Back to Journals » International Journal of Nanomedicine » Volume 19
Toxoplasma gondii-Derived Exosomes: A Potential Immunostimulant and Delivery System for Tumor Immunotherapy Superior to Toxoplasma gondii
Authors Zhao LX, Sun Q, Wang C, Liu JJ, Yan XR, Shao MC, Yu L, Xu WH , Xu R
Received 20 June 2024
Accepted for publication 23 October 2024
Published 22 November 2024 Volume 2024:19 Pages 12421—12438
DOI https://doi.org/10.2147/IJN.S483626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Lai-Xi Zhao,1,* Qiong Sun,2,* Chong Wang,3,* Jia-Jia Liu,1 Xiao-Rong Yan,1 Meng-Ci Shao,1 Li Yu,3 Wen-Hua Xu,1 Rui Xu1
1College & Hospital of Stomatology, Anhui Medical University, Key Laboratory of Oral Diseases Research of Anhui Province, Hefei, 230032, People’s Republic of China; 2Department of Stomatology, Anhui Province Direct Subordinate Hospital, Hefei, 230601, People’s Republic of China; 3Department of Microbiology and Parasitology, School of Basic Medical Sciences, Anhui Medical University, Anhui Province Key Laboratory of Zoonoses, The Provincial Key Laboratory of Zoonoses of High Institutions in Anhui, Hefei, Anhui Province, 230032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen-Hua Xu; Rui Xu, Email [email protected]; [email protected]
Abstract: Immunotherapies such as immune checkpoint blockade (ICB) therapy and chimeric antigen receptor T-cell (CAR-T) therapy have ushered in a new era of tumor treatment. However, most patients do not benefit from immunotherapy due to limitations such as narrow indications, low response rates, and high rates of adverse effects. Toxoplasma gondii (T. gondii), a specialized intracellular protozoan, can modulate host immune responses by inhibiting or stimulating cytokines. The ability of T. gondii to enhance an organism’s immune response was found to have a direct anti-tumor effect and enhance the sensitivity of patients with tumors to ICB therapy. However, the application of T. gondii for tumor therapy faces several challenges, such as biosafety concerns. Exosomes, a subtype of extracellular vesicle that contains active components such as proteins, nucleic acids, and lipids, have become effective therapeutic tools for various diseases, including tumors. Parasites, such as T. gondii, mediate the communication of pathogens with immune cells and modulate host cellular immune responses through exosomes. Growing evidence indicates that T. gondii-derived exosomes mediate communication between pathogens and immune cells, modulate host immune responses, and have great potential as new tools for tumor therapy. In this review, we highlight recent advances in isolation and identification techniques, profiling analysis, host immunomodulatory mechanisms, and the role of T. gondii-derived exosomes in tumor immunotherapy. Additionally, we emphasize the potential of T. gondii-derived exosomes as delivery platform to enhance anti-tumor efficacy in combination with other therapies. This review proposes that T. gondii-derived exosomes may serve as a novel tool for tumor immunotherapy owing to their ability to activate host immune function and properties such as high modifiability, stability, and low toxicity. This work will assist in promoting the application of parasite exosomes in tumor therapy.
Keywords: Toxoplasma gondii, exosomes, tumor, immune regulation, immunotherapy
Corrigendum for this paper has been published.
Graphical Abstract:

Introduction
Tumors are serious threats to human health and are important obstacles to increasing human life expectancy.1 Traditional tumor treatments focus on directly killing tumors through physical removal, chemotherapeutic drugs, or radiation,2,3 which have poor efficacy in advanced tumors and side effects on vital organs.4 In recent years, immunotherapies have emerged rapidly and have provided new pathways for tumor treatment.4 Immunotherapies, including cancer vaccines,5 chimeric antigen receptor T-cell (CAR-T) therapy,6 nonspecific immunomodulator therapy,7 and immune checkpoint blockade (ICB) therapy,8,9 intervene in multiple stages of tumor immune evasion by activating host effector T cells and enhancing anti-tumor immune responses, thereby achieving treatment of or even curing tumors.10 However, immunotherapies are in the early stages of development and have several shortcomings. The low clinical response rate leaves a large number of patients without benefit.11,12 The tumor location is an important factor affecting anti-tumor strategies,13 but current tumor immunotherapies are generally not organ-specific, leading to various adverse effects, including central system complications,14 cytotoxicity,15 and inflammation.16 Therefore, new strategies for implementing tumor immunotherapy to improve efficacy and safety remain to be explored.
Toxoplasma gondii (T. gondii) is an obligate intracellular eukaryotic parasite able to infect humans and other warm-blooded animals. T. gondii invades organisms and repeatedly proliferates in infected cells, leading to massive host cell destruction and causing disease.17,18 In recent years, the role of T. gondii in modulating host immunity and anti-tumor activity has attracted widespread attention. Numerous studies have indicated that T. gondii parasitizes the host and induces vigorous immune responses, which have anti-tumor effects.19 This effect results from the ability of T. gondii to activate antigen processing, antigen presentation, and tumor antigen-specific CD8+ T cells.20,21 Despite numerous studies confirming the potential of T. gondii for immunomodulatory and anti-tumor applications, several serious obstacles to live T. gondii use as a clinical tumor therapeutic tool exist. First, T. gondii must be cultured in living cells to maintain its activity and must be isolated and extracted before use, which greatly increases the time and capital costs. Secondly, directly using live parasites and products for treatment involves biosafety concerns and ethical issues. Live replicating T. gondii strains are unlikely to be suitable for tumor therapy, as tumor patients are immunocompromised and thus more vulnerable to infection. Once a nosocomial T. gondii infection occurs, it may lead to a variety of adverse effects in the patients. Symptoms such as dizziness and vomiting in milder cases, and retinal necrosis in more serious cases.22 In contrast, non-replicating attenuated T. gondii strains are more likely to be used for tumor therapy.23 However, the attenuated T. gondii strains are significantly inhibited in their ability to replicate by special processing, making them difficult to produce in large quantities. Both of their treatments are often invasive, with unpredictable risks of nosocomial T. gondii infection.24 Therefore, safer, more convenient, and more cost-effective tumor immunotherapy strategies based on T. gondii need to be developed.
Exosomes are nanoscale membrane-bound vesicles formed by phospholipid bilayers encapsulating various components,25 that include membrane proteins, metabolic enzymes, cytoplasmic and nuclear proteins, signaling molecules, a variety of nucleic acids,26 lipids, and metabolites.27,28 Exosomes interact with receptor cells by directly signaling through ligand/receptor interactions or enter receptor cells and regulate receptor cell function by membrane fusion, endocytosis, and macropinocytosis.29,30 Exosomes of different origins play distinct roles in normal physiological processes such as the immune response, cell proliferation, and inflammation, as well as in various stages of disease, such as tumors.31,32 Exosomes naturally, abundantly, and stably exist in a series of body fluid samples, contain specific substances derived from parental cells such as tumor cells, and their detection is simpler and more specific than that of traditional tumor markers. Thus, exosomes show great potential in tumor body fluid biopsy techniques.33 Exosomes are also characterized by low toxicity and immunogenicity, and artificially modified engineered exosomes have become a focus of attention as loaded tumor-targeted drug delivery vehicles and cancer vaccines.34,35 Recently, research has shown that parasites such as T. gondii can secrete exosomes, facilitating communication with host cells.36,37 T. gondii-derived exosomes can regulate host immune responses by inducing macrophage polarization and stimulating T cells.38,39 Compared with live T. gondii, T. gondii-derived exosomes have significant advantages in terms of biosafety and convenience. Thus, T. gondii-derived exosomes have great potential as novel tools for tumor treatment.
Hence, in this paper, we highlight recent advances in isolation, profiling analysis, and host immunomodulatory mechanisms of T. gondii-derived exosomes and the role of T. gondii-derived exosomes in tumor immunotherapy. Additionally, we emphasize the potential of T. gondii-derived exosomes as delivery platform to enhance anti-tumor efficacy in combination with other therapies. This review will assist in promoting the application of parasite exosomes in tumor therapy.
Immunomodulatory and Anti-Tumor Effects of Toxoplasma gondii
Toxoplasma gondii
T. gondii is one of the most common parasites worldwide, infecting nearly one-third of the global population. T. gondii invades organs such as the heart through the circulatory system, resulting in chronic infection, and is able to cross biologically restrictive barriers such as the blood‒brain barrier (BBB) and placental barrier to enter immune-privileged sites.40,41 Although T. gondii infection is asymptomatic or presents with recurrent ocular lesions, lymphadenopathy, etc, in most immunocompetent individuals, immunocompromised individuals can develop life-threatening central nervous system infections after repeated chronic infections.40,42 Studies have consistently shown the immunomodulatory and anti-tumor functions of T. gondii. In the 1970s, researchers reported the phenomenon of growth restriction of solid tumors in mice infected with T. gondii,43,44 which attracted widespread attention. Subsequently, studies on T. gondii and its products have increased, further revealing the immunomodulatory and anti-tumor effects of T. gondii.
Toxoplasma gondii-Mediated Immune Regulation
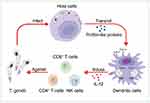
T. gondii triggers innate immune responses mediated by macrophages, dendritic cells, neutrophils, monocytes, and natural killer (NK) cells during the acute phase of host infection.45 Subsequent generation of specific immune responses against T. gondii infection relies on cells such as NK cells, CD4+ T cells, and CD8+ T cells, which produce interferon-γ (IFN-γ).42,46 Innate immunity serves as the first defense of the host after infection with pathogens. As an important part of innate immunity, Toll-like receptors (TLRs) recognize microbial components of pathogen-associated molecular patterns (PAMPs) and initiate subsequent immune responses.47 After infection of the host by T. gondii, Profilin-like proteins, which can be recognized by TLR11 and TLR12 of the TLR family in mice, are released and ultimately induce the production of interleukin 12 (IL-12) in mouse dendritic cells.48,49 A significant reduction in IL-12 production by dendritic cells can be observed in T. gondii-infected mouse models after knocking out important molecules in TLR-related pathways.50 TLR11 and TLR12 are not expressed by human genes; however, human TLR7 and TLR9 can recognize RNA and DNA derived from T. gondii, respectively,51 and may accordingly induce host cells to produce high levels of proinflammatory cytokines, including IL-12 and TNF-α. IL-12 produced by dendritic cells, macrophages, etc, induces massive proliferation of NK cells,52 CD4+ T cells, and CD8+ T cells against T. gondii infection (Figure 1).53,54 In addition to these cells, antigen-presenting cells such as monocytes also play an important role in T. gondii infection. Upon recognition of T. gondii antigens by these antigen-presenting cells, genes encoding chemokines, such as CCL2 and CXCL2, are induced, resulting in massive production of chemokines that drive neutrophils and monocytes toward infected sites.55 Furthermore, innate lymphoid cells Group 1 (ILCs1), including NK cells, produce Th1-type cytokines, such as IFN-γ and tumor necrosis factor-α (TNF-α), to counteract T. gondii infections via the oral route.56 The mechanisms described above favor the organism over T. gondii infection, and the potential application of this host immunomodulatory capacity due to T. gondii infection in tumor therapy has drawn the attention of researchers.
Toxoplasma gondii and Tumor Immunotherapy
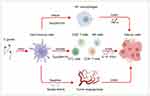
T. gondii triggers a vigorous immune response in the host to control self-replication and facilitate its survival and proliferation within host. Such vigorous immune responses can also inhibit the progression of tumors (Figure 2).57 After infecting host immune cells, the T. gondii Me49 strain induces the production of certain soluble factors that exert strong and systemic anti-tumor angiogenic effects, leading to severe hypoxia within the tumor tissues, which in turn inhibits the growth of B16F10 melanoma cells in experimental animal models.58 A subsequent study found that the survival rate of Lewis lung carcinoma-loaded mice significantly increased after infection with the T. gondii Me49 strain. In this study, researchers not only observed significant inhibition of tumor angiogenesis in mice but also significant increases in IFN-γ-related mRNA expression levels, CD8+ T-cell percentages, and cytotoxic T lymphocyte (CTL) counts, suggesting that the T. gondii Me49 strain achieves anti-tumor effects by modulating host immunity.59 Recently, another study found that the T. gondii Me49 strain can stimulate dendritic cells to produce exosomes, which achieve inhibition of tumor growth in hormonal mice by suppressing macrophage polarization toward the M2 type.60 In addition to the T. gondii Me49 strain, researchers have developed the T. gondii nonreplicating avirulent uracil auxotroph vaccine strain (CPS). Uridine-5’-mono-phosphate (UMP) is necessary for nucleic acid synthesis by T. gondii. UMP production can be blocked by blocking T. gondii synthetic pyrimidines, which leads to the inability of T. gondii replication. Modified strains become T. gondii CPS strains characterized by low toxicity.23,61 After treatment with the T. gondii CPS strain, B16F10 melanoma-loaded mice showed significantly increased counts and activity of CD8+ T cells, while primary tumors gradually regressed.21 T. gondii CPS strains are not specific to melanoma, they show similar inhibitory effects on ovarian,62 pancreatic,63 and breast cancers.64 Immune cells such as CD8+ T cells and NK cells and immune-related substances such as IL-12, IFN-γ, and chemokine receptor 3 (CCR3) play key roles in this anti-tumor effect. Subsequently, the anti-tumor effect of T. gondii CPS strains was shown to be based on the secretion of rhoptry protein (ROP) or dense granule protein (GRA), and knockout of genes related to these two proteins resulted in a significant reduction of the anti-tumor effect.24 Researchers also observed similar effects on the products of treated T. gondii as well. Back in the 1990s, researchers discovered the anti-tumor effect of T. gondii lysate antigen (TLA). After using T. gondii lysate antigen on YAC-1 lymphoma cell-loaded mice, a significant enhancement of the cytotoxic response was observed.65 Treating mice with TLA before tumor emergence can delay tumor initiation. Based on histopathological observations, the TLA-treated group showed significant tumor necrosis and infiltration of lymphocytes and neutrophils compared with groups without TLA treatment.66 Later, researchers found that TLA had an inhibitory effect on mouse colon cancer CT26 cells. After treating mice with TLA, they observed a significant reduction in the expression of the serum metastasis marker TIMP-1 and enhanced serum IL-12 and MyD88 signaling pathway expression in mouse macrophages.67 Many experiments have shown that the TLA-induced maturation of dendritic cells exerts anti-tumor effects through specific immune functions.68,69 T. gondii virulence-associated dense granule protein (ToxoGRA15II) can induce host macrophage polarization to the M1 type and downregulation of matrix metalloproteinase-9 (MMP-9) and MMP-2, which in turn inhibits Hepa1-6 tumor cell metastasis and invasion.70 ToxoGRA15II also induces polarized M1 cells to produce large amounts of TNF-α, which can inhibit liver fibrosis and sarcoidosis.71 In addition, since ToxoGRA15II is nontoxic to mammals, its potential value as a novel tool to modulate host immunity and anti-tumor is noteworthy. These studies have documented the immunomodulatory and anti-tumor effects of T. gondii through both cellular and animal experiments. In summary, T. gondii can transform tumors from “cold” to “hot” by awakening the host’s immune response and destroying the immunosuppressive tumor microenvironment, ultimately reducing the tumor size and inhibiting tumor metastasis. However, because of various obstacles such as biosafety issues and economic burdens, the clinical application of T. gondii requires more research support.
Immunomodulatory and Anti-Tumor Effects of T. gondii-Derived Exosomes
T. gondii-Derived Exosomes
Exosomes were first isolated from tumor cell lines in the 1880s and were initially described as vesicles with 5’-nucleotidase activity.72 Subsequent research has shown that exosomes are nanoscale vesicles containing various components, such as proteins, nucleic acids, lipids, and metabolites, with diameters ranging from 30 nm to 150 nm; these vesicles are released from intracellular multivesicular bodies (MVBs).73 Exosomes are produced by various cells and are involved in a wide range of physiological activities, such as cell-to-cell communication and functional regulation.28 Exosomes play an important role in the generation, diagnosis, and treatment of tumors, infectious diseases, and cardiovascular diseases.74,75 Recent studies have reported that numerous parasites, such as Schistosoma japonicum,76 Leishmania protozoa,77 Trypanosoma brucei,78 Plasmodium falciparum, Neospora caninum,79 and many others, can communicate with host cells by secreting exosomes and delivering virulence factors, drug resistance factors, and differentiation factors, thus modulating the immune responses of hosts.80,81 T. gondii can also produce exosomes. Some researchers have successfully identified T. gondii-derived exosomes and analyzed them using proteomics, revealing several classical exosomal proteins.82 Further studies on T. gondii-derived exosomes using transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) revealed that T. gondii-derived exosomes range from 10 to 150 nm in diameter, averaging approximately 50 nm. The specific markers of T. gondii-derived exosomes, recombinant lysosomal associated membrane protein 3 (CD63), heat shock protein 70 (Hsp70), and surface antigen 1 (SAG1, also named P30), were also detected using protein blotting.83
Isolation and Identification of T. gondii-Derived Exosomes
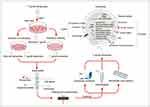
Exploring exosomes is highly important for related life science research. The prerequisites for exploring exosomes are isolation and characterization techniques with high purity and yield. Exosomes derived from different sources carry distinct bioactive substances,28,84 and differ in size; thus, different isolation techniques are required.85 Traditional isolation techniques include ultracentrifugation-based methods,86,87 size-based methods74 and precipitation,88 as well as immunoaffinity capture, which is based on the binding of aptamers or antibodies to specific proteins in exosomes.89 Currently, ultracentrifugation, which has the advantages of simplicity and quantity, is the gold standard for isolating exosomes and is suitable for large samples. However, some studies have shown that ultracentrifugation may destroy exosome vesicles and interfere with subsequent research.90 In summary, traditional exosome isolation techniques are cumbersome, require special equipment, and are costly and time-consuming. Researchers often combine these techniques in complementary manners, for example, by combining ultrafiltration with size-exclusion chromatography (SEC), which allows for the extraction of higher-purity free exosome concentrates.91,92 In recent years, novel technologies have emerged, providing new possibilities for exosome isolation and characterization. Microfluidic chip technology is based on a chip containing numerous microtubules that can handle microvolume fluid.93 In the field of exosome isolation and identification, synthesized microfluidic chip systems combining ultracentrifugation, immunoaffinity capture methods, and other technologies have emerged.94,95 These methods are convenient, automated, and applicable for the analysis of minor samples due to their low throughput. Some researchers have achieved markerless detection of PD-L1 in exosomes using surface plasmon resonance (SPR) biochips.96 Proper culture of T. gondii and obtaining supernatants that contain T. gondii-derived exosomes is essential for their isolation. Two common techniques determine the purity and yield of T. gondii-derived exosomes.82,97 First, the host cells are infected with T. gondii tachyzoites. After a period of coculture, T. gondii is separated from the cells by centrifugation. Then, activated T. gondii is introduced into medium without exosomes, and the supernatant is collected for subsequent isolation after the appropriate time. Second, based on the former method, without separating activated T. gondii from the host cells, the culture is continued, and the supernatant containing exosomes is collected for subsequent isolation (Figure 3). The latter technique enables specialized intracellular parasitism of T. gondii and can maintain T. gondii activity for a long time, resulting in a greater yield of exosomes. However, this approach results in lower purity because host cells can also secrete exosomes. For the isolation of T. gondii-derived exosomes from the supernatant, the most used methods include centrifugation and exosome sorting kits. Exosome sorting kits are based on polymer precipitation technology and allow easier handling, lack special equipment, as well as higher recovery rates than centrifugation.98 After repeated centrifugation and concentration of the supernatants of T. gondii cocultured with cells in combination with specialized kits, exosome samples meeting the requirements for subsequent experiments can be extracted. However, problems such as lower purity due to nonexosome contamination still cannot be solved. The use of emerging technologies such as microfluidic microarrays with high purity and high recovery rates for the isolation and characterization of T. gondii-derived exosomes is a new direction for future research. After extracting T. gondii-derived exosomes, the concentration, ultrastructure, particle size, and markers of exosomes must be determined using techniques such as nanoparticle tracking and analysis (NTA), TEM, scanning electron microscopy (SEM) and Western blotting (WB) for subsequent analysis.99,100
Composition of T. gondii-Derived Exosomes
The immunomodulatory functions of T. gondii-derived exosomes rely on their contents of proteins, nucleic acids, and lipids. Omics analysis of T. gondii-derived exosomes will contribute to unraveling their biological functions. T. gondii-derived exosomes contain various protein components, including ROP proteins, GRA proteins, microneme proteins (MIC), surface antigen (SAG), cytoskeletal proteins, heat shock proteins (HSP), and Rab proteins.37,82 These proteins widely participate in several physiological processes, such as invading host cells and regulating host immunity. Some researchers analyzed proteins of exosomes derived from T. gondii, T. gondii-infected human foreskin fibroblasts (HFFs), and T. gondii-uninfected HFFs utilizing NTA, qubit fluorometric quantification, and other proteomics-related techniques.82 Notably, 346, 69, and 15 proteins were successfully identified as specific to exosomes from the three sources, respectively, and 216 proteins were common. A comparison of the 216 common proteins with the EVpedia database revealed that 24.7% of proteins are among the top 100 proteins in the EVpedia database,101 including classical exosome protein markers such as extracellular matrix glycoproteins, calcium-binding proteins, fiber-associated proteins, membrane-bound protein family members, HSP70, and CD63. Later, another researcher successfully identified the protein composition of T. gondii-derived exosomes, which consisted of 3 MIC proteins, 11 GRA proteins, 16 ROP proteins, 12 SAG proteins, 3 hSP proteins, 5 Rab proteins, 10 kinases, 3 phosphatases, 7 proteases, and 215 other proteins (Figure 3).102
T. gondii-derived exosomes also contain abundant nucleic acid components (Figure 3), including miRNAs, but their types, quantities, and functions remain unclear.37,103 Current research focuses on exosomes derived from host immune cells infected with T. gondii. T. gondii stimulates host dendritic cells to produce exosomes, which contain various mRNAs and miRNAs. After exosomes are internalized by recipient cells, these RNAs further influence biological processes such as immune responses in the organism by regulating gene expression. Several researchers have extracted and purified exosomal RNA from T. gondii (PRU strain) tachyzoites that infected HFFs using ultracentrifugation and an exosome sorting kit and analyzed it with microarrays. A total of 222 differentially expressed RNAs were found, and miR-23b was highly expressed. miR-23b can regulate the immune response in host cells by inhibiting the expression of the cytokine IL-17.104 Some researchers have observed that exosomes derived from rat myoblast L6 cells infected with T. gondii (RH strain) tachyzoites contain 64 differentially expressed miRNAs. Eleven of these miRNAs are related to the cell cycle and proliferation and regulate various cell cycle-related genes, such as cytochalasin D2.105 In recent years, another researcher performed a high-throughput analysis of exosomes derived from DC cells infected with tachyzoites of T. gondii (RH strain) and found 3434 more miRNAs than exosomes from DC cells not infected with T. gondii. Further bioinformatics analyses revealed that 12 of these stably enriched miRNAs were extensively involved in host innate immune regulatory processes.26
Studies on the lipid composition of exosomes are limited. According to the ExoCarta database, exosome membranes contain 1116 lipids, including cholesterol, sphingomyelin, and phosphatidic acid (PA), in varying quantities.106 The total amounts and components of exosomal lipids vary among different subtypes and are closely related to their original cells.107 A study revealed that exosomes from sheep erythrocyte culture supernatants contained high levels of sphingomyelin, which is specific to the plasma membranes of sheep erythrocytes.108 Exosomes secreted by B cells contain lipid components such as lysophosphatidic acid (LPA) and cholesterol (Figure 3).109,110 Studies on the lipid composition of T. gondii-derived exosomes are lacking, but researchers have hypothesized that T. gondii-derived exosomes contain specific lipid fractions from T. gondii. However, related issues remain to be explored.
During the invasion of host cells by T. gondii, the various components mentioned above are loaded into exosomes and then involved in host cell adhesion, invasion, and intracellular localization. Examples include proteins such as MIC2 and MIC4, which are involved in the adhesion process;111,112 ROP and GRA proteins, which collectively participate in the formation of intracellular parasitophorous vacuoles;113 and RON proteins and AMA1 proteins, which form kinetic linkages during T. gondii exosome internalization and serve to exclude host plasma membranes.114
Immunomodulatory Activity of T. gondii-Derived Exosomes
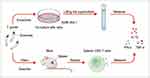
A few studies have investigated the immunomodulatory function of T. gondii-derived exosomes. Some researchers successfully isolated and identified T. gondii-derived exosomes using TEM, NTA, and WB techniques and cocultured high concentrations of T. gondii-derived exosomes with a mouse macrophage line (RAW264.7). Finally, the extraction and analysis of the supernatants revealed that macrophages secreted significantly more IL-12, TNF-α, and IFN-γ but secreted significantly less IL-10. Researchers also immunized BALB/c mice with T. gondii RH strain tachyzoite exosomes (Exo-R) and T. gondii Me49 strain tachyzoite exosomes (Exo-M). High levels of IgG2α were detected in serum from both groups of mice, and the levels of IgG2α were significantly higher than those of IgG1, suggesting that T. gondii-derived exosomes induced the onset of a Th1-type cellular immune response in mice. Moreover, the percentages of CD8+ T cells in the spleens of exosome-treated mice and the concentrations of IFN-γ and IL-12 in the supernatants of splenic lymphocyte cultures were significantly higher than those in the controls, which supports the view that T. gondii-derived exosomes stimulate humoral and cellular immunity in mice (Figure 4).83 Subsequent experiments on the function of T. gondii-derived exosomes in macrophages using similar methods revealed a significant increase in the concentrations of IL-12, IFN-γ, and TNF-α secreted by macrophages under the effect of T. gondii-derived exosomes, which was shown to be the result of exosomes triggering innate immunity by activating the c-Jun N-terminal kinase (JNK) signaling pathway.97 In recent years, some researchers have also immunized mice with purified T. gondii-derived exosomes and found that IFN-γ and TNF-α levels were significantly increased in brain and spleen cells, and the mortality rate of T. gondii-infected mice was also significantly decreased.115 Another study revealed that T. gondii secretes exosomes after host invasion, and the exosomes present T. gondii antigens to T cells either directly or via antigen-presenting cells, which activate T cells and immune responses.39
Immunomodulatory Effect of T. gondii-Infected Cell-Derived Exosomes
Other studies on the immunomodulatory effect of T. gondii-associated exosomes have focused on exosomes derived from infected host cells.60,116 After invading the host, T. gondii can stimulate the production of exosomes by dendritic cells, macrophages, monocytes, and even tumor cells. In 2004, researchers stimulated host dendritic cells to produce exosomes using soluble T. gondii antigens and attempted to prepare cell-free vaccines based on exosomes. After injecting exosomes into mice, a significant increase in IL-2 and IFN-γ levels in mouse splenocytes was observed, suggesting the generation of vigorous Th1-type cellular immune responses and a favorable anti-infective effect.117 After phagocytosis of T. gondii, macrophages can release exosomes with T. gondii antigens, and these exosomes activate other macrophages to release cytokines, such as TNF-α, and destroy pathogens.118 The T. gondii-infected human monocyte cell line THP-1 also releases exosomes that stimulate uninfected THP-1 cells to produce TNF-α and other proinflammatory mediators to protect against infection.118 Exosomes derived from infected dendritic cells of pregnant maternal mice protect murine fetuses from vertically transmitted infections, and cystic loads in the brains of pups were found to be significantly reduced. This effect was accompanied by strong immune responses and changes in the expression levels of numerous cytokines in the serum of mice.119 In recent years, several studies have attempted to validate the therapeutic effect of exosomes secreted by dendritic cells infected with the T. gondii Me49 strain (Me49-DC-Exos) in mouse colorectal cancer models. Significant inhibition of tumor growth was observed, and subsequent experiments verified that Me49-DC-Exos achieved tumor inhibition by regulating the proportion of myeloid-derived suppressor cells through the suppression of signal transducer activator of transcription 3 (STAT3).116 Researchers followed up with experiments comparing the effects of uninfected dendritic cell exosomes (DC-Exos) with those of Me49-DC-Exos in colon cancer-bearing mice. Tumor growth was significantly inhibited in the Me49-DC-Exo group compared to the DC-Exo group, and the proportion of M2 macrophages in mouse blood decreased markedly. In subsequent cellular experiments, this outcome resulted from exosomes regulating SOCS1 gene expression, and an increase in SOCS1 gene expression increased the proportion of M1 macrophages.60 Recently, several researchers have extracted exosomes from T. gondii-infected human hepatoblastoma cells, treated them with alum adjuvant, and performed immune induction experiments on cerebral cyst-loaded mice. The expression of IFN-γ and IL-4 and the numbers of CD4+ and CD8+ T lymphocytes were significantly elevated in mice, suggesting the successful induction of humoral immunity and mixed Th 1/Th 2 cell immunity and a marked reduction in the brain cyst load by 75% in mice.120 These findings provide new insights into tumor immunotherapy. The ability of T. gondii-derived exosomes to modulate host immune responses confers their potential as tumor immunotherapy vaccines (Figure 5). In addition, T. gondii-derived exosomes may also affect the host cell cycle and proliferation. Researchers have found that L6 cells infected with T. gondii RH strains have a decreased proliferative capacity and an increased proportion of cells in S phase or G2/M phase.105
Potential of T. gondii-Derived Exosomes in Tumor Immunotherapy
Although few relevant studies are available, the potential of T. gondii-derived exosomes in tumor immunotherapy remains noteworthy. First, numerous studies have reported the role of T. gondii in immune modulation and tumor therapy. As mentioned above, direct use of attenuated T. gondii strains or T. gondii extracts such as TLA, GRA, and ROP can stimulate host immune cells, including dendritic cells, macrophages, CD8+ T cells, and NK cells, and induce significantly increased expression of cytokines and other factors, such as IL-12, IFN-γ, and TNF-α; such strong immune responses can simultaneously inhibit tumor progression.24,67 Essentially, this effect stems from T. gondii’s Profilin-like proteins, ToxoGRA15II, RNA, and other immunogenic contents.48,70 These components, when recognized by host immune cells, activate a series of immune responses, such as subsequent antigen processing and antigen presentation, which in turn play an anti-tumor role. The composition and function of exosomes are closely related to their original cells. During the formation of exosomes, associated molecules in the cytoplasm, such as nucleic acids, lipids, and proteins, are endocytosed and transferred to early endosomes. Early endosomes mature and differentiate into late MVBs or endosomes, which are degraded after binding with lysosomes or fusing with plasma membranes and are subsequently released to extracellular sites to form exosomes.121,122 In summary, exosomes contain specific substances, such as proteins from the cells of origin, which could provide T. gondii-derived exosomes with immunomodulatory and anti-tumor effects similar to those of living T. gondii strains.
As an anti-tumor tool, T. gondii-derived exosomes possess numerous advantages over live T. gondii strains. For example, as exosome extraction and identification techniques continuously develop, obtaining high-precision and high-purity target exosomes becomes simpler.123 Compared to live T. gondii strains, exosomes can be collected and preserved in large quantities upfront without longer-term live cell maintenance cultures to maintain their activity. In addition, T. gondii-derived exosomes are significantly superior to live T. gondii strains in terms of biosafety. T. gondii contains other unidentified components, and even after attenuation, direct use in patients may still have unforeseen adverse effects. Exosomes are composed of phospholipid bilayers, which can be easily accessed by receptor cells through ligand–receptor binding or direct membrane fusion, endocytosis, and even phagocytosis.124,125 They can also cross biological barriers such as the BBB, intestinal barrier, and placental barrier to reach specific sites and therefore could be used in various drug delivery routes.126 Exosomes, as natural carriers of intercellular information, possess high stability, low toxicity, and low immunogenicity in the circulation, as well as excellent modifiability; thus, they can be used for the loading and transportation of therapeutic drugs as novel drug delivery platforms and have high potential as immunomodulatory and anti-tumor agents.127,128 In summary, the significant potential of T. gondii-derived exosomes in the field of tumor immunotherapy merits attention.
Prospects of T. gondii-Derived Exosomes in Tumor Therapy
Immunotherapy or chemotherapy drugs need to be delivered precisely to target cells to achieve therapeutic efficacy and avoid the adverse effects associated with whole-body drug administration. However, deep tumors remain difficult to treat pharmacologically due to the presence of biological barriers such as the BBB. In addition, the nutritional blood vessels of tumors are mostly distributed more densely around the periphery of tumors, making it difficult to transport drugs through the circulation to the center of tumors, which affects drug efficacy.129 Some researchers have found that some chemotherapy drugs are poorly targeted when conducting tumor chemotherapy, leading to decreased anti-tumor effects and an increased incidence of various adverse reactions, such as neurotoxicity130 and cardiovascular toxicity.131 Research on drug delivery systems (DDSs) to improve drug efficacy is emerging. DDSs are designed to efficiently deliver drug molecules to targets and reduce the incidence of adverse reactions. Popularly utilized DDSs include nanomaterials, liposomes,132 and hydrogels.133
In recent years, studies have consistently documented the potential of engineered exosomes with appropriate modifications as drug carriers in various oncology therapies.127,134 Engineered exosomes, as novel drug carriers for tumor therapy, offer long half-lives, better biocompatibility, low toxicity, greater tissue targeting ability, and high modifiability compared to current well-established DDSs, such as liposomes and polymer nanoparticles,135,136 and have relatively fewer adverse effects.137 The introduction of anti-tumor drugs into exosomes by electroporation and other techniques to obtain nanoplatforms, the so-called Trojan horse, which can cross biological barriers and penetrate deeply into tumor tissues, dramatically improves the therapeutic effect of these drugs.138 Biomodification of exosomes is also possible using targeting peptides139 or aptamers,140 which can endow exosomes with significantly higher tumor affinity and specific targeting capabilities, further enhancing their efficacy and preventing adverse reactions. Accordingly, researchers have developed exosomes expressing characteristic exosomal membrane proteins and loaded them with drugs such as doxorubicin (Dox) by electroporation. In experiments in which tumor-loaded mice were treated, these exosomes exhibited significant tumor growth inhibition without remarkable toxicity.141,142
T. gondii-derived exosomes possess multiple advantages from exosomes, as they inherit the characteristics, components, and ability of T. gondii to modulate the host immune response, which can overcome various shortcomings of existing DDSs and has promising applications in tumor therapy (Figure 6). T. gondii-derived exosomes can activate host antigen presentation, stimulate host immune cell secretion, weaken the immunosuppressive microenvironment, and greatly increase the expression of cytokines such as IL-12, TNF-α, and IFN-γ. When applied for tumor immunotherapy, engineered T. gondii-derived exosomes can accurately and efficiently deliver drugs such as PD-1 monoclonal antibody, Dox, and RNA to target cells. In addition, these exosomes weaken the immunosuppressive microenvironment induced by tumor cells, restore the anti-tumor effects of T cells and other immune cells, and transform tumors from “cold” to “hot”, resulting in efficient anti-tumor effects on deep tumors. However, there are still challenges that need to be overcome on the path to clinical translation of T. gondii-derived exosomes. Firstly, both existing T. gondii culture techniques are flawed (Figure 3). Co-culture with host cells results in exosomes from host cells being mixed in the collected supernatant. Individual culturing of T. gondii reduces the activity of T. gondii, leading to a reduction in the quality and quantity of exosomes secreted by T. gondii. Secondly, T. gondii can be divided into various subtypes; therefore, it is necessary to investigate the distinct host immunomodulatory functions of exosomes from different subtypes of T. gondii for the sake of safer clinical application. In addition, because T. gondii-derived exosomes contain a variety of antigenic components from T. gondii, they acquire the ability to modulate host immunity while inevitably potentially causing adverse reactions in the host. Therefore, more research is necessary to investigate the specific T. gondii antigenic components in T. gondii-derived exosomes and their mechanisms of host immunomodulatory effects. Pursuing the goal of achieving precise sorting of T. gondii-derived exosomes, and even attenuating or removing specific antigenic components. In addition, with further research on T. gondii-derived exosomes and advances in related production technologies, the use of laboratory-produced DDSs containing T. gondii components may be able to activate the host immune response while offering a better biosafety profile. Finally, because therapy with T. gondii-derived exosomes relies on an intact immune system, it may be significantly less effective when used in tumor patients with severe immunocompromise. To date, research on the application of T. gondii-derived exosomes remains in the exploratory stage. T. gondii-derived exosomes may become an excellent tool for tumor therapy in the future.
Conclusion
The emergence of tumor immunotherapy has brought a new pathway for tumor treatment but also confronts obstacles such as low response rates and adverse effects. The ability of T. gondii to modulate host immunity and combat tumors has been demonstrated in some studies, making it a possible tool for adjuvant tumor immunotherapy. However, T. gondii cultures are technically sensitive and suffer from biosafety concerns, limiting the availability of applications in tumor immunotherapy. In order to address these problems, finding new ways to utilize T. gondii has become critical. Evidence provided herein suggests that T. gondii can also secrete exosomes and that they may be a potent tool to assist in tumor immunotherapy. Firstly, T. gondii exosomes are characterized by low toxicity and immunogenicity. Secondly, it can be collected in large quantities in advance without long-term live cell culture. Finally, T. gondii exosomes contain fewer unidentified components than live T. gondii strains, making their biosafety performance superior. Based on these, the modified engineered T. gondii exosomes platform has a longer half-life, better biocompatibility, and better targetability. Such platforms can load anti-tumor drugs (such as PD-1 monoclonal antibody, Dox, and RNA) across biological barriers (such as the BBB) to precisely reach the tumor sites. Meanwhile, it can also stimulate immune cells, increase the expression of various cytokines, relieve the tumor-induced immunosuppressive microenvironment, and ultimately enhance the anti-tumor efficacy. Further work is required to bring engineered T. gondii exosomes into clinical application. Firstly, researchers are needed to examine and minimize the cytotoxicity and immunogenicity of T. gondii-derived exosomes. Secondly, existing exosome isolation and extraction techniques have been applied most frequently to various cells and relatively few to T. gondii. Thus, to increase the purity and yield of the exosomes obtained from T. gondii, it is necessary to further test the ability of T. gondii to secrete exosomes, as well as to develop the isolation and extraction techniques suitable for T. gondii. Thirdly, more animal experiments should then be carried out to clarify the specific mechanism of the immunomodulatory function of engineered T. gondii-derived exosomes on organisms, to assess their anti-tumor and biosafety properties, and to study their stability within the blood circulation, pharmacokinetics, and pharmacodynamics. Finally, the criteria for approval for marketing can only be met after a series of rigorously designed clinical trials with the cooperation of clinicians. In conclusion, continued understanding, experimentation, and application of the immune response regulation mechanisms and potential as carriers of anti-tumor drugs of T. gondii exosomes can provide new perspectives in tumor immunotherapy.
Abbreviations
BBB, Blood‒brain barrier; CCR3, Chemokine receptor 3; CAR-T, Chimeric antigen receptor T-cell; JNK C-Jun N-terminal kinase; CTL, Cytotoxic T lymphocyte; DC-Exos, Dendritic cell exosomes; GRA, Dense granule protein; Dox, Doxorubicin; DDSs, Drug delivery systems; Me49-DC-Exos, Exosomes secreted by dendritic cells infected with the T. gondii Me49 strain; HSP, Heat shock proteins; HFFs, Human foreskin fibroblasts; ICB, Immune checkpoint blockade; ILCs1, Innate lymphoid cells Group 1; IFN-γ, Interferon-γ; IL-12 Interleukin 12; LPA, Lysophosphatidic acid; MMP-9, Matrix metalloproteinase-9; MIC, Microneme proteins; MVBs, Multivesicular bodies; NTA, Nanoparticle tracking analysis; NK, Natural killer; PAMPs, Pathogen-associated molecular patterns; PA, Phosphatidic acid; CD63 Recombinant lysosomal associated membrane protein 3; ROP, Rhoptry protein; SEM, Scanning electron microscopy; SEC, Size-exclusion chromatography; SAG, Surface antigen; SAG1, Surface antigen 1 (also named P30); SPR, Surface plasmon resonance; TLA, T. gondii lysate antigen; Exo-M, T. gondii Me49 strain tachyzoite exosomes; CPS T. gondii nonreplicating avirulent uracil auxotroph vaccine strain; Exo-R T. gondii RH strain tachyzoite exosomes; ToxoGRA15II T. gondii virulence-associated dense granule protein; TLRs, Toll-like receptors; T. gondii, Toxoplasma gondii; STAT3, Transcription 3; TEM, Transmission electron microscopy; TNF-α, Tumor necrosis factor-α; UMP, Uridine-5’-mono-phosphate; WB, Western blotting.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Fund of Anhui Institute of Translational Medicine (2023zhyx-C90), the Basic and Clinical Cooperative Research and Promotion Program of Anhui Medical University (2023xkjT046), the National Natural Science Foundation of China (82072304), and the Health Research Program of Anhui (AHWJ2022b001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502. doi:10.1038/s41569-020-0348-1
3. Banfill K, Giuliani M, Aznar M, et al. Cardiac Toxicity of Thoracic Radiotherapy: existing Evidence and Future Directions. J Thorac Oncol. 2021;16:216–227. doi:10.1016/j.jtho.2020.11.002
4. Tang J, Pearce L, O’Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018;17:783–784. doi:10.1038/nrd.2018.167
5. Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15:28. doi:10.1186/s13045-022-01247-x
6. Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–648. doi:10.1038/s41586-023-05707-3
7. Zhang L, Zhao J, Hu X, et al. A Peritumorally Injected Immunomodulating Adjuvant Elicits Robust and Safe Metalloimmunotherapy against Solid Tumors. Adv Mater. 2022: 34:e2206915. doi:10.1002/adma.202206915
8. Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther. 2022;7:331. doi:10.1038/s41392-022-01136-2
9. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi:10.1038/s41422-020-0343-4
10. Rui R, Zhou L, He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. 2023;14:1212476. doi:10.3389/fimmu.2023.1212476
11. Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33:3293–3304. doi:10.1200/jco.2015.61.1509
12. Manca P, Raez LE, Salzberg M, Sanchez J, Hunis B, Rolfo C. The value of immunotherapy in head and neck cancer. Expert Opin Biol Ther. 2019;19:35–43. doi:10.1080/14712598.2019.1556637
13. Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity. 2020;52:17–35. doi:10.1016/j.immuni.2019.12.011
14. Vogrig A, Muñiz-Castrillo S, Joubert B, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psych. 2020;91:772–778. doi:10.1136/jnnp-2020-323055
15. Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495–508. doi:10.1038/s41573-021-00259-5
16. Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 2021;184:1575–1588. doi:10.1016/j.cell.2021.02.011
17. Kochanowsky JA, Koshy AA. Toxoplasma gondii. Curr Biol. 2018; 28:R770–r771. doi:10.1016/j.cub.2018.05.035
18. Elsheikha HM, Marra CM, Zhu XQ. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin Microbiol Rev. 2021;34:e00115–00119. doi:10.1128/cmr.00115-19
19. Chen J, Liao W, Peng H. Toxoplasma gondii infection possibly reverses host immunosuppression to restrain tumor growth. Front Cell Infect Microbiol. 2022;12:959300. doi:10.3389/fcimb.2022.959300
20. Dupont CD, Christian DA, Selleck EM, et al. Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS Pathog. 2014; 10:e1004047. doi:10.1371/journal.ppat.1004047
21. Baird JR, Byrne KT, Lizotte PH, et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190:469–478. doi:10.4049/jimmunol.1201209
22. Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol. 2021;19:467–480. doi:10.1038/s41579-021-00518-7
23. Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415:926–929. doi:10.1038/415926a
24. Fox BA, Sanders KL, Rommereim LM, Guevara RB, Bzik DJ. Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity. PLoS Genet. 2016; 12:e1006189. doi:10.1371/journal.pgen.1006189
25. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi:10.1146/annurev-biochem-013118-111902
26. Li DL, Zou WH, Deng SQ, Peng HJ. Analysis of the Differential Exosomal miRNAs of DC2.4 Dendritic Cells Induced by Toxoplasma gondii Infection. Int J Mol Sci. 2019;20:5506. doi:10.3390/ijms20215506
27. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi:10.1186/s12943-019-0991-5
28. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi:10.1126/science.aau6977
29. Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334–10347. doi:10.1128/jvi.01310-13
30. Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi:10.1074/jbc.M112.445403
31. Maus RLG, Jakub JW, Nevala WK, et al. Human Melanoma-Derived Extracellular Vesicles Regulate Dendritic Cell Maturation. Front Immunol. 2017;8:358. doi:10.3389/fimmu.2017.00358
32. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi:10.1016/j.ccell.2016.10.009
33. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi:10.1038/nrclinonc.2017.14
34. Lee NK, Kothandan VK, Kothandan S, Byun Y, Hwang SR. Exosomes and Cancer Stem Cells in Cancer Immunity: current Reports and Future Directions. Vaccines. 2021;9:441. doi:10.3390/vaccines9050441
35. Buscail L. Pancreatic cancer: exosomes for targeting KRAS in the treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2017;14:636–638. doi:10.1038/nrgastro.2017.113
36. Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. doi:10.3402/jev.v4.28533
37. Silva VO, Maia MM, Torrecilhas AC, et al. Extracellular vesicles isolated from Toxoplasma gondii induce host immune response. Parasite Immunol. 2018;40:e12571. doi:10.1111/pim.12571
38. Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. doi:10.15252/embr.201439363
39. Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi:10.1038/ni854
40. Lourido S. Toxoplasma gondii. Trends Parasitol. 2019;35:944–945. doi:10.1016/j.pt.2019.07.001
41. Harker KS, Ueno N, Lodoen MB. Toxoplasma gondii dissemination: a parasite’s journey through the infected host. Parasite Immunol. 2015;37:141–149. doi:10.1111/pim.12163
42. Sasai M, Pradipta A, Yamamoto M. Host immune responses to Toxoplasma gondii. Int Immunol. 2018;30:113–119. doi:10.1093/intimm/dxy004
43. Conley FK, Remington JS. Nonspecific inhibition of tumor growth in the central nervous system: observations of intracerebral ependymoblastoma in mice with chronic Toxoplasma infection. J Natl Cancer Inst. 1977;59:963–973. doi:10.1093/jnci/59.3.963
44. Conley FK. Influence of chronic Toxoplasma infection on ethylnitrosourea-induced central nervous system tumors in rats. Cancer Res. 1980;40:1240–1244.
45. Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi:10.1038/nrmicro2858
46. Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi:10.1128/iai.62.7.2818-2824.1994
47. Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116–133. doi:10.3109/08830185.2013.774391
48. Koblansky AA, Jankovic D, Oh H, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38:119–130. doi:10.1016/j.immuni.2012.09.016
49. Plattner F, Yarovinsky F, Romero S, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi:10.1016/j.chom.2008.01.001
50. Scanga CA, Aliberti J, Jankovic D, et al. Cutting edge: myD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi:10.4049/jimmunol.168.12.5997
51. Andrade WA, Souza Mdo C, Ramos-Martinez E, et al. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13:42–53. doi:10.1016/j.chom.2012.12.003
52. Mahmoudzadeh S, Nozad Charoudeh H, Marques CS, Bahadory S, Ahmadpour E. The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: a mini-review. Parasitol Res. 2021;120:2303–2309. doi:10.1007/s00436-021-07204-w
53. Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90:6115–6119. doi:10.1073/pnas.90.13.6115
54. Khan IA, Matsuura T, Kasper LH. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–1642. doi:10.1128/iai.62.5.1639-1642.1994
55. Ma JS, Sasai M, Ohshima J, et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. 2014;211:2013–2032. doi:10.1084/jem.20131272
56. Klose CSN, Flach M, Möhle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi:10.1016/j.cell.2014.03.030
57. Lima TS, Lodoen MB. Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii. Front Cell Infect Microbiol. 2019;9:103. doi:10.3389/fcimb.2019.00103
58. Hunter CA, Yu D, Gee M, et al. Cutting edge: systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J Immunol. 2001;166:5878–5881. doi:10.4049/jimmunol.166.10.5878
59. Kim JO, Jung SS, Kim SY, et al. Inhibition of Lewis lung carcinoma growth by Toxoplasma gondii through induction of Th1 immune responses and inhibition of angiogenesis. J Korean Med Sci. 2007:S38–46. doi:10.3346/jkms.2007.22.S.S38
60. Zhu S, Lu J, Lin Z, et al. Anti-Tumoral Effect and Action Mechanism of Exosomes Derived From Toxoplasma gondii-Infected Dendritic Cells in Mice Colorectal Cancer. Front Oncol. 2022;12:870528. doi:10.3389/fonc.2022.870528
61. Fox BA, Bzik DJ. Avirulent uracil auxotrophs based on disruption of orotidine-5’-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun. 2010;78:3744–3752. doi:10.1128/iai.00287-10
62. Baird JR, Fox BA, Sanders KL, et al. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013;73:3842–3851. doi:10.1158/0008-5472.Can-12-1974
63. Sanders KL, Fox BA, Bzik DJ. Attenuated Toxoplasma gondii Stimulates Immunity to Pancreatic Cancer by Manipulation of Myeloid Cell Populations. Cancer Immunol Res. 2015;3:891–901. doi:10.1158/2326-6066.Cir-14-0235
64. Xu LQ, Yao LJ, Jiang D, et al. A uracil auxotroph Toxoplasma gondii exerting immunomodulation to inhibit breast cancer growth and metastasis. Parasit Vectors. 2021;14:601. doi:10.1186/s13071-021-05032-6
65. Yang MP, Goitsuka R, Ono K, Suzuki N, Hasegawa A. Effect of Toxoplasma lysate antigen (TLA) on feline cytotoxicity against FeLV positive lymphoma cells. Nihon Juigaku Zasshi. 1990;52:735–742. doi:10.1292/jvms1939.52.735
66. Miyahara K, Yokoo N, Sakurai H, et al. Antitumor activity of Toxoplasma lysate antigen against methylcholanthrene-induced tumor-bearing rats. J Vet Med Sci. 1992;54:221–228. doi:10.1292/jvms.54.221
67. Pyo KH, Jung BK, Xin CF, Lee YW, Chai JY, Shin EH. Prominent IL-12 production and tumor reduction in athymic nude mice after Toxoplasma gondii lysate antigen treatment. Korean J Parasitol. 2014;52:605–612. doi:10.3347/kjp.2014.52.6.605
68. Motamedi M, Arab S, Moazzeni SM, Khamis Abadi M, Hadjati J. Improvement of a dendritic cell-based therapeutic cancer vaccine with components of Toxoplasma gondii. Clin Vaccine Immunol. 2009;16:1393–1398. doi:10.1128/cvi.00199-09
69. Boghozian R, Saei A, Mirzaei R, et al. Identification of Toxoplasma gondii protein fractions induce immune response against melanoma in mice. Apmis. 2015;123:800–809. doi:10.1111/apm.12420
70. Li Y, Poppoe F, Chen J, et al. Macrophages Polarized by Expression of ToxoGRA15(II) Inhibit Growth of Hepatic Carcinoma. Front Immunol. 2017;8:137. doi:10.3389/fimmu.2017.00137
71. Xie Y, Wen H, Yan K, et al. Toxoplasma gondii GRA15(II) effector-induced M1 cells ameliorate liver fibrosis in mice infected with Schistosomiasis japonica. Cell Mol Immunol. 2018;15:120–134. doi:10.1038/cmi.2016.21
72. Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi:10.1016/0005-2736(81)90512-5
73. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi:10.1038/nri855
74. Guo J, Wu C, Lin X, et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J Extracell Vesicles. 2021;10:e12145. doi:10.1002/jev2.12145
75. Neves KB, Rios FJ, Sevilla-Montero J, Montezano AC, Touyz RM. Exosomes and the cardiovascular system: role in cardiovascular health and disease. J Physiol. 2023;601:4923–4936. doi:10.1113/JP282054
76. Zhu L, Zhao J, Wang J, et al. MicroRNAs Are Involved in the Regulation of Ovary Development in the Pathogenic Blood Fluke Schistosoma japonicum. PLoS Pathog. 2016: 12:e1005423. doi:10.1371/journal.ppat.1005423
77. Zauli RC, de Souza Perez IC, de Morais ACC, et al. Extracellular Vesicles Released by Leishmania (Leishmania) amazonensis Promastigotes with Distinct Virulence Profile Differently Modulate the Macrophage Functions. Microorganisms. 2023;11:2973. doi:10.3390/microorganisms11122973
78. Szempruch AJ, Sykes SE, Kieft R, et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell. 2016;164:246–257. doi:10.1016/j.cell.2015.11.051
79. Mota CM, Oliveira AC, Davoli-Ferreira M, et al. Neospora caninum Activates p38 MAPK as an Evasion Mechanism against Innate Immunity. Front Microbiol. 2016;7:1456. doi:10.3389/fmicb.2016.01456
80. Chen JG, Liu SC, Nie Q, et al. Exosome-derived long noncoding RNAs: mediators of host-Plasmodium parasite communication. Wiley Interdiscip Rev RNA. 2023;15:e1808. doi:10.1002/wrna.1808
81. Ang JXD, Kadir KA, Mohamad DSA, et al. New vectors in northern Sarawak, Malaysian Borneo, for the zoonotic malaria parasite, Plasmodium knowlesi. Parasit Vectors. 2020;13:472. doi:10.1186/s13071-020-04345-2
82. Wowk PF, Zardo ML, Miot HT, Goldenberg S, Carvalho PC, Mörking PA. Proteomic profiling of extracellular vesicles secreted from Toxoplasma gondii. Proteomics. 2017;17. doi:10.1002/pmic.201600477
83. Li Y, Liu Y, Xiu F, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int J Nanomed. 2018;13:467–477. doi:10.2147/ijn.S151110
84. Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol. 2018;9:738. doi:10.3389/fimmu.2018.00738
85. Shu S, Yang Y, Allen CL, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9:1692401. doi:10.1080/20013078.2019.1692401
86. Langevin SM, Kuhnell D, Orr-Asman MA, et al. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16:5–12. doi:10.1080/15476286.2018.1564465
87. Ter-ovanesyan D, Norman M, Lazarovits R, et al. Framework for rapid comparison of extracellular vesicle isolation methods. Elife. 2021;10:e70725. doi:10.7554/eLife.70725
88. Kamei N, Nishimura H, Matsumoto A, et al. Comparative study of commercial protocols for high recovery of high-purity mesenchymal stem cell-derived extracellular vesicle isolation and their efficient labeling with fluorescent dyes. Nanomedicine. 2021;35:102396. doi:10.1016/j.nano.2021.102396
89. Mondal SK, Whiteside TL. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol Biol. 2021;2265:305–321. doi:10.1007/978-1-0716-1205-7_23
90. Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509. doi:10.3402/jev.v4.29509
91. Benedikter BJ, Bouwman FG, Vajen T, et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep. 2017;7:15297. doi:10.1038/s41598-017-15717-7
92. Turner NP, Abeysinghe P, Kwan Cheung KA, et al. A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis. Proteomes. 2022;10:19. doi:10.3390/proteomes10020019
93. Mark D, Haeberle S, Roth G, von Stetten F, Zengerle R. Microfluidic lab-on-A-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39:1153–1182. doi:10.1039/b820557b
94. Kang YT, Niu Z, Hadlock T, et al. On-Chip Biogenesis of Circulating NK Cell-Derived Exosomes in Non-Small Cell Lung Cancer Exhibits Antitumoral Activity. Adv Sci. 2021;8:2003747. doi:10.1002/advs.202003747
95. Bathini S, Pakkiriswami S, Ouellette RJ, Ghosh A, Packirisamy M. Magnetic particle based liquid biopsy chip for isolation of extracellular vesicles and characterization by gene amplification. Biosens Bioelectron. 2021;194:113585. doi:10.1016/j.bios.2021.113585
96. Zhang J, Zhu Y, Guan M, et al. Isolation of circulating exosomes and identification of exosomal PD-L1 for predicting immunotherapy response. Nanoscale. 2022;14:8995–9003. doi:10.1039/d2nr00829g
97. Li Y, Xiu F, Mou Z, et al. Exosomes derived from Toxoplasma gondii stimulate an inflammatory response through JNK signaling pathway. Nanomedicine. 2018;13:1157–1168. doi:10.2217/nnm-2018-0035
98. Tang YT, Huang YY, Zheng L, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834–844. doi:10.3892/ijmm.2017.3080
99. Miron RJ, Zhang Y. Understanding exosomes: part 1-Characterization, quantification and isolation techniques. Periodontol 2000. 2023;94:231–256. doi:10.1111/prd.12520
100. Altıntaş Ö, Saylan Y. Exploring the Versatility of Exosomes: a Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal Chem. 2023;95:16029–16048. doi:10.1021/acs.analchem.3c02224
101. Kim DK, Kang B, Kim OY, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.20384
102. Ramírez-Flores CJ, Cruz-Mirón R, Mondragón-Castelán ME, González-Pozos S, Ríos-Castro E, Mondragón-Flores R. Proteomic and structural characterization of self-assembled vesicles from excretion/secretion products of Toxoplasma gondii. J Proteomics. 2019;208:103490. doi:10.1016/j.jprot.2019.103490
103. Quiarim TM, Maia MM, da Cruz AB, Taniwaki NN, Namiyama GM, Pereira-Chioccola VL. Characterization of extracellular vesicles isolated from types I, II and III strains of Toxoplasma gondii. Acta Trop. 2021;219:105915. doi:10.1016/j.actatropica.2021.105915
104. Pope SM, Lässer C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.22484
105. Kim MJ, Jung BK, Cho J, et al. Exosomes Secreted by Toxoplasma gondii-Infected L6 Cells: their Effects on Host Cell Proliferation and Cell Cycle Changes. Korean J Parasitol. 2016;54:147–154. doi:10.3347/kjp.2016.54.2.147
106. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688–692. doi:10.1016/j.jmb.2015.09.019
107. Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. doi:10.1038/s41556-018-0040-4
108. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–9420.
109. Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–1265. doi:10.4049/jimmunol.165.3.1259
110. Möbius W, Ohno-Iwashita Y, van Donselaar EG, et al. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50:43–55. doi:10.1177/002215540205000105
111. Brecht S, Carruthers VB, Ferguson DJ, et al. The toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J Biol Chem. 2001;276:4119–4127. doi:10.1074/jbc.M008294200
112. Jewett TJ, Sibley LD. The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J Biol Chem. 2004;279:9362–9369. doi:10.1074/jbc.M312590200
113. Clough B, Frickel EM. The Toxoplasma Parasitophorous Vacuole: an Evolving Host-Parasite Frontier. Trends Parasitol. 2017;33:473–488. doi:10.1016/j.pt.2017.02.007
114. Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 2005; 1:e17. doi:10.1371/journal.ppat.0010017
115. Maia MM, da Cruz AB, Taniwaki NN, et al. Immunization with extracellular vesicles excreted by Toxoplasma gondii confers protection in murine infection, activating cellular and humoral responses. Int J Parasitol. 2021;51:559–569. doi:10.1016/j.ijpara.2020.11.010
116. Lu J, Wei N, Zhu S, et al. Exosomes Derived From Dendritic Cells Infected With Toxoplasma gondii Show Antitumoral Activity in a Mouse Model of Colorectal Cancer. Front Oncol. 2022;12:899737. doi:10.3389/fonc.2022.899737
117. Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72:4127–4137. doi:10.1128/iai.72.7.4127-4137.2004
118. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi:10.1182/blood-2007-03-079152
119. Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–1757. doi:10.1016/j.vaccine.2009.01.022
120. Tawfeek GM, Abou-El-Naga IF, Hassan EME, Sabry D, Meselhey RA, Younis SS. Protective efficacy of Toxoplasma gondii infected cells-derived exosomes against chronic murine toxoplasmosis. Acta Trop. 2023;248:107041. doi:10.1016/j.actatropica.2023.107041
121. Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi:10.1034/j.1600-0854.2002.30502.x
122. Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1744:438–454. doi:10.1016/j.bbamcr.2005.04.004
123. Gao J, Li A, Hu J, Feng L, Liu L, Shen Z. Recent developments in isolating methods for exosomes. Front Bioeng Biotechnol. 2022;10:1100892. doi:10.3389/fbioe.2022.1100892
124. Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi:10.1111/j.1600-0854.2010.01041.x
125. Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi:10.1242/jcs.074088
126. Pofali P, Mondal A, Londhe V. Exosome as a Natural Gene Delivery Vector for Cancer Treatment. Curr Cancer Drug Targets. 2020;20:821–830. doi:10.2174/1568009620666200924154149
127. Zhang M, Hu S, Liu L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8:124. doi:10.1038/s41392-023-01382-y
128. Liu J, Ren L, Li S, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–2797. doi:10.1016/j.apsb.2021.01.001
129. Zhang W, Chen H, Ding L, et al. Trojan Horse Delivery of 4,4’-Dimethoxychalcone for Parkinsonian Neuroprotection. Adv Sci. 2021;8:2004555. doi:10.1002/advs.202004555
130. Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, Phase 3 trial. Lancet Oncol. 2021;22:702–715. doi:10.1016/s1470-2045(21)00079-6
131. Ransom D, Wilson K, Fournier M, et al. Final results of Australasian Gastrointestinal Trials Group Arctic study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 2014;25:117–121. doi:10.1093/annonc/mdt479
132. Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 2013;24:682–689. doi:10.1016/j.copbio.2013.04.014
133. Basso J, Miranda A, Nunes S, et al. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels. 2018;4:62. doi:10.3390/gels4030062
134. Yang Q, Li S, Ou H, et al. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnology. 2024;22:41. doi:10.1186/s12951-024-02298-7
135. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
136. van der Koog L, Gandek TB, Nagelkerke A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: a Comparison of Composition, Pharmacokinetics, and Functionalization. Adv Healthc Mater. 2022; 11:e2100639. doi:10.1002/adhm.202100639
137. Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014; 306:C621–633. doi:10.1152/ajpcell.00228.2013
138. Xu Z, Huang H, Xiong X, et al. A near-infrared light-responsive extracellular vesicle as a”Trojan horse” for tumor deep penetration and imaging-guided therapy. Biomaterials. 2021;269:120647. doi:10.1016/j.biomaterials.2020.120647
139. Roth L, Agemy L, Kotamraju VR, et al. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31:3754–3763. doi:10.1038/onc.2011.537
140. Ishiguro K, Yan IK, Lewis-Tuffin L, Patel T. Targeting Liver Cancer Stem Cells Using Engineered Biological Nanoparticles for the Treatment of Hepatocellular Cancer. Hepatol Commun. 2020;4:298–313. doi:10.1002/hep4.1462
141. Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi:10.1016/j.biomaterials.2013.11.083
142. Wang C, Li N, Li Y, et al. Engineering a HEK-293T exosome-based delivery platform for efficient tumor-targeting chemotherapy/internal irradiation combination therapy. J Nanobiotechnology. 2022;20:247. doi:10.1186/s12951-022-01462-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review
Kong C, Chen X
International Journal of Nanomedicine 2022, 17:6427-6446
Published Date: 16 December 2022