjd.15703
6. Vičić M, Kaštelan M, Brajac I, Sotošek V, Massari LP. Current concepts of psoriasis immunopathogenesis. Int J Mol Sci. 2021;22(21):11574. doi:10.3390/ijms222111574
7. Liu Z, Liu M, Cao Z, Qiu P, Song G. Phosphodiesterase-4 inhibitors: a review of current developments (2013–2021). Expert Opin Ther Pat. 2022;32(3):261–278. doi:10.1080/13543776.2022.2026328
8. Drakos A, Vender R, Torres T. Topical roflumilast for the treatment of psoriasis. Expert Rev Clin Immunol. 2023;19(9):1053–1062. doi:10.1080/1744666x.2023.2219897
9. Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012;35(3):197–210. doi:10.4103/2319-4170.106152
10. Kwak HJ, Song JS, Heo JY, Yang SD, Nam JY, Cheon HG. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear factor-kappaB, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase activation. J Pharmacol Exp Ther. 2005;315(3):1188–1195. doi:10.1124/jpet.105.092056
11. Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43(1):167–188. doi:10.1385/cbb:43:1:167
12. Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arterioscler Thromb Vasc Biol. 2008;28(3):s39–42. doi:10.1161/atvbaha.107.160226
13. Harel F, Finnerty V, Authier S, Pelletier-Galarneau M. Comparison of two dipyridamole infusion protocols for myocardial perfusion imaging in subjects with low likelihood of significant obstructive coronary artery disease. J Nucl Cardiol. 2020;27(5):1820–1828. doi:10.1007/s12350-018-01478-x
14. Cabrini MR, Sezen SF, Lagoda G, et al. Fibrotic protein expression profiles in penile tissue of patients with erectile dysfunction. Urology. 2013;82(4):
15. Coeugniet E, Bendtzen K, Bendixen G. Leucocyte migration inhibitory activity of concanavalin-A-stimulated human lymphocytes. Modification by dipyridamole, lysine-acetylsalicylate and heparin. Acta Med Scand. 1976;199(1–2):99–104. doi:10.1111/j.0954-6820.1976.tb06698.x
16. Dong H, Osmanova V, Epstein PM, Brocke S. Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated lymphocytes. Biochem Biophys Res Commun. 2006;345(2):713–719. doi:10.1016/j.bbrc.2006.04.143
17. Insel PA, Murray F, Yokoyama U, et al. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166(2):447–456. doi:10.1111/j.1476-5381.2012.01847.x
18. Rapalli VK, Waghule T, Gorantla S, Dubey SK, Saha RN, Singhvi G. Psoriasis: pathological mechanisms, current pharmacological therapies, and emerging drug delivery systems. Drug Discov Today. 2020;25(12):2212–2226. doi:10.1016/j.drudis.2020.09.023
19. Lapteva M, Santer V, Mondon K, et al. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J Control Release. 2014;196:9–18. doi:10.1016/j.jconrel.2014.09.021
20. Pinto MF, Moura CC, Nunes C, Segundo MA, Costa Lima SA, Reis S. A new topical formulation for psoriasis: development of methotrexate-loaded nanostructured lipid carriers. Int J Pharm. 2014;477(1–2):519–526. doi:10.1016/j.ijpharm.2014.10.067
21. Mao KL, Fan ZL, Yuan JD, et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf B. 2017;160:704–714. doi:10.1016/j.colsurfb.2017.10.029
22. Makuch S, Dróżdż M, Makarec A, Ziółkowski P, Woźniak M. An update on photodynamic therapy of psoriasis-current strategies and nanotechnology as a future perspective. Int J Mol Sci. 2022;23(17). doi:10.3390/ijms23179845
23. Barradas TN, Senna JP, Cardoso SA, de Holanda ESKG, Elias Mansur CR. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater Sci Eng C. 2018;92:245–253. doi:10.1016/j.msec.2018.06.049
24. Langasco R, Tanrıverdi ST, Özer Ö, et al. Prolonged skin retention of clobetasol propionate by bio-based microemulsions: a potential tool for scalp psoriasis treatment. Drug Dev Ind Pharm. 2018;44(3):398–406. doi:10.1080/03639045.2017.1395458
25. Pukale SS, Sharma S, Dalela M, et al. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020;115:393–409. doi:10.1016/j.actbio.2020.08.020
26. Soares A, Gonçalves LMO, Ferreira RDS, et al. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr Polym. 2020;243:116498. doi:10.1016/j.carbpol.2020.116498
27. Lin Z, Xi L, Chen S, et al. Uptake and trafficking of different sized PLGA nanoparticles by dendritic cells in imiquimod-induced psoriasis-like mice model. Acta pharmaceutica Sinica B. 2021;11(4):1047–1055. doi:10.1016/j.apsb.2020.11.008
28. Abd-Alhussain GK, Alatrakji M, Ahmed SJ, Fawzi HA. Efficacy of oral insulin nanoparticles for the management of hyperglycemia in a rat model of diabetes induced with streptozotocin. J Med Life. 2024;17(2):217–225. doi:10.25122/jml-2023-0355
29. Shores LS, Kelly SH, Hainline KM, Suwanpradid J, MacLeod AS, Collier JH. Multifactorial design of a supramolecular peptide Anti-IL-17 vaccine toward the treatment of psoriasis. Front Immunol. 2020;11:1855. doi:10.3389/fimmu.2020.01855
30. Lin ZC, Hwang TL, Huang TH, Tahara K, Trousil J, Fang JY. Monovalent antibody-conjugated lipid-polymer nanohybrids for active targeting to desmoglein 3 of keratinocytes to attenuate psoriasiform inflammation. Theranostics. 2021;11(10):4567–4584. doi:10.7150/thno.56995
31. Chen D, Wan Z, Zhou Y, et al. Chapter 8 - Controllable synthesis of lanthanide upconversion nanomaterials through impurity doping. In: Grumezescu AM, editor. Fabrication and Self-Assembly of Nanobiomaterials. William Andrew Publishing; 2016:211–241.
32. Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3):102–110. doi:10.1016/j.cocis.2005.06.004
33. Lochhead R. Basic physical sciences for the formulation of cosmetic products. Cosmet Sci Technol. 2017;39–76.
34. Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys. 2006;18(41):R635. doi:10.1088/0953-8984/18/41/R01
35. Drakontis CE, Amin S. Biosurfactants: formulations, properties, and applications. Curr Opin Colloid Interface Sci. 2020;48:77–90. doi:10.1016/j.cocis.2020.03.013
36. Ghosh PK, Murthy RS. Microemulsions: a potential drug delivery system. Curr Drug Deliv. 2006;3(2):167–180. doi:10.2174/156720106776359168
37. Lapasin R, Grassi M, Coceani N. Effects of polymer addition on the rheology of o/w microemulsions. Rheologica Acta. 2001;40(2):185–192. doi:10.1007/s003970000151
38. Hussain A, Samad A, Singh SK, et al. Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Delivery. 2016;23(2):642–647. doi:10.3109/10717544.2014.933284
39. Khullar R, Saini S, Seth N, Rana A. Emulgels: a surrogate approach for topically used hydrophobic drugs. Int J Pharm Bio Sci. 2011;1(3):117–128.
40. Barry BW. Breaching the skin’s barrier to drugs. Nat Biotechnol. 2004;22(2):165–167. doi:10.1038/nbt0204-165
41. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109–131. doi:10.4155/tde.10.16
42. Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials. 2011;32(23):5417–5426. doi:10.1016/j.biomaterials.2011.04.006
43. Gupta SK. Formulation and evaluation of nanoemulsion based nanoemulgel of aceclofenac. J Pharm Sci Res. 2020;12(4):524–532.
44. Anand K, Ray S, Rahman M, et al. Nano-emulgel: emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent Pat Antiinfect Drug Discov. 2019;14(1):16–35. doi:10.2174/1574891x14666190717111531
45. El-Kayal M, Hatem S. A comparative study between nanostructured lipid carriers and invasomes for the topical delivery of luteolin: design, optimization and pre-clinical investigations for psoriasis treatment. J Drug Deliv Sci Technol. 2024;97:105740. doi:10.1016/j.jddst.2024.105740
46. Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2022;33(1):111–120. doi:10.1080/09546634.2020.1729335
47. Dalibor M, Fabrizio S, Ian PH. Topical moisturisers for the management of psoriasis vulgaris. In: Shahin A, editor. Psoriasis. IntechOpen; 2022:
48. Maded ZK, Sfar S, Taqa GAA, Lassoued MA, Ben Hadj Ayed O, Fawzi HA. Development and optimization of dipyridamole- and roflumilast-loaded nanoemulsion and nanoemulgel for enhanced skin permeation: formulation, characterization, and in vitro assessment. Pharmaceuticals. 2024;17(6):803. doi:10.3390/ph17060803
49. Patel J, Patel B, Banwait H, Parmar K, Patel M. Formulation and evaluation of topical aceclofenac gel using different gelling agent. Int J Drug Dev Res. 2011;3(1):156–164.
50. Akhter S, Jain GK, Ahmad FJ, et al. Investigation of nanoemulsion system for transdermal delivery of domperidone: ex-vivo and in vivo studies. Curr Nanosci. 2008;4(4):381–390. doi:10.2174/157341308786306071
51. Haider F, Khan BA, Khan MK. Formulation and evaluation of topical linezolid nanoemulsion for open incision wound in diabetic animal model. AAPS Pharm Sci Tech. 2022;23(5):129. doi:10.1208/s12249-022-02288-8
52. Zeng YC, Li S, Liu C, et al. Soluplus micelles for improving the oral bioavailability of scopoletin and their hypouricemic effect in vivo. Acta Pharmacol Sin. 2017;38(3):424–433. doi:10.1038/aps.2016.126
53. Dodelet-Devillers A, Zullian C, Beaudry F, et al. Physiological and pharmacokinetic effects of multilevel caging on Sprague Dawley rats under ketamine-xylazine anesthesia. Exp Anim. 2016;65(4):383–392. doi:10.1538/expanim.16-0026
54. Underwood W, Anthony R AVMA guidelines for the euthanasia of animals: 2020 edition.
55. Kaoud RM, Alwan MH, Amran M, Fawzi HA. Design and optimization of pantoprazole sodium mucoadhesive hydrogel microcapsules for the healing of peptic ulcers. Article. Pharmacia. 2024;71:1–14. doi:10.3897/pharmacia.71.e118323
56. Obaid KA, Fawzi HA. Evaluation of empagliflozin efficacy as a promising anti-aging treatment in mice: in-vivo study. Article. Pharmacia. 2024;71e116184. doi:10.3897/pharmacia.71.e116184
57. Khafaji AW, Al-Zubaidy AA, Farhood IG, Fawzi HA. Effects of topical isoxsuprine ointment on imiquimod-induced psoriasiform skin inflammation in mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024. doi:10.1007/s00210-024-03359-2
58. Parmar KM, Jagtap CS, Katare NT, Dhobi M, Prasad SK. Development of a psoriatic-like skin inflammation rat model using imiquimod as an inducing agent. Indian J Pharmacol. 2021;53(2):125–131. doi:10.4103/ijp.IJP_506_19
59. Shahine Y, El-Aal SAA, Reda AM, et al. Diosmin nanocrystal gel alleviates imiquimod-induced psoriasis in rats via modulating TLR7,8/NF-κB/micro RNA-31, AKT/mTOR/P70S6K milieu, and Tregs/Th17 balance. Inflammopharmacology. 2023;31(3):1341–1359. doi:10.1007/s10787-023-01198-w
60. Smajlović A, Haverić A, Alić A, et al. Molecular and histopathological profiling of imiquimod induced dermatosis in Swiss Wistar rats: contribution to the rat model for novel anti-psoriasis treatments. Mol Biol Rep. 2021;48(5):4295–4303. doi:10.1007/s11033-021-06445-3
61. van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi:10.4049/jimmunol.0802999
62. Palamara F, Meindl S, Holcmann M, Lührs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173(5):3051–3061. doi:10.4049/jimmunol.173.5.3051
63. Almudaris SA, Gatea FK. Effects of topical Ivermectin on imiquimod-induced Psoriasis in mouse model – novel findings. Pharmacia. 2024;71:1–14. doi:10.3897/pharmacia.71.e114753
64. Shimo T, Takahara Y, Noguchi Y, Mukawa A, Kato H, Ito Y. [Comparative toxicity test of dexamethasone valerate (DV-17) and other steroid ointments in rats]. J Toxicol Sci. 1982;7(Suppl 1):15–33. doi:10.2131/jts.7.supplementi_15
65. Kimura M, Tarumoto Y, Nakane S, Otomo S. Comparative toxicity study of hydrocortisone 17-butyrate 21-propionate (HBP) ointment and other topical corticosteroids in rats. Drugs Exp Clin Res. 1986;12(8):643–652.
66. Carvalho MFP, Pereira CSB, Fregnani JH, Ribeiro Fd AQ. Comparative histological study on wound healing on rat’s skin treated with Mitomycin C or Clobetasol propionate. Acta Cirúrgica Brasileira. 2015;30:593–597.
67. Diehl KH, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21(1):15–23. doi:10.1002/jat.727
68. Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor Protocols. 2014;2014(6):655–658. doi:10.1101/pdb.prot073411
69. Ali Z, Robert Zibert J, Dahiya P, et al. Mild-to-moderate severity of psoriasis may be assessed remotely based on photographs and self-reported extent of skin involvement. JAAD Int. 2023;11:129–136. doi:10.1016/j.jdin.2023.02.004
70. Baker BS, L FRY. The immunology of psoriasis. British Journal of Dermatology. 1992;126(1):1–9. doi:10.1111/j.1365-2133.1992.tb08394.x
71. Mohammed SS, Kadhim HM, Al-Sudani IM, Musatafa WW. Anti-inflammatory effects of topically applied Azilsartan in a mouse model of imiquimod-induced psoriasis. Int J Drug Deliv Technol. 2022;12:1249–1255. doi:10.25258/ijddt.12.3.53
72. Luo DQ, Wu HH, Zhao YK, Liu JH, Wang F. Original research: different imiquimod creams resulting in differential effects for imiquimod-induced psoriatic mouse models. Exp Biol Med. 2016;241(16):1733–1738. doi:10.1177/1535370216647183
73. Al-juhaishi AMR, Al-Zubaidy AAK, Al-Mousawy JMM. Effects of Montelukast on imiquimod-induced model of psoriasis in mice. Natural Volatiles & Essential Oils Journal. 2021;8(6):3160–3171.
74. Thamer MS, Yahya MQ. The effect of lenalidomide ointment on TNF-α tissue levels in mice with imiquimod-induced psoriasis. J Fac Med Baghdad. 2023;64(4):252–260. doi:10.32007/jfacmedbagdad.6441959
75. Abed FM, Rahawi AM, Al-Sabaawy HB, Dark MJ, Abduljawaad AN. Pathological and immunohistochemical findings of prostate glands from clinically normal dogs. Am J Anim Vet Sci. 2023;18(4):317–326. doi:10.3844/ajavsp.2023.317.326
76. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/bf03193146
77. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–306. doi:10.4103/0976-500x.119726
78. Festing MFW. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47(1):5–14. doi:10.1093/ilar.47.1.5
79. Weber A, Zimmermann C, Mausberg AK, Kieseier BC, Hartung HP, Hofstetter HH. Induction of pro-inflammatory cytokine production in thymocytes by the immune response modifiers Imiquimod and Gardiquimod™. Int Immunopharmacol. 2013;17(2):427–431. doi:10.1016/j.intimp.2013.06.023
80. Hao Y, Peng B, Che D, et al. Imiquimod-related dermatitis is mainly mediated by mast cell degranulation via Mas-related G-protein coupled receptor B2. Int Immunopharmacol. 2020;81:106258. doi:10.1016/j.intimp.2020.106258
81. Varma SR, Sivaprakasam TO, Mishra A, Prabhu S, Rafiq M, Rangesh P. Imiquimod-induced psoriasis-like inflammation in differentiated human keratinocytes: its evaluation using curcumin. Eur J Pharmacol. 2017;813:33–41. doi:10.1016/j.ejphar.2017.07.040
82. Torsekar R, Gautam MM. Topical therapies in psoriasis. Indian Dermatol Online J. 2017;8(4):235–245. doi:10.4103/2229-5178.209622
83. Alam MS, Ali MS, Alam N, et al. Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. J Appl Pharm Sci. 2012;2(10):148–158.
84. Herrmann ML, Schleyerbach R, Kirschbaum BJ. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000;47(2–3):273–289. doi:10.1016/s0162-3109(00)00191-0
85. Kaltwasser JP. Leflunomide in psoriatic arthritis. Autoimmun Rev. 2007;6(8):511–514. doi:10.1016/j.autrev.2006.12.001
86. Ahmad MZ, Mohammed AA, Algahtani MS, Mishra A, Ahmad J. Nanoscale topical pharmacotherapy in management of psoriasis: contemporary research and scope. J Funct Biomater. 2022;14(1):19. doi:10.3390/jfb14010019
87. Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186(3):1495–1502. doi:10.4049/jimmunol.1001001
88. Ahmed N, Al-Zubaidy A, Qasim B. Effect of topical dipyridamole gel in comparison with clobetasol on induced psoriasis in mice. Int J Drug Deliv Technol. 2021;2(11):524–529.
89. Fabregat I, Moreno-Càceres J, Sánchez A, et al. TGF-β signalling and liver disease. Febs j. 2016;283(12):2219–2232. doi:10.1111/febs.13665
90. Han G, Williams CA, Salter K, Garl PJ, Li AG, Wang XJ. A role for TGFbeta signaling in the pathogenesis of psoriasis. J Investigate Dermatol. 2010;130(2):371–377. doi:10.1038/jid.2009.252
91. Flisiak I, Zaniewski P, Rogalska-Taranta M, Chodynicka B. Effect of psoriasis therapy on VEGF and its soluble receptors serum concentrations. J Eur Acad Dermatol Venereol. 2012;26(3):302–307. doi:10.1111/j.1468-3083.2011.04053.x
92. Kyttaris VC, Zhang Z, Kampagianni O, Tsokos GC. Calcium signaling in systemic lupus erythematosus T cells: a treatment target. Arthritis Rheumatism. 2011;63(7):2058–2066. doi:10.1002/art.30353
93. Rapalli VK, Singhvi G, Dubey SK, Gupta G, Chellappan DK, Dua K. Emerging landscape in psoriasis management: from topical application to targeting biomolecules. Biomed Pharmacother. 2018;106:707–713. doi:10.1016/j.biopha.2018.06.136
94. Lana D, Ugolini F, Melani A, Nosi D, Pedata F, Giovannini MG. The neuron-astrocyte-microglia triad in CA3 after chronic cerebral hypoperfusion in the rat: protective effect of dipyridamole. Experimental Gerontology. 2017;96:46–62. doi:10.1016/j.exger.2017.06.006
95. Cordoro KM. Roflumilast for chronic plaque psoriasis. JAMA. 2022;328(11):1049–1050. doi:10.1001/jama.2022.14663
96. Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358(3):413–422. doi:10.1124/jpet.116.232819
97. Pixley JN, Schaetzle T, Feldman SR. A review of topical roflumilast for the treatment of plaque psoriasis. Ann Pharmacother. 2023;57(8):966–969. doi:10.1177/10600280221137750
98. Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. New Engl J Med. 2020;383(3):229–239. doi:10.1056/NEJMoa2000073
99. Pincelli C, Schafer PH, French LE, Augustin M, Krueger JG. Mechanisms underlying the clinical effects of apremilast for psoriasis. J Drugs Dermatol. 2018;17(8):835–840.
100. Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19(8):734–740. doi:10.36849/jdd.2020.5370
101. Baye J. Roflumilast (daliresp): a novel phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. P T. 2012;37(3):149–161.
102. Blumenthal DK. Pharmacodynamics: molecular mechanisms of drug action. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. McGraw-Hill Education; 2017.
103. Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Frontiers in Pharmacology. 2018;9:1048. doi:10.3389/fphar.2018.01048
104. Tonel G, Conrad C, Laggner U, et al. Cutting edge: a critical functional role for IL-23 in psoriasis. J Immunol. 2010;185(10):5688–5691. doi:10.4049/jimmunol.1001538
105. Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297(1):267–279.
106. Burgin AB, Magnusson OT, Singh J, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28(1):63–70. doi:10.1038/nbt.1598
107. Park HJ, Lee JH, Park YH, et al. Roflumilast ameliorates airway hyperresponsiveness caused by diet-induced obesity in a Murine model. Am J Respir Cell Mol Biol. 2016;55(1):82–91. doi:10.1165/rcmb.2015-0345OC
108. Cortijo J, Iranzo A, Milara X, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol. 2009;156(3):534–544. doi:10.1111/j.1476-5381.2008.00041.x
109. Hohlfeld JM, Schoenfeld K, Lavae-Mokhtari M, et al. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial. Pulm Pharmacol Therap. 2008;21(4):616–623. doi:10.1016/j.pupt.2008.02.002
110. Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi:10.1136/thx.2006.075937
111. Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbröker D, Bethke TD. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–571. doi:10.1016/s0140-6736(05)67100-0
112. Popa C, van den Hoogen FH, Radstake TR, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheumatic Dis. 2007;66(11):1503–1507. doi:10.1136/ard.2006.066191
113. Park JH, Park YJ, Kim SK, et al. Histopathological differential diagnosis of psoriasis and seborrheic dermatitis of the scalp. Ann Dermatol. 2016;28(4):427–432. doi:10.5021/ad.2016.28.4.427
114. Johansen C, Funding AT, Otkjaer K, et al. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176(3):1431–1438. doi:10.4049/jimmunol.176.3.1431
115. Fanti PA, Dika E, Vaccari S, Miscial C, Varotti C. Generalized psoriasis induced by topical treatment of actinic keratosis with imiquimod. Int J Dermatol. 2006;45(12):1464–1465. doi:10.1111/j.1365-4632.2006.02980.x
116. Chiricozzi A, Nograles KE, Johnson-Huang LM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9(2):e90284. doi:10.1371/journal.pone.0090284
117. Kawakita T, Landy HJ. Surgical site infections after cesarean delivery: epidemiology, prevention and treatment. Matern Health Neonatol Perinatol. 2017;3:12. doi:10.1186/s40748-017-0051-3
118. Page CP, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol. 2012;12(3):275–286. doi:10.1016/j.coph.2012.02.016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.