Back to Journals » Journal of Pain Research » Volume 18
Trajectories of mHealth-Tracked Mental Health and Their Predictors in Female Chronic Pelvic Pain Disorders
Authors Leventhal EL , Nukavarapu N, Elhadad N, Bakken SR, Elovitz MA, Hirten RP, Rodrigues J , Danieletto M, Landell K, Ensari I
Received 22 November 2024
Accepted for publication 28 January 2025
Published 26 February 2025 Volume 2025:18 Pages 899—913
DOI https://doi.org/10.2147/JPR.S499102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Emily L Leventhal,1,2 Nivedita Nukavarapu,1,2 Noemie Elhadad,3 Suzanne R Bakken,4 Michal A Elovitz,5 Robert P Hirten,1,2,6 Jovita Rodrigues,1,2 Matteo Danieletto,1,2 Kyle Landell,1,2 Ipek Ensari1,2
1Windreich Department of Artificial Intelligence and Human Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 2Hasso Plattner Institute for Digital Health Mount Sinai, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 3Department of Biomedical Informatics, Columbia University Irving Medical Center, New York, NY, USA; 4Columbia University School of Nursing, Columbia University Irving Medical Center, New York, NY, USA; 5Department of Obstetrics, Gynecology and Reproductive Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 6The Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Correspondence: Ipek Ensari, Department of Artificial Intelligence and Human Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA, Email [email protected]
Background: Female chronic pelvic pain disorders (CPPDs) affect 1 in 7 women worldwide and are characterized by psychosocial comorbidities, including a reduced quality of life and 2– 10-fold increased risk of depression and anxiety. Despite its prevalence and morbidity, CPPDs are often inadequately managed with few patients experiencing relief from any medical intervention. Characterizing mental health symptom trajectories and lifestyle predictors of mental health is a starting point for enhancing patient self-efficacy in managing symptoms. Here, we investigate the association between mental health, pain, and physical activity (PA) in females with CPPD and demonstrate a method for handling multi-modal mobile health (mHealth) data.
Methods: The study sample included 4270 person-level days and 799 person-level weeks of data from CPPD participants (N=76). Participants recorded PROMIS global mental health (GMH) and physical functioning and pain weekly for 14 weeks using a research mHealth app, and moderate-to-vigorous PA (MVPA) was passively collected via activity trackers.
Data Analysis: We used penalized functional regression (PFR) to regress weekly GMH-T (GMH-T) on MVPA and weekly pain outcomes while adjusting for baseline measures, time in study, and the random intercept of the individual. We converted 7-day MVPA data into a single smooth using spline basis functions to model the potential non-linear relationship.
Results: MVPA was a significant, curvilinear predictor of GMH-T (F=18.989, p< 0.001), independent of pain measures and prior psychiatric diagnosis. Physical functioning was positively associated with GMH-T, while pain was negatively associated with GMH-T (B=2.24, B=− 1.16, respectively; p< 0.05).
Conclusion: These findings suggest that engaging in MVPA is beneficial to the mental health of females with CPPD. Additionally, this study demonstrates the potential of ambulatory mHealth-based data combined with functional models for delineating inter-individual and temporal variability.
Keywords: chronic pelvic pain, digital health, functional data modeling, global mental health
Introduction
Described as a “neglected reproductive health morbidity”, chronic pelvic pain (CPP) is a highly debilitating condition that affects between 5.7% and 26.6% of women worldwide.1–3 CPP, which encompasses complex female CPP disorders (CPPDs) such as endometriosis, adenomyosis, and fibroids, is characterized by non-cyclic pain in the pelvis or abdomen that lasts for at least 6 months and leads to functional disability or the necessity for medical intervention.3–5 Its severity is underscored by its associated physical, psychological, and emotional, and social consequences.4,5
Previous research indicates that CPPDs have a strong psychosocial impact on individuals. For example, individuals with CPPDs are more likely to experience reduced quality of life, emotional well-being, productivity, and sexual function compared to the general population.3,6–8 Additionally, CPPD patients have a significantly higher risk of comorbid psychiatric disorders.4,9,10 For example, individuals with CPPDs have been reported to experience depressive disorders and anxiety disorders at a prevalence of 2 to 10 times that of the general population.4,11–13 Because chronic pain is linked to mental health problems, the investigation of modifiable predictors of mental well-being in individuals with CPPDs is a starting point for comprehensively managing CPPDs.14 Here, we focus on female-specific CPPDs, as being female is associated with a higher burden of anxiety and depressive disorders.15
Despite its prevalence and burden, female CPPDs are often inadequately managed, with less than half of patients experiencing pain relief from any medical treatment.9,16 Patient self-management, which aims to equip the individual with tools to actively manage pain and its effects on physical and emotional function, is a common chronic pain care model intervention, and it has been associated with significant improvement in symptoms.17–19 Some examples of patient self-management include relaxation techniques, symptom tracking to identify triggers, mindfulness, and behavioral factors (eg, sleep hygiene).20–22 Further, Center for Disease Control (CDC) guidelines state that non-opioid and non-pharmacologic therapies should be prioritized for chronic pain management.23,24 Non-pharmacological self-management strategies, especially those that target mental health outcomes of CPPD patients, are needed for effective personalized treatment of CPPD. Psychosocial interventions can be effective at improving mental health outcomes for those with chronic pain.25 However, psychosocial support that requires mental health professionals, such as cognitive behavioral therapy or biofeedback, may not be accessible to everyone. Here, we investigate how physical activity (PA) patterns of individuals with CPPDs may affect mental health outcomes and serve as an additional method to improve mental well-being in female CPPDs.
PA and exercise, defined as planned, structured, and repetitive PA with the goal of improving health or fitness, have been demonstrated to be effective pain self-management strategies for both reducing pain severity and improving psychological function in chronic pain patients.18,26 Experts recommend that chronic pain patients exercise on a regular schedule on the premise that avoiding activity during pain and increasing intensity later may lead to pain flares.4 Importantly, exercise is a modifiable behavior that can also improve pain self-efficacy, defined as the confidence in one’s ability to function effectively while in pain, which is associated with improved quality of life.27,28 Further, for chronic pain patients with comorbid psychiatric conditions, exercise may improve mood, depression, and anxiety symptoms.29,30 A previous study with individuals with endometriosis estimated a small but statistically significant favorable effect of exercise on pain severity.31 However, this study relied on self-reported exercise, which is limited in its ability to capture more granular PA parameters (eg, step counts, intensity level).31 Another meta-analysis reported that a combination of exercise and patient education was effective in reducing pain and disability in pregnant women with low back and/or pelvic pain.32 While most of the evidence connecting PA to psychosocial improvement has been from other chronic pain conditions, yoga has previously been demonstrated to be efficacious in improving pain and quality of life for patients with endometriosis.33,34 The impact of broader PA on mental health in patients with CPPDs specifically remains to be investigated, with a focus on using longitudinal data to capture potentially meaningful trends over time.
Accordingly, this study aims to characterize the patterns of association between longitudinal self-reported mental health symptoms and their predictors in female CPPDs, with a focus on modifiable lifestyle factors. Specifically, this overall aim includes the investigation of 1) between- and within-individual fluctuations in weekly self-reported mental health, and 2) possible modifiable and trait predictors of weekly mental health. We hypothesized that there would be significant variability in the mental health both between and within individuals and that PA would be a positive non-linear predictor of mental health.
Methods
Study Design and Procedures
The study design and procedures were approved by the IRB of the Icahn School of Medicine at Mount Sinai (ISMMS; IRB# STUDY-22-01002). CPPDs and their symptomatic patterns are notably heterogeneous in clinical presentation both between patients and within-individuals over time.35 Capturing these fluctuations under ecologically valid circumstances can help improve our understanding of the dynamic unfolding of these symptoms and their potential predictors. This is a secondary analysis of the data from an ongoing larger study that aims to design, develop, and evaluate CPPD-specific mHealth measures from patient generated health data with high complexity and temporality using non-linear distributed lag and functional data modeling (NIH/NICHD: R01HD108263). It uses an observational study design to collect 90 days of data on patient self-tracked symptoms via a research mHealth app (ehive36) and passively collected activity data using activity trackers from participants. All participants used the ehive research study app to provide baseline and weekly data on overall health, symptoms, well-being, and health behaviors, as well as to receive prompts and reminders about the study.36 Participants were instructed to wear a Fitbit for the duration of the study.
Study Sample
The study sample included individuals who met the following eligibility criteria for the parent study: 1) female assigned at birth (individuals with female reproductive anatomy who menstruate), 2) between the ages of 18 and 64, 3) self-reported CPPD based on clinician diagnosis, 4) experience of CPP for at least 6 months, and 5) ability to read and write in English. Exclusion criteria include: 1) current pregnancy, a birth in the past 6 months, or planning pregnancy during the months of the study and 2) currently active major diseases or comorbidities (eg, active cancer, acute coronary syndrome within the past 3 months, heart failure, chronic kidney disease, etc.). Participants were recruited from all campuses of the Mount Sinai Health System (MSHS) and Columbia University Irving Medical Center (CUIMC) via Email advertisements and on the myChart by EPIC mobile app for MSHS patients. Participants in this study were recruited between April 2023 and July 2024. We included all participants who had PA and questionnaire data at the time of the analysis (July 2024).
Enrollment
Interested patients reached out to the study coordinator at Mount Sinai for screening and enrollment, after which they were onboarded and oriented to the study app and data collection protocols. All participants were mailed a Fitbit Inspire 2 device and instructed to use for the duration of the study (90 days). Participants were remunerated $15 for every 2 weeks of data collection and $20 for the final week (ie, up to $120 in total for completing 90 days of data collection). All participants provided informed consent prior to enrolling in the study.
Study Measures
Primary Outcomes
Self-reported mental health was assessed every week using the PROMIS Global Mental Health Questionnaire (GMH; 2a, v1.2).37 PROMIS global mental health scores have been previously validated as a metric for estimating self-reported mental health in chronic conditions.37 Further, GMH has been demonstrated to be significantly decreased in patients with CPP.38 The GMH includes 2 questions: 1) “In general, how would you rate your mental health, including your mood and your ability to think?” 2) “In general, how would you rate your satisfaction with your social activities and relationships?” Both questions have a 5-point multiple choice response scale (1-not at all, 5-very much) and the responses are added to compute the total score on the GMH (range 2–10). Higher scores represent better mental health.37 The two-item GMH survey provides a brief measure of mental health that has been demonstrated to be both reliable and have construct validity.37 Scores from the GMH survey have been positively associated with other self-reported outcomes including overall quality of life and physical functioning and negatively correlated with fatigue, anxiety, anger, depressive symptoms, and chronic conditions (eg, liver disease, kidney disease, hypertension, etc.).37 We converted raw GMH scores to population-standardized GMH scores (T-scores) according to the PROMIS Global Health scoring manual by standardizing the raw total score to a mean of 50 and a standard deviation (SD) of 10.39 GMH T-scores (GMH-T) are further categorized as excellent (>55), very good (48–55), good (40–47), fair (29–39), and poor (<29).40
Predictors
Physical Activity
Daily minutes of moderate-to-vigorous intensity PA (MVPA) and step counts were obtained from the wrist-worn Fitbit devices. MVPA has been previously associated with improved mental health outcomes in populations with chronic pain.29 Tracking MVPA minutes is a common approach to studying the PA and its effects among individuals with chronic pain.41
Participants were instructed to wear their devices continuously for the study duration. The study app (ehive) allows the user to link their account with their Fitbit device,36 which enables regular daily data syncing on the backend of the app. Fitbit uses its proprietary algorithms for detection of step counts and activity intensities. We collected 6341 days of physical activity data for 78 participants. For wear time validation, we relied on the commonly used standard “10-hour minimum wear” rule, in which a valid day is defined as at least 10 hours of non-zero activity counts.42–44 Ten hours of wear has been shown to be sufficient to estimate total daily physical activity during non-sleep time.45 There were 4301 valid days of Fitbit data for 76 participants. Days with unrealistically low activity counts (eg, <500 steps in a day; n=14) were removed in accordance with similar cutoffs that have been used in the past to define a valid day, although we used a more conservative cutoff.42,43 This resulted in 4287 days of physical activity data for 76 participants. If there were more than 7 days of Fitbit data in between survey responses (ie, if a participant waited more than 7 days before completing the next survey), we only considered the first 7 days of Fitbit activity data to avoid sparsity in the penalized functional regression (PFR) model (described below). Seventy-seven days of activity data measured more than 7 days after a survey response were removed for this reason. The final dataset had 4270 days of data for 76 participants.
Physical Functioning
Weekly physical functioning scores were measured using the PROMIS physical function survey (4a, v1.0).46 Physical functioning is the self-reported capability of performing everyday physical activities. PROMIS physical function scores have been demonstrated to be inversely correlated with pelvic pain among patients with CPP.38 The score evaluates the functioning of upper extremities, lower extremities, central regions, and activities of daily living. The 4-item PROMIS survey assesses the extent to which individuals find difficulty with physical tasks (5-without any difficulty to 1-unable to do). Scores range from 4 to 20, with higher scores indicating better physical functioning. We used the physical functioning T-scores in the analyses, which are standardized to a mean of 50 and a SD of 10 based on a representative population distribution.46
Pain
We measured weekly pain levels using the VAS pain intensity item from the short-form McGill Pain Questionnaire (MPQ-VAS).47 The MPQ-VAS asks participants to rate the intensity of their present pain intensity on a scale of 0 (no pain) to 100 (worst imaginable pain).48 This type of VAS-based pain assessment is commonly used as a standard practice in clinical settings to evaluate patient pain status and treatment outcomes.49,50 VAS has additionally been benchmarked and validated as a measure of pain intensity in chronic pain.48
Other Covariates
Data on personal demographics and general health were collected via a baseline questionnaire on the ehive app. We collected age, marital status, ethnicity, and employment status from the demographic survey. In addition, we used prior psychiatric diagnosis (“Have you ever been diagnosed with a psychiatric diagnosis by a provider?”) as a covariate from the general health survey.
Data Analysis
Descriptive and Bivariate Analyses
First, we performed descriptive analyses and investigated bivariate associations between the weekly measured survey items. Given the repeated-measures design, we used both person-level means (ie, a participant’s mean score across the 14 weeks) and overall sample means (ie, mean of means) when necessary to report the overall study average scores from the daily (ie, steps, MVPA) and weekly (ie, pain, physical functioning T-score, GMH-T) measures. To analyze the GMH-T, we converted the mean GMH-T for each participant to its corresponding GMH category (eg, fair, good, excellent, etc.), and computed the percent of participants in each category.40 To evaluate sample GMH-T and physical functioning T-scores against known population means, we used a one-sample T-test to compare the sample means to the population means. We then computed repeated-measures correlations between GMH-T, physical functioning, MPQ-VAS, and the sum of MVPA over 7 days using the rmcorr R package, which evaluates the within-individual association of paired measurements taken two or more times longitudinally.51,52
Multivariable Regression Analysis of GMH Predictors
In the context of health behaviors such as PA, data from mobile health (mHealth) technologies (eg, smartphone apps, trackers) combined with longitudinal analytic techniques can help elucidate symptom associations with psychosocial outcomes in CPP.10,31 For example, there may be non-linear associations and cumulative effects in these longitudinal data that are not possible to capture via linear modeling approaches. Flexible techniques are particularly useful when working with mHealth data that typically differ in sampling frequencies, missingness patterns, modality, and temporal complexity.
Functional regression models, which are a part of the family of generalized additive models (GAMs), constitute one such approach.53 In a functional regression framework, the entire data curve is considered as the unit of analysis, instead of discrete data points in a set of longitudinal data. This is particularly useful for handling PA data from wearables, rather than aggregating multiple data points per individual,54 as they allow investigating the associations between scalar and functional variables with different time intervals. One example scenario is the consideration of continuous or daily PA data with weekly self-reported survey data, in a repeated-measures design. This results in a data structure where each weekly questionnaire corresponds to 7 days of PA data leading up to the survey data. A functional regression model considers the PA data as a weekly data curve rather than aggregating the entire week into a summary score and thus preserves the temporal pattern within the data. This can reveal important information that may be lost otherwise, such as periods of inactivity or bursts of activity, which could be related to mental health.55
To investigate the potential predictors of GMH-T scores at the week level, we implemented PFR modeling using the R refund library.54 PFR models are flexible in numerous ways that are particularly useful for the data in this study. First, they allow for entire data curves to be units of analysis as opposed to individual data points. Next, they accommodate different sampling intervals in the outcomes vs predictors, ie, week-level outcome (eg, GMH-T) and week-level (eg, pain, physical functioning) and day-level (eg, MVPA) predictors. Instead of aggregating multiple day-level MVPA values for each week, this feature of PFR allows for the preservation of temporal variability in MVPA over a week. Third, it allows specification of random intercepts (ie, individual participants), which is useful for both accommodating a repeated measures design and for investigating potential between- vs within-individual variability in the outcome of interest (ie, GMH-T scores).
We regressed GMH-T on MVPA while considering MPQ-VAS, PROMIS physical functioning, age, marital status, employment status, and prior psychiatric diagnosis. We further adjusted for time in study using month-level cyclical encoding, in which each date is mapped into a cyclic coordinate system using sine-cosine waves and allows the models to infer the distance between dates based on their sine-cosine coordinates. We converted 7-day MVPA data into smooths with up to 7 knots using the tensor product basis function56 to model the potential non-linear relationship between GMH-T and daily PA. We similarly included the time covariate as a functional smooth with up to 7 knots.54 We scaled MPQ-VAS, PROMIS physical functioning, and age by mean-centering each variable and dividing by its standard deviation. We included participant and week in study as random effects. Finally, other categorical variables (ie, psychiatric diagnosis, employment status, and marital status) were included as person-level linear covariates.54 We used a generalized additive model as the fitter to estimate the model and restricted maximum likelihood as the smoothing parameter estimation method, which are the default recommended methods for the function.54
Results
Study Sample
Participants (n=76) provided 799 weeks of survey and 4270 days of activity data in total for analysis. Participants had a mean age of 35 years and were mostly employed (76%). Most participants identified as White (42%) or Hispanic or Latino (17%). In our sample, 28% had at least one prior diagnosis of a psychiatric condition, including anxiety and mood disorders (Table 1). The CPPD diagnoses included endometriosis (N=51), adenomyosis (N=1), uterine fibroids (N=2), interstitial cystitis (N=1), inflammatory bowel syndrome (3), and inflammatory pelvic dysfunction (N=1).
 |
Table 1 Study Sample Demographics |
Descriptive and Bivariate Analyses
The overall sample means of the scores from the daily and weekly measures are reported in Table 2. Thirty-nine percent of the participants, on average, reported scores that corresponded to “fair” mental health, with another 39% of the participants on average reporting “good” mental health (Table 2). The mean GMH-T was 42.166 (95% CI: 40.363–43.969), which is 7.83 SDs below the population mean (ie, M=50, “very good”)37 and significantly different (t=−8.658, p < 0.001; Figure 1). The mean physical functioning T-score was 45.19 (95% CI: 43.52–46.853), which is 0.48 SDs below the population mean (ie, M=50; Figure 1; t = −5.758, p < 0.001).
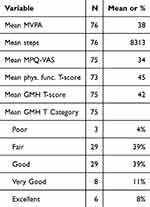 |
Table 2 Average Weekly and Daily Measures Across the Study. The Average Was Taken of the Participant Means for Each Repeated Measure |
To characterize the PA patterns in the sample, we compared participants’ activity levels to the published recommendations and CDC/HHS PA guidelines for adults with respect to steps and MVPA.57–59 On average, participants accumulated 8313 steps and 38 minutes of MVPA per day (Table 2). Forty-three percent of the sample engaged in fewer than 7500 daily steps, which is the lower threshold recommended for being considered “sufficiently active” (Figure 2A). 58,59 Similarly, 40.9% accumulated fewer than 150 minutes of weekly MVPA recommended by the PA Guidelines (Figure 2B).60
To inspect the bivariate associations between weekly measures, we computed repeated measures correlations between GMH-T and the other variables. GMH-T was positively correlated with weekly MVPA (p<0.05), and physical function T-score (p<0.01), while they were negatively correlated with MPQ-VAS (p<0.001; Figure 3). Weekly MVPA was additionally positively correlated with physical functioning T-score (p<0.05) but was not significantly correlated with MPQ-VAS.
PFR Model
We fitted a PFR model to the data to investigate cumulative and non-linear effects of MVPA on the weekly GMH-T. The best fitting final model explained 72.6% of the deviance in GMH-T (adjusted R2=0.65). The smooth of MVPA and time on GMH-T indicated a significant non-linear relationship (Table S1; Table S2; F=18.99, edf=2.23, p<0.001). Predicted GMH-T increased with increasing daily MVPA minutes (Figure 4A). Over time, the largest positive effect of MVPA on predicted GMH-T as reported at the end of the week was a few days prior (~day 4). The positive effect of MVPA on GMH-T reported at the end of the week diminished after day 4, suggesting the positive effects of MVPA lagged by a couple of days. Weekly MPQ-VAS was a significant negative predictor of GMH-T (B=−1.16, SE=0.50, t=−2.34, p<0.05), while physical functioning T-score was a significant positive predictor of GMH-T (Figure 4B and Table S3; B=2.24, SE=0.598, t=3.75, p<0.001). For demographic factors, age was negatively associated with GMH-T (B=−1.20, SE=0.46, t=−2.58, p<0.05), while being employed and married were positively associated with GMH-T (B=4.01, SE=1.09, t=3.67, p<0.001; B=3.60, SE=0.86, t=4.20, p<0.001). Prior psychiatric diagnosis was not a significant predictor of weekly GMH. The random effect of participant was significant (Figure 4C; edf=33.43, F=2.76, p<0.001). The random effect of week and the cyclically encoded sine and cosine functions of month were not significant.
Discussion
In this study, we leverage ambulatory mHealth-tracked mental health, pain, and PA data to characterize longitudinal self-reported mental health patterns of individuals with CPPDs. Our results indicate a positive, non-linear relationship between MVPA and mental health, independent of prior psychiatric diagnosis or other pain-related factors, with considerable variability both between and within participants over time. To our knowledge, this study provides the first evidence of the positive effect of PA on mental health in females with CPPDs using repeated measures data collected in real time. We further report lower scores of mental health and physical functioning compared to the general population, as well as lower PA levels than those recommended by the PA guidelines.
Our study sample had a 28% incidence of prior psychiatric conditions and lower average GMH scores compared to the reference population norms from PROMIS.39 Chronic pain, and specifically CPP, has been established as a strong predisposing factor for psychiatric conditions. The association of chronic pain and mental health is due to both the psychosocial impact of chronic pain and the common neurobiological vulnerabilities and genetic factors between chronic pain and mood.4,9,13,61 CPPD patients with comorbid psychiatric conditions are more likely to incur higher health care costs, experience lower quality of life, endure increased disability, and are more likely to be prescribed opioids.4 Additionally, our findings add to the literature documenting the worsened mental health of CPPD patients as a whole compared to the general population.4,13,37 In the 2019 National Health Interview Survey, those with chronic pain had a 23.9% prevalence of co-occurring anxiety and/or depression symptoms, whereas the population without chronic pain had a prevalence of 4.9%.13 Given the high incidence of psychiatric co-morbidities and the generally low mental health among CPPD patients, it is important to treat mental health as part of comprehensive chronic pain management and continue to determine ways to aid patients to manage their symptoms. As such, here, we investigated how lifestyle factors may modify the association of CPPDs with poorer mental health outcomes.
Our findings suggest that many females with CPPDs do not reach nationally recommended PA levels, and moreover, that engaging in MVPA is beneficial for the mental health of CPPD patients. The PA levels of this sample are consistent with previous studies suggesting that individuals with CPPDs have lower PA levels,62 though data on CPPDs are scarce. One longitudinal study using accelerometers indicated that MVPA negatively mediated the relationship between chronic pain and risk of mental disorders, although this study did not focus on CPP.29 Increased MVPA in individuals with chronic pain was associated with decreased anxiety and depression symptoms, whereas light intensity PA did not have this effect.13 Additionally, strategies combining exercise and other interventions have been demonstrated to be promising. For example, a combined intervention of exercise and telephone-delivered cognitive behavioral therapy (CBT) had positive effects in patients with chronic widespread pain.63 While previous studies have established the connection between MVPA and mental health in chronic pain, this is the first study to establish the relationship between PA and mental health in the context of CPPDs by using passively obtained data from activity trackers.31
Our findings further indicate that increased pain is associated with worsened GMH, while increased physical functioning was associated with improved GMH. Though pain and depression or anxiety have been noted to have a bidirectional relationship, there is more evidence that pain is a risk factor for mental health problems than the inverse.4 Additionally, a longitudinal study focused on musculoskeletal conditions reported that improvements in physical functioning were associated with improved anxiety symptoms, although it was not associated with improved depression symptoms.64 The relationship between physical functioning and mental health in CPPDs has not been well defined to this point; however, one previous longitudinal study on endometriosis reported that functional pain disability did not predict later emotional distress.65
With respect to demographic factors as potential predictors, increased age was associated with worsened GMH, while prior psychiatric diagnosis was not a significant predictor. Age may be a proxy for years of experience with the chronic pain condition or severity of the condition. In this study, we did not have a survey item assessing time of initial diagnosis, although this may be possible in the future by linking mobile health studies with electronic health records (EHRs). Over time, chronic pain may become more difficult to treat due to structural and functional neuroplastic changes that eventually become irreversible and insensitive to treatment.61 From a psychosocial standpoint, the economic consequences of health care costs and loss of productivity may accumulate over time.61 It will be important to assess how length of time of living with chronic pain impacts mental health in the future. Our findings indicated that prior psychiatric condition, including mood and anxiety disorders, was not a significant predictor of GMH. This suggests that those who are at higher risk for poor mental health outcomes with a comorbid psychiatric disorder can also benefit from these favorable effects of PA. However, none of the participants had a debilitating psychiatric condition at the time of study participation, as per study exclusion criteria. Nevertheless, we do not have data on whether or not participants were actively receiving mental health care for their condition, so we can not rule out it as a potential protective factor.
We observed substantial between- and within-individual variability in mental health scores in the sample, underscoring the importance of personalized approaches to care. Predicted average GMH-T varied greatly between individuals as shown by the random intercepts. CPPDs are notoriously heterogeneous in pain symptomatology, and it follows that mental health would exhibit similar variability among and within participants.10 As such, it is important to use individualized approaches, such as that which may be achieved with mHealth, to comprehensively understand the complexity of CPP. Due to their heterogeneous clinical presentation and differing etiologies, CPPDs are often non-responsive to treatment, and a personalized approach is necessary for the successful management of CPPD. To better understand how to manage the mental health of CPPD patients, we should continue to study modifiable lifestyle factors, as was done here with PA, that may alter the poor mental health outcomes associated with CPP. This study demonstrates the potential of using ambulatory mHealth-based data combined with functional data methods to delineate inter-individual and temporal variability in symptoms of chronic conditions.
There are numerous strengths of this work. First, we focus on a patient population that has been under-studied (ie, CPPDs) and currently still not well understood as a cluster of disorders with overlapping symptomatology. While endometriosis, the most common underlying primary diagnosis for a CPPD, has been receiving more attention recently, our sample also included those less-studied CPPDs (eg, adenomyosis, fibroids, inflammatory pelvic disease). Next, the implementation of functional data methods and generalized additive modeling using smooths provide robust and flexible approaches for handling the complex patient-generated health data from mHealth technologies. The PFR models in this context facilitate the evaluation of complex relationships between outcomes and their predictors in instances where the data sampling frequency differs between the outcomes and predictors, or between different predictors. This work demonstrates a method for analyzing longitudinal, continuous wearable data combined with actively tracked survey data at different intervals. As such, it may be used as a framework for future mHealth interventions focused on patient self-management to elucidate the effects of PA on other factors. As mHealth use is becoming more ubiquitous for conducting research, expanding upon the available methods will enable fully harnessing the information from these data. Third, our analyses were based on frequently sampled prospective data of up to 14 weeks from the study participants. This is a strength of the data design as most studies to date are limited to convenience samples of retrospective data with much less frequency of data points. The study design could enable personalized intervention strategies in the future if methods are used to predict mental health outcomes in individual participants. Finally, it is the first study to date that has demonstrated a positive effect of MVPA on global mental health in female CPPDs, suggesting it may be recommended as an additional self-management strategy for mental health in those with CPPDs.
Nevertheless, we acknowledge the limitations of this study. Although we had 799 person-level weeks for analysis, 76 participants are a relatively modest sample size in comparison to large, nationally representative cohort studies. Similarly, the sample was somewhat homogeneous with respect to demographic factors including employment status and education levels. Specifically, our cohort was mostly employed (76%), whereas individuals with CPPDs have significantly lower employment rates compared to the general population.6 Third, in this study, a consumer-grade device, Fitbit, was chosen as a tool to measure PA in patients with CPPDs. Some studies have demonstrated that Fitbit devices under- or over-estimate PA compared to medical-grade devices (ie, Actigraph), while others indicated that Fitbits have fair to good agreement with these devices.41–43,66 While not the gold standard for activity tracking, Fitbit offers advantages especially for conducting longitudinal studies, including ease of wear and charging for participants, which aid participant adherence, user interface, and relative ease of syncing with other mobile Apps and devices for data extraction.67,68 These factors support the broader aim of facilitating patient self-management with devices that are both accessible and familiar to many individuals. Despite our careful inspection of the missing data and implementing cautious filtering criteria to prevent potentially erroneous inference from the data, Fitbit’s proprietary algorithms do not always enable as informed decisions regarding the missing data as do some other devices, such as research grade trackers that allow access to the raw acceleration data. To circumvent these issues, we conducted a series of sensitivity analyses to assess the pattern of missingness in the data, as well as the possible influence of missingness on the model results. Results (not reported herein) indicated no significant bias, suggesting a missing-at-random (MAR) pattern or change in model point estimates. Finally, most of the participants had endometriosis as their primary CPPD, therefore we are not able to delineate differences in mental health trajectories among different disorders within CPPD. Similarly, our dataset included those with a diagnosis of CPPD, and as such we did not have data from those who are undiagnosed yet symptomatic. This might have limited the extent of the outcomes observed in the findings. Future studies can benefit from investigating this question to compare the associations and to obtain a broader picture of experiences from individuals with chronic pelvic pain. To this end, inclusion of those who are symptomatic but have not received a formal diagnosis can enhance the understanding of the association between PA behavior and mental health outcomes.
Conclusions
mHealth-enabled direct patient input and passive tracking via wearables enables the capturing of real-world data to improve our understanding of inter-individual and temporal variability in mental health symptoms and factors that may improve mental health. By leveraging patient-tracked mental health and pain outcomes combined with passively obtained activity data from CPPD patients, we demonstrate a positive, non-linear relationship between PA and mental health in female CPPDs. From a methodological perspective, our work demonstrates an approach for handling complex longitudinal mHealth and activity data and may be used as a framework for future mHealth interventions for chronic pain centered on patient self-management. Additionally, from a clinical lens, the positive relationship between PA and mental health suggests that MVPA may be suggested as a feasible and accessible way to positively modulate mental health in individuals with CPPDs.
Data Sharing Statement
The data collection for the parent grant is currently ongoing. After completion of the active grant period, the data produced in the present study will be made available upon reasonable request to the corresponding author.
Ethics Approval and Informed Consent
The study was approved by the Institutional Review Board (IRB) of the Icahn School of Medicine at Mount Sinai (IRB# STUDY-22-01002) and all participants provided informed consent. This study complies with the Declaration of Helsinki.
Acknowledgments
This paper has been uploaded to [medRxiv] as a preprint: [https://www.medrxiv.org/content/10.1101/2024.09.25.24314368v2].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD108263. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additionally, this research was supported by the T32 grant 5T32GM146636 (ELL).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Latthe P, Latthe M, Say L, Gülmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6(1):177. doi:10.1186/1471-2458-6-177
2. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014;17(2;3):E141–E147. doi:10.36076/ppj.2014/17/E141
3. Hutton D, Mustafa A, Patil S, et al. The burden of chronic pelvic pain (CPP): costs and quality of life of women and men with CPP treated in outpatient referral centers. PLoS One. 2023;18(2):e0269828. doi:10.1371/journal.pone.0269828
4. Till SR, As-Sanie S, Schrepf A. Psychology of chronic pelvic pain: prevalence, neurobiological vulnerabilities, and treatment. Clin Obstet Gynecol. 2019;62(1):22–36. doi:10.1097/GRF.0000000000000412
5. Ayorinde AA, Macfarlane GJ, Saraswat L, Bhattacharya S. Chronic pelvic pain in women: an epidemiological perspective. Womens Health. 2015;11(6):851–864. doi:10.2217/whe.15.30
6. Facchin F, Buggio L, Ottolini F, Barbara G, Saita E, Vercellini P. Preliminary insights on the relation between endometriosis, pelvic pain, and employment. Gynecol Obstet Invest. 2019;84(2):190–195. doi:10.1159/000494254
7. Ferrero S, Esposito F, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Quality of sex life in women with endometriosis and deep dyspareunia. Fertil Steril. 2005;83(3):573–579. doi:10.1016/j.fertnstert.2004.07.973
8. Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynecol. 2004;25(2):123–133. doi:10.1080/01674820400002279
9. Meltzer-Brody S, Leserman J. Psychiatric comorbidity in women with chronic pelvic pain. CNS Spectr. 2011;16(2):29–35. doi:10.1017/S1092852912000156
10. Till SR, Nakamura R, Schrepf A, As-Sanie S. Approach to diagnosis and management of chronic pelvic pain in women. Obstet Gynecol Clin North Am. 2022;49(2):219–239. doi:10.1016/j.ogc.2022.02.006
11. Lorençatto C, Petta CA, José Navarro M, Bahamondes L, Matos A. Depression in women with endometriosis with and without chronic pelvic pain. Acta Obstet Gynecol Scand. 2006;85(1):88–92. doi:10.1080/00016340500456118
12. Meira E, Siqueira-Campos V, Da Luz RA, De Deus JM, Zangiacomi Martinez E, Conde DM. Anxiety and depression in women with and without chronic pelvic pain: prevalence and associated factors. J Pain Res. 2019;Volume 12:1223–1233. doi:10.2147/JPR.S195317
13. La Rosa JS D, Brady BR, Ibrahim MM, et al. Co-occurrence of chronic pain and anxiety/depression symptoms in U.S. adults: prevalence, functional impacts, and opportunities. Pain. 2024;165(3):666–673. doi:10.1097/j.pain.0000000000003056
14. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The Link between Depression and Chronic Pain: neural Mechanisms in the Brain. Neural Plast. 2017;2017:1–10. doi:10.1155/2017/9724371
15. Patwardhan V, Gil GF, Arrieta A, et al. Differences across the lifespan between females and males in the top 20 causes of disease burden globally: a systematic analysis of the global burden of disease study 2021. Lancet Public Health. 2024;9(5):e282–e294. doi:10.1016/S2468-2667(24)00053-7
16. Green-top Guideline. The Initial Management of Chronic Pelvic Pain. 2012.
17. Kerns RD, Burgess DJ, Coleman BC, et al. Self-management of chronic pain: psychologically guided core competencies for providers. Pain Med. 2022;23(11):1815–1819. doi:10.1093/pm/pnac083
18. Leonardi M, Horne AW, Vincent K, et al. Self-management strategies to consider to combat endometriosis symptoms during the COVID-19 pandemic. Hum Reprod Open. 2020;2020(2):hoaa028. doi:10.1093/hropen/hoaa028
19. Mann EG, LeFort S, VanDenKerkhof EG. Self-management interventions for chronic pain. Pain Manag. 2013;3(3):211–222. doi:10.2217/pmt.13.9
20. Díaz-Mohedo E, Carrillo-León AL, Calvache-Mateo A, Ptak M, Romero-Franco N, Carlos-Fernández J. App-Mohedo®: a mobile app for the management of chronic pelvic pain. A design and development study. Int J Med Inf. 2024;186:105410. doi:10.1016/j.ijmedinf.2024.105410
21. Zeidan F, Vago DR. Mindfulness meditation–based pain relief: a mechanistic account. Ann N Y Acad Sci. 2016;1373(1):114–127. doi:10.1111/nyas.13153
22. Whale K, Gooberman‐Hill R. the importance of sleep for people with chronic pain: current insights and evidence. JBMR Plus. 2022;6(7):e10658. doi:10.1002/jbm4.10658
23. Nonopioid Therapies for Pain Management. Overdose Prevention. CDC.
24. CDC. Clinical practice guideline for prescribing opioids for pain. United States; MMWR:2022.
25. Ariza-Mateos MJ, Cabrera-Martos I, Ortiz-Rubio A, Torres-Sánchez I, Rodríguez-Torres J, Valenza MC. Effects of a patient-centered graded exposure intervention added to manual therapy for women with chronic pelvic pain: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100(1):9–16. doi:10.1016/j.apmr.2018.08.188
26. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;2020(2). doi:10.1002/14651858.CD011279.pub3
27. Gilanyi YL, Wewege MA, Shah B, et al. Exercise increases pain self-efficacy in adults with nonspecific chronic low back pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2023;53(6):335–342. doi:10.2519/jospt.2023.11622
28. Kalapurakkel S, A. Carpino E, Lebel A, E. Simons L. “pain can’t stop me”: examining pain self-efficacy and acceptance as resilience processes among youth with chronic headache. J Pediatr Psychol. 2015;40(9):926–933. doi:10.1093/jpepsy/jsu091
29. Chen J, Fang X, Zhang F. The associations of chronic pain and 24-h movement behaviors with incident mental disorders: evidence from a large-scale cohort study. BMC Medicine. 2024;22(1). doi:10.1186/s12916-024-03534-5
30. Herring MP. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101. doi:10.1001/archinternmed.2011.696
31. Ensari I, Lipsky-Gorman S, Horan EN, Bakken S, Elhadad N. Associations between physical exercise patterns and pain symptoms in individuals with endometriosis: a cross-sectional mHealth-based investigation. BMJ Open. 2022;12(7):e059280. doi:10.1136/bmjopen-2021-059280
32. Diez‐Buil H, Hernandez‐Lucas P, Leirós‐Rodríguez R, Echeverría‐García O. Effects of the combination of exercise and education in the treatment of low back and/or pelvic pain in pregnant women: systematic review and meta‐analysis. Int J Gynecol Obstet. 2024;164(3):811–822. doi:10.1002/ijgo.15000
33. Gonçalves AV, Barros NF, Bahamondes L. The practice of hatha yoga for the treatment of pain associated with endometriosis. J Altern Complement Med. 2017;23(1):45–52. doi:10.1089/acm.2015.0343
34. Saxena R, Gupta M, Shankar N, Jain S, Saxena A. Effects of yogic intervention on pain scores and quality of life in females with chronic pelvic pain. Int J Yoga. 2017;10(1):9. doi:10.4103/0973-6131.186155
35. Ensari I, Pichon A, Lipsky-Gorman S, Bakken S, Elhadad N. Augmenting the clinical data sources for enigmatic diseases: a cross-sectional study of self-tracking data and clinical documentation in endometriosis. Appl Clin Inform. 2020;11(05):769–784. doi:10.1055/s-0040-1718755
36. Hirten RP, Danieletto M, Landell K, et al. Development of the ehive digital health app: protocol for a centralized research platform. JMIR Res Protoc. 2023;12:e49204. doi:10.2196/49204
37. Hays RD, Schalet BD, Spritzer KL, Cella D. Two-item PROMIS® global physical and mental health scales. J Patient-Rep Outcomes. 2017;1(1):2. doi:10.1186/s41687-017-0003-8
38. Fenton BW, Palmieri P, Diantonio G, Vongruenigen V. Application of patient-reported outcomes measurement information system to chronic pelvic pain. J Minim Invasive Gynecol. 2011;18(2):189–193. doi:10.1016/j.jmig.2010.12.001
39. Global Health Scoring Manual. 2021.
40. Elsman EBM, Roorda LD, Crins MHP, Boers M, Terwee CB. Dutch reference values for the patient-reported outcomes measurement information system scale v1.2 - global health (PROMIS-GH). J Patient-Rep Outcomes. 2021;5(1):38. doi:10.1186/s41687-021-00314-0
41. Grunberg VA, Greenberg J, Mace RA, Bakhshaie J, Choi KW, Vranceanu AM. Fitbit activity, quota-based pacing, and physical and emotional functioning among adults with chronic pain. J Pain. 2022;23(11):1933–1944. doi:10.1016/j.jpain.2022.07.003
42. Master H, Annis J, Huang S, et al. Association of step counts over time with the risk of chronic disease in the all of us research program. Nat Med. 2022;28(11):2301–2308. doi:10.1038/s41591-022-02012-w
43. Perry AS, Annis JS, Master H, et al. Association of longitudinal activity measures and diabetes risk: an analysis from the national institutes of health all of us research program. J Clin Endocrinol Metab. 2023;108(5):1101–1109. doi:10.1210/clinem/dgac695
44. Beagle AJ, Tison GH, Aschbacher K, Olgin JE, Marcus GM, Pletcher MJ. Comparison of the physical activity measured by a consumer wearable activity tracker and that measured by self-report: cross-sectional analysis of the health eheart study. JMIR MHealth UHealth. 2020;8(12):e22090. doi:10.2196/22090
45. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi:10.1249/mss.0b013e31815a51b3
46. Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE. The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67(5):516–526. doi:10.1016/j.jclinepi.2013.10.024
47. Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi:10.1016/0304-3959(87)91074-8
48. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;
49. Lukacz ES, Lawrence JM, Burchette RJ, Luber KM, Nager CW, Galen Buckwalter J. The use of visual analog scale in urogynecologic research: a psychometric evaluation. Am J Obstet Gynecol. 2004;191(1):165–170. doi:10.1016/j.ajog.2004.04.047
50. Creinin MD. Pain associated with cervical priming for first-trimester surgical abortion: a randomized controlled trial. Obstet Gynecol. 2021;138(4):680. doi:10.1097/AOG.0000000000004552
51. Bakdash JZ, Marusich LR. Repeated Measures Correlation. Front Psychol. 2017;8:456. doi:10.3389/fpsyg.2017.00456
52. RStudio Team. RStudio: integrated development for R. 2020. Available from: http://www.rstudio.com/.
53. Muller HG, Wu Y, Yao F. Continuously additive models for nonlinear functional regression. Biometrika. 2013;100(3):607–622. doi:10.1093/biomet/ast004
54. Goldsmith J, Bobb J, Crainiceanu CM, Caffo B, Reich D. Penalized Functional Regression. J Comput Graph Stat. 2011;20(4):830–851. doi:10.1198/jcgs.2010.10007
55. Manning JR, Notaro GM, Chen E, Fitzpatrick PC. Fitness tracking reveals task-specific associations between memory, mental health, and physical activity. Sci Rep. 2022;12(1):13822. doi:10.1038/s41598-022-17781-0
56. Mixed GAM computation vehicle with automatic smoothness estimation, 2000:1.9–1. doi:10.32614/CRAN.package.mgcv.
57. Leavitt MO, Downing GJ. Physical activity guidelines for Americans. Cancer. 2008;113(7 Suppl):1724–1727. doi:10.1002/cncr.23641
58. Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab. 2013;38(2):100–114. doi:10.1139/apnm-2012-0235
59. Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting “how many steps are enough? Med Sci Sports Exerc. 2008;40(7):S537–S543. doi:10.1249/MSS.0b013e31817c7133
60. Olson RD, Vaux-Bjerke A, Quam JB, et al. Physical Activity Guidelines for Americans.
61. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011;12(7):996–1004. doi:10.1111/j.1526-4637.2011.01187.x
62. Sachs MK, Dedes I, El-Hadad S, et al. Physical activity in women with endometriosis: less or more compared with a healthy control? Int J Environ Res Public Health. 2023;20(17):6659. doi:10.3390/ijerph20176659
63. McBeth J, Prescott G, Scotland G, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med. 2012;172(1):48. doi:10.1001/archinternmed.2011.555
64. Zhang W, Singh SP, Clement A, Calfee RP, Bijsterbosch JD, Cheng AL. Improvements in physical function and pain interference and changes in mental health among patients seeking musculoskeletal care. JAMA Network Open. 2023;6(6):e2320520. doi:10.1001/jamanetworkopen.2023.20520
65. Dowding C, Mikocka‐Walus A, Skvarc D, et al. The temporal effect of emotional distress on psychological and physical functioning in endometriosis: a 12‐month prospective study. Appl Psychol Health Well-Being. 2023;15(3):901–918. doi:10.1111/aphw.12415
66. Imboden MT, Nelson MB, Kaminsky LA, Montoye AH. Comparison of four fitbit and jawbone activity monitors with a research-grade ActiGraph accelerometer for estimating physical activity and energy expenditure. Br J Sports Med. 2018;52(13):844–850. doi:10.1136/bjsports-2016-096990
67. Polhemus A, Sieber C, Haag C, et al. Non-equivalent, but still valid: establishing the construct validity of a consumer fitness tracker in persons with multiple sclerosis. PLOS Digit Health. 2023;2(1):e0000171. doi:10.1371/journal.pdig.0000171
68. Silva GS, Yang H, Collins JE, Losina E. Validating fitbit for evaluation of physical activity in patients with knee osteoarthritis: do thresholds matter? ACR Open Rheumatol. 2019;1(9):585–592. doi:10.1002/acr2.11080
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.